Climate Change Promotes the Large-Scale Population Growth of Grapholita molesta (Busck) (Lepidoptera: Tortricidae) within Peach Orchards in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of G. molesta
2.2. Climate Data
2.3. Data Analysis
3. Results
3.1. The Population Dynamics of G. molesta in China
3.2. Temperature and Precipitation Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bale, J.; Masters, G.; Hodkinson, I.; Awmack, C.; Bezemer, T.M.; Brown, V.; Butterfield, J.; Buse, A.; Coulson, J.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Musolin, D.L. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Glob. Chang. Biol. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Zhang, H.; Liu, J.; Jiang, Y.; Wyckhuys, K.A.G.; Wu, K. Global warming modifies long-distance migration of an agricultural insect pest. J. Pest. Sci. 2020, 93, 569–581. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022; p. 3056. [Google Scholar] [CrossRef]
- Juroszek, P.; von Tiedemann, A. Plant pathogens, insect pests and weeds in a changing global climate: A review of approaches, challenges, research gaps, key studies and concepts. J. Agric. Sci. 2013, 151, 163–188. [Google Scholar] [CrossRef]
- Chaudhry, G.U. The development and fecundity of the oriental fruit moth, Grapholita (Cydia) molesta (Busck) under controlled temperatures and humidities. Bull. Entomol. Res. 1956, 46, 869–898. [Google Scholar] [CrossRef]
- Harrington, R.; Fleming, R.; Woiwood, I.P. Climate change impacts on insect management and conservation in temperate regions: Can they be predicted? Agric. Entomol. 2001, 3, 233–240. [Google Scholar] [CrossRef]
- Ladányi, M.; Horváth, L. A review of the potential climate change impact on insect populations—General and agricultural aspects. Appl. Ecol. Environ. Res. 2010, 8, 143–152. [Google Scholar] [CrossRef]
- Estay, S.A.; Lima, M.; Labra, F.A. Predicting insect pest status under climate change scenarios: Combining experimental data and population dynamics modelling. J. Appl. Entomol. 2009, 133, 491–499. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Zimmermann, N.E.; Dullinger, S. Elevational rear edges shifted at least as much as leading edges over the last century. Glob. Ecol. Biogeogr. 2019, 28, 533–543. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef] [PubMed]
- Nufio, C.R.; Buckley, L.B. Grasshopper phenological responses to climate gradients, variability, and change. Ecosphere 2019, 10, e02866. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Boggs, C.L. The fingerprints of global climate change on insect populations. Curr. Opin. Insect Sci. 2016, 17, 69–73. [Google Scholar] [CrossRef]
- Ouyang, F.; Hui, C.; Ge, S.; Men, X.Y.; Zhao, Z.H.; Shi, P.J.; Zhang, Y.S.; Li, B.L. Weakening density dependence from climate change and agricultural intensification triggers pest outbreaks: A 37-year observation of cotton bollworms. Ecol. Evol. 2014, 4, 3362–3374. [Google Scholar] [CrossRef] [PubMed]
- Kirk, H.; Dorn, S.; Mazzi, D. Worldwide population genetic structure of the oriental fruit moth (Grapholita molesta), a globally invasive pest. BMC Ecol. 2013, 13, 12. [Google Scholar] [CrossRef]
- Myers, C.T.; Hull, L.A.; Krawczyk, G. Effects of orchard host plants (apple and peach) on development of oriental fruit moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2007, 100, 421–430. [Google Scholar] [CrossRef]
- Monteiro, L.B.; Souza, A.; Argenton, J. Mating disruption with low density diffusers for the management of oriental fruit moths (lepidoptera: Tortricidae) in apple orchards under subtropical climate in southern Brazil. Rev. Bras. De Frutic. 2013, 35, 1007–1016. [Google Scholar] [CrossRef][Green Version]
- Neven, L.G.; Kumar, S.; Yee, W.L.; Wakie, T. Current and Future Potential Risk of Establishment of Grapholita molesta (Lepidoptera: Tortricidae) in Washington State. Environ. Entomol. 2018, 47, 448–456. [Google Scholar] [CrossRef]
- Cao, L.J.; Li, B.Y.; Chen, J.C.; Zhu, J.Y.; Hoffmann, A.A.; Wei, S.J. Local climate adaptation and gene flow in the native range of two co-occurring fruit moths with contrasting invasiveness. Mol. Ecol. 2021, 30, 4204–4219. [Google Scholar] [CrossRef]
- Torriani, M.V.G.; Mazzi, D.; Hein, S.; Dorn, S. Structured populations of the oriental fruit moth in an agricultural ecosystem. Mol. Ecol. 2010, 19, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.D.B.; Kuhn, T.M.A.; Monteiro, L.B. Oviposition behavior of Grapholita molesta Busck (Lepidoptera: Tortricidae) at different temperatures. Neotrop. Entomol. 2011, 40, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Fang, A.; Wu, J.; Xu, X. Effects of short-term heat treatment on adult’s reproduction and longevity of oriental fruit moth, Grapholita molesta Busck. J. Fruit Sci. 2019, 36, 486–492. (In Chinese) [Google Scholar] [CrossRef]
- Jin, L.; Lin, H.; Wang, X.; Yue, L.; Li, J. Effects of climatic condition of spring on the population dynamics of Grapholita molesta (Busck). Plant Prot. 2014, 40, 169–173. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, X.; Hoffmann, A.A.; Zhang, B.; Ma, C. Are adult life history traits in oriental fruit moth affected by a mild pupal heat stress? J. Insect Physiol. 2017, 102, 36–41. [Google Scholar] [CrossRef]
- Scrantona, K.; Amarasekarea, P. Predicting phenological shifts in a changing climate. Proc. Natl. Acad. Sci. USA 2017, 114, 13212–13217. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 9783319242750. [Google Scholar]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; ISBN 9780387983554. [Google Scholar]
- Kiritani, K. Different effects of climate change on the population dynamics of insects. Appl. Entomol. Zool. 2013, 48, 97–104. [Google Scholar] [CrossRef]
- Ferrer, A.; Mazzi, D.; Dorn, S. Stay cool, travel far: Cold-acclimated oriental fruit moth females have enhanced flight performance but lay fewer eggs. Entomol. Exp. Appl. 2014, 151, 11–18. [Google Scholar] [CrossRef]
- Ferrer, A.; Dorn, S.; Mazzi, D. Cross-generational effects of temperature on flight performance, and associated life-history traits in an insect. J. Evol. Biol. 2013, 26, 2321–2330. [Google Scholar] [CrossRef]
- Fitzgerald, J.L.; Stuble, K.L.; Nichols, L.M.; Diamond, S.E.; Wentworth, T.R.; Pelini, S.L.; Gotelli, N.J.; Sanders, N.J.; Dunn, R.R.; Penick, C.A. Abundance of spring- and winter-active arthropods declines with warming. Ecosphere 2021, 12, e03473. [Google Scholar] [CrossRef]
- Notter-Hausmann, C.; Dorn, S. Relationship between behavior and physiology in an invasive pest species: Oviposition site selection and temperature-dependent development of the oriental fruit moth (Lepidoptera: Tortricidae). Environ. Entomol. 2010, 39, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kaspari, M.; Clay, N.A.; Lucas, J.; Yanoviak, S.P.; Kay, A. Thermal adaptation generates a diversity of thermal limits in a rainforest ant community. Glob. Chang. Biol. 2015, 21, 1092–1102. [Google Scholar] [CrossRef]
- Pincebourde, S.; Casas, J. Narrow safety margin in the phyllosphere during thermal extremes. Proc. Natl. Acad. Sci. USA 2019, 116, 5588–5596. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.L.; Li, Y.P.; Wu, J.X. Characterization of heat shock protein 90, 70 and their transcriptional expression patterns on high temperature in adult of Grapholita molesta (Busck). Insect Sci. 2014, 21, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, X.; Zhang, W.; Zhang, B.; Ma, C.S. Dynamics of heat shock protein responses to thermal stress changes after metamorphosis in a lepidopteran insect. Arch. Insect Biochem. Physiol. 2021, 107, 1–12. [Google Scholar] [CrossRef]
- Damos, P.; Bonsignore, C.P.; Gardi, F.; Avtzis, D.N. Phenological responses and a comparative phylogenetic insight of Anarsia lineatella and Grapholita molesta between distinct geographical regions within the Mediterranean basin. J. Appl. Entomol. 2014, 138, 528–538. [Google Scholar] [CrossRef]
- Amarasekare, P.; Coutinho, R.M. Effects of temperature on intraspecific competition in ectotherms. Am. Nat. 2014, 184, E50–E65. [Google Scholar] [CrossRef]
- Ntiri, E.S.; Calatayud, P.A.; van Den Berg, J.; Schulthess, F.; Ru, B.P.L. Influence of temperature on intra- and interspecific resource utilization within a community of Lepidopteran Maize Stemborers. PLoS ONE 2016, 11, e0148735. [Google Scholar] [CrossRef]
- Estay, S.A.; Mauricio, L.; Francisco, B. The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 2014, 123, 131–140. [Google Scholar] [CrossRef]
- Leckey, E.H.; Smith, D.M.; Nufio, C.R.; Fornash, K.F. Oak-insect herbivore interactions along a temperature and precipitation gradient. Acta Oecol. 2014, 61, 1–8. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Csóka, G.; Hirka, A.; Björkman, C. Forest insects and climate change: Long-term trends in herbivore damage. Ecol. Evol. 2013, 3, 4183–4196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Grayson, K.L.; Johnson, D.K. Novel insights on population and range edge dynamics using an unparalleled spatiotemporal record of species invasion. J. Anim. Ecol. 2018, 87, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Riedl, H.; Croft, B.A.; Howitt, A.J. Forecasting codling moth phenology based on pheromone trap catches and physiological-time models. Can. Entomol. 1976, 108, 449–460. [Google Scholar] [CrossRef]
- Bernardi, D.; Lazzari, J.C.; Andreazza, F.; Mayer, N.A.; Botton, M.; Nava, D.E. Susceptibility, Oviposition Preference, and Biology of Grapholita molesta (Lepidoptera: Tortricidae) in Prunus Spp. Rootstock Genotypes. Environ. Entomol. 2017, 46, 871–877. [Google Scholar] [CrossRef][Green Version]
- Najar-Rodriguez, A.J.; Galizia, C.G.; Stierle, J.; Dorn, S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exp. Biol. 2010, 213, 3388–3397. [Google Scholar] [CrossRef]
- Kong, W.; Wang, Y.; Guo, Y.; Chai, X.; Li, J.; Ma, R. Importance of Preovipositional Period of an Oligophagous Moth in Predicting Host Suitability. J. Econ. Entomol. 2020, 113, 222–229. [Google Scholar] [CrossRef]
- Cammell, M.E.; Knight, J.D. Effects of climatic change on the population dynamics of crop pests. Adv. Ecol. Res. 1992, 22, 117–162. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
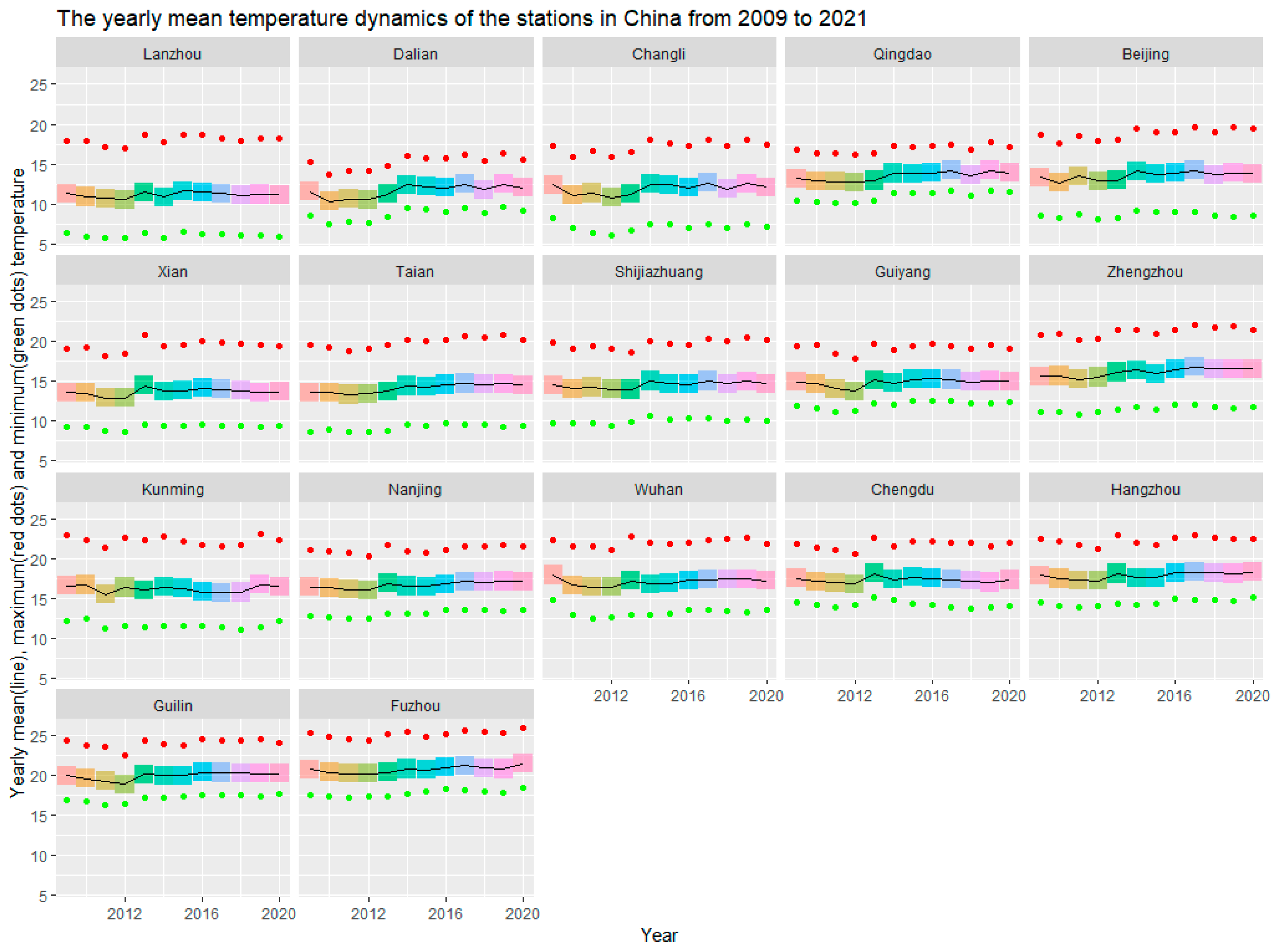


| Region and Station | Generations | Time | Time of Peak Abundance |
|---|---|---|---|
| Northeast China (Dalian) | 2~4 | Early Apr to late Sep | Early to mid-May, late June, mid- to late July, late August |
| North China (Beijing, Changli, Qingdao, Shijiazhuang and Taian) | 3~5 | Early Apr to early Oct | Early to mid-May, late June, mid-July, mid-August, mid- to late September |
| Northwest China (Lanzhou and Xian) | 4~5 | Early Apr to early Oct | Early to mid-May, mid-June, early July, mid-August, mid-September |
| Central China (Zhengzhou and Wuhan) | 4~5 | Mid Mar to late Oct | Late March, late May, mid- to late June, late July, early September |
| Southwest China (Chengdu, Kunming and Guiyang) | 4~5 | Early Mar to mid Oct | Mid- to late May, mid- to late June, late July, early August, late August, early September, mid-September |
| East China (Hangzhou and Nanjing) | 5~6 | Late Mar to early Oct | Mid to late April, late May, late June, late July, late August, mid- to late September |
| South China (Fuzhou and Guilin) | 6~7 | Early Mar to late Oct | Early March, late April, mid-May, mid-June, mid-July, mid-August, mid- to late September |
| Predictors | npar | AIC | BIC | logLik | Deviance | χ2 | Df | Pr (>χ2) |
|---|---|---|---|---|---|---|---|---|
| Null | 6 | 4434.4 | 4465.0 | −2211.2 | 4422.4 | |||
| Mean temperature | 7 | 4335.9 | 4371.7 | −2161.0 | 4321.9 | 100.4 | 1.0 | <0.001 |
| Minimum temperature | 7 | 4335.3 | 4371.0 | −2160.6 | 4321.3 | 0.7 | 0.0 | |
| Maximum temperature | 7 | 4349.9 | 4385.6 | −2167.9 | 4335.9 | 0.0 | 0.0 | |
| Mean precipitation | 7 | 4431.2 | 4467.0 | −2208.6 | 4417.2 | 0.0 | 0.0 | |
| Mean night precipitation | 7 | 4429.5 | 4465.3 | −2207.8 | 4415.5 | 1.7 | 0.0 | |
| Mean day precipitation | 7 | 4434.6 | 4470.3 | −2210.3 | 4420.6 | 0.0 | 0.0 | |
| Monthly precipitation | 7 | 4430.9 | 4466.7 | −2208.5 | 4416.9 | 3.6 | 0.0 | |
| Monthly day precipitation | 7 | 4434.7 | 4470.5 | −2210.4 | 4420.7 | 0.0 | 0.0 | |
| Monthly night precipitation | 7 | 4430.6 | 4466.3 | −2208.3 | 4416.6 | 4.1 | 0.0 |
| Predictors | npar | AIC | BIC | logLik | Deviance | χ2 | Df | Pr (>χ2) |
|---|---|---|---|---|---|---|---|---|
| Mean temperature + mean night precipitation | 8 | 4331.2 | 4372.0 | −2157.6 | 4315.2 | |||
| Maximum temperature + mean night precipitation | 8 | 4339.9 | 4380.8 | −2162.0 | 4323.9 | 0.0 | 0.0 | |
| Minimum temperature + mean night precipitation | 8 | 4334.2 | 4375.0 | −2159.1 | 4318.2 | 5.7 | 0.0 | |
| Mean temperature × mean night precipitation | 9 | 4326.8 | 4372.7 | −2154.4 | 4308.8 | 9.4 | 1.0 | p < 0.01 |
| Maximum temperature × mean night precipitation | 9 | 4333.7 | 4379.7 | −2157.9 | 4315.7 | 0.0 | 0.0 | |
| Minimum temperature × mean night precipitation | 9 | 4333.0 | 4378.9 | −2157.5 | 4315.0 | 0.7 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Peng, Q.; Wang, S.; Zhang, F.; Guo, X.; Jiang, Q.; Huang, N.; Li, H. Climate Change Promotes the Large-Scale Population Growth of Grapholita molesta (Busck) (Lepidoptera: Tortricidae) within Peach Orchards in China. Agronomy 2022, 12, 2954. https://doi.org/10.3390/agronomy12122954
Li H, Peng Q, Wang S, Zhang F, Guo X, Jiang Q, Huang N, Li H. Climate Change Promotes the Large-Scale Population Growth of Grapholita molesta (Busck) (Lepidoptera: Tortricidae) within Peach Orchards in China. Agronomy. 2022; 12(12):2954. https://doi.org/10.3390/agronomy12122954
Chicago/Turabian StyleLi, Hongchen, Qiulian Peng, Su Wang, Fan Zhang, Xiaojun Guo, Quan Jiang, Ningxing Huang, and Hu Li. 2022. "Climate Change Promotes the Large-Scale Population Growth of Grapholita molesta (Busck) (Lepidoptera: Tortricidae) within Peach Orchards in China" Agronomy 12, no. 12: 2954. https://doi.org/10.3390/agronomy12122954
APA StyleLi, H., Peng, Q., Wang, S., Zhang, F., Guo, X., Jiang, Q., Huang, N., & Li, H. (2022). Climate Change Promotes the Large-Scale Population Growth of Grapholita molesta (Busck) (Lepidoptera: Tortricidae) within Peach Orchards in China. Agronomy, 12(12), 2954. https://doi.org/10.3390/agronomy12122954







