Abstract
Little is known about how Indian farming practices affect German chamomile (Matricaria chamomilla L.). This study examines the effects of the sowing date and spacing of plants on flower productivity, essential oil concentration, and the composition of German chamomile grown in the arid zone of Rajasthan, India. In a factorial randomized block design (FRBD), the treatments consisted of four sowing dates (15 and 25 October, 5 and 15 November) and three spacings of plants (20 × 10 cm, 30 × 10 cm, and 40 × 10 cm). The dried flower yield (Kg ha−1), fresh flower yield (Kg ha−1), and number of flowers per plant of chamomile crop were significantly affected by the varying spacing of plants and the date of sowing. The highest values for dry weight, fresh weight, and number of flowers were obtained from the second date of sowing (25 October) with 40 × 10 cm geometry. Likewise, the highest values for total oil (12.44%) and essential oil (0.94%) contents were also obtained from the D2P3 combination (D2—sowing date 25 October, P3—40 × 10 cm spacing of plants). GC/MS analyses of the samples showed that p-menth-1-en-4-ol, acetate, cis-alpha-farnesene, anethole+estragol, 1,4-cyclohexadiene,1-methyl-4-(1-methylethyl)- and 3,6-dihydro-4-(4-methyl-3-pentenyl)-1,2-dithiin were the main identified compounds in the essential oil of chamomile fresh flowers. The treatments altered the quality profile of the essential oils in general. The principal components of chamomile essential oil were significantly affected by the D2P3 treatment. The findings of this study add to our understanding of how to grow high-quality chamomile flowers in arid regions.
1. Introduction
The discovery of formulated drugs and their greater availability to people are of benefit in the speedy control of illnesses, but medicinal plants are still widely used due to their economic viability and historical importance. According to the WHO [], 70% to 95% of developing-country populations use medicinal herbs for primary care. Many herbal medicines are perceived as being green and healthy, having few side effects, and being simple to obtain; additionally, they generate income. As a result, interest in using natural ingredients rather than synthetic chemicals has increased. The uncontrolled harvesting of natural populations and the growth of urban areas are both factors that pose a threat to the diversity of medicinal plants. The cultivation of these species can decrease the stress imposed on their wild populations and, as a result, save them from extinction []. Since the cultivation of medicinal plants allows for more precise control over the quality of the targeted bioactive components, this is recommended []. This strategy also enables the creation of consistent plant material at regular intervals in the requisite quantities, which can then be harvested as needed. Chamomile (Matricaria chamomilla L.) is a common medicinal plant that has a growing demand globally []. To meet the increased demand for chamomile, it is important to increase flower yield, expand cultivation zones, and improve quality.
Chamomile (Matricaria chamomilla L.) is an annual herb that grows to a height of about one meter and produces branched stems, double feathery leaves, and heads that are very delicate. The arsenal of the essential oils industry will soon be augmented with the inclusion of chamomile as a new crop. Matricaria chamomilla L., which belongs to the family Asteraceae, is more often known as German chamomile. Its natural habitat is in Europe and Asia. The countries of Argentina, Egypt, France, Germany, Hungary, and Yugoslavia are the primary producers of the chamomile essential oil []. Fresh or partially dried flowers and plant stalks are used in the steam distillation process that is used to produce chamomile essential oil []. The regions of Jammu and Kashmir, Uttar Pradesh, and Assam in India are currently the most important for the growth of this crop. Now, on an experimental basis, it is also being introduced into western Rajasthan and other parts of southern India for cultivation.
Both the pharmaceutical and cosmetic industries make use of chamomile oil. The essential oil of chamomile has been shown to alleviate inflammation, inhibit bacteria, accelerate healing, induce digestion, and calm spasms []. Additionally, it is utilized for the production of high-end liqueurs and perfumes as a flavoring component [,]. It is employed in pomades as well as pain balms. Dry flowers, when used to make herbal tea, can heal coughs and colds while also promoting bile and stomach flow. Soothing sedatives and digestive infusions are widely consumed across Europe [].
The use of essential oils in the food industry is also increasing. Natural antioxidants originating from plants and prepared as extracts should be used more. According to Jovanovic et al. [], this is because synthetic antioxidants have harmful and carcinogenic side effects. Plant extracts inhibit lipid and molecular oxidation. Due to their reducing potential, phenolic compounds have antioxidant action and neutralize free radicals, superoxide anion, nitric oxide, and peroxides []. Herbs are utilized as natural food preservatives. Many spices and herbs contain antibacterial activity, which is important for the food sector in reducing disease development [,,].
Sesquiterpene gives chamomile oil its blue color [,]. It contains matricine, beta-farnesene, alpha-bisabolol, bisabolol oxides A and B, chamazulene, and (Z)-en-yn-dicycloethers [,]. Essential oil content in dried flowers varies from 0.3% to 1.3%, depending on genotype/cultivar, edaphic, and climatic circumstances []. Sashidhara et al. [] reported that chamomile essential oil contains chamazulene (5–24%), α-(-)-bisabolol (24–41.5%), bisabolone oxide (2–7%), bisabolol oxide A (1–36.2%), and bisabolol oxide B (3.6–20.42%). Tirillini et al. [] discovered 77 chamomile components that make up 99% of the oil.
German chamomile grows in many soils and climates but favors cool weather. It is a 4–6-month crop grown by transplanting or direct sowing seeds. The main field gets one-month-old seedlings. Growers sometimes employ transplants to get more even and early production. However, transplanting is a costly and labor-intensive practice. Plant growth and productivity are also influenced by nursery management and seedling age at transplantation []. Chamomile flowers continuously, and multiple harvests give higher yields than a single harvest mid-flowering. To our knowledge, there is no information available on the impact of plant spacing and sowing date on flower production as well as the quantity and quality of chamomile essential oil. Genetic background and environment affect chamomile plants’ shape, morphology, physiology, essential oil content, and quality. Growing conditions also affect essential oil content and quality []. From this perspective, the management of environmental and growing conditions is crucial. A higher yield can be achieved by adjusting factors such as as planting date, plant density, etc. []. Chamomile should be sown as early as possible in the winter in order to enable sufficient growth prior to flowering because there is a possibility that differences in yield-contributing aspects could affect the production of chamomile flowers []. It is believed that late sowing reduces plant size and blooms []. In India’s hot arid zone, there has been no research conducted on the cultivation of chamomile or the impact of horticulture practices on the plant’s growth, yield, essential oil content, and composition. It is essential to find out the optimal growing conditions for this species in order to be able to suggest it to farmers in India’s hot arid zone. Therefore, the present study is carried out to test chamomile for commercial production under Indian hot arid conditions, including its effective date of sowing and the spacing of plants to optimize the yield of chamomile flowers and essential oil content and composition.
2. Materials and Methods
2.1. Field Experiment
This study was conducted in the Rabi season of 2020–2021 and 2021–2022 at the Agricultural Research Station at Mandor, Jodhpur. Its coordinates are 26°15′26.45″ N; 73°00′73.29″ E—231 m a.s.l. The soil is loamy-sandy in texture, slightly alkaline (pH 7.8), and contains 0.13% organic carbon, 184 kg ha−1 available nitrogen, 22 kg ha−1 available phosphorus, and 325 kg ha−1 available potassium. The field experiment was laid out in a factorial randomized block design (FRBD) and replicated thrice (Figure 1). Conventional soil preparation was carried out with plowing and harrowing, aiming to maintain an even soil surface. The experiment constituted twelve treatment combinations, viz., four dates of owing (15 and 25 October, 5 and 15 November) and three spacings of plants (20 × 10, 30 × 10, and 40 × 10 cm). The date of sowing and the spacing of plant layouts with the twelve treatment combinations are represented in Table 1.

Figure 1.
Chamomile (Matricaria chamomilla L.) field trial during experimental years 2020–2021 and 2021–2022.

Table 1.
Treatment details with sowing date and spacing of plants of chamomile (Matricaria chamomilla L.).
Seeds of a chamomile variety (M. chamomilla L.) (Mandor selection-1) were collected from the Agriculture University, Jodhpur, Rajasthan, India, and planted on field plots that had been prepared in accordance with the treatment. The plots were 2 × 2.4 m and planted as per treatment seed distance; 240, 160, and 120 plants were maintained in plots for 20 × 10, 30 × 10 and 40 × 10 cm, respectively. Emerging plants were removed to obtain proper spacing between plants. All plots were fertilized (60 N:40P2O5:20K2O) uniformly by using urea, SSP (single super phosphate), and MOP (muriate of potash) as N, P, and K sources, respectively. Nitrogen was applied in two equal split doses, i.e., as a basal dose and one month after sowing. Whole amounts of P and K sources were applied as a basal dose only. All plots were irrigated completely (flood irrigation method) and uniformly. About 8 irrigations were applied frequently. Weed management was done thrice during the vegetative and reproductive phases by hand. Chenopodium murale L., Chenopodium album L., Rumex dentatus L., Launea asplenifolia L., Cynodon dactylon L., Cyperus rotundus, and Asphodelus tenuifolius L. were the noticed weed flora in the chamomile experimental field. Flowers that had reached their maximum blooming potential were selected for harvesting from all plots. Flowers were harvested three times in 7 days intervals. The initial harvests occurred on 1 March, 11 March, 21 March, and 1 April for sowing on 15 October, 25 October, 5 November, and 15 November, respectively. All harvested flowers from each plot were separated carefully. After harvesting, the fresh weight of the flowers was measured. Thereafter, chamomile flowers were air-dried. Chamomile flowers were spread on a paper sheet in a single layer in a dark, dry, and well-ventilated room at 30 °C and left for 5–7 days. After drying, flowers were weighed and stored in airtight containers with treatment and replication labels, similar to field experiments. The weekly agro-meteorological parameters for the years 2020–2021 and 2021–2022 are presented on charts (Figure 2).
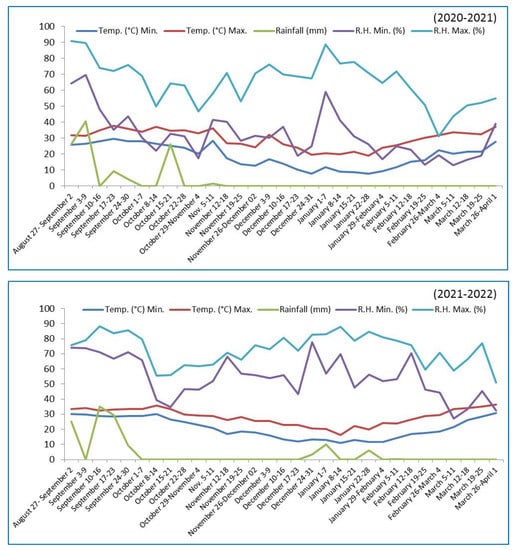
Figure 2.
Weekly meteorological parameters during the Rabi seasons of 2020–2021 and 2021–2022.
2.2. Total Oil Extraction Method
For oil extraction, 15 g of air-dried powdered flowers from three replications of each treatment were subjected to soxhlet extraction for 2 h using a soxhlet solvent extraction system (BUCHI India Pvt. Ltd., Mumbai, India). The extraction solvent (150 mL of n-hexane) was continuously cycled through the cellulose extraction thimbles for 2 h by boiling at 150 °C and condensation, with the oil being collected in the hot solvent. The solvent was removed and allowed to dry in a desiccator. The total oil content of all the treatments is given as a percentage (w/w).
2.3. Essential Oil Extraction Method
Hydro-distillation: Using a Clevenger-extraction apparatus (Borosil Glass Works Ltd., Mumbai, India) and a 30 g sample of dried flowers for each treatment in 500 mL of distilled water, the chamomile oil was extracted in triplicate and brought to a boil for 6 h []. The vapor mixture of water and essential oil was condensed, and oil and bioactive compounds were separated from water through a separating funnel. Before GC/MS analysis, extracts were dried over anhydrous MgSO4. After weighing the dried essential oil extract, the yield in percent of dried flowers was estimated. The samples were stored in amber-colored dark-sealed vials at 4 °C until they were analyzed.
2.4. Gas Chromatography/Mass Spectrometry Analysis (GC/MS)
GC/MS analysis was performed on a gas chromatograph system (Agilent 7693 GC, San Diego, CA, USA)with an HP5-MS capillary column (30 × 0.25 mm; with coating thickness 0.25 µm) connected to an Agilent- Mass Selective Detector (MSD). Helium gas was used as the carrier gas, with a flow rate of 1.0 mL/min. Samples were analyzed under the following GC/MS conditions: injector temperature was 250 °C, transfer line to MSD temperature was 250 °C, oven temperature started at 50 °C and held there for 3 min; programmed from 50 to 180 °C at 10 °C/min and from 180 to 280 °C at 45 °C/min, held for 2 min; the split was 10:1; ionization EI 70 eV. Standard references were run alone and together to detect the retention time and peak area of each constituent of the chamomile essential oil.
2.5. Identification of Compounds
Chemstation software (Agilent technologies, San Diego, CA, USA) was used to find out the retention indices and peak area of all the constituentsBased on a comparison of the volatile components in chamomile essential oil’s retention time (min), peak area, peak height, and mass spectral patterns with those found in the computer library (National Institute of Standards and Technology, Gaithersburg, MD, USA) connected to the GC/MS instrument, the volatile compounds were identified. The results obtained have been tabulated.
2.6. Statistical Analysis
Using Minitab v17.1.0 statistical software, all the collected data from the experiment were used to analyze the mean values of three replications of each treatment of the field experiment. To group information between the mean values of the data obtained from the field experiment Tukey’s test and 95% confidence (p ≤ 0.05) were used. Additionally, the data were analyzed using MSTATC v6.1 statistical software. To compare the means of the treatments, the least significant difference (LSD) at the 5% level was utilized.
3. Results
Air temperature, the extent of incident solar radiation, and rainfall represent the most significant limiting meteorological factors affecting the expression of the vegetative growth of crops. Here, chamomile flower production was studied by experimenting with four different sowing dates and three row spacings to expose the crop to different environmental conditions throughout its growth and developmental cycle. By combining these distinct treatments, it was possible to gain a better understanding of how chamomile responds to weather conditions both phonological and productively.
3.1. Effect of Different Sowing Dates and Spacings of Plants on Fresh and Dried Flower Yield and Number of Flowers of Chamomile
The dried flower yield (Kg ha−1), fresh flower yield (Kg ha−1), and number of flowers per plant of chamomile crop were significantly affected by the varying spacing of plants and the date of sowing (Figure 3 and Supplementary Table S1). The range of dried flower yield, fresh flower yield, and number of flowers per plant was from 277 to 463 Kg ha−1, 1610 to 2235 Kg ha−1, and 125–269, respectively, during the year 2020–21. The highest values for dried flower yield (463 Kg ha−1), fresh flower yield (2235 Kg ha−1), and number of flowers per plant (269) were obtained from the second date of sowing (25 October) during the year 2020–2021. Similar trends were also observed during 2021–2022. The spacing of plants of 40 × 10cm recorded significantly higher dry weight, fresh weight, and number of flowers compared to 20 × 10 and 30 × 10 cm spacings for both years.
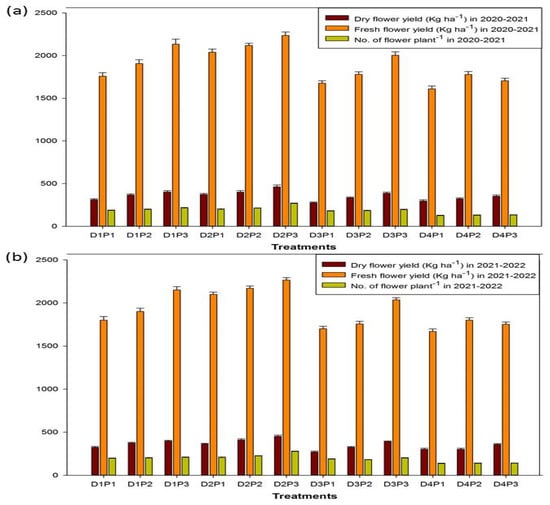
Figure 3.
Effect of spacing of plants and date of sowing on the yield of chamomile (Matricaria chamomilla L.) during 2020–2021 (a) and 2021–2022 (b).
3.2. Effect of Different Sowing Dates and Spacings of Plants on Essential Oil and Total Oil Contents
During the experimental year 2020–2021, the hydro-distillation of the air-dried chamomile flowers collected from varying spacing of plants gave dark-blue-colored essential oil in the range of 0.48% to 0.94% (w/w). However, the total oil percentage varied from 6.97% to 12.44%. The highest values for total and essential oil content were obtained from D2P3 (sowing date 25 October and spacing of plants 40 × 10 cm). The second best treatment in terms of total oil (11.04%) was D2P2, followed by D3P2, whereas after D2P3, the essential oil content was higher in D1P3. The last planting date, i.e., 15 November, showed decreasing trends (Table 2). Similar trends were also observed during 2021–2022 with respect to total oil content and essential oil content. These findings confirm that the planting date and plant spacing have an effect on the total and essential oil contents of chamomile flowers.

Table 2.
Effect of date of sowing and spacing of plants on essential oil and total oil contents of chamomile (Matricaria chamomilla L.) during the year 2020–2021 and 2021–2022.
3.3. Effect of Different Sowing Dates and Spacings of Plants on the Essential Oil Profile of Chamomile
The composition of the attained essential oil of chamomile is presented in Table 3. In total, twenty-four components were identified from the chamomile oil obtained from flowers harvested from plants sowed on the four dates and in three crop geometries. The samples’ GC/MS analyses reveal that the oxygen-containing monoterpene p-menth-1-en-4-ol, ace-tate (91.3%), sesquiterpene cis-alpha-farnesene (12.47%), 1,4-cyclohexadiene, 1-methyl-4-(1-methylethyl) (10.27%), and 3,6-dihydro-4-(4-methyl-3-pentenyl)-1,2- In contrast, the maximum amount of anethole+estragol (11.16%) was found with the D4P1 treatment. Minor oil components include monoterpene cymene (0.01 to 0.06%) and geranyl acetate (0.01 to 0.05%), an intermediate of the isoprenoid biosynthetic pathway. The date of sowing and plant spacing were found to affect the composition of essential oils. The findings show that for the major compounds of the essential oil, except for anethole + estragol, the D2P3 treatment had a significant effect, where the sowing date was 25 October and the spacing of plants was 40 × 10 cm. There was no discernible pattern in the impact of various plant spacings and sowing dates on the composition of essential oils. The treatments generally altered the essential oil composition of chamomile and its constituents.

Table 3.
Effect of date of sowing and spacing of plants on the essential chamomile oil profile (percentage of essential oil) in GC/MS in the experimental year 2020–2021.
4. Discussion
Chamomile is a medicinal plant possessing great economic and social importance. The agro-morphological monitoring and management of environmental factors are very crucial to its production and quality [,]. Present findings clearly demonstrate that the sowing date and spacing of plants have a significant effect on all of the agro-morphological characteristics of the yield and quality of chamomile flowers (Figure 3 and Table 2). The second sowing date, i.e., 25 October, yielded the highest values for the traits measured, while all other sowing dates showed declining trends.In terms of the spacing of plants, the 40 × 10 cm arrangement complemented the date of sowing and came up best among the treatments. According to Mohammad et al. [], the growth and yield of chamomile crops are significantly impacted by the sowing dates. It is due to the reason that the whole plant cycle adapts better to diurnal variations in the air currents and temperatures, resulting in better growth and higher crop yields. Our results are also corroborated by the study of da Silva et al. [], who reported that sowing dates affect the flower yield and essential oil content (%) of chamomile because of interference in the thermal time regarding the plastochron and flower buds. Ebadi et al. [] also found a significant effect of the date of sowing and seedling levels on flower yields and essential oil content and composition. By adjusting the basics of the growing system, such as planting time, density, and the use of specific elicitors, the crop yield can be increased []. In our study, we found a positive correlation between dried flower yield and essential oil content (r = 0.7). Our results are corroborated by Rathore and Kumar [], who also found a positive correlation (r = 0.74) between dried flower yield and essential oil content. In addition, Rathore and Kumar [] also discovered that agronomic interventions, such as planting time, have a massive impact on the agro-morphological and yield attributes of chamomile. Early sowing (20 November) of German chamomile in the western Himalayan region of India resulted in noticeably better agromorphological and yield characteristics.
Different row spacings, spatial layouts, and plant densities affect German chamomile’s flower yield and oil content. In varying environmental conditions, a 10 to 80 cm row spacing is optimal [,]. Row spacing affects flower head yields per hectare and per plant. Tadesse and Chala [] achieved the highest dried flower head yields of 517.2 and 586.7 Kg ha−1 in Ethiopia using a 40 × 40 cm spacing of plants. Kanjilal and Singh [] in Assam, India, found that German chamomile yielded more when planted with a 30 × 30 cm spacing in December. In April in Lublin, Poland, 35 and 45 cm row spacings gave the maximum crop yields []. In Iran, Pirzad et al. [] obtained the highest dried flower head (1241 Kg ha−1), seed (765 Kg ha−1), essential oil (8057 g/ha), and total biomass (2716 Kg ha−1) yields with a 10 × 30 cm spacing, whereas the harvest index of the essential oil was highest with a 5 × 30 cm spacing. Arslan et al. [] from Turkey observed that a 15 cm row spacing generated the best gross profit, while increasing row spacing decreases profitability.
Total oil and essential oil content percentages showed a significant increase under the D2P3 treatment, followed by D1P3. A similar trend was witnessed in the case of the spacing of plants. Such a result may be associated with the lower air temperature during the reproductive phase, causing an increase in the flower yield. According to Jamshidi [] and Singh et al. [], the time period of flower plucking affects the chamomile essential oil yield. Our two years of data revealed that the essential oil concentration ranged from 0.49% to 0.92%. Salamon et al. [] discovered essential oil yields ranging from 0.24% to 1.90%, which is consistent with our findings. Tsivelika et al. [] also found 0.4–0.7% essential oil in several chamomile cultivars. According to Formisano et al. [], various factors, including ploidy, genotype, geographic origin, environmental factors, agricultural practices, and capitulum development stage, influence the yield of essential oils.
Many reports have shown alterations in total and essential oil quantity, with minimal or no change in oil quality [,,]. Our results demonstrate an improvement in oil quality as well. Axtell and Fairman [] reported that a greater day length increases essential oil content and quality, which is in agreement with our results. p-menth-1-en-4-ol, acetate or terpinene 4-acetate is an isomer of the monoterpene alcohol. It possesses gastroprotective activity and has been found to be effective against gastric ulcers by the probable means of cytoprotective mechanisms [,]. In our study, p-menth-1-en-4-ol, acetate was found as a novel component of chamomile essential oil (Supplementary Figure S1). It was reported that cis-α-farnesene, a sesquiterpene found in the oil of chamomile, has potential antifungal activity []. Stanojevic et al. [] reported 9.3% of α-farnesene in the essential oil obtained from chamomile flowers, which is in the range of the current study. Alireza [] reported that an essential oil rich in monoterpenes and sesquiterpenes showed potential antifungal activity against Bacillus cereus, Staphylococcus aureus, and Proteus vulgaris. Overall, terpenes are the major constituent in the total essential oil of chamomile and are also responsible for its characteristic color [].
5. Conclusions
Based on these results, it is inferred that chamomile can be successfully cultivated in hot arid regions, which would be of great socioeconomic potential for producers in western India, if the sowing is in the last week of October (25 October), with a 40 × 10 cm spacing of plants. Forty centimeters of row spacing allow the optimum production of chamomile flowers, providing better crop canopy and space for crop management. Under such crop practice and geometry, chamomile presents a gross yield that allows good sustainability, with the additional benefit of good oil quantity and quality for rural producers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12122912/s1, Figure S1: Mass spectra of p-Menth-1-en-4-ol, acetate obtained from GC-MS; Table S1: Effect of spacing of plants and date of sowing on yield of chamomile (Matricaria chamomilla) during 2020–2021 and 2021–2022.
Author Contributions
Conceptualization, methodology, investigation, resources, data curation, project administration, M.L.M.; software, validation, writing—original draft preparation, D.S. and A.V.; formal analysis, S.N.S.; funding acquisition, writing—review and editing, A.A., A.A.A.-O., A.Z.D. and M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs and to the Research Chair of the Prince Sultan Bin Abdulaziz International Prize for Water.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was supported by the Deanship of Scientific Research, King Saud University, through the Vice Deanship of Scientific Research Chairs and the Research Chair of the Prince Sultan Bin Abdulaziz International Prize for Water. The authors are thankful to ARS Mandor, AU Jodhpur, ICAR-CAZRI, Jodhpur, and ICAR-NRCSS, Ajmer, for providing support during the research period.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. The world medicines situation 2011. In Traditional Medicines: Global Situation, Issues and Challenges; WHO: Geneva, Switzerland, 2011; 12p. [Google Scholar]
- Saglam, C.; Atakisi, I.; Turhan, H.; Kaba, S.; Arslanoglu, F.; Onemli, F. Effect of propagation method, plant density, and age on lemon balm (Melissa officinalis) herb and oil yield. N. Z. J. Crop. Hortic. 2004, 32, 419–423. [Google Scholar] [CrossRef]
- Franz, C.H. Why scientific research into the cultivation of medicinal and aromatic plants. Acta Hort. ISHS 1983, 132, 13–14. [Google Scholar]
- CorrêaJúnior, C.; Scheffer, M.C. As plantasmedicinais, aromáticas e condimentares e aagricultura familiar. HorticulturaBrasileira 2014, 32, 376. [Google Scholar]
- Jeshni, M.G.; Mousavinik, M.; Khammari, I.; Rahimi, M. The changes of yield and essential oil components of German Chamomile (Matricariarecutita L.) under application of phosphorus and zinc fertilizers and drought stress conditions. J. Saudi. Soc. Agric. Sci. 2017, 16, 60–65. [Google Scholar]
- Ristic, M.; Ðokic, D. Chemical Composition and Other Properties of Chamomile, Chamomile (Chamomilla erecutita L. Rauch), Monograph Study; Institute of Medicinal Plant Research “Dr Josif Panèic”: Belgrade, Serbia, 1996; Volume 197, p. 137. [Google Scholar]
- Srivastava, J.K.; Gupta, S. Chamomile: A herbal agent for treatment of diseases of the elderly. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Academic Press: Cambridge, MA, USA, 2015; pp. 171–183. [Google Scholar]
- Shams-ardakani, M.; Ghannadi, A.; Rahimzadeh, A. Volatile Constituents of Matricaria chamomilla L. from Isfahan, Iran. Analysis 2006, 2, 57–60. [Google Scholar]
- Heidari, M.; Sarani, S. Growth, biochemical components and ion content of Chamomile (Matricaria chamomilla L.) under salinity stress and iron deficiency. J. Saudi Soc. Agric. Sci. 2012, 11, 37–42. [Google Scholar] [CrossRef]
- Shrivastava, L.I.; Johri, A.K. Chamomile. In Advances in Horticulture: Volume 11, Medicinal and Aromatic Crops; Chadha, K.L., Gupta, R., Eds.; Malhotra Publishing House: New Delhi, India, 1995; pp. 790–804. [Google Scholar]
- Jovanovic, A.A.; Zundic, G.M.; Šavikin, K.P.; Cujic, N.; Bukara, K.; Levic, S.; Bugarski, B.M. Antioxidant activity of ethanolic extracts of Thymus serpyllum. In Proceedings of the IV International Congress of Engineering, Environment and Materials in Processing Industry, Jahorina, Republic of Srpska, Bosnia and Hercegovina, 4–6 March 2015; pp. 444–452. [Google Scholar]
- Ozen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Kalaba, V.; Marjanovic-Balaban, Z.; Stijepic, M.; Glušac, J.; Kalaba, D. Antimicrobial activity of selected essential oils against of Staphylococcus aureus compared with antimicrobial drugs. In Proceedings of the II International Congress Food Technology Quality and Safety, Novi Sad, Servia, 28–30 October 2014; pp. 434–439. [Google Scholar]
- Kalaba, V.; Glušac, J.; Stijepic, M.; Kalaba, D.; ÐurdjevicMiloševic, D. Antimicrobial activity of Hypericum perforatum essential oil. Qual. Life 2015, 6, 45–52. [Google Scholar] [CrossRef][Green Version]
- Kalaba, V.; Kalaba, D. Comparative effects of essential oils on growth of Escherichia coli. Carpathian J. Food Sci. Technol. 2014, 6, 5–8. [Google Scholar]
- Baghalian, K.; Haghiry, A.; Naghavi, M.R.; Mohammadi, A. Effect of saline irrigation water on agronomical and phytochemical characters of chamomile (Matricariarecutita L.). Sci. Hortic. 2008, 116, 437–441. [Google Scholar] [CrossRef]
- Baghalian, K.; Abdoshah, S.; Khalighi-sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricariarecutita L.). Plant Physiol. Biochem. 2011, 49, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tsivelika, N.; Sarrou, E.; Gusheva, K.; Pankou, C.; Koutsos, T.; Chatzopoulou, P.; Mavromatis, A. Phenotypic variation of wild Chamomile (Matricaria chamomilla L.) populations and their evaluation for medicinally important essential oil. Biochem. Syst. Ecol. 2018, 80, 21–28. [Google Scholar] [CrossRef]
- Rezaeih, K.A.P.; Gurbuz, B.; Uyanik, M.; Rahimi, A.; Arslan, N. Volatile constituents variability in Matricaria chamomilla L. from Ankara, Turkey. J. Essent. Oil-Bear. Plants 2015, 18, 255–260. [Google Scholar] [CrossRef]
- Salamon, I. Effect of the internal and external factors on yield and qualitative quantitative characteristics of chamomile essential oil. In Proceedings of the First International Symposium on Chamomile Research, Development and Production, Presov, Slovakia, 7–10 June 2006; pp. 45–64. [Google Scholar]
- Sashidhara, K.V.; Verma, S.R.; Ram, P. Essential oil composition of Matricariarecotita L. from the lower region of the Himalayas. Flavor Fragr. J. 2006, 21, 274–276. [Google Scholar] [CrossRef]
- Tirillini, B.; Pagiotti, R.; Menghini, L.; Pintore, G. Esseential oil composition of ligulae and tubular flowers and receptacle from wild Chamomilla rectita (L.) Rausch. Grown in Italy. J. Essent. Oil Res. 2006, 18, 42–45. [Google Scholar] [CrossRef]
- Pasuquin, E.; Lafarge, T.; Tubana, B. Transplanting young seedlings in irrigated rice fields: Early and high tiller production enhanced grain yield. Field Crops Res. 2008, 105, 141–155. [Google Scholar] [CrossRef]
- Deans, S.G.; Svoboda, K.P. Biological activity of plant volatile oils. In Volatile Oil Crops: Their Biology, Biochemistry and Production; Hay, R.K.M., Warterman, P.G., Eds.; Longman Group: London, UK, 1993; pp. 97–112. [Google Scholar]
- Franz, C.H.; Vömel, A.; Hölzl, J. Variation in the essential oil of Matricaria chamomilla L. depending on plant age and stage of development. Acta Hort. ISHS 1978, 73, 229–238. [Google Scholar] [CrossRef]
- Hadi, M.H.S.; Noormohammadi, G.; Masoud Sinaki, J.; Khodabandeh, N.; Yasa, N.; Darzi, M.T. Effects of planting time and plant density on flower yield and active substance of Chamomile (Matricaria chamomilla L.). In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Guenther, E. The Essential Oils; Robert E. Krieger Publishing Co.: New York, NY, USA, 1950; Volume IV. [Google Scholar]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef]
- Mohammad, R.; Hamid, S.; Um, A.; Norbert, K.de.; Patrick, V.D. Effects of planting date and seedling age on agro-morphological characteristics, essential oil content and composition of German chamomile (Matricaria chamomilla L.) grown in Belgium. Ind. Crops Prod. 2010, 31, 45–152. [Google Scholar] [CrossRef]
- da Silva, J.R.; Heldwein, A.B.; Puhl, A.J.; do Amarante, A.A.; Salvadé, D.M.; Leonardi, M.; da Rocha, L. Phenology and productive performance of chamomile in sowing dates and spacing between plants. Comun. Sci. 2020, 11, e3285. [Google Scholar] [CrossRef]
- Ebadi, M.T.; Azizi, M.A.J.I.D.; Omidbaigi, R.; HassanzadehKhayyat, M. The effect of sowing date and seeding levels on quantitative and qualitative yield of chamomile (Matricariarecutita L.) CV. Presov. Iran. J. Med. Aromat. Plants Res. 2009, 25, 296–308. [Google Scholar]
- Gorni, P.H.; Pacheco, A.C.; Moro, A.L.; Silva, J.F.A.; Moreli, R.R.; de Miranda, G.R.; Pelegrini, J.M.; Spera, K.D.; Junior, J.L.B.; da Silva, R.M.G. Salicylic acid foliar application increases biomass, nutrient assimilation, primary metabolites and essential oil content in Achillea millefolium L. Sci. Hortic. 2020, 270, 109436. [Google Scholar] [CrossRef]
- Rathore, S.; Kumar, R. Agronomic interventions affect the growth, yield, and essential oil composition of German chamomile (Matricaria chamomilla L.) in the western Himalaya. Ind. Crops Prod. 2021, 171, 113873. [Google Scholar] [CrossRef]
- Wagner, T. Chamomile production in Slovenia. Acta Hortic. 1993, 344, 476–478. [Google Scholar] [CrossRef]
- Salamon, I. Chamomile a medicinal plant. In The Herb, Spice and Medicinal Plant Digest; Springer: New York, NY, USA, 1992; Volume 10, pp. 1–4. [Google Scholar]
- Tadesse, N.; Chala, M. Influence of Plant Population Density on Growth and Yield of Chamomile (Matricaria chamomilla L.) at Wondo Genet, South Ethiopia. Adv. Crop. Sci. Technol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Kanjilal, P.B.; Singh, R.S. Effect of spacing and planting time on Chamomile performance. J. Agric. Sci. 2000, 70, 631–637. [Google Scholar]
- Kwiatkowski, C.A. Yield and quality of chamomile (Chamomilla recutita (L.) Rausch.) raw material depending on selected foliar sprays and plant spacing. Acta Sci. Pol. Hortorum Cultus. 2015, 14, 143–156. [Google Scholar]
- Pirzad, A.; Shakiba, M.R.; Zehtab-Salmasi, S.; Mohammadi, S.A.; Hadi, H.; Darvishzadeh, R. Effects of irrigation regime and plant density on harvest index of German chamomile (Matricaria chamomilla L.). Aust. J. Agric. Eng. 2011, 2, 120–126. [Google Scholar]
- Arslan, D.; Bayraktar, O.V.; Temel, M.; Bayram, E. Economical analysis of chamomile (Matricariarecutita L.) cultivars, flower yields which are obtained from different sowing times and row spacing. J. Agric. Sci. 2019, 25, 129–136. [Google Scholar]
- Jamshidi, K. Effects of row spacing and plant density on quantitative aspects of chamomile flower. Iran. J. Agri. Sci. 2000, 31, 203–210. [Google Scholar]
- Salamon, I. Chamomile biodiversity of the essential oil qualitative-quantitative char-acteristics. In Innovations in Chemical Biology; Springer: Dordrecht, The Netherlands, 2009; pp. 83–90. [Google Scholar]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy). Ind. Crops Prod. 2015, 63, 256–263. [Google Scholar] [CrossRef]
- EL-Hefny, M.; Abo Elgat, W.A.A.; Al-Huqail, A.A.; Ali, H.M. Essential and recovery oils from Matricaria chamomilla flowers as environmentally friendly fungicides against four fungi isolated from cultural heritage objects. Processes 2019, 7, 809. [Google Scholar] [CrossRef]
- Puhl, A.J.; Heldwein, A.B.; da Silva, J.R.; Salvadé, D.M.; da Rocha, L.; Leonardi, M.; Meus, K.V. Yield of chamomile capitula and essential oil in competition with weeds in different spacings and sowing dates. Comun. Sci. 2021, 12, e3608. [Google Scholar] [CrossRef]
- Axtell, B.L.; Fairman, R.M. Minor oil crops (Part III. Essential oils). FAO Agric. Services Bulletin. 1992, 94, vii + 241p. [Google Scholar]
- Matsunaga, T.; Hasegawa, C.; Kawasuji, T.; Suzuki, H.; Saito, H. Isolation of the antiulcer compound in essential oil from the leaves of Cryptomeria japonica. Biol. Pharm. Bull. 2000, 23, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.H.; Cardoso, M.S.; Menezes, C.T.; Silva, J.P.; De Sousa, D.P.; Batista, J.S. Gastroprotective activity of α-terpineol in two experimental models of gastric ulcer in rats. DARU J. Pharm. Sci. 2011, 19, 277–281. [Google Scholar]
- Pauli, A.; Schilcher, H. Specific selection of essential oil compounds for treatment of children’s infection disease. Pharma 2004, 1, 1–30. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.). J. Essent. Oil-Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Alireza, M. Antimicrobial activity and chemical composition of essential oils of chamomile from Neyshabur. Iran. J. Med. Plants Res. 2012, 6, 820–824. [Google Scholar]
- Salamon, I.; Ghanavati, M.; Khazaei, H. Chamomile biodiversity and essential oil qualitative-quantitative characteristics in Egyptian production and Iranian landraces. Emir. J. Food Agric. 2010, 22, 59–64. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).