Early Detection of Coffee Leaf Rust Caused by Hemileia vastatrix Using Multispectral Images
Abstract
1. Introduction
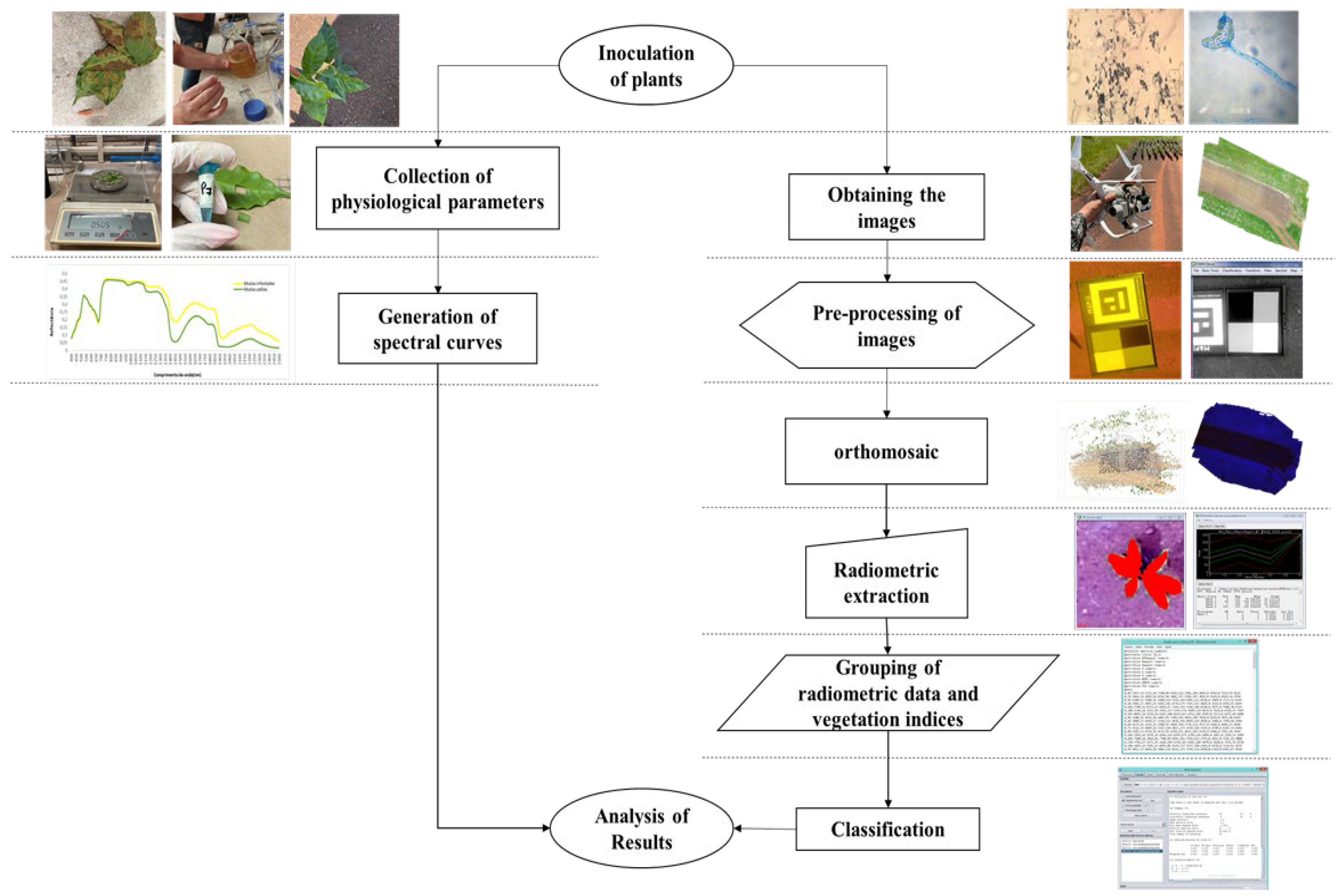
2. Material and Methods
2.1. Inoculation of Coffee Seedlings with H. vastatrix
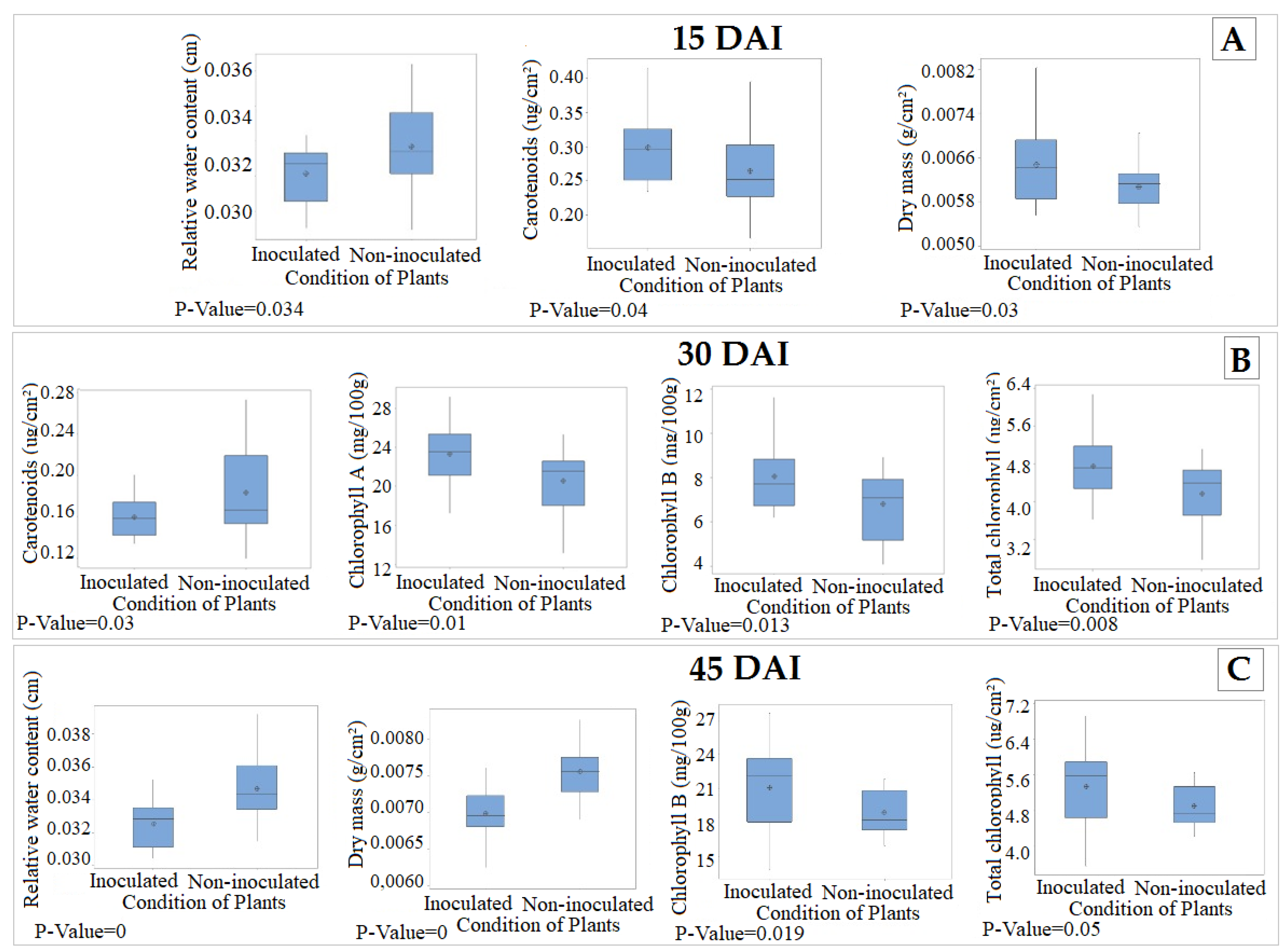
2.2. Determination of Biochemical and Structural Parameters of Leaves for Hyperspectral Characterization
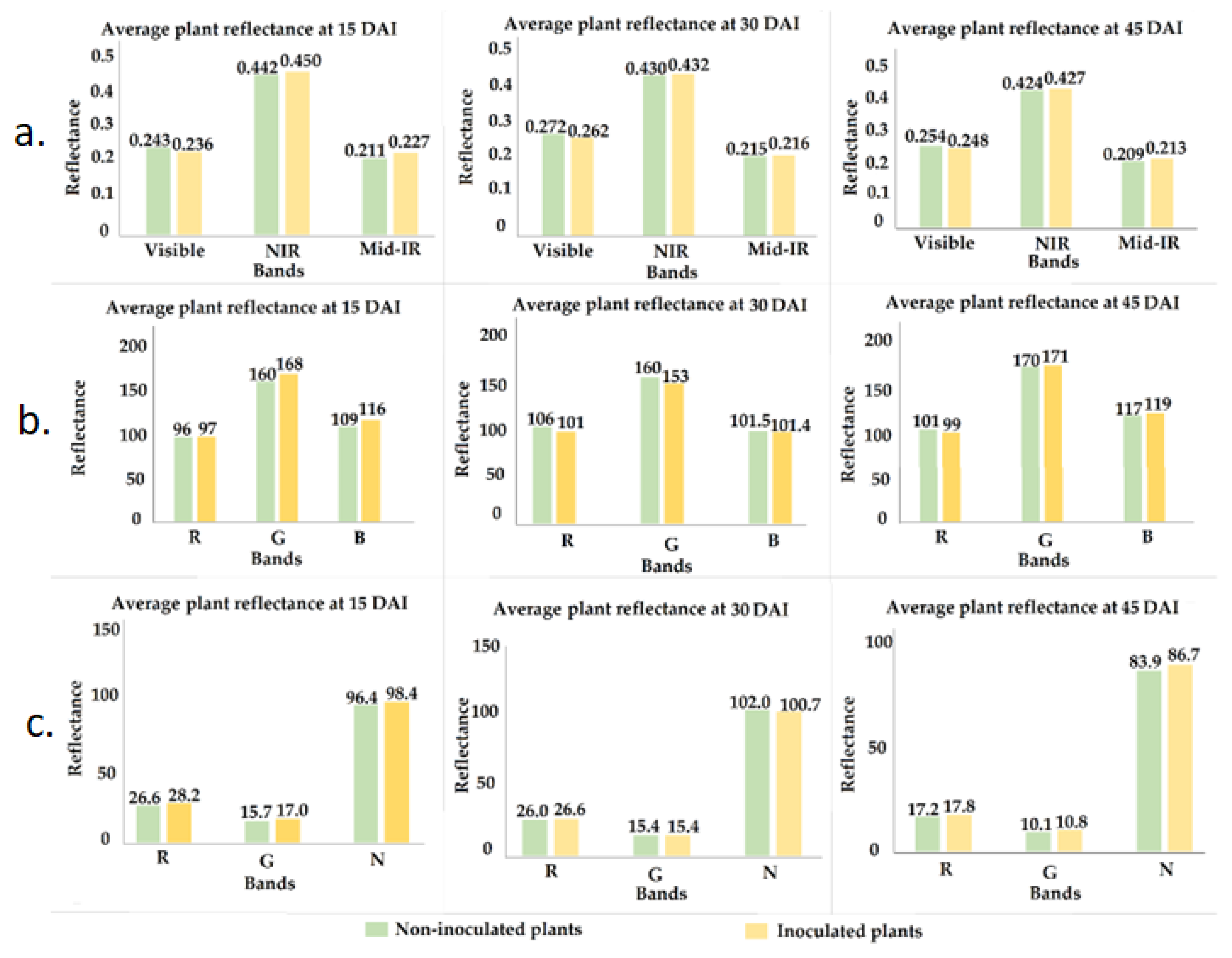
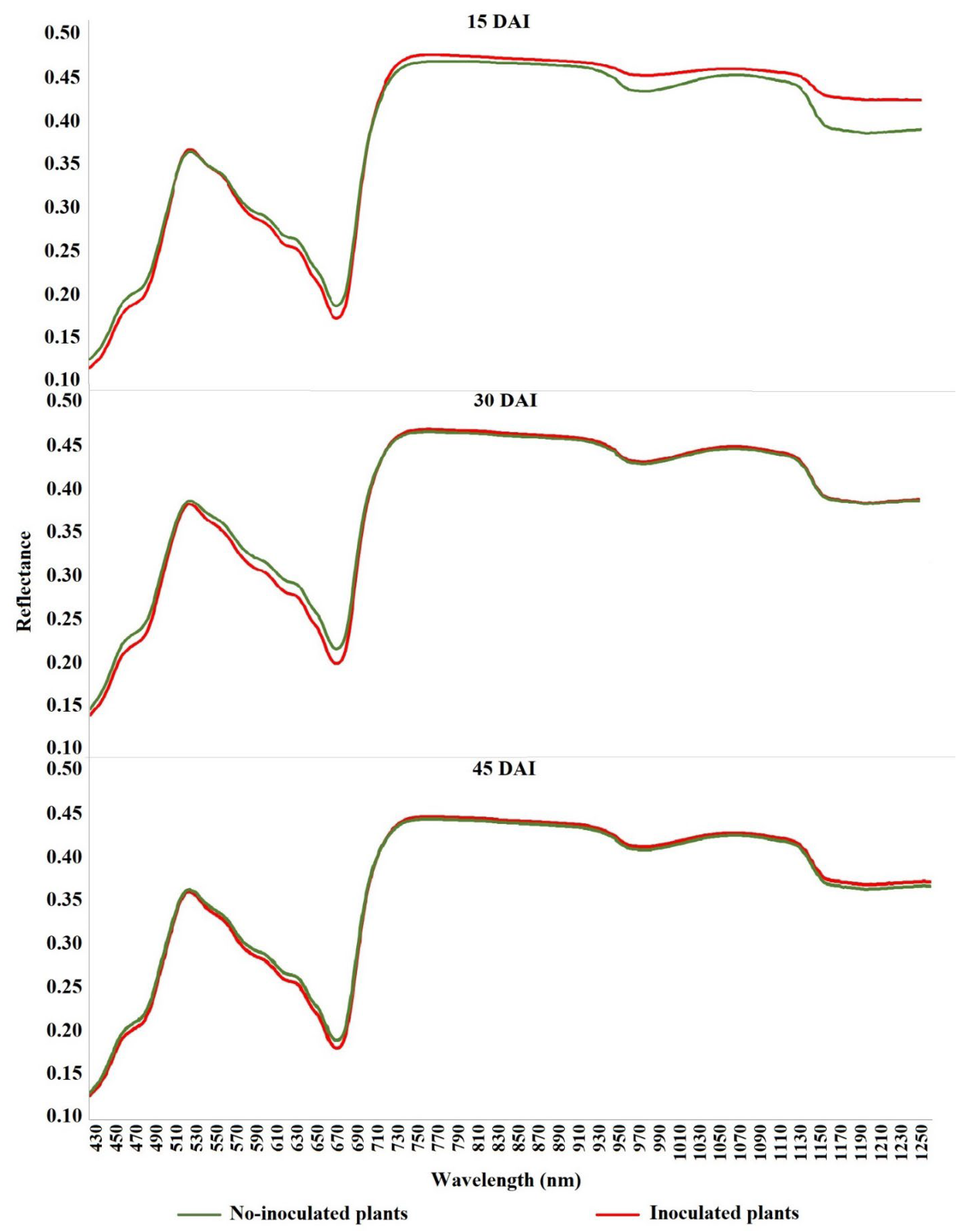
2.3. Image Acquisition
2.4. Image Pre-Processing
2.4.1. Radiometric Calibration
2.4.2. Radiometric Normalization
2.5. Extraction of Radiometric Data
2.6. Calculation of Vegetation Indices
2.7. Supervised Classification
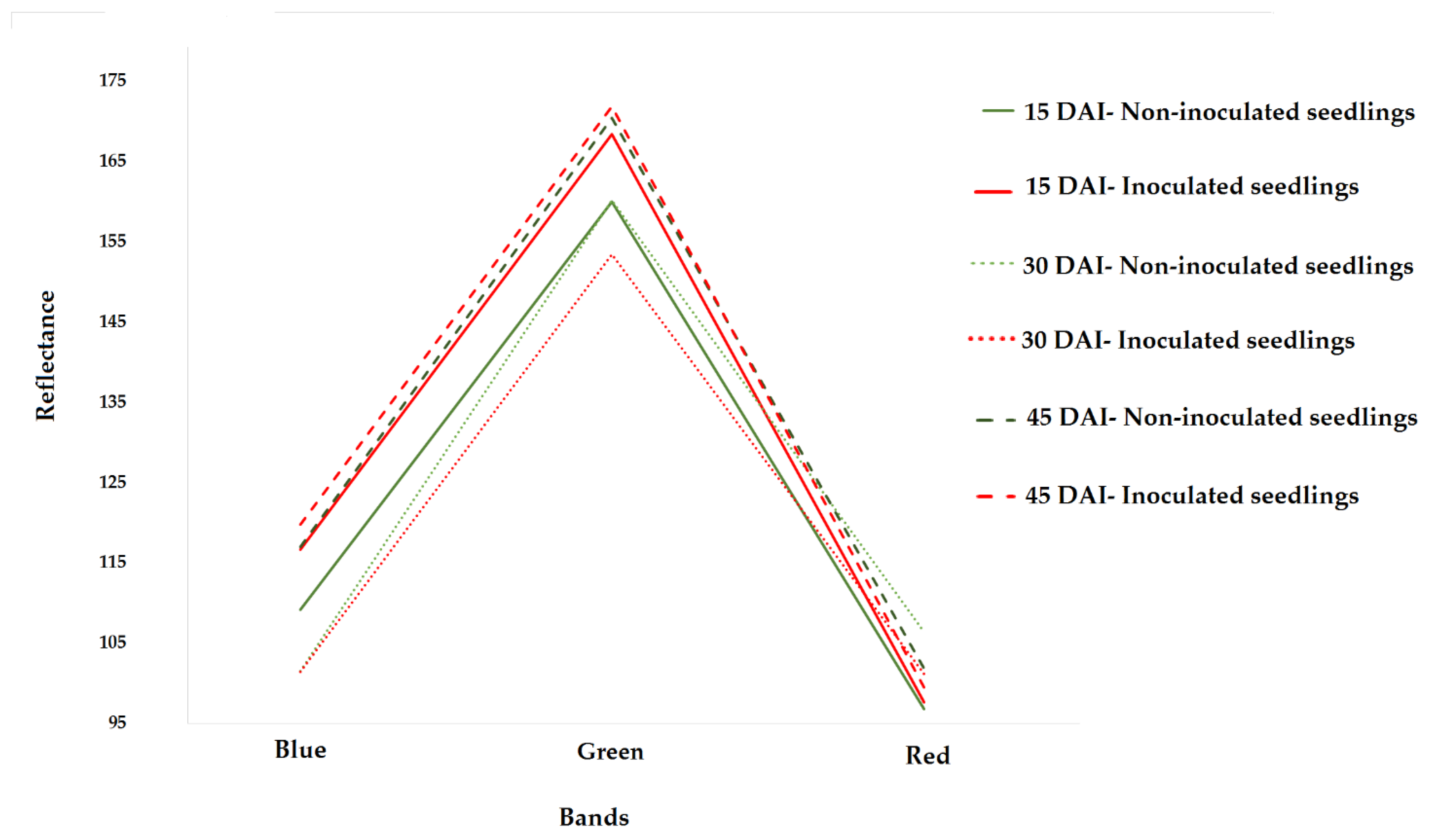
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Vossen, H.; Bertrand, B.; Charrier, A. Next generation variety development for sustainable production of arabica coffee (Coffea arabica L.): A review. Euphytica 2015, 204, 243–256. [Google Scholar] [CrossRef]
- Motisi, N.; Bommel, P.; Leclerc, G.; Robin, M.; Aubertot, J.; Butron, A.; Treminio, E.; Avelino, J. Improved forecasting of coffee leaf rust by qualitative modeling: Design and expert validation of the ExpeRoya model. Agric. Syst. 2021, 197, 103–129. [Google Scholar] [CrossRef]
- Cerda, R.; Avelino, J.; Gary, C.; Tixier, P.; Lechevallier, E. Primary and secondary yield losses caused by pests and diseases: Assessment and modeling in coffee. PLoS ONE 2017, 12, e0169133. [Google Scholar] [CrossRef]
- De Moraes, S.A. A Ferrugem do Cafeeiro: Importância, Condições Predisponentes, Evolução e Situação no Brasil; Instituto Agronômico: Londrina, Brazil, 1983; p. 50. [Google Scholar]
- Garçon, C.L.P.; Zambolim, L.; Vale, F.X.R.; Mizubuti, E.S.G.; Altmann, T.; Paiva, S.B. Modelo de previsão da ferrugem (Hemileia vastatrix Berk. & Br.) do cafeeiro (Coffea arabica L.). In I Simpósio de Pesquisa dos Cafés do Brasil; Resumos Expandidos: Poços de Caldas, Brazil, 2000; Volume 1, pp. 230–234. Available online: http://www.sbicafe.ufv.br/handle/123456789/640 (accessed on 23 March 2022).
- Chalfoun, S.M.; de Lima, R.D. Influência do clima sobre a incidência de doenças infecciosas. Inf. Agropecuário 1986, 12, 31–36. [Google Scholar]
- Jorge, L.A.C.; Inamasu, R.Y. Detecção do greening dos citrus por imagens multiespectrais. In Agricultura de Precisão: Resultados de um Novo Olhar; Bernardi, A.C.C., Ed.; Embrapa: Brasília, Brazil, 2014; pp. 180–190. [Google Scholar]
- Lan, Y.; Huang, Z.; Deng, X.; Zhu, Z.; Huang, H.; Zheng, Z.; Lian, B.; Zeng, G.; Tong, Z. Comparison of machine learning methods for citrus greening detection on UAV multispectral images. Comput. Electron. Agric. 2020, 171, 105–234. [Google Scholar] [CrossRef]
- Santos, L.M.; Ferraz, G.A.F.; Santana, L.S.; Barbosa, B.D.S.; Xavier, L.A.G.; Andrade, M.T. Índice de Vegetação (ExGR) Aplicado a Imagens rgb Obtidas por UAV para Detecção de Doença em Cafeeiros, Proceedings of the X Simpósio de Pesquisa dos Cafés do Brasil, Vitoria, Brazil, 8 November 2019; Centro de Convenções de Vitoria: Vitoria, Brazil, 2019; ISSN 1984-9249. [Google Scholar]
- Pires, M.S.O.; Alves, M.C.; Pozza, E.A. Multispectral radiometric characterization of coffee rust epidemic in different irrigation management systems. Int. J. Appl. Earth Obs. Geoinf. 2020, 86, 102016. [Google Scholar] [CrossRef]
- Saleem, M.H.; Potgieter, J.; Arif, K.M. Plant Disease Detection and Classification by Deep Learning. Plants 2019, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Katsuhama, N.; Imai, M.; Naruse, N.; Takahashi, Y. Discrimination of areas infected with coffee leaf rust using a vegetation index. Remote Sens. Lett. 2018, 9, 1186–1194. [Google Scholar] [CrossRef]
- Carmo, G.J.S.; Castoldi, R.; Martins, G.D.; Jacinto, A.C.P.; Tebaldi, N.D.; Charlo, H.C.O.; Zampiroli, R. Detection of Lesions in Lettuce Caused by Pectobacterium carotovorum subsp. carotovorum by Supervised Classification Using Multispectral Images. Can. J. Remote Sens. 2022, 48, 144–157. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Fazuoli, L.C.; Carvalho, C.H.S.; Carvalho, G.R.; Filho, O.G.; Pereira, A.A.; Bartholo, G.F.; Moura, W.M.; Silvarolla, M.B.; Braghini, M.T. Cultivares de Café Arábica de Porte Alto. Cultivares de Café: Origem, Características e Recomendações; Embrapa: Brasília, Brazil, 2008; pp. 227–252. [Google Scholar]
- Zambolim, L.; Chaves, M.C. Efeito de baixas temperaturas e do binomio temperatura-umidade relativa sobre a viabilidade dos uredósporos de Hemileia vastatrix Berk. Et Br. E Uromyces Phaseolitypica Arth. Exp. 1974, 17, 151–184. [Google Scholar]
- Shein, R.D.; Rotem, J. Temperature and humidity effects on uredospore viability. Mycologia 1965, 57, 397–403. [Google Scholar] [CrossRef]
- Schaepman-Strub, G.; Schaepman, M.E.; Painter, T.H.; Dangel, S.; Martonchik, J.V. Reflectance quantities in optical remote sensing—Definitions and case studies. Remote Sens. Environ. 2006, 103, 27–42. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Jiang, J.; Comar, A.; Burger, P.; Bancal, P.; Weiss, M.; Baret, F. Estimation of leaf traits from reflectance measurements: Comparison between methods based on vegetation indices and several versions of the PROSPECT model. Plant Methods 2018, 14, 23. [Google Scholar] [CrossRef]
- De Souza Echer, M.P.; Martins, F.R.; Pereira, E.B. A importância dos dados de cobertura de nuvens e de sua variabilidade: Metodologias para aquisição de dados. Rev. Bras. Ensino Física 2006, 28, 341–352. [Google Scholar] [CrossRef]
- Khanna, R.; Sa, I.; Nieto, J.; Siegwart, R. On field radiometric calibration for multispectral cameras. In Proceedings of the IEEE International Conference on Robotics and Automation, Marina Bay Sands Convention Centre, Singapore, 29 May–3 June 2017; pp. 6503–6509. [Google Scholar] [CrossRef]
- Hall, F.G.; Strebel, D.E.; Nickeson, J.E.; Goetz, S.J. Radiometric rectification: Toward a common radiometric response among multidate, multisensor images. Remote Sens. Environ. 1991, 35, 11–27. [Google Scholar] [CrossRef]
- Ponzoni, F.J.; Shimabukuro, Y.E.; Kuplich, T.M. Sensoriamento Remoto da Vegetação, 2nd ed.; Oficina de Textos: São Paulo, Brazil, 2012; Volume 1, p. 176. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Rouse, J.W.; Hass, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third ERTS Symposium, Washington, DC, USA, 10–14 December 1973; pp. 309–317. [Google Scholar]
- Zarate-Valdez, J.L.; Whiting, M.L.; Lampinen, B.D.; Metcalf, S.; Ustin, S.; Brown, P.H. Prediction of leaf area index in almonds by vegetation indexes. Comput. Electron. Agric. 2012, 85, 24–32. [Google Scholar] [CrossRef]
- Hunt, E.R.J.; Hively, W.D.; Fujikawa, S.; Linden, D.; Daughtry, C.S.; McCarty, G. Acquisition of nir-green-blue digital photographs from unmanned aircraft for crop monitoring. Remote Sens. 2010, 2, 290–305. [Google Scholar] [CrossRef]
- Perroca, M.G.; Gaidzinski, R.R. Avaliando a confiabilidade interavaliadores de um instrumento para classificação de pacientes: Coeficiente Kappa. Rev. Esc. Enferm. USP 2003, 37, 72–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Emerson, R.; Lewis, C.M. The Dependence of the Quantum Yield of Chlorella Photosynthesis on Wave Length of Light. Am. J. Bot. 1943, 30, 165–178. [Google Scholar] [CrossRef]
- Chemura, A.; Mutanga, O.; Dube, T. Separability of Coffee Leaf Rust Infection Levels with Machine Learning Methods at Sentinel-2 MSI Spectral Resolutions. In Precision Agriculture; Springer: New York, NY, USA, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. Photosynthetic activity of far-red light in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1708, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.R. Sensoriamento Remoto do Ambiente: Uma Perspectiva em Recursos Terrestres; Parêntese: São José dos Campos, Brazil, 2011. [Google Scholar]
- Velásquez, D.; Sánchez, A.; Sarmiento, S.; Toro, M.; Maiza, M.; Sierra, B. A Method for Detecting Coffee Leaf Rust through Wireless Sensor Networks, Remote Sensing, and Deep Learning: Case Study of the Caturra Variety in Colombia. Appl. Sci. 2020, 10, 697. [Google Scholar] [CrossRef]
- Da Silva Soares, R.R. Estudo de Propriedades da Clorofila a e da Feofitina a Visando a Terapia Fotodinâmica. Master’s Thesis, Universidade Estadual de Maringá, Maringá, Brazil, 2006. [Google Scholar]
- Björn, L.O.; Papageorgiou, G.C.; Blankenship, R.E. A viewpoint: Why chlorophyll a? Photosynth. Res. 2009, 99, 85–98. [Google Scholar] [CrossRef]
- Moreira, M.A. Fundamentos do Sensoriamento Remoto e Metodologias de Aplicação, 3rd ed.; Universidade Federal de Viçosa: Viçosa, Brazil, 2005; p. 320. [Google Scholar]
- Martins, G.D. Caracterização espectral e espacial de áreas infestadas por nematoides e Migdolus fryanus em cultura canavieira. Master’s Thesis, Universidade Estadual Paulista (Unesp), São Paulo, Brazil, 2013. [Google Scholar]
- Terentev, A.; Dolzhenko, V.; Fedotov, A.; Eremenko, D. Current state of Hyperspectral remote sensing for early plant disease detection: A rewiew. Sensors 2022, 22, 757. [Google Scholar] [CrossRef]




| Vegetation Index | Equation | Application | Source |
|---|---|---|---|
| Green Normalized Difference Vegetation Index (GNDVI) | Chlorophyll, LAI, biomass, N uptake, and productivity | [26] | |
| Normalized Difference Vegetation Index (NDVI) | Biomass, LAI, productivity, and photosynthetically active radiation | [27,28] | |
| Triangular Greenness Index (TGi) | Chlorophyll | [29] |
| 15 DAI | ||||||||
|---|---|---|---|---|---|---|---|---|
| SVM | RN | |||||||
| Classified Subsets | EO (%) | EC (%) | OA | K | EO (%) | EC (%) | OA | K |
| RGN | 20 | 20 | 80 | 0.6 | 25 | 25 | 75 | 0.5 |
| RGN, NDVI, and GNDVI | 20 | 20 | 80 | 0.6 | 25 | 25 | 75 | 0.5 |
| RGB | 20 | 35 | 72 | 0.4 | 25 | 35 | 70 | 0.4 |
| RGB and TGI | 20 | 45 | 67 | 0.3 | 25 | 40 | 67 | 0.3 |
| Evaluation Period | Algorithm | RGN | RGN, NDVI, and GNDVI | RGB | RGB and TGI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PredictionReal | Non-Inoculated | Inoculated | Non-Inoculated | Inoculated | Non-Inoculated | Inoculated | Non-Inoculated | Inoculated | ||
| 15 DAI | SVM | non-inoculated | 16 | 4 | 16 | 4 | 16 | 4 | 16 | 4 |
| inoculated | 4 | 16 | 4 | 16 | 7 | 13 | 9 | 11 | ||
| 15 DAI | RN | non-inoculated | 15 | 5 | 15 | 5 | 15 | 5 | 15 | 5 |
| inoculated | 5 | 15 | 5 | 15 | 7 | 13 | 8 | 12 | ||
| 30 DAI | SVM | non-inoculated | 9 | 11 | 8 | 12 | 17 | 3 | 15 | 5 |
| inoculated | 7 | 13 | 3 | 17 | 3 | 17 | 4 | 15 | ||
| 30 DAI | RN | non-inoculated | 18 | 2 | 16 | 4 | 17 | 3 | 15 | 5 |
| inoculated | 8 | 12 | 9 | 11 | 5 | 15 | 4 | 16 | ||
| 45 DAI | SVM | non-inoculated | 13 | 7 | 12 | 8 | 18 | 2 | 17 | 3 |
| inoculated | 5 | 15 | 8 | 12 | 18 | 2 | 18 | 2 | ||
| 45 DAI | RN | non-inoculated | 19 | 1 | 19 | 1 | 18 | 2 | 18 | 2 |
| inoculated | 16 | 4 | 16 | 4 | 15 | 5 | 16 | 4 | ||
| 30 DAI | ||||||||
|---|---|---|---|---|---|---|---|---|
| SVM | RN | |||||||
| Classified Subsets | EO (%) | EC (%) | OA | K | EO (%) | EC (%) | OA | K |
| RGN | 55 | 35 | 55 | 0.1 | 10 | 40 | 76 | 0.5 |
| RGN, NDVI, and GNDVI | 60 | 15 | 62 | 0.2 | 20 | 45 | 67 | 0.3 |
| RGB | 15 | 15 | 85 | 0.7 | 25 | 15 | 80 | 0.6 |
| RGB and TGI | 25 | 20 | 77 | 0.5 | 25 | 20 | 77 | 0.5 |
| 45 DAI | ||||||||
|---|---|---|---|---|---|---|---|---|
| SVM | RN | |||||||
| Classified Subsets | EO (%) | EC (%) | OA | K | EO (%) | EC (%) | OA | K |
| RGN | 35 | 25 | 70 | 0,4 | 5 | 80 | 57.5 | 0.1 |
| RGN, NDVI, and GNDVI | 40 | 40 | 60 | 0,2 | 5 | 80 | 57.5 | 0.1 |
| RGB | 10 | 90 | 50 | 0 | 10 | 75 | 57.5 | 0.1 |
| RGB and TGI | 15 | 90 | 47 | 0 | 10 | 80 | 55 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, A.d.S.; Vieira, B.S.; Bezerra, T.A.; Martins, G.D.; Siquieroli, A.C.S. Early Detection of Coffee Leaf Rust Caused by Hemileia vastatrix Using Multispectral Images. Agronomy 2022, 12, 2911. https://doi.org/10.3390/agronomy12122911
Soares AdS, Vieira BS, Bezerra TA, Martins GD, Siquieroli ACS. Early Detection of Coffee Leaf Rust Caused by Hemileia vastatrix Using Multispectral Images. Agronomy. 2022; 12(12):2911. https://doi.org/10.3390/agronomy12122911
Chicago/Turabian StyleSoares, Analis da Silva, Bruno Sérgio Vieira, Thalita Almeida Bezerra, George Deroco Martins, and Ana Carolina Silva Siquieroli. 2022. "Early Detection of Coffee Leaf Rust Caused by Hemileia vastatrix Using Multispectral Images" Agronomy 12, no. 12: 2911. https://doi.org/10.3390/agronomy12122911
APA StyleSoares, A. d. S., Vieira, B. S., Bezerra, T. A., Martins, G. D., & Siquieroli, A. C. S. (2022). Early Detection of Coffee Leaf Rust Caused by Hemileia vastatrix Using Multispectral Images. Agronomy, 12(12), 2911. https://doi.org/10.3390/agronomy12122911







