Agro-Morphological and Biochemical Characterization of Korean Sorghum (Sorghum bicolor (L.) Moench) Landraces
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sorghum Materials, Cultivation and Preparation
2.3. Extraction of Seed Samples
2.4. Determination of Total Tannin Content (TTC)
2.5. Determination of Total Phenolic Content (TPC)
2.6. Determination of Antioxidant Activities
2.6.1. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.6.2. 2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) Diammonium Radical Cation (ABTS•+) Scavenging Activity
2.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7. Statistical Analysis
3. Results and Discussion
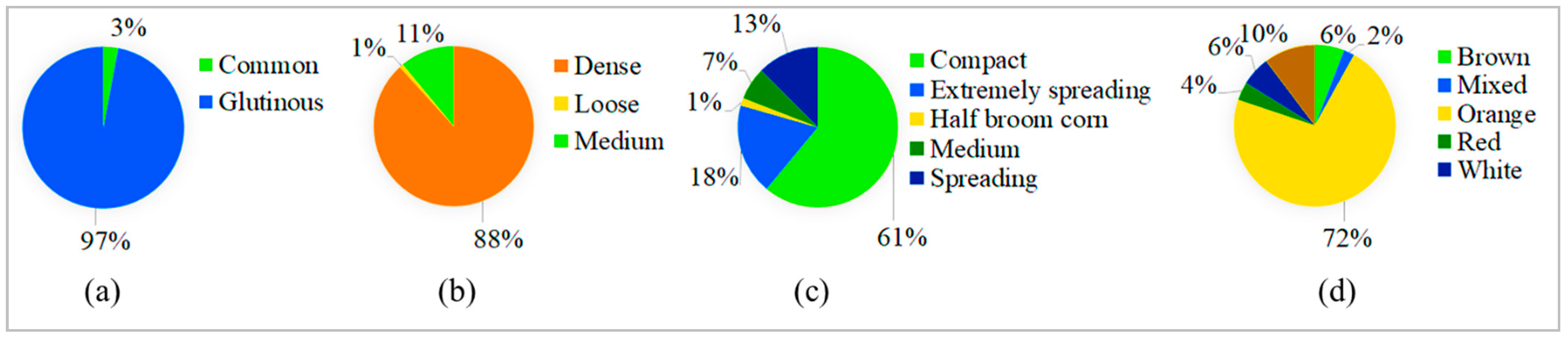
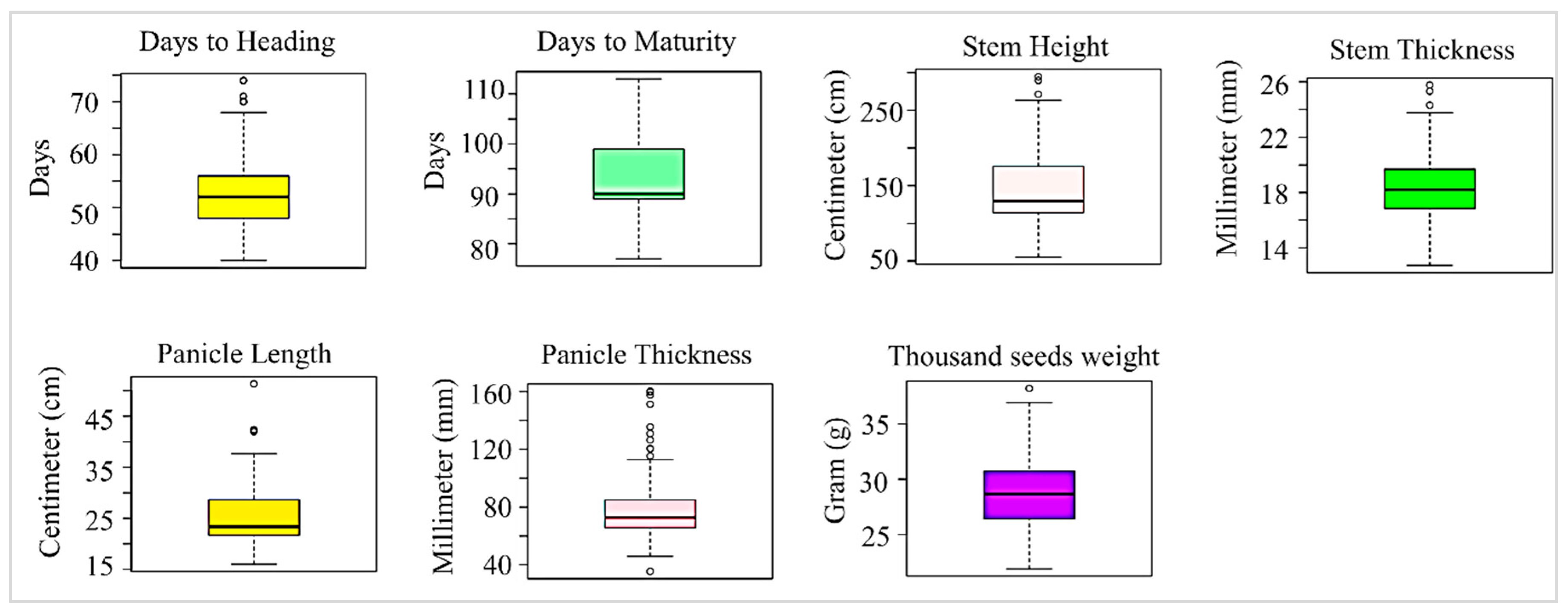
3.1. Agro-Morphological Characteristics
3.2. Biochemical Contents and Antioxidant Activities
3.2.1. Selected Landraces with Unique Seed-Related Characteristics
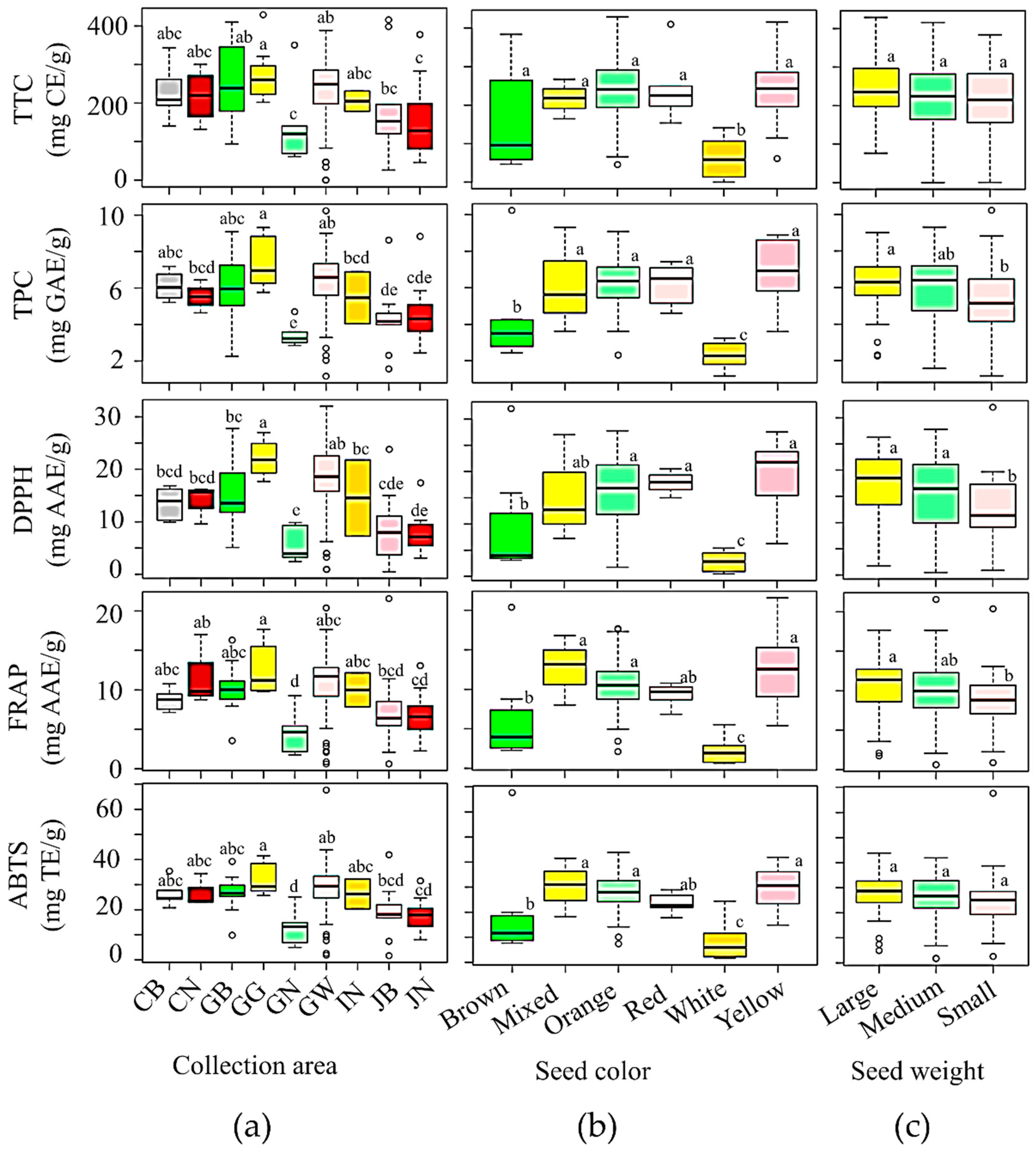
3.2.2. Effect of Collection Area on Biochemical Contents and Antioxidant Activities
3.2.3. Effects of Seed Color and Weight on Biochemical Contents and Antioxidant Activities
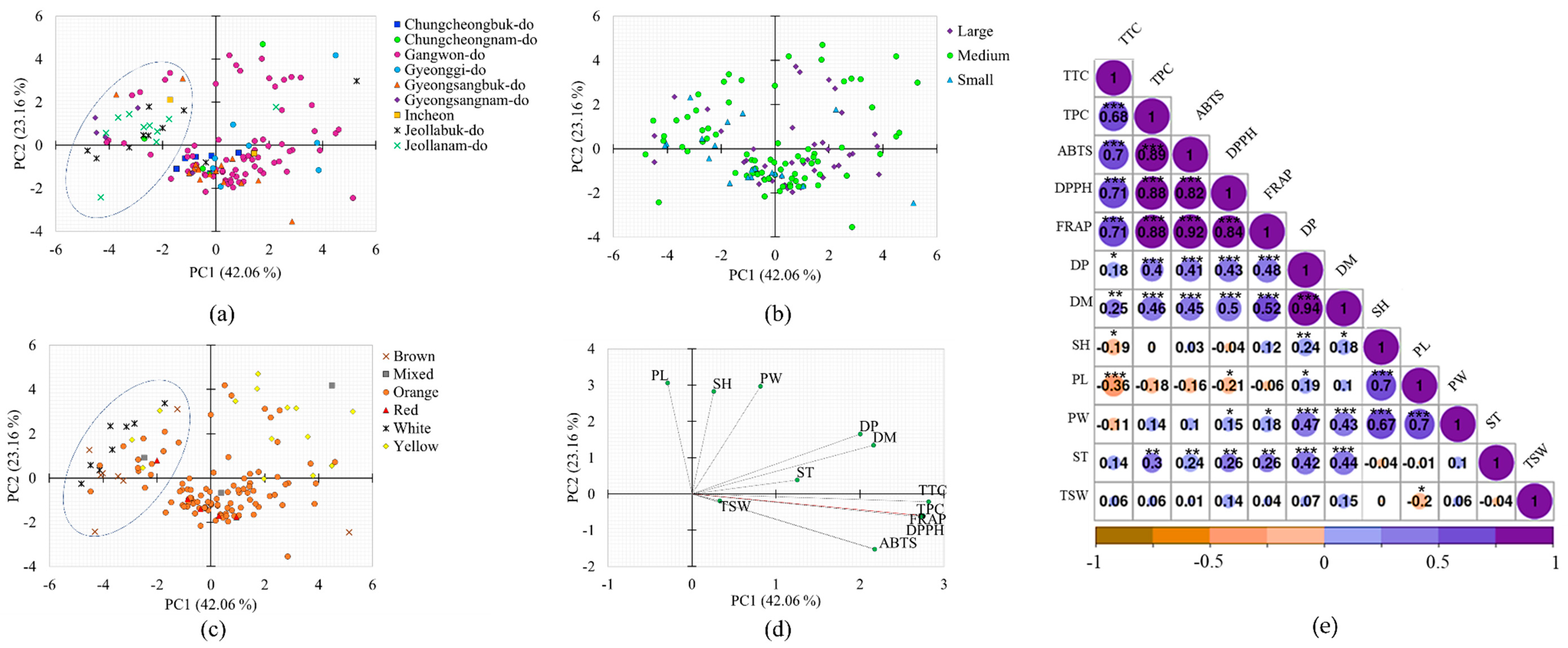
3.3. Principal Component (PCA) and Correlation Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davidson, R.M.; Gowda, M.; Moghe, G.; Lin, H.; Vaillancourt, B.; Shiu, S.H.; Jiang, N.; Robin Buell, C. Comparative transcriptomics of three Poaceae species reveals patterns of gene expression evolution. Plant J. 2012, 71, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.L.; Awika, J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations Statistical Database; FAO: Rome, Italy, 2020; Available online: www.fao.org/faostat (accessed on 18 October 2022).
- Kolozsvári, I.; Kun, Á.; Jancsó, M.; Palágyi, A.; Bozán, C.; Gyuricza, C. Agronomic Performance of Grain Sorghum (Sorghum bicolor (L.) Moench) Cultivars under Intensive Fish Farm Effluent Irrigation. Agronomy 2022, 12, 1185. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum Grain: From Genotype, Nutrition, and Phenolic Profile to Its Health Benefits and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic Compositions and Antioxidant Activities Differ Significantly among Sorghum Grains with Different Applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef]
- Aruna, C.; Visarada, K.B.R.S. Other industrial uses of sorghum. In Breeding Sorghum for Diverse End Uses; Woodhead Publishing: Sawston, UK, 2018; pp. 271–292. [Google Scholar] [CrossRef]
- Tian, X.; Engel, B.A.; Qian, H.; Hua, E.; Sun, S.; Wang, Y. Will reaching the maximum achievable yield potential meet future global food demand? J. Clean. Prod. 2021, 294, 126285. [Google Scholar] [CrossRef]
- Mundia, C.W.; Secchi, S.; Akamani, K.; Wang, G. A Regional Comparison of Factors Affecting Global Sorghum Production: The Case of North America, Asia and Africa’s Sahel. Sustainability 2019, 11, 2135. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Kumar, A.A.; Ramesh, S.; Reddy, P.S. Sorghum Genetic Enhancement for Climate Change Adaptation. In Crop Adaptation to Climate Change, 1st ed.; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, A.E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 326–339. [Google Scholar]
- Hou, F.; Su, D.; Xu, J.; Gong, Y.; Zhang, R.; Wei, Z.; Chi, J.; Zhang, M. Enhanced Extraction of Phenolics and Antioxidant Capacity from Sorghum (Sorghum bicolor L. Moench) Shell Using Ultrasonic-Assisted Ethanol–Water Binary Solvent. J. Food Process. Preserv. 2016, 40, 1171–1179. [Google Scholar] [CrossRef]
- Shehzad, T.; Okuizumi, H.; Kawase, M.; Okuno, K. Development of SSR-based sorghum (Sorghum bicolor (L.) Moench) diversity research set of germplasm and its evaluation by morphological traits. Genet. Resour. Crop Evol. 2009, 56, 809–827. [Google Scholar] [CrossRef]
- Bouargalne, Y.; Ben Mrid, R.; Bouchmaa, N.; Zouaoui, Z.; Benmrid, B.; Kchikich, A.; El Omari, R.; Kabach, I.; Mohamed, N. Genetic diversity for agromorphological traits, phytochemical profile, and antioxidant activity in Moroccan sorghum ecotypes. Sci. Rep. 2022, 12, 5895. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Li, Z.; Leng, C.; Lu, C.; Luo, H.; Liu, Y.; Wu, X.; Liu, Z.; Shang, L.; Jing, H.-C. Sorghum breeding in the genomic era: Opportunities and challenges. Theor. Appl. Genet. 2021, 134, 1899–1924. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, S.; Hammer, G.L.; Broad, I.; Harland, P.; McLean, G. Modelling environmental effects on phenology and canopy development of diverse sorghum genotypes. Field Crops Res. 2009, 111, 157–165. [Google Scholar] [CrossRef]
- Kardeş, Y.M.; Kaplan, M.; Kale, H.; Yılmaz, M.F.; Karaman, K.; Temizgül, R.; Akar, T. Biochemical composition of selected lines from sorghum (Sorghum bicolor L.) landraces. Planta 2021, 254, 26. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef]
- Tari, I.; Laskay, G.; Takács, Z.; Poór, P. Response of sorghum to abiotic stresses: A review. J. Agron. Crop Sci. 2013, 199, 264–274. [Google Scholar] [CrossRef]
- Sarshad, A.; Talei, D.; Torabi, M.; Rafiei, F.; Nejatkhah, P. Morphological and biochemical responses of Sorghum bicolor (L.) Moench under drought stress. SN Appl. Sci. 2021, 3, 81. [Google Scholar] [CrossRef]
- Abdelhalim, T.S.; Kamal, N.M.; Hassan, A.B. Nutritional potential of wild sorghum: Grain quality of Sudanese wild sorghum genotypes (Sorghum bicolor L. Moench). Food Sci. Nutr. 2019, 7, 1529–1539. [Google Scholar] [CrossRef]
- Chen, B.; Wang, C.; Wang, P.; Zhu, Z.; Xu, N.; Shi, G.; Yu, M.; Wang, N.; Li, J.; Hou, J.; et al. Genome-wide association study for starch content and constitution in sorghum (Sorghum bicolor (L.) Moench). J. Integr. Agric. 2019, 18, 2446–2456. [Google Scholar] [CrossRef]
- Mengistu, G.; Shimelis, H.; Laing, M.; Lule, D.; Mathew, I. Genetic variability among Ethiopian sorghum landrace accessions for major agro-morphological traits and anthracnose resistance. Euphytica 2020, 216, 113. [Google Scholar] [CrossRef]
- Badigannavar, A.; Girish, G.; Ganapathi, T.R. Genetic variation for seed phosphorus and yield traits in Indian sorghum landraces and varieties. Crop J. 2015, 3, 358–365. [Google Scholar] [CrossRef][Green Version]
- Nagesh Kumar, M.V.; Ramya, V.; Govindaraj, M.; Sameer Kumar, C.V.; Maheshwaramma, S.; Gokenpally, S.; Prabhakar, M.; Krishna, H.; Sridhar, M.; Venkata Ramana, M.; et al. Harnessing Sorghum Landraces to Breed High-Yielding, Grain Mold-Tolerant Cultivars with High Protein for Drought-Prone Environments. Front. Plant Sci. 2021, 12, 659874. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Lee, S.Y.; Lee, H.S.; Ma, K.H.; Gwag, J.G.; Kim, Y.G.; Lee, J.R. Development of microsatellite markers at the National Agrobiodiversity Center in Korea for the genetic assessment of underutilized crops. Plant Genet. Resour. Characterisation Util. 2014, 12, 125–129. [Google Scholar] [CrossRef]
- Choe, M.; Do-Yeon, K.; Jee-Yeon, K.; Seok-Bo, S.; Han, S.-I.; Chu, J. Heterosis and Combining Ability of F1 Hybrid Grain Sorghum in Korea (Sorghum bicolor L.). Korean J. Breed. Sci. 2019, 51, 367–375. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, Y.-S. The Contents of Phytosterols, Squalene, and Vitamin E and the Composition of Fatty Acids of Korean Landrace Setaria italica and Sorghum bicolar Seeds. Korean J. Plant Resour. 2013, 26, 663–672. [Google Scholar] [CrossRef]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2021, 337, 127970. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A Critical Evaluation of the Vanillin Reaction as an Assay for Tannin in Sorghum Grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Choi, Y.M.; Yoon, H.; Shin, M.J.; Lee, Y.; Hur, O.S.; Lee, B.C.; Ha, B.K.; Wang, X.; Desta, K.T. Metabolite contents and antioxidant activities of soybean (Glycine max (L.) Merrill) seeds of different seed coat colors. Antioxidants 2021, 10, 1210. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Molecular breeding and the impacts of some important genes families on agronomic traits, a review. Genet. Resour. Crop Evol. 2021, 68, 1709–1730. [Google Scholar] [CrossRef]
- Diallo, C.; Rattunde, H.W.F.; Gracen, V.; Touré, A.; Nebié, B.; Leiser, W.; Dzidzienyo, D.K.; Sissoko, I.; Danquah, E.Y.; Diallo, A.G.; et al. Genetic diversification and selection strategies for improving sorghum grain yield under phosphorous-deficient conditions in West Africa. Agronomy 2019, 9, 742. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, K.; Li, Z.; Wang, J.; Zhang, F.; Wu, H.; Zhang, Z.; Lu, F.; Wang, Y.; Duan, Y.; et al. Transcriptomic analysis of starch accumulation patterns in different glutinous sorghum seeds. Sci. Rep. 2022, 12, 11133. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, D.W.; Weibel, D.E. Inheritance and Genetic Relationships of Six Endosperm Types in Sorghum. Crop Sci. 1972, 12, 378–382. [Google Scholar] [CrossRef]
- Malambo, L.; Popescu, S.C.; Horne, D.W.; Pugh, N.A.; Rooney, W.L. Automated detection and measurement of individual sorghum panicles using density-based clustering of terrestrial lidar data. ISPRS J. Photogramm. Remote Sens. 2019, 149, 1–13. [Google Scholar] [CrossRef]
- Tolk, J.A.; Schwartz, R.C. Do more seeds per panicle improve grain sorghum yield? Crop Sci. 2017, 57, 490–496. [Google Scholar] [CrossRef]
- Rhodes, D.H.; Hoffmann, L.; Rooney, W.L.; Ramu, P.; Morris, G.P.; Kresovich, S. Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L.) Moench] germplasm. J. Agric. Food Chem. 2014, 62, 10916–10927. [Google Scholar] [CrossRef]
- Choi, S.C.; Kim, J.M.; Lee, Y.G.; Kim, C. Antioxidant Activity and Contents of Total Phenolic Compounds and Anthocyanins According to Grain Colour in Several Varieties of Sorghum bicolor (L.) Moench. Cereal Res. Commun. 2019, 47, 228–238. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Zhao, Y. Phenolic compounds in whole grain sorghum and their health benefits. Foods 2021, 10, 1921. [Google Scholar] [CrossRef]
- Kenga, R.; Alabi, S.O.; Gupta, S.C. Combining ability studies in tropical sorghum (Sorghum bicolor (L.) Moench). Field Crops Res. 2004, 88, 251–260. [Google Scholar] [CrossRef]
- Shukla, S.; Felderhoff, T.J.; Saballos, A.; Vermerris, W. Field Crops Research The relationship between plant height and sugar accumulation in the stems of sweet sorghum (Sorghum bicolor (L.) Moench). Field Crops Res. 2017, 203, 181–191. [Google Scholar] [CrossRef]
- Qingshan, L.; Dahlberg, J.A. Chinese sorghum genetic resources. Econ. Bot. 2001, 55, 401–425. [Google Scholar] [CrossRef]
- Amelework, B.; Shimelis, H.; Laing, M. Genetic variation in sorghum as revealed by phenotypic and SSR markers: Implications for combining ability and heterosis for grain yield. Plant Genet. Resour. Characterisation Util. 2017, 15, 335–347. [Google Scholar] [CrossRef]
- vom Brocke, K.; Trouche, G.; Weltzien, E.; Barro-Kondombo, C.P.; Gozé, E.; Chantereau, J. Participatory variety development for sorghum in Burkina Faso: Farmers’ selection and farmers’ criteria. Field Crops Res. 2010, 119, 183–194. [Google Scholar] [CrossRef]
- Yoon, S.-T.; Jeong, I.-H.; Han, T.-K.; Kim, Y.-J.; Yu, J.-B.; Yang, G.; Ye, M.-H.; Baek, S.-W.; Kim, K.-W. Evaluation of Crop Characteristics of Sorghum (Sorghum bicolor L.) Germplasm for the Selection of Excellent Resources. Korean J. Plant Resour. 2016, 29, 479–494. [Google Scholar] [CrossRef][Green Version]
- Ghimire, B.K.; Seo, J.W.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Comparative study on seed characteristics, antioxidant activity, and total phenolic and flavonoid contents in accessions of Sorghum bicolor (L.) Moench. Molecules 2021, 26, 3964. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, T.S.; Abdelhalim, N.S.; Kamal, N.M.; Mohamed, E.E.; Hassan, A.B. Exploiting the potential of Sudanese sorghum landraces in biofortification: Physicochemical quality of the grain of sorghum (Sorghum bicolor L. Moench) landraces. Food Chem. 2021, 337, 127604. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Zhu, S.; Sun, M.; Zhou, X. Understanding the flavor signature of the rice grown in different regions of China via metabolite profiling. J. Sci. Food Agric. 2022, 102, 3010–3020. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Hirata, S.; Yamada, T.; Sawad, Y.; ElSyaed, M.; Yamada, Y.; Sato, M.; Hira, Y.M.; Shigyo, M. Comprehensive Metabolite Profiling in Genetic Resources of Garlic (Allium sativum L.) Collected from Different Geographical Regions. Molecules 2021, 26, 1415. [Google Scholar] [CrossRef]
- Davis, H.; Su, X.; Shen, Y.; Xu, J.; Wang, D.; Scott Smith, J.; Aramouni, F.; Wang, W. Phenotypic Diversity of Colored Phytochemicals in Sorghum Accessions with Various Pericarp Pigments, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128137680. [Google Scholar]
- Han, L.; Chen, J.; Mace, E.S.; Liu, Y.; Zhu, M.; Yuyama, N.; Jordan, D.R.; Cai, H. Fine mapping of qGW1, a major QTL for grain weight in sorghum. Theor. Appl. Genet. 2015, 128, 1813–1825. [Google Scholar] [CrossRef]
- Sarfraz, Z.; Shah, M.M.; Iqbal, M.S.; Nazir, M.F.; Al-Ashkar, I.; Rehmani, M.I.A.; Shahid Iqbal, M.; Ullah, N.; El Sabagh, A. Rendering Multivariate Statistical Models for Genetic Diversity Assessment in A-Genome Diploid Wheat Population. Agronomy 2021, 11, 12339. [Google Scholar] [CrossRef]
- Moraes, É.A.; Marineli, R.D.S.; Lenquiste, S.A.; Steel, C.J.; de Menezes, C.B.; Queiroz, V.A.V.; Maróstica Júnior, M.R. Sorghum flour fractions: Correlations among polysaccharides, phenolic compounds, antioxidant activity and glycemic index. Food Chem. 2015, 180, 116–123. [Google Scholar] [CrossRef]
| IT-Number | DP (Days) | DM (Days) | SH (cm) | ST (mm) | PL (cm) | PW (mm) | TSW (g) |
|---|---|---|---|---|---|---|---|
| IT028365 | 50 | 90 | 120.33 ± 6.02 ah-as | 23.78 ± 0.98 a-d | 20.17 ± 2.02 u-ab | 70.59 ± 7.53 q-aj | 26.50 ± 0.36 bb-be |
| IT100010 | 46 | 82 | 150.00 ± 5.72 u-y | 15.26 ± 0.73 ah-as | 25.67 ± 3.79 j-s | 86.90 ± 7.26 j-t | 29.83 ± 0.12 z-ad |
| IT100018 | 46 | 82 | 169.33 ± 1.25 r-s | 15.22 ± 0.21 ah-as | 27.83 ± 2.25 g-n | 76.04 ± 3.25 n-ag | 31.47 ± 0.62 o-p |
| IT100024 | 42 | 83 | 152.67 ± 5.44 u-x | 15.96 ± 0.41 ad-ar | 28.00 ± 1.00 g-n | 82.79 ± 11.65 k-x | 29.37 ± 0.34 ad-ah |
| IT100045 | 46 | 82 | 95.33 ± 5.73 ax-ba | 22.04 ± 0.77 c-h | 31.33 ± 1.15 d-h | 62.70 ± 2.66 y-al | 24.20 ± 0.16 br-bt |
| IT100046 | 46 | 83 | 115.67 ± 2.05 aj-au | 18.81 ± 0.57 j-ae | 29.83 ± 1.04 e-j | 64.30 ± 7.20 v-al | 25.00 ± 0.90 bl-bo |
| IT100047 | 48 | 90 | 121.33 ± 3.40 ah-as | 21.75 ± 0.12 c-j | 23.33 ± 0.58 o-y | 74.20 ± 2.92 n-ai | 32.47 ± 0.17 f-i |
| IT100073 | 46 | 83 | 182.33 ± 3.09 m-r | 17.77 ± 0.47 p-al | 31.33 ± 1.53 d-h | 73.83 ± 2.75 o-ai | 27.70 ± 0.45 aq-av |
| IT100074 | 50 | 91 | 109.67 ± 3.86 ap-ax | 20.04 ± 0.98 f-u | 22.33 ± 1.15 q-aa | 72.61 ± 2.10 o-ai | 31.47 ± 0.12 o-p |
| IT100090 | 46 | 83 | 177.33 ± 3.30 o-r | 16.82 ± 0.94 x-ap | 26.33 ± 0.58 i-q | 71.47 ± 2.39 p-aj | 24.37 ± 0.34 bq-bs |
| IT100143 | 62 | 101 | 249.67 ± 4.50 c-e | 19.61 ± 0.02 g-y | 29.33 ± 2.89 f-k | 88.03 ± 5.48 i-r | 22.93 ± 0.05 bu |
| IT100177 | 46 | 83 | 159.33 ± 8.65 s-u | 16.37 ± 0.00 z-aq | 37.67 ± 2.08 c | 81.57 ± 14.54 k-aa | 26.83 ± 0.05 ay-bb |
| IT103099 | 40 | 78 | 109.67 ± 2.87 ap-ax | 12.72 ± 1.46 as | 21.00 ± 1.73 t-aa | 45.94 ± 3.84 al-am | 27.10 ± 0.16 aw-az |
| IT103452 | 48 | 85 | 177.00 ± 2.16 o-r | 15.78 ± 1.87 ae-ar | 23.33 ± 1.15 o-y | 90.73 ± 10.50 i-p | 29.17 ± 0.17 ae-ai |
| IT103496 | 48 | 90 | 192.33 ± 8.18 l-n | 17.77 ± 0.96 p-al | 30.33 ± 0.58 e-i | 99.48 ± 5.34 f-k | 30.40 ± 0.29 t-y |
| IT103970 | 42 | 83 | 193.67 ± 6.94 l-m | 14.55 ± 0.27 am-as | 24.67 ± 0.58 l-u | 68.15 ± 6.67 s-aj | 30.00 ± 0.22 x-aa |
| IT104574 | 56 | 93 | 168.67 ± 8.96 r-s | 18.54 ± 0.88 l-af | 37.67 ± 4.51 c | 120.50 ± 12.72 b-e | 28.17 ± 0.57 an-ar |
| IT104594 | 46 | 82 | 100.33 ± 1.70 au-ba | 21.28 ± 0.45 d-n | 29.33 ± 1.53 f-k | 71.44 ± 3.57 p-aj | 27.00 ± 0.08 ax-ba |
| IT104963 | 46 | 83 | 178.00 ± 2.83 n-r | 20.08 ± 0.09 f-u | 35.00 ± 3.00 c-d | 91.50 ± 8.57 i-o | 28.73 ± 0.26 ai-al |
| IT113294 | 42 | 83 | 139.67 ± 3.86 w-ae | 16.25 ± 1.27 ab-aq | 21.00 ± 1.00 t-aa | 69.77 ± 10.44 q-aj | 38.20 ± 1.73 a |
| IT134976 | 48 | 90 | 141.67 ± 2.36 w-ab | 18.47 ± 0.32 l-af | 27.33 ± 1.53 g-o | 79.47 ± 3.46 l-ac | 30.83 ± 3.12 q-t |
| IT158264 | 50 | 91 | 145.67 ± 4.19 u-z | 18.13 ± 0.53 o-aj | 23.00 ± 1.00 o-z | 73.04 ± 2.72 o-ai | 27.63 ± 5.12 as-av |
| IT158265 | 52 | 91 | 122.33 ± 3.68 ag-ar | 17.77 ± 0.39 p-al | 24.00 ± 1.73 m-w | 55.02 ± 4.47 ai-al | 27.47 ± 5.50 au-ax |
| IT162843 | 53 | 95 | 119.67 ± 10.66 ah-as | 19.68 ± 0.92 g-y | 23.33 ± 2.08 o-y | 73.43 ± 3.79 o-ai | 31.80 ± 6.22 k-o |
| IT162877 | 53 | 90 | 78.67 ± 4.50 bb | 18.46 ± 0.30 l-af | 21.67 ± 1.53 m-w | 58.08 ± 3.33 af-al | 29.07 ± 6.12 af-aj |
| IT180549 | 50 | 82 | 242.67 ± 2.05 d-f | 15.31 ± 0.39 ah-as | 35.00 ± 5.29 r-aa | 85.32 ± 9.17 j-u | 24.10 ± 8.22 bs-bt |
| IT180614 | 50 | 90 | 120.33 ± 2.05 ah-as | 16.73 ± 0.37 x-ap | 23.00 ± 3.61 c-d | 62.05 ± 2.42 z-al | 24.63 ± 8.05 bo-br |
| IT185760 | 56 | 99 | 129.33 ± 0.94 aa-al | 16.43 ± 0.16 z-aq | 21.33 ± 1.53 o-z | 64.26 ± 1.64 v-al | 28.10 ± 8.16 ao-as |
| IT185796 | 42 | 77 | 205.67 ± 7.36 j-l | 13.57 ± 0.19 aq-as | 35.00 ± 1.00 c-d | 75.01 ± 6.02 n-ah | 28.03 ± 8.17 ao-as |
| IT185807 | 49 | 86 | 186.83 ± 3.97 m-o | 14.99 ± 0.61 al-as | 29.92 ± 1.28 e-j | 81.74 ± 1.72 k-z | 26.92 ± 8.10 ay-bb |
| IT185812 | 50 | 83 | 206.33 ± 6.65 j-l | 13.53 ± 0.60 aq-as | 32.67 ± 2.08 d-f | 88.00 ± 21.34 i-r | 22.37 ± 8.09 bv |
| IT185813 | 42 | 82 | 202.67 ± 2.49 j-l | 15.38 ± 0.73 ag-as | 29.00 ± 1.00 f-l | 75.10 ± 7.43 n-ah | 24.07 ± 8.12 bs-bt |
| IT185816 | 42 | 77 | 194.33 ± 2.05 l-m | 13.16 ± 0.91 ar-as | 31.33 ± 1.53 d-h | 74.82 ± 5.13 n-ah | 26.53 ± 8.12 ba-bd |
| IT195442 | 62 | 99 | 144.67 ± 5.73 u-aa | 20.15 ± 0.36 f-t | 22.67 ± 2.08 p-z | 80.21 ± 6.46 l-ab | 30.83 ± 9.12 q-t |
| IT208560 | 47 | 84 | 174.67 ± 4.11 o-r | 14.08 ± 0.76 ao-as | 31.67 ± 2.08 d-g | 87.80 ± 7.99 i-s | 29.43 ± 0.25 ab-ag |
| IT208562 | 62 | 101 | 289.67 ± 6.85 a | 18.90 ± 0.65 j-ad | 51.33 ± 3.06 a | 99.63 ± 11.69 f-k | 25.33 ± 0.05 bj-bm |
| IT208566 | 56 | 95 | 256.33 ± 17.21 c-d | 20.69 ± 0.16 e-p | 37.67 ± 5.13 c | 83.56 ± 4.10 k-v | 27.27 ± 0.12 av-ay |
| IT208567 | 50 | 91 | 112.67 ± 3.68 am-av | 17.51 ± 0.18 q-an | 22.67 ± 1.15 p-z | 63.62 ± 4.64 w-al | 36.90 ± 0.08 b |
| IT208568 | 50 | 84 | 171.67 ± 0.47 p-s | 17.09 ± 0.29 t-an | 31.67 ± 0.58 d-g | 74.42 ± 9.67 n-ai | 29.07 ± 0.31 af-aj |
| IT208901 | 53 | 91 | 213.00 ± 14.31 i-j | 17.81 ± 0.62 p-al | 35.50 ± 2.60 c-d | 86.64 ± 7.19 j-t | 25.20 ± 0.08 bk-bn |
| IT221619 | 62 | 104 | 294.67 ± 10.50 a | 23.34 ± 0.03 a-e | 31.67 ± 1.53 d-g | 95.76 ± 5.89 g-m | 27.67 ± 2.17 ar-av |
| IT230297 | 56 | 97 | 138.33 ± 6.24 x-af | 18.90 ± 0.76 j-ad | 23.00 ± 2.00 o-z | 73.58 ± 5.21 o-ai | 32.23 ± 3.12 h-k |
| IT235850 | 50 | 90 | 139.33 ± 10.21 w-ae | 19.95 ± 0.74 f-v | 26.33 ± 2.52 i-q | 77.14 ± 4.11 m-ag | 26.50 ± 3.33 bb-be |
| IT235856 | 62 | 101 | 250.00 ± 21.21 c-e | 22.76 ± 0.34 b-f | 37.33 ± 4.93 c | 102.99 ± 11.63 e-j | 30.87 ± 3.12 q-t |
| IT251882 | 62 | 101 | 185.00 ± 2.45 m-q | 20.05 ± 0.90 f-u | 23.00 ± 1.00 o-z | 106.43 ± 7.40 e-i | 33.97 ± 5.17 d |
| IT262553 | 53 | 95 | 167.67 ± 2.05 r-t | 17.60 ± 0.43 q-al | 21.67 ± 2.08 r-aa | 71.05 ± 4.01 q-aj | 31.90 ± 6.08 j-o |
| IT262554 | 50 | 90 | 131.67 ± 9.03 z-aj | 22.03 ± 0.61 c-h | 20.67 ± 1.15 t-aa | 74.15 ± 4.58 n-ai | 29.30 ± 6.08 ae-ah |
| IT262557 | 62 | 104 | 157.33 ± 3.30 s-v | 20.19 ± 0.85 f-s | 23.67 ± 1.53 n-x | 71.51 ± 6.23 p-aj | 32.00 ± 6.08 i-n |
| IT262566 | 53 | 93 | 129.33 ± 7.36 aa-al | 13.62 ± 0.66 aq-as | 24.67 ± 4.04 l-u | 70.35 ± 5.94 q-aj | 26.10 ± 6.16 bd-bg |
| IT262570 | 50 | 93 | 154.67 ± 1.89 t-w | 16.81 ± 0.18 x-ap | 20.00 ± 1.00 v-ab | 63.58 ± 4.43 w-al | 23.87 ± 6.09 bt |
| IT262576 | 48 | 90 | 125.67 ± 1.70 ac-ap | 17.89 ± 0.52 p-al | 20.67 ± 2.52 t-aa | 61.23 ± 5.98 ab-al | 25.57 ± 6.25 bh-bk |
| IT264998 | 53 | 91 | 150.67 ± 0.47 u-x | 20.23 ± 0.81 f-s | 25.67 ± 1.53 j-s | 74.81 ± 2.89 n-ah | 25.67 ± 6.12 bg-bk |
| IT270343 | 50 | 90 | 121.33 ± 1.89 ah-as | 18.54 ± 0.94 l-af | 22.67 ± 1.53 p-z | 57.32 ± 2.35 ag-al | 25.00 ± 7.08 bl-bo |
| IT270346 | 48 | 85 | 126.67 ± 4.03 ab-an | 20.58 ± 0.28 e-q | 22.00 ± 1.00 q-aa | 74.93 ± 4.92 n-ah | 25.43 ± 7.12 bj-bl |
| IT270349 | 56 | 91 | 115.33 ± 4.50 ak-au | 18.93 ± 0.18 j-ad | 22.67 ± 1.15 p-z | 58.59 ± 5.66 ae-al | 24.90 ± 7.08 bm-bp |
| IT270366 | 70 | 109 | 93.33 ± 2.49 ay-ba | 22.60 ± 0.71 b-g | 28.00 ± 1.00 g-n | 76.32 ± 7.73 n-ag | 25.00 ± 7.08 bl-bo |
| IT278444 | 50 | 93 | 109.67 ± 5.31 ap-ax | 19.18 ± 0.46 h-ac | 22.67 ± 0.58 p-z | 69.80 ± 5.59 q-aj | 28.07 ± 7.12 ao-as |
| IT278445 | 53 | 93 | 114.00 ± 3.27 ak-av | 16.73 ± 0.49 x-ap | 23.00 ± 2.00 o-z | 77.97 ± 3.62 m-ae | 29.60 ± 7.08 aa-ae |
| IT286399 | 52 | 90 | 113.00 ± 2.94 am-av | 16.40 ± 0.36 z-aq | 21.67 ± 1.15 r-aa | 65.56 ± 1.05 u-ak | 23.07 ± 8.12 bu |
| IT286403 | 52 | 90 | 127.67 ± 3.09 ab-am | 17.39 ± 0.77 r-an | 18.67 ± 2.31 z-ab | 52.26 ± 4.79 aj-al | 32.10 ± 8.14 h-m |
| IT286412 | 53 | 91 | 140.67 ± 0.94 w-ae | 17.19 ± 0.75 s-an | 18.00 ± 1.00 aa-ab | 69.33 ± 6.31 q-aj | 30.43 ± 8.12 t-x |
| IT286423 | 50 | 90 | 108.33 ± 4.99 aq-ay | 18.55 ± 0.10 l-af | 20.67 ± 2.08 t-aa | 67.63 ± 3.82 t-aj | 28.07 ± 8.12 ao-as |
| IT286424 | 52 | 90 | 105.33 ± 8.34 as-ba | 17.93 ± 0.90 p-al | 20.67 ± 1.15 t-aa | 75.08 ± 8.85 n-ah | 25.00 ± 8.08 bl-bo |
| IT286446 | 53 | 91 | 132.67 ± 7.04 z-ai | 21.98 ± 0.55 c-i | 23.00 ± 1.00 o-z | 80.22 ± 1.33 l-ab | 29.43 ± 8.05 ac-ag |
| IT286448 | 70 | 109 | 212.33 ± 6.13 i-j | 17.75 ± 0.31 p-al | 42.33 ± 2.52 b | 151.42 ± 6.65 a | 28.78 ± 8.12 ai-al |
| IT297192 | 62 | 101 | 130.00 ± 8.16 a-ak | 13.92 ± 0.20 ap-as | 21.33 ± 1.53 s-aa | 78.55 ± 4.73 m-ad | 29.63 ± 9.52 aa-ae |
| IT300032 | 53 | 91 | 125.33 ± 3.40 ad-ap | 17.77 ± 0.44 p-al | 20.00 ± 0.00 v-ab | 59.10 ± 1.93 ad-al | 29.90 ± 0.08 z-ac |
| IT300088 | 56 | 93 | 122.33 ± 2.05 ag-ar | 17.77 ± 0.11 p-al | 21.33 ± 2.31 s-aa | 57.81 ± 3.67 ag-al | 28.90 ± 0.16 ah-ak |
| IT320893 | 56 | 95 | 110.00 ± 6.38 ao-ax | 15.04 ± 1.27 ak-as | 19.67 ± 3.21 w-ab | 63.11 ± 8.82 x-al | 27.93 ± 2.12 ap-au |
| IT320897 | 53 | 90 | 107.67 ± 5.56 aq-az | 14.54 ± 0.42 an-as | 18.00 ± 2.00 aa-ab | 64.50 ± 4.18 v-al | 30.90 ± 2.08 q-t |
| IT320898 | 70 | 106 | 224.67 ± 10.87 h-i | 14.90 ± 1.41 al-as | 34.00 ± 3.61 c-e | 126.51 ± 16.19 b-d | 35.30 ± 2.16 c |
| IT322510 | 56 | 100 | 141.00 ± 8.60 w-ad | 17.56 ± 0.54 q-an | 21.33 ± 1.53 s-aa | 66.36 ± 1.75 u-ak | 30.20 ± 2.22 u-z |
| IT322513 | 50 | 90 | 117.00 ± 2.94 ai-at | 16.35 ± 0.15 aa-aq | 25.00 ± 1.00 k-t | 68.18 ± 1.23 s-aj | 26.67 ± 2.12 az-bc |
| IT322530 | 48 | 88 | 120.00 ± 4.55 ah-as | 19.80 ± 0.78 f-x | 24.33 ± 1.15 m-v | 66.23 ± 6.20 u-ak | 30.97 ± 2.17 q-s |
| IT322531 | 48 | 90 | 126.00 ± 4.08 ab-ao | 21.46 ± 0.87 d-l | 22.67 ± 1.53 p-z | 67.96 ± 7.41 t-aj | 33.90 ± 2.16 d |
| IT322533 | 50 | 88 | 113.33 ± 5.25 al-av | 19.32 ± 0.35 h-ab | 23.33 ± 1.53 o-y | 66.60 ± 4.08 u-ak | 27.13 ± 2.12 aw-az |
| IT322546 | 50 | 90 | 123.00 ± 4.90 af-aq | 17.53 ± 0.11 q-an | 20.33 ± 1.53 u-aa | 61.91 ± 7.01 aa-al | 27.30 ± 2.16 av-ay |
| IT322549 | 70 | 106 | 92.67 ± 1.89 az-ba | 25.76 ± 0.35 a | 26.00 ± 2.78 i-r | 113.10 ± 2.54 d-g | 25.70 ± 2.16 bg-bj |
| IT322554 | 52 | 91 | 108.00 ± 5.89 aq-ay | 15.33 ± 0.88 ag-as | 20.33 ± 0.58 u-aa | 59.89 ± 5.65 ac-al | 30.90 ± 2.08 q-t |
| IT322555 | 62 | 105 | 121.33 ± 0.94 ah-as | 17.10 ± 0.90 t-an | 23.33 ± 0.58 o-y | 86.51 ± 12.07 j-t | 32.13 ± 2.17 h-l |
| IT322558 | 68 | 109 | 129.33 ± 6.13 aa-al | 17.58 ± 0.20 q-am | 20.67 ± 1.15 t-aa | 83.32 ± 3.96 k-w | 29.13 ± 2.17 ae-aj |
| IT322570 | 62 | 105 | 106.67 ± 4.64 ar-ba | 15.17 ± 0.25 aj-as | 20.67 ± 1.15 t-aa | 58.18 ± 4.85 ae-al | 31.67 ± 2.17 l-o |
| IT322571 | 63 | 105 | 109.67 ± 3.30 ap-ax | 17.34 ± 0.79 r-an | 21.67 ± 1.53 r-aa | 67.98 ± 7.64 t-aj | 30.57 ± 2.17 r-v |
| IT322572 | 52 | 90 | 110.67 ± 3.30 an-ax | 19.12 ± 0.20 h-ac | 22.67 ± 2.31 p-z | 62.53 ± 2.20 y-al | 24.83 ± 2.12 bn-bq |
| IT322578 | 52 | 90 | 111.67 ± 3.40 am-aw | 19.10 ± 0.77 h-ac | 23.00 ± 1.73 o-z | 57.60 ± 3.66 ag-al | 29.53 ± 2.05 aa-af |
| IT322580 | 57 | 101 | 149.67 ± 2.87 u-y | 20.72 ± 0.64 e-p | 22.00 ± 1.00 q-aa | 71.31 ± 10.06 p-aj | 31.03 ± 2.12 p-r |
| IT322613 | 74 | 113 | 96.33 ± 2.05 aw-ba | 20.38 ± 0.93 f-r | 28.33 ± 1.53 g-m | 72.75 ± 5.08 o-ai | 26.03 ± 2.17 be-bh |
| IT322621 | 56 | 99 | 145.67 ± 4.19 u-z | 19.93 ± 0.67 f-w | 25.00 ± 1.73 k-t | 71.97 ± 1.29 o-aj | 26.03 ± 2.17 be-bh |
| IT322622 | 52 | 99 | 127.67 ± 3.40 ab-am | 21.29 ± 0.68 d-n | 23.33 ± 0.58 o-y | 76.57 ± 4.43 m-ag | 28.20 ± 2.22 an-aq |
| IT329008 | 50 | 90 | 116.67 ± 0.47 ai-at | 19.37 ± 0.23 h-aa | 22.00 ± 1.73 q-aa | 67.70 ± 2.78 t-aj | 28.70 ± 2.08 ai-am |
| IT329026 | 48 | 90 | 120.67 ± 2.87 ah-as | 18.53 ± 0.11 l-af | 23.33 ± 0.58 o-y | 69.60 ± 2.58 q-aj | 28.53 ± 2.12 ak-ao |
| IT329047 | 63 | 105 | 243.00 ± 1.63 d-f | 18.94 ± 0.23 j-ad | 26.33 ± 4.16 i-q | 110.10 ± 6.12 d-h | 29.43 ± 2.12 ac-ag |
| IT329048 | 62 | 106 | 170.67 ± 9.88 q-s | 15.20 ± 0.85 ai-as | 42.00 ± 1.00 b | 135.68 ± 11.22 b | 28.10 ± 2.16 ao-as |
| IT329049 | 57 | 100 | 256.67 ± 15.46 c-d | 16.87 ± 0.51 v-ap | 37.33 ± 5.13 c | 159.93 ± 24.19 a | 26.30 ± 2.29 bc-bf |
| IT329050 | 53 | 91 | 233.33 ± 17.00 f-h | 17.76 ± 0.85 p-al | 33.67 ± 1.53 c-e | 130.97 ± 13.41 b-c | 29.60 ± 2.08 a-ae |
| IT329053 | 47 | 88 | 141.33 ± 4.92 w-ac | 18.65 ± 0.25 l-ae | 24.67 ± 2.52 l-u | 58.85 ± 3.17 ad-al | 32.30 ± 2.16 g-j |
| IT329056 | 56 | 98 | 135.00 ± 6.16 y-ah | 18.62 ± 0.19 l-ae | 22.33 ± 1.53 q-aa | 82.50 ± 6.57 k-x | 34.10 ± 2.16 d |
| IT329063 | 52 | 90 | 110.33 ± 2.49 ao-ax | 18.08 ± 0.50 o-ak | 20.00 ± 0.00 v-ab | 57.76 ± 3.83 ag-al | 28.13 ± 2.17 ao-as |
| IT329064 | 52 | 90 | 114.67 ± 1.70 ak-av | 18.27 ± 0.46 m-ah | 19.67 ± 0.58 w-ab | 59.06 ± 6.24 ad-al | 28.63 ± 2.12 aj-an |
| IT329074 | 56 | 93 | 114.00 ± 7.87 ak-av | 17.79 ± 0.96 p-al | 24.00 ± 2.65 m-w | 77.81 ± 7.09 m-af | 28.23 ± 2.12 am-ap |
| IT329076 | 47 | 88 | 126.67 ± 2.36 ab-an | 18.24 ± 0.29 n-ai | 23.33 ± 2.52 o-y | 71.70 ± 3.14 p-aj | 22.03 ± 2.12 bv-bw |
| IT329077 | 63 | 105 | 174.33 ± 12.50 o-r | 24.32 ± 0.73 a-c | 24.00 ± 2.65 m-w | 73.69 ± 7.32 o-ai | 30.50 ± 2.16 s-w |
| IT329078 | 50 | 90 | 130.00 ± 3.27 aa-ak | 18.38 ± 0.90 m-ag | 24.00 ± 2.65 m-w | 75.83 ± 6.20 n-ag | 25.50 ± 2.16 bi-bk |
| IT329082 | 53 | 90 | 125.00 ± 3.56 ae-ap | 18.84 ± 0.91 j-ae | 26.00 ± 1.00 i-r | 68.06 ± 3.69 t-aj | 27.70 ± 2.16 aq-av |
| IT329085 | 70 | 107 | 203.33 ± 4.71 j-l | 18.75 ± 1.32 j-ae | 30.00 ± 2.00 e-j | 93.49 ± 15.44 h-n | 27.47 ± 2.12 au-ax |
| IT329089 | 48 | 90 | 95.67 ± 6.65 ax-ba | 21.32 ± 0.48 d-m | 28.00 ± 1.00 g-n | 55.74 ± 11.74 ah-al | 21.90 ± 2.08 bw |
| IT329090 | 47 | 88 | 112.00 ± 1.63 am-av | 16.62 ± 0.59 y-ap | 20.67 ± 1.15 t-aa | 57.75 ± 3.78 ag-al | 24.47 ± 2.17 bp-bs |
| IT329108 | 56 | 98 | 240.67 ± 8.99 e-g | 18.18 ± 0.32 o-aj | 37.33 ± 5.51 c | 157.67 ± 8.11 a | 27.50 ± 2.08 at-aw |
| IT329120 | 56 | 98 | 112.33 ± 5.25 am-av | 17.66 ± 0.82 p-al | 22.00 ± 1.00 q-aa | 65.71 ± 4.62 u-ak | 32.57 ± 2.09 f-h |
| IT329124 | 53 | 90 | 103.00 ± 3.56 at-ba | 18.54 ± 0.71 l-af | 21.67 ± 2.08 r-aa | 63.87 ± 4.87 v-al | 32.73 ± 2.21 f-g |
| IT331874 | 52 | 90 | 120.00 ± 4.24 ah-as | 18.54 ± 0.56 l-af | 23.00 ± 1.00 o-z | 66.09 ± 3.00 u-ak | 27.97 ± 3.12 ap-at |
| IT331878 | 56 | 95 | 55.33 ± 4.11 bc | 17.36 ± 0.64 r-an | 16.00 ± 2.00 a-b | 35.50 ± 3.60 am | 27.07 ± 3.17 aw-az |
| IT331882 | 47 | 88 | 127.00 ± 8.04 ab-am | 16.85 ± 0.48 w-ap | 22.33 ± 2.08 q-aa | 68.96 ± 7.34 q-aj | 32.87 ± 3.54 e-f |
| IT331889 | 50 | 90 | 113.67 ± 3.30 al-av | 17.28 ± 0.62 s-an | 19.00 ± 2.65 y-zb | 58.52 ± 3.06 ae-al | 29.93 ± 3.05 y-zb |
| IT331894 | 47 | 88 | 108.33 ± 2.87 aq-ay | 17.73 ± 0.90 p-al | 19.33 ± 0.58 x-ab | 58.10 ± 3.56 ae-al | 28.33 ± 3.31 al-ap |
| IT331896 | 48 | 90 | 125.33 ± 4.64 ad-ap | 17.03 ± 0.86 u-ao | 21.67 ± 1.53 r-aa | 67.22 ± 8.47 t-ak | 26.00 ± 3.08 bf-bh |
| IT331899 | 48 | 88 | 127.00 ± 2.45 ab-am | 19.04 ± 0.35 h-ac | 22.67 ± 0.58 p-z | 67.80 ± 7.06 t-aj | 24.47 ± 3.05 bp-bs |
| IT331904 | 57 | 100 | 137.67 ± 9.84 x-ag | 21.46 ± 0.87 d-l | 27.33 ± 2.08 g-o | 84.99 ± 7.80 j-u | 26.30 ± 3.08 bc-bf |
| IT331907 | 62 | 100 | 243.67 ± 10.66 d-f | 19.71 ± 0.48 g-x | 26.00 ± 3.61 i-r | 103.28 ± 16.24 e-j | 33.30 ± 3.16 e |
| IT331921 | 62 | 105 | 143.33 ± 1.25 v-aa | 21.69 ± 0.98 c-k | 22.33 ± 3.21 q-aa | 67.46 ± 6.86 t-aj | 34.03 ± 3.12 d |
| IT331922 | 62 | 104 | 102.00 ± 2.83 at-ba | 23.43 ± 0.11 a-e | 24.67 ± 3.51 l-u | 82.13 ± 12.36 k-y | 30.13 ± 3.12 v-z |
| IT331936 | 70 | 109 | 99.33 ± 0.94 av-ba | 25.32 ± 0.68 ab | 27.00 ± 1.00 h-p | 99.56 ± 9.56 f-k | 25.93 ± 3.05 bf-bi |
| IT331937 | 47 | 90 | 114.00 ± 2.16 ak-va | 17.53 ± 0.08 q-an | 22.00 ± 2.65 q-aa | 65.64 ± 4.55 u-ak | 32.87 ± 3.12 e-f |
| IT331938 | 57 | 99 | 137.33 ± 4.11 x-ag | 18.99 ± 0.42 i-ad | 21.67 ± 1.53 r-aa | 88.22 ± 5.34 i-q | 30.00 ± 3.08 x-aa |
| IT331962 | 47 | 90 | 122.67 ± 3.30 af-ar | 18.17 ± 0.02 o-aj | 20.00 ± 2.00 q-aa | 63.06 ± 11.44 x-al | 30.03 ± 3.09 w-aa |
| IT331963 | 47 | 90 | 120.67 ± 1.70 ah-as | 15.27 ± 0.92 ah-as | 21.67 ± 2.08 r-aa | 68.49 ± 6.70 r-aj | 28.93 ± 3.05 ag-ak |
| IT331978 | 47 | 84 | 186.33 ± 2.62 m-p | 15.54 ± 0.55 af-as | 30.33 ± 1.53 e-i | 88.16 ± 3.94 i-r | 31.63 ± 3.26 m-o |
| IT331988 | 62 | 101 | 174.00 ± 9.93 o-r | 17.25 ± 0.16 s-an | 34.00 ± 0.00 c-e | 160.43 ± 25.98 a | 31.13 ± 3.12 p-q |
| IT332014 | 62 | 104 | 263.33 ± 4.71 b-c | 19.45 ± 0.10 h-z | 30.00 ± 2.00 e-j | 113.09 ± 11.40 d-g | 30.67 ± 3.26 q-u |
| IT332024 | 56 | 95 | 196.67 ± 17.00 k-m | 18.66 ± 0.66 l-ae | 22.67 ± 2.31 p-z | 73.75 ± 3.28 o-ai | 29.80 ± 3.08 z-ad |
| IT332042 | 47 | 88 | 114.33 ± 4.03 ak-av | 18.64 ± 0.25 l-ae | 23.00 ± 1.73 o-z | 71.07 ± 2.36 q-aj | 29.00 ± 3.08 ag-ak |
| IT332046 | 56 | 98 | 184.67 ± 11.12 m-q | 19.62 ± 0.97 g-y | 27.00 ± 2.65 h-p | 88.53 ± 8.46 i-q | 31.67 ± 3.12 l-o |
| IT340260 | 74 | 109 | 271.33 ± 13.82 b | 23.33 ± 0.04 a-e | 26.00 ± 3.46 i-r | 115.44 ± 12.22 c-f | 27.97 ± 4.25 ap-at |
| IT340261 | 71 | 109 | 228.50 ± 6.36 g-h | 21.13 ± 0.88 d-o | 37.50 ± 3.12 c | 120.62 ± 5.66 b-e | 25.40 ± 4.07 bj-bl |
| ITK276521 | 47 | 88 | 209.00 ± 7.87 j-k | 16.13 ± 0.73 ac-aq | 34.67 ± 2.08 c-d | 98.08 ± 7.60 f-l | 31.60 ± 2.08 n-o |
| IT231310 | 47 | 90 | 108.00 ± 6.53 aq-ay | 18.72 ± 0.36 k-ae | 22.33 ± 3.21 q-aa | 69.63 ± 1.79 q-aj | 28.90 ± 3.08 ah-ak |
| Nampungchal | 56 | 98 | 132.00 ± 3.56 z-ai | 18.53 ± 0.33 l-af | 22.33 ± 2.08 q-aa | 88.16 ± 5.45 i-r | 30.63 ± 2.09 r-u |
| Wheatland | 47 | 88 | 77.00 ± 0.82 bb | 21.04 ± 0.54 d-o | 21.33 ± 2.31 s-aa | 47.95 ± 3.83 ak-am | 37.93 ± 2.17 a |
| Sodamchal | 62 | 104 | 91.67 ± 2.49 ba | 24.52 ± 0.38 a-c | 27.00 ± 1.00 h-p | 81.13 ± 7.89 k-aa | 30.53 ± 3.21 r-v |
| Total range | 40–74 | 77–113 | 55.33–294.67 | 12.72–25.76 | 16.00–51.33 | 35.50–160.43 | 21.90–38.20 |
| Total mean | 53.48 | 93.10 | 147.83 | 18.40 | 25.65 | 78.31 | 28.71 |
| CV (%) | 13.62 | 8.41 | 32.49 | 13.76 | 22.65 | 27.67 | 10.80 |
| IT-Number | TTC (mg CE/g) | TPC (mg GAE/g) | ABTS (mg TE/g) | DPPH (mg AAE/g) | FRAP (mg AAE/g) |
|---|---|---|---|---|---|
| IT028365 | 179.94 ± 5.09 av-aw | 7.65 ± 0.07 n | 24.15 ± 1.19 ap-av | 13.93 ± 0.17 aq-as | 10.91 ± 0.11 w-z |
| IT100010 | 140.75 ± 4.48 bd-be | 3.24 ± 0.01 bk | 6.87 ± 0.40 bl-bm | 3.22 ± 0.05 bi-bj | 2.17 ± 0.03 b-c |
| IT100018 | 120.16 ± 1.61 bf | 3.01 ± 0.05 bl | 4.92 ± 0.28 bn | 2.44 ± 0.10 bk | 1.76 ± 0.05 b-c |
| IT100024 | 45.76 ± 0.00 bn | 3.81 ± 0.04 bi | 10.06 ± 0.73 bj | 4.49 ± 0.05 bg | 3.43 ± 0.01 az-ba |
| IT100045 | 83.46 ± 0.35 bi-bj | 3.29 ± 0.07 bk | 7.68 ± 0.26 bl | 3.24 ± 0.09 bi-bj | 2.26 ± 0.09 bb-bc |
| IT100046 | 47.00 ± 2.30 bn | 3.72 ± 0.03 bi-bj | 10.07 ± 0.65 bj | 4.01 ± 0.05 bh | 3.25 ± 0.12 az-ba |
| IT100047 | 180.93 ± 3.00 av-aw | 6.00 ± 0.01 am-ap | 20.97 ± 0.19 ay-ba | 9.76 ± 0.20 ax-az | 6.95 ± 0.20 au-av |
| IT100073 | 80.73 ± 1.05 bj | 5.10 ± 0.04 ba | 17.51 ± 0.81 bd-be | 8.11 ± 0.32 bb | 6.04 ± 0.12 aw |
| IT100074 | 140.50 ± 3.35 b-e | 6.05 ± 0.07 al-an | 20.75 ± 0.26 ay-ba | 10.29 ± 0.13 az | 7.55 ± 0.12 at-au |
| IT100090 | 91.64 ± 1.95 bh-bi | 5.86 ± 0.04 ao-ar | 20.58 ± 0.45 az-ba | 10.27 ± 0.20 az | 7.14 ± 0.12 au-av |
| IT100143 | 282.86 ± 1.95 r-v | 8.83 ± 0.11 e-f | 31.51 ± 1.08 t-x | 17.41 ± 0.26 za-ac | 13.07 ± 0.48 m-o |
| IT100177 | 66.16 ± 1.15 bl-bm | 4.64 ± 0.02 bd | 16.07 ± 0.40 bf-bg | 6.67 ± 0.09 bd-be | 4.96 ± 0.12 ax-ay |
| IT103099 | 377.60 ± 3.79 e | 2.44 ± 0.04 bn | 8.03 ± 0.07 bk-bl | 3.08 ± 0.02 bj | 2.27 ± 0.04 bb-bc |
| IT103452 | 122.64 ± 4.05 bf | 4.32 ± 0.06 be | 18.44 ± 0.38 bc-bd | 7.92 ± 0.09 bb | 5.97 ± 0.23 aw |
| IT103496 | 76.45 ± 2.13 bj-bk | 3.99 ± 0.06 bh | 16.65 ± 0.15 be-bf | 7.99 ± 0.32 bb | 5.48 ± 0.18 aw-ax |
| IT103970 | 151.91 ± 4.05 bb-bc | 4.26 ± 0.07 be-bf | 17.30 ± 0.28 bd-bf | 3.66 ± 0.00 bh-bi | 6.01 ± 0.07 aw |
| IT104574 | 114.89 ± 3.28 bf-bg | 4.11 ± 0.09 bf-bh | 17.73 ± 0.57 bc-be | 6.24 ± 0.07 be | 5.39 ± 0.19 aw-ax |
| IT104594 | 46.94 ± 1.15 bn | 2.70 ± 0.01 bm | 9.25 ± 0.20 bj-bk | 15.88 ± 0.21 ak-am | 2.86 ± 0.03 ba-bb |
| IT104963 | 61.32 ± 0.18 bl-bm | 3.60 ± 0.02 bj | 14.86 ± 0.41 bg-bh | 9.86 ± 0.13 ax-ay | 5.43 ± 0.17 aw-ax |
| IT113294 | 119.97 ± 0.93 bf | 2.31 ± 0.03 bn-bo | 7.39 ± 0.36 bl-bm | 1.75 ± 0.00 bl | 2.12 ± 0.01 bc |
| IT134976 | 344.81 ± 12.25 g-i | 5.92 ± 0.11 an-aq | 32.62 ± 0.14 p-t | 13.65 ± 0.10 ar-as | 13.71 ± 0.33 k-m |
| IT158264 | 162.52 ± 2.27 ay-ba | 4.84 ± 0.01 bb-bc | 25.29 ± 0.38 aj-ap | 9.93 ± 0.12 ax-ay | 10.51 ± 0.59 y-ab |
| IT158265 | 224.06 ± 0.90 ai-am | 4.89 ± 0.03 bb | 26.67 ± 0.23 ah-ak | 10.03 ± 0.17 ax-ay | 10.77 ± 0.17 x-aa |
| IT162843 | 398.27 ± 2.08 c | 4.09 ± 0.05 bf-bh | 22.03 ± 0.03 aw-ay | 8.85 ± 0.14 ba | 8.45 ± 0.15 an-as |
| IT162877 | 26.24 ± 1.23 bo | 1.57 ± 0.02 br | 1.64 ± 0.01 bo | 0.48 ± 0.01 bn | 0.63 ± 0.02 bd |
| IT180549 | 197.05 ± 3.31 ar-au | 5.11 ± 0.02 ba | 27.28 ± 0.14 af-ai | 11.11 ± 0.10 aw | 11.40 ± 0.33 t-x |
| IT180614 | 350.38 ± 2.73 gh | 4.70 ± 0.01 bc-bd | 25.11 ± 0.46 al-aq | 9.33 ± 0.20 az-ba | 9.28 ± 0.10 af-al |
| IT185760 | 217.93 ± 2.74 al-am | 5.63 ± 0.05 as-av | 31.07 ± 0.14 u-y | 12.68 ± 0.01 at | 13.17 ± 0.12 m-o |
| IT185796 | 69.09 ± 1.23 bk-bl | 2.86 ± 0.03 bl-bm | 13.23 ± 0.12 bi | 3.91 ± 0.03 bh | 4.67 ± 0.20 ay |
| IT185807 | 164.88 ± 1.20 ax-az | 3.62 ± 0.04 bj | 18.23 ± 0.40 b-d | 7.27 ± 0.18 b-c | 8.01 ± 0.28 aq-at |
| IT185812 | 186.88 ± 2.24 au-av | 4.73 ± 0.06 bb-bd | 24.62 ± 0.24 an-at | 9.52 ± 0.15 ay-az | 10.24 ± 0.16 za-ad |
| IT185813 | 154.72 ± 0.59 az-bc | 4.08 ± 0.06 bf-bh | 20.24 ± 0.23 ba-bb | 7.81 ± 0.13 bb | 8.54 ± 0.12 an-ar |
| IT185816 | 132.16 ± 2.66 be | 4.64 ± 0.03 bd | 23.05 ± 0.42 au-ax | 9.58 ± 0.04 ay-az | 9.69 ± 0.05 ac-ai |
| IT195442 | 209.30 ± 1.75 an-ap | 6.21 ± 0.07 aj-al | 33.17 ± 0.10 o-r | 14.53 ± 0.13 ao-ap | 14.39 ± 0.23 ij |
| IT208560 | 83.33 ± 1.01 bi-bj | 2.91 ± 0.05 bl | 13.30 ± 0.66 bi | 5.38 ± 0.23 bf | 5.54 ± 0.11 aw-ax |
| IT208562 | 195.93 ± 1.68 as-au | 6.45 ± 0.04 ac-ah | 34.42 ± 0.18 m-o | 15.43 ± 0.27 am-an | 17.02 ± 0.20 d-f |
| IT208566 | 108.77 ± 6.85 bg | 4.24 ± 0.21 be-bf | 19.97 ± 1.01 ba-bb | 8.26 ± 0.56 bb | 8.79 ± 0.56 ak-ap |
| IT208567 | 171.99 ± 6.12 aw-ay | 5.23 ± 0.05 ay-ba | 27.30 ± 0.37 af-ai | 11.04 ± 0.22 aw | 11.37 ± 0.08 t-x |
| IT208568 | 198.72 ± 1.04 aq-at | 3.98 ± 0.05 bh | 19.02 ± 0.45 bb-bc | 6.99 ± 0.24 bc-bd | 7.84 ± 0.32 as-at |
| IT208901 | 178.53 ± 2.13 av-aw | 4.04 ± 0.04 bg-bh | 20.33 ± 0.03 ba-bb | 7.25 ± 0.04 bc | 7.84 ± 0.39 as-at |
| IT221619 | 196.35 ± 1.68 ar-au | 6.67 ± 0.01 x-aa | 36.21 ± 0.44 j-k | 16.02 ± 0.22 ai-al | 17.30 ± 0.30 c-f |
| IT230297 | 265.22 ± 3.43 yz | 6.24 ± 0.03 ai-ak | 33.34 ± 0.08 n-q | 14.18 ± 0.01 ap-aq | 14.41 ± 0.29 i-j |
| IT235850 | 241.58 ± 9.33 ad-ag | 7.76 ± 0.26 m-n | 37.16 ± 0.83 i-j | 17.23 ± 0.67 aa-ad | 12.00 ± 0.38 r-t |
| IT235856 | 209.41 ± 4.10 an-ap | 7.46 ± 0.12 o | 33.34 ± 0.64 n-q | 16.82 ± 0.34 ad-ag | 11.90 ± 0.40 r-u |
| IT251882 | 264.96 ± 4.97 yz | 9.00 ± 0.05 c-d | 43.88 ± 0.20 b | 24.83 ± 0.86 f | 15.20 ± 1.02 h |
| IT262553 | 149.72 ± 2.06 bc-bd | 5.55 ± 0.09 au-av | 24.90 ± 0.52 al-ar | 10.85 ± 0.22 aw | 8.28 ± 0.22 ap-as |
| IT262554 | 146.97 ± 2.37 bc-bd | 5.52 ± 0.04 av-aw | 25.55 ± 0.17 aj-ap | 10.94 ± 0.12 aw | 7.86 ± 0.21 as-at |
| IT262557 | 199.50 ± 1.17 ap-at | 7.33 ± 0.04 o-q | 35.54 ± 0.37 k-m | 16.59 ± 0.17 ae-ah | 11.43 ± 0.39 t-x |
| IT262566 | 223.16 ± 5.06 aj-am | 6.01 ± 0.09 am-ap | 27.71 ± 0.21 ae-ah | 12.04 ± 0.13 au | 8.56 ± 0.28 am-ar |
| IT262570 | 282.29 ± 9.34 s-v | 6.47 ± 0.04 ab-ah | 32.31 ± 0.08 p-u | 13.65 ± 0.12 ar-as | 9.26 ± 0.13 af-am |
| IT262576 | 194.55 ± 1.35 at-au | 5.21 ± 0.07 ay-ba | 24.41 ± 0.18 ao-au | 9.92 ± 0.06 ax-ay | 7.16 ± 0.32 au-av |
| IT264998 | 261.94 ± 1.03 yz-ab | 7.18 ± 0.03 q-s | 35.39 ± 0.30 k-m | 15.88 ± 0.14 ak-am | 10.76 ± 0.28 x-aa |
| IT270343 | 295.76 ± 2.37 p-q | 7.76 ± 0.06 m-n | 38.71 ± 0.32 g-h | 17.24 ± 0.00 aa-ad | 11.96 ± 0.09 r-t |
| IT270346 | 288.34 ± 4.12 q-t | 7.22 ± 0.03 q-r | 35.97 ± 0.28 j-l | 15.93 ± 0.02 aj-am | 11.33 ± 0.31 t-x |
| IT270349 | 383.50 ± 5.09 d-e | 10.23 ± 0.08 a | 67.60 ± 1.06 a | 31.99 ± 0.16 a | 20.38 ± 0.31 b |
| IT270366 | 0.48 ± 0.00 bp | 1.17 ± 0.01 bs | 2.56 ± 0.14 bo | 0.89 ± 0.02 bm-bn | 0.89 ± 0.01 bd |
| IT278444 | 209.68 ± 3.83 an-ap | 7.26 ± 0.07 p-r | 38.23 ± 3.18 hi | 15.92 ± 0.15 aj-am | 11.19 ± 0.13 u-y |
| IT278445 | 277.06 ± 1.35 u-x | 8.24 ± 0.04 j | 39.87 ± 0.22 e-g | 18.61 ± 0.13 x | 13.54 ± 0.42 l-n |
| IT286399 | 350.22 ± 4.87 g-h | 3.61 ± 0.02 bj | 27.24 ± 0.53 ag-ai | 11.37 ± 0.16 av-aw | 7.93 ± 0.47 ar-at |
| IT286403 | 227.28 ± 4.49 ai-al | 6.16 ± 0.08 ak-am | 25.77 ± 0.90 aj-ao | 12.58 ± 0.07 at | 8.60 ± 0.39 ak-ar |
| IT286412 | 260.29 ± 2.72 za-ab | 6.61 ± 0.07 y-ad | 26.16 ± 0.54 ai-am | 13.43 ± 0.17 as | 10.29 ± 0.26 za-ac |
| IT286423 | 178.05 ± 5.39 av-aw | 5.92 ± 0.13 an-aq | 25.22 ± 2.36 ak-ap | 12.18 ± 0.14 at-au | 8.98 ± 0.03 ai-ap |
| IT286424 | 179.98 ± 6.65 av-aw | 5.97 ± 0.07 an-ap | 26.25 ± 0.32 ai-al | 12.29 ± 0.02 at-au | 8.82 ± 0.10 aj-ap |
| IT286446 | 253.96 ± 3.50 aa-ac | 7.67 ± 0.03 n | 34.65 ± 0.32 l-n | 17.28 ± 0.18 aa-ad | 12.27 ± 0.17 p-r |
| IT286448 | 267.16 ± 12.85 x-z | 9.31 ± 0.12 b | 41.50 ± 0.36 c-d | 27.00 ± 0.81 c | 16.80 ± 0.57 f-g |
| IT297192 | 231.28 ± 1.85 ag-aj | 6.89 ± 0.04 u-w | 32.24 ± 0.75 p-u | 21.80 ± 0.22 mn | 12.16 ± 0.34 q-s |
| IT300032 | 293.24 ± 2.52 p-r | 6.29 ± 0.07 ah-ak | 29.25 ± 0.65 za-ad | 19.94 ± 0.31 tu | 10.77 ± 0.13 x-aa |
| IT300088 | 220.08 ± 2.96 ak-am | 5.07 ± 0.06 ba | 21.77 ± 0.26 ax-az | 14.61 ± 0.06 ao-ap | 7.81 ± 0.10 as-at |
| IT320893 | 300.13 ± 5.94 o-p | 5.45 ± 0.04 av-ax | 23.41 ± 0.18 ar-aw | 15.95 ± 0.29 aj-am | 8.75 ± 0.16 ak-ap |
| IT320897 | 262.66 ± 10.61 y-aa | 6.81 ± 0.04 v-x | 30.89 ± 0.35 u-y | 21.40 ± 0.06 no | 12.27 ± 0.16 p-r |
| IT320898 | 161.00 ± 3.92 az-bb | 5.47 ± 0.19 av-ax | 22.10 ± 0.60 aw-ay | 16.31 ± 0.49 ag-ak | 9.31 ± 0.33 af-ak |
| IT322510 | 230.64 ± 2.39 ah-ak | 6.60 ± 0.01 za-ad | 28.40 ± 0.63 ac-ag | 21.01 ± 0.23 o-q | 11.78 ± 0.26 r-u |
| IT322513 | 281.40 ± 1.26 s-v | 7.19 ± 0.04 q-r | 35.51 ± 0.16 k-m | 22.59 ± 0.06 kl | 13.55 ± 0.19 l-m |
| IT322530 | 343.04 ± 2.18 h-i | 5.45 ± 0.02 h-i | 24.09 ± 0.73 ap-av | 16.26 ± 0.22 ah-ak | 9.00 ± 0.26 ai-ao |
| IT322531 | 300.29 ± 3.06 o-p | 5.86 ± 0.05 ao-ar | 26.07 ± 0.19 ai-an | 17.70 ± 0.03 z-aa | 9.82 ± 0.02 ab-ag |
| IT322533 | 243.45 ± 0.91 ac-af | 5.58 ± 0.04 at-av | 23.16 ± 0.12 at-ax | 16.25 ± 0.04 ah-ak | 9.95 ± 0.09 ab-af |
| IT322546 | 299.01 ± 0.99 o-p | 6.64 ± 0.12 x-ac | 28.47 ± 0.62 ac-ag | 20.72 ± 0.16 p-s | 11.90 ± 0.42 r-u |
| IT322549 | 329.11 ± 2.75 j-k | 8.58 ± 0.06 h | 40.45 ± 0.26 d-f | 23.55 ± 0.05 g-i | 17.58 ± 0.63 c-e |
| IT322554 | 218.16 ± 1.96 al-an | 6.59 ± 0.10 za-ae | 29.71 ± 0.30 y-ad | 21.09 ± 0.06 o-p | 12.23 ± 0.34 p-r |
| IT322555 | 277.55 ± 8.10 t-w | 7.90 ± 0.10 k-m | 36.81 ± 0.51 jk | 23.42 ± 0.09 g-i | 15.23 ± 0.09 h |
| IT322558 | 308.13 ± 4.01 n-o | 8.57 ± 0.01 h | 39.54 ± 0.25 e-h | 23.51 ± 0.04 g-i | 17.64 ± 0.14 c-d |
| IT322570 | 229.52 ± 2.84 ah-ak | 6.76 ± 0.07 w-z | 29.26 ± 0.39 za-ad | 21.85 ± 0.18 m-n | 12.37 ± 0.17 p-r |
| IT322571 | 270.51 ± 1.04 w-z | 7.42 ± 0.03 o-p | 33.52 ± 1.12 n-p | 22.79 ± 0.25 j-k | 13.92 ± 0.36 j-l |
| IT322572 | 214.79 ± 1.71 am-ao | 6.46 ± 0.09 ab-ah | 28.75 ± 0.82 ab-af | 19.67 ± 0.08 u-v | 11.19 ± 0.08 u-y |
| IT322578 | 313.26 ± 4.24 m-n | 6.79 ± 0.12 w-y | 31.71 ± 0.94 r-w | 21.27 ± 0.13 o | 12.30 ± 0.08 p-r |
| IT322580 | 428.95 ± 4.73 a | 8.77 ± 0.10 e-g | 40.80 ± 0.61 c-e | 23.57 ± 0.02 g-h | 17.68 ± 0.48 c |
| IT322613 | 0.12 ± 0.00 bp | 2.04 ± 0.02 bp | 1.84 ± 0.05 bo | 0.95 ± 0.01 bm-bn | 0.63 ± 0.03 bd |
| IT322621 | 174.29 ± 4.34 aw-ax | 5.71 ± 0.02 ar-au | 25.28 ± 0.37 aj-ap | 17.04 ± 0.06 ab-ae | 9.14 ± 0.15 ag-an |
| IT322622 | 248.90 ± 2.83 ac-ae | 6.89 ± 0.10 u-w | 31.90 ± 0.76 q-v | 22.46 ± 0.33 k-l | 12.71 ± 0.71 o-q |
| IT329008 | 282.70 ± 6.18 r-v | 7.37 ± 0.11 o-q | 29.36 ± 1.41 za-ad | 20.30 ± 0.21 s-t | 11.03 ± 0.15 v-y |
| IT329026 | 266.55 ± 1.78 y-z | 6.64 ± 0.01 x-ac | 24.87 ± 0.30 al-as | 18.60 ± 0.13 x | 9.72 ± 0.26 ac-ah |
| IT329047 | 252.18 ± 9.59 ab-ac | 8.67 ± 0.21 f-h | 38.30 ± 0.76 hi | 27.55 ± 0.48 b | 15.29 ± 0.94 h |
| IT329048 | 233.93 ± 12.59 af-ai | 8.40 ± 0.02 i | 34.40 ± 0.27 m-o | 25.79 ± 0.26 e | 14.79 ± 0.16 hi |
| IT329049 | 266.55 ± 3.20 y-z | 7.20 ± 0.05 q-r | 28.42 ± 0.19 ac-ag | 20.94 ± 0.10 o-q | 11.71 ± 0.21 r-v |
| IT329050 | 151.08 ± 3.68 bb-bc | 6.40 ± 0.07 ae-ai | 23.67 ± 0.25 aq-av | 17.41 ± 0.11 z-ac | 9.85 ± 0.11 ab-ag |
| IT329053 | 409.72 ± 8.46 b | 7.43 ± 0.12 o-p | 28.95 ± 0.88 aa-ae | 20.54 ± 0.35 q-s | 10.77 ± 0.41 x-aa |
| IT329056 | 353.76 ± 11.25 g | 7.87 ± 0.02 l-m | 32.73 ± 0.87 p-t | 22.79 ± 0.34 j-k | 11.82 ± 0.16 r-u |
| IT329063 | 249.76 ± 1.05 ac-ad | 7.09 ± 0.04 r-t | 26.78 ± 0.48 ah-aj | 19.33 ± 0.05 v | 10.31 ± 0.21 za-ac |
| IT329064 | 197.43 ± 4.41 aq-au | 6.50 ± 0.11 aa-ag | 22.69 ± 0.51 av-ax | 16.53 ± 0.28 ae-ai | 9.68 ± 0.22 ac-ai |
| IT329074 | 335.03 ± 6.28 ij | 8.81 ± 0.02 e-f | 36.08 ± 0.62 jk | 26.99 ± 0.11 c | 15.23 ± 0.19 h |
| IT329076 | 238.61 ± 0.79 ae-ah | 7.00 ± 0.05 s-u | 28.27 ± 0.31 ad-ag | 18.28 ± 0.27 x-y | 9.90 ± 0.27 ab-af |
| IT329077 | 320.82 ± 3.76 k-m | 8.89 ± 0.09 d-e | 36.18 ± 0.64 jk | 26.28 ± 0.28 d | 14.23 ± 0.19 i-k |
| IT329078 | 201.95 ± 1.83 ap-at | 6.91 ± 0.11 u-w | 26.76 ± 0.36 ah-aj | 17.68 ± 0.25 z-aa | 10.18 ± 0.12 aa-ae |
| IT329082 | 193.88 ± 1.27 at-au | 6.77 ± 0.13 w-z | 24.71 ± 0.15 am-as | 16.86 ± 0.25 ac-af | 9.49 ± 0.45 ae-aj |
| IT329085 | 33.67 ± 1.24 bo | 2.31 ± 0.01 bn-bo | 24.44 ± 0.21 ao-au | 3.93 ± 0.19 bh | 2.09 ± 0.10 bc |
| IT329089 | 156.90 ± 2.69 az-bc | 4.20 ± 0.08 be-bg | 14.13 ± 0.68 bh-bi | 8.94 ± 0.14 ba | 5.11 ± 0.15 ax-ay |
| IT329090 | 249.43 ± 5.65 ac-ad | 5.31 ± 0.06 ax-az | 18.37 ± 0.22 bc-bd | 11.83 ± 0.17 au-av | 7.01 ± 0.14 au-av |
| IT329108 | 171.92 ± 2.57 aw-ay | 6.44 ± 0.11 ad-ah | 23.62 ± 0.35 aq-av | 16.46 ± 0.14 af-aj | 9.56 ± 0.38 ad-ai |
| IT329120 | 296.27 ± 1.58 p-q | 8.04 ± 0.15 k-l | 31.62 ± 0.48 s-w | 21.92 ± 0.08 m-n | 12.27 ± 0.17 p-r |
| IT329124 | 283.19 ± 0.00 r-u | 6.38 ± 0.07 af-aj | 24.05 ± 0.28 ap-av | 15.69 ± 0.20 al-am | 8.49 ± 0.18 an-as |
| IT331874 | 365.79 ± 0.79 f | 8.06 ± 0.09 k | 33.04 ± 0.15 o-s | 21.90 ± 0.28 m-n | 12.33 ± 0.23 p-r |
| IT331878 | 346.50 ± 6.44 g-h | 9.08 ± 0.04 c | 39.22 ± 0.27 f-h | 27.77 ± 0.13 b | 16.31 ± 0.23 g |
| IT331882 | 323.87 ± 3.38 k-l | 6.05 ± 0.07 al-ao | 30.35 ± 0.36 w-aa | 21.27 ± 0.12 o | 10.51 ± 0.22 y-ab |
| IT331889 | 272.22 ± 4.46 v-y | 5.93 ± 0.07 an-aq | 29.84 ± 0.22 y-ac | 20.85 ± 0.33 o-r | 9.81 ± 0.19 ab-ag |
| IT331894 | 342.15 ± 4.57 h-i | 5.36 ± 0.02 aw-ay | 25.12 ± 0.77 al-aq | 18.30 ± 0.15 x-y | 8.59 ± 0.25 al-ar |
| IT331896 | 346.59 ± 2.61 g-h | 6.60 ± 0.01 y-ad | 30.47 ± 0.16 v-z | 23.01 ± 0.01 i-k | 12.33 ± 0.19 p-r |
| IT331899 | 224.65 ± 6.13 ai-am | 5.15 ± 0.08 az-ba | 21.89 ± 0.22 ax-az | 17.95 ± 0.43 y-z | 8.68 ± 0.18 ak-aq |
| IT331904 | 318.37 ± 7.54 l-m | 7.33 ± 0.12 o-q | 32.72 ± 0.29 p-t | 23.69 ± 0.05 g-h | 14.72 ± 0.30 h-i |
| IT331907 | 285.36 ± 3.45 r-u | 6.99 ± 0.02 t-v | 31.80 ± 0.38 r-w | 23.54 ± 0.04 g-i | 13.31 ± 0.19 l-o |
| IT331921 | 198.21 ± 1.76 aq-at | 6.65 ± 0.08 x-ab | 29.71 ± 0.86 y-ad | 23.15 ± 0.09 h-j | 12.03 ± 0.29 q-t |
| IT331922 | 334.88 ± 0.87 i-j | 7.13 ± 0.04 r-t | 30.97 ± 0.26 u-y | 23.55 ± 0.08 g-i | 12.92 ± 0.10 n-p |
| IT331936 | 388.25 ± 7.24 d | 7.99 ± 0.07 k-l | 36.99 ± 0.35 i-j | 23.69 ± 0.01 g-h | 16.99 ± 0.24 e-f |
| IT331937 | 206.90 ± 1.53 ao-ar | 5.57 ± 0.12 at-av | 26.20 ± 0.42 ai-am | 19.24 ± 0.13 v-w | 9.68 ± 0.20 ac-ai |
| IT331938 | 265.83 ± 2.39 y-z | 6.45 ± 0.06 ac-ah | 28.74 ± 0.70 ab-af | 22.57 ± 0.12 k-l | 11.76 ± 0.17 r-u |
| IT331962 | 282.52 ± 2.39 r-v | 6.35 ± 0.10 ag-aj | 28.41 ± 0.73 ac-ag | 22.10 ± 0.06 l-m | 11.48 ± 0.46 s-w |
| IT331963 | 224.48 ± 7.33 ai-am | 5.25 ± 0.03 ay-ba | 22.06 ± 0.21 aw-ay | 17.66 ± 0.14 z-aa | 8.34 ± 0.20 ao-as |
| IT331978 | 153.66 ± 0.50 ba-bc | 4.62 ± 0.01 bd | 17.80 ± 0.11 bc-be | 15.01 ± 0.08 an-ao | 6.84 ± 0.13 av |
| IT331988 | 197.32 ± 3.70 aq-au | 5.82 ± 0.05 ap-ar | 23.39 ± 0.36 as-aw | 19.58 ± 0.09 u-v | 9.06 ± 0.12 ah-an |
| IT332014 | 239.74 ± 5.67 ad-ah | 6.66 ± 0.01 x-ab | 30.15 ± 0.26 x-ab | 23.01 ± 0.15 i-k | 12.72 ± 0.15 o-q |
| IT332024 | 253.94 ± 7.18 aa-ac | 6.59 ± 0.14 za-ae | 28.58 ± 0.72 ac-ag | 22.74 ± 0.16 j-k | 12.26 ± 0.25 p-r |
| IT332042 | 291.57 ± 10.51 p-s | 5.75 ± 0.11 aq-at | 25.30 ± 0.45 aj-ap | 19.36 ± 0.14 v | 9.06 ± 0.46 ah-an |
| IT332046 | 206.19 ± 4.11 ao-as | 5.76 ± 0.05 aq-as | 25.67 ± 0.21 aj-ao | 20.35 ± 0.08 r-t | 9.92 ± 0.20 ab-af |
| IT340260 | 285.53 ± 1.96 r-u | 6.56 ± 0.02 aa-af | 29.40 ± 0.40 za-ad | 22.62 ± 0.14 j-l | 11.72 ± 0.44 r-v |
| IT340261 | 415.58 ± 1.00 b | 8.62 ± 0.01 gh | 41.89 ± 0.79 c | 23.80 ± 0.01 g | 21.56 ± 0.52 a |
| K276521 | 93.95 ± 1.53 bh | 2.25 ± 0.06 bo | 9.79 ± 0.45 bj | 5.10 ± 0.07 bf | 3.57 ± 0.03 az |
| IT231310 | 208.06 ± 17.61 an-aq | 4.85 ± 0.38 bb-bc | 30.66 ± 2.47 v-z | 14.01 ± 1.35 aq-ar | 9.76 ± 0.99 ac-ah |
| Nampungchal | 323.36 ± 0.73 k-l | 6.31 ± 0.03 ag-ak | 39.85 ± 0.42 e-g | 18.79 ± 0.17 wx | 13.72 ± 0.35 k-m |
| Wheatland | 58.61 ± 2.15 bm | 1.82 ± 0.14 bq | 6.14 ± 0.31 bm-bn | 1.05 ± 0.05 bm | 1.90 ± 0.07 bc |
| Sodamchal | 241.15 ± 3.30 ad-ag | 5.74 ± 0.07 aq-at | 36.79 ± 0.17 jk | 17.57 ± 0.33 z-ab | 12.41 ± 0.33 p-r |
| Total range | 0.12–428.95 | 1.17–10.23 | 1.64–67.60 | 0.48–31.99 | 0.63–21.56 |
| Total mean | 224.35 | 5.96 | 26.41 | 15.55 | 10.06 |
| CV (%) | 41.12 | 29.93 | 36.13 | 44.61 | 40.04 |
| Days to Maturity | Thousand-Seed Weight | Total Tannin Content | Total Phenolic Content | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Landrace | >105 Days | Landrace | >35.00 g | Landrace | >350 mg CE/g | Landrace | >8.5 mg GAE/g | |||
| IT322613 | 113 | IT113294 | 38.20 | IT322580 | 428.95 | IT270349 | 10.23 | |||
| IT331936 | 109 | IT208567 | 36.90 | IT340261 | 415.58 | IT286448 | 9.31 | |||
| IT340260 | 109 | IT320898 | 35.30 | IT329053 | 409.72 | IT331878 | 9.08 | |||
| IT270366 | 109 | <23.00 g | IT162843 | 398.27 | IT251882 | 9.00 | ||||
| IT340261 | 109 | IT100143 | 22.93 | IT331936 | 388.25 | IT329077 | 8.89 | |||
| IT286448 | 109 | IT185812 | 22.37 | IT270349 | 383.50 | IT100143 | 8.83 | |||
| IT322558 | 109 | IT329076 | 22.03 | IT103099 | 377.60 | IT329074 | 8.81 | |||
| IT329085 | 107 | IT329089 | 21.90 | IT331874 | 365.79 | IT322580 | 8.77 | |||
| IT322549 | 106 | IT329056 | 353.76 | IT329047 | 8.67 | |||||
| IT329048 | 106 | IT180614 | 350.38 | IT340261 | 8.62 | |||||
| IT320898 | 106 | IT286399 | 350.22 | IT322549 | 8.58 | |||||
| <80 days | <50 mg CE/g | IT322558 | 8.57 | |||||||
| IT103099 | 78 | IT100046 | 47.00 | <2.5 mg GAE/g | ||||||
| IT185796 | 77 | IT104594 | 46.94 | IT103099 | 2.44 | |||||
| IT185816 | 77 | IT100024 | 45.76 | IT113294 | 2.31 | |||||
| IT329085 | 33.67 | IT329085 | 2.31 | |||||||
| IT162877 | 26.24 | K276521 | 2.25 | |||||||
| IT270366 | 0.48 | IT322613 | 2.04 | |||||||
| IT322613 | 0.12 | IT162877 | 1.57 | |||||||
| IT270366 | 1.17 | |||||||||
| Wheatland | 88 | 37.93 | 58.61 | 1.82 | ||||||
| Nampungchal | 98 | 30.63 | 323.36 | 6.31 | ||||||
| Sodamchal | 104 | 30.53 | 241.15 | 5.74 | ||||||
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| TTC | 10.21 | 6.78 | 3.48 | 0.05 |
| TPC | 16.37 | 1.05 | 2.93 | 0.02 |
| ABTS | 16.10 | 1.03 | 4.62 | 0.16 |
| DPPH | 16.00 | 1.16 | 1.16 | 0.70 |
| FRAP | 17.16 | 0.13 | 4.23 | 0.03 |
| Days to panicle | 8.67 | 7.96 | 16.26 | 0.15 |
| Days to maturity | 10.09 | 5.30 | 18.52 | 0.03 |
| Stem height | 0.14 | 23.19 | 10.22 | 1.62 |
| Stem thickness | 3.40 | 0.43 | 23.97 | 16.33 |
| Panicle length | 0.18 | 27.17 | 6.42 | 1.29 |
| Panicle width | 1.44 | 25.68 | 1.28 | 1.85 |
| Thousand seed weight | 0.24 | 0.11 | 6.90 | 77.76 |
| Eigenvalue | 5.05 | 2.78 | 1.29 | 1.06 |
| Variability (%) | 42.06 | 23.16 | 10.73 | 8.86 |
| Cumulative (%) | 42.06 | 65.21 | 75.94 | 84.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Choi, Y.-M.; Shin, M.-J.; Yoon, H.; Wang, X.; Lee, Y.; Yi, J.; Desta, K.T. Agro-Morphological and Biochemical Characterization of Korean Sorghum (Sorghum bicolor (L.) Moench) Landraces. Agronomy 2022, 12, 2898. https://doi.org/10.3390/agronomy12112898
Lee S, Choi Y-M, Shin M-J, Yoon H, Wang X, Lee Y, Yi J, Desta KT. Agro-Morphological and Biochemical Characterization of Korean Sorghum (Sorghum bicolor (L.) Moench) Landraces. Agronomy. 2022; 12(11):2898. https://doi.org/10.3390/agronomy12112898
Chicago/Turabian StyleLee, Sukyeung, Yu-Mi Choi, Myoung-Jae Shin, Hyemyeong Yoon, Xiaohan Wang, Yoonjung Lee, Jungyoon Yi, and Kebede Taye Desta. 2022. "Agro-Morphological and Biochemical Characterization of Korean Sorghum (Sorghum bicolor (L.) Moench) Landraces" Agronomy 12, no. 11: 2898. https://doi.org/10.3390/agronomy12112898
APA StyleLee, S., Choi, Y.-M., Shin, M.-J., Yoon, H., Wang, X., Lee, Y., Yi, J., & Desta, K. T. (2022). Agro-Morphological and Biochemical Characterization of Korean Sorghum (Sorghum bicolor (L.) Moench) Landraces. Agronomy, 12(11), 2898. https://doi.org/10.3390/agronomy12112898





