Influence Evaluation of Enzyme Treatments on Aroma Profile of White Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Vine Growing Cultural Conditions and Winemaking Processes
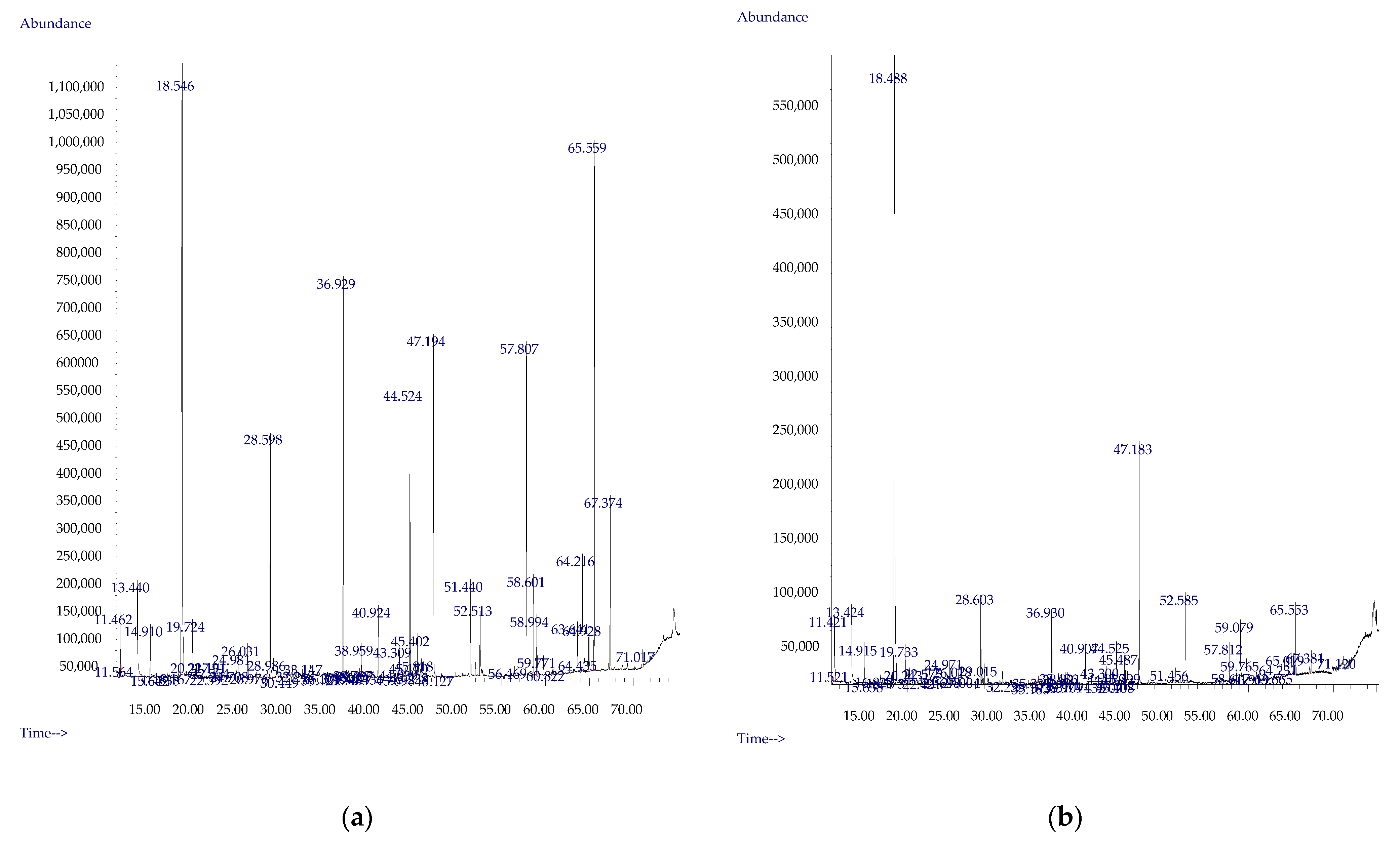
2.2. Volatile Compounds Determination
2.3. Sensory Analysis
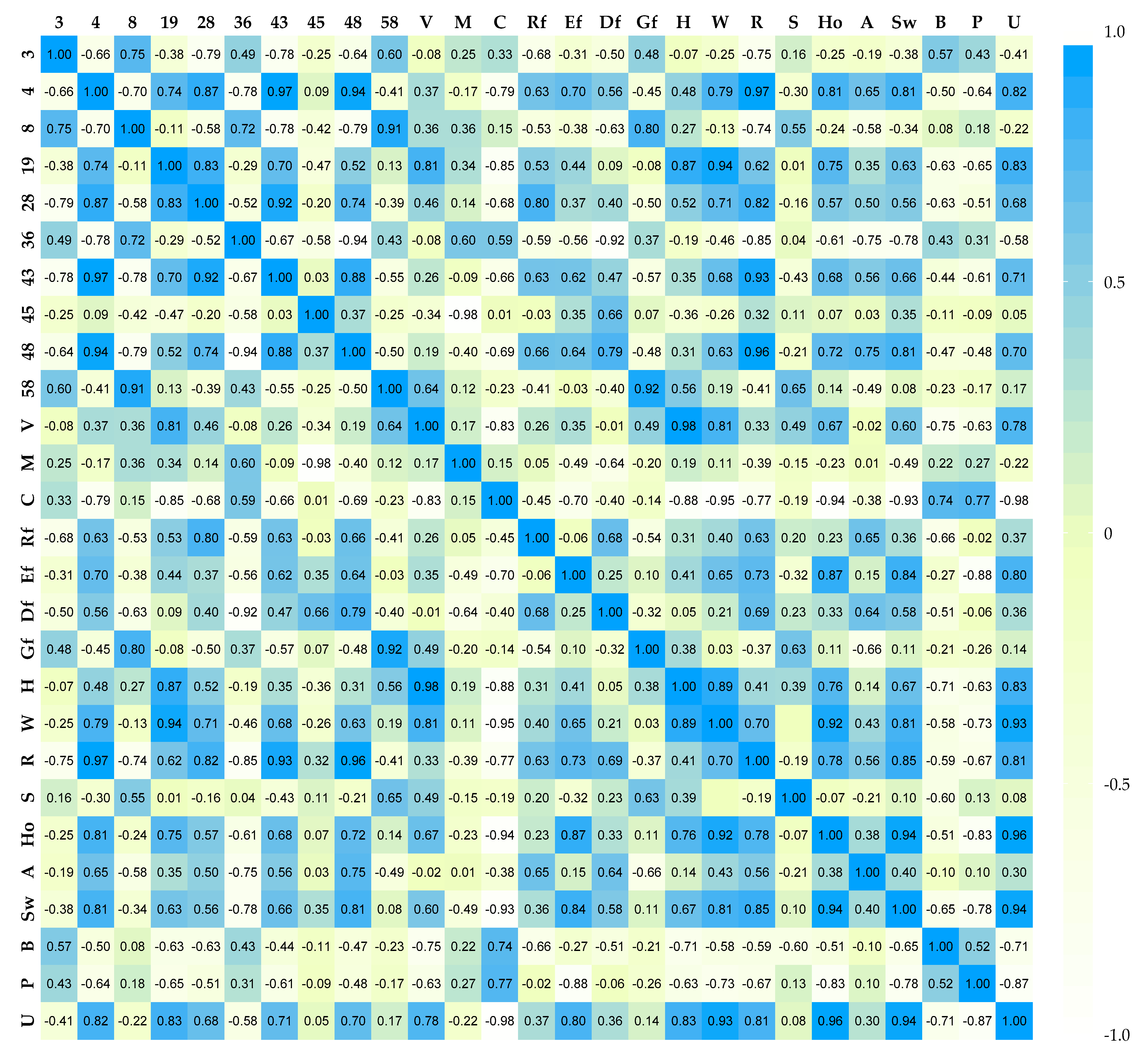
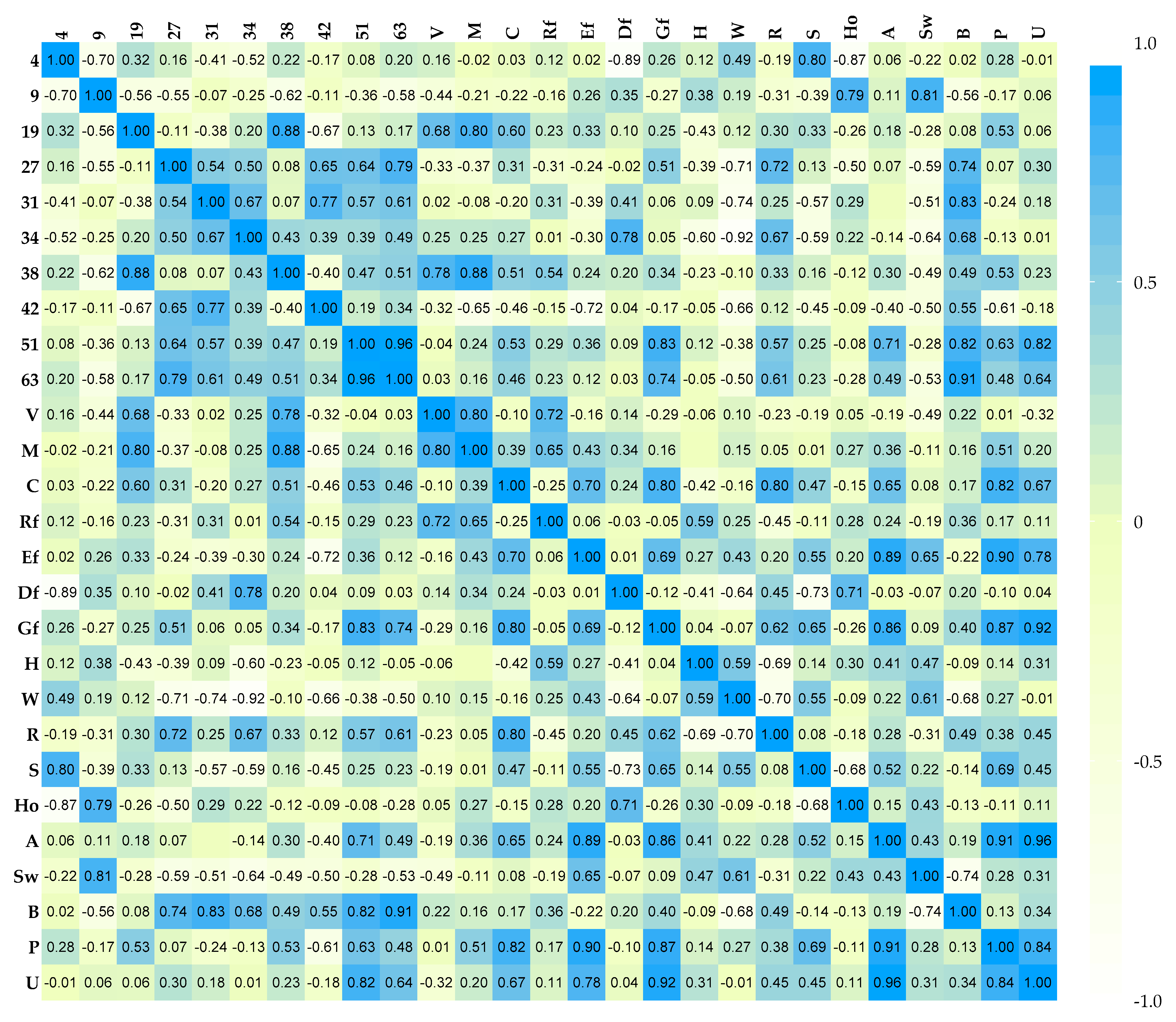
2.4. Statistics
3. Results
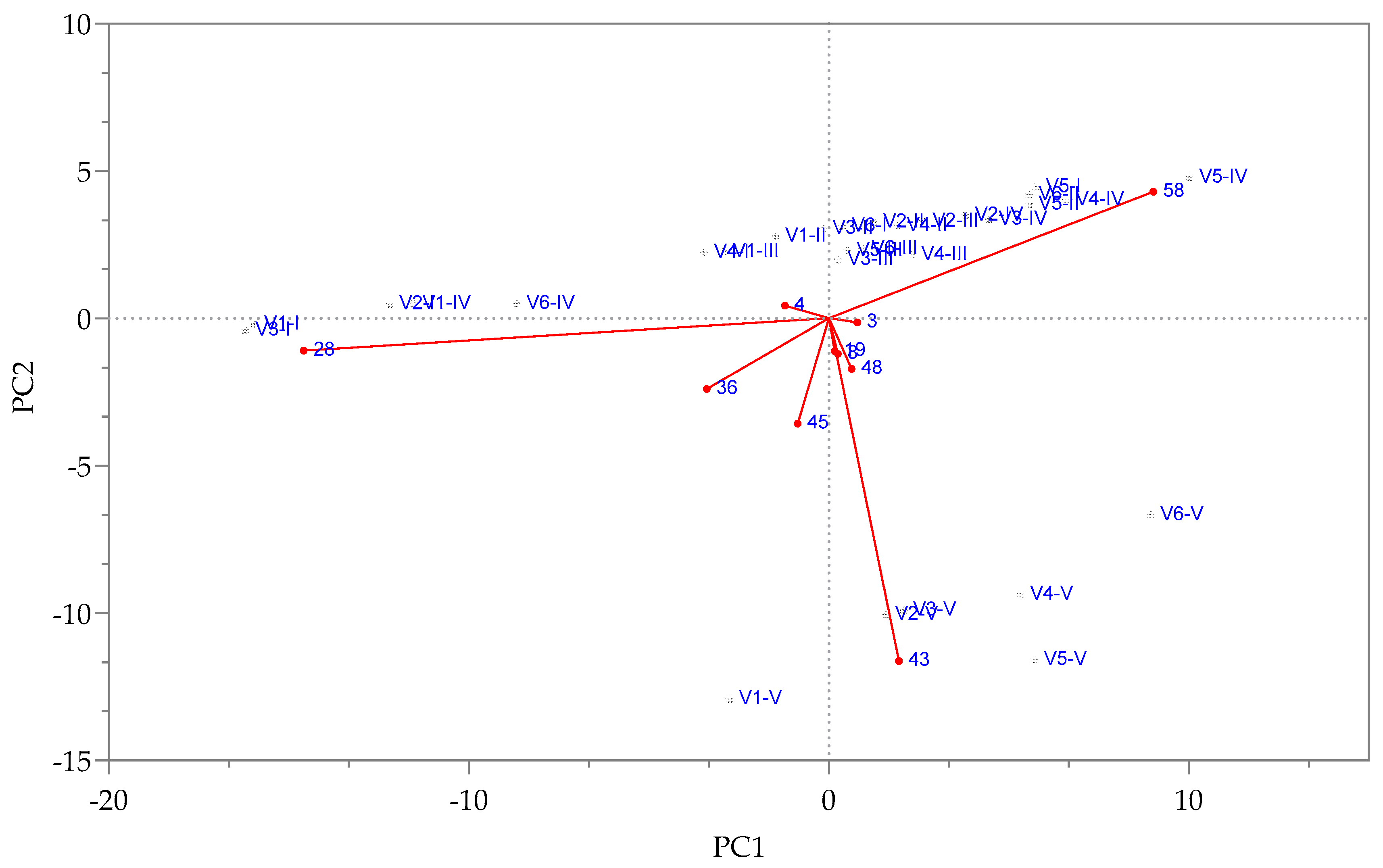
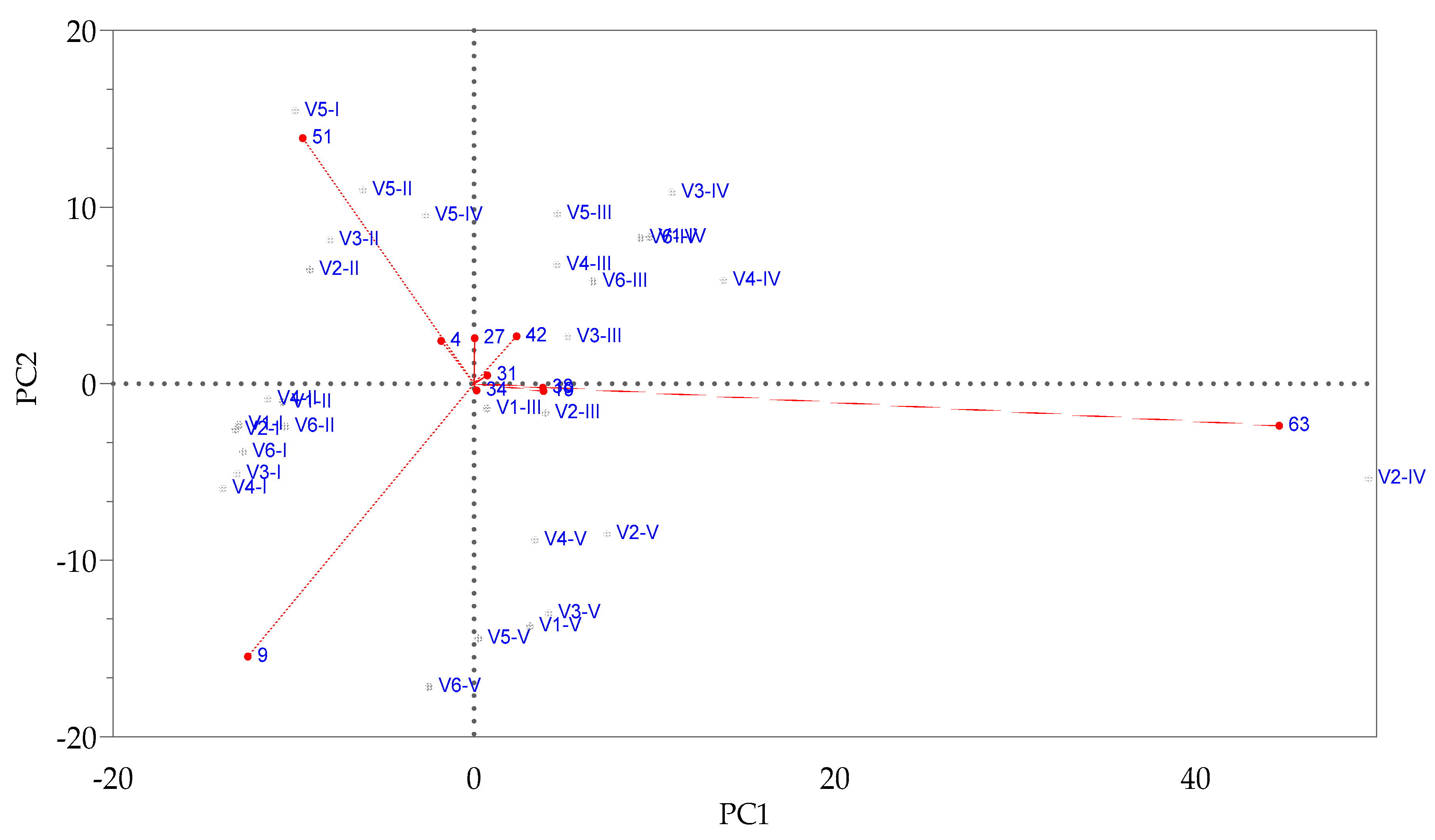
3.1. The Influence of Enzymes on Volatile Compound Fraction
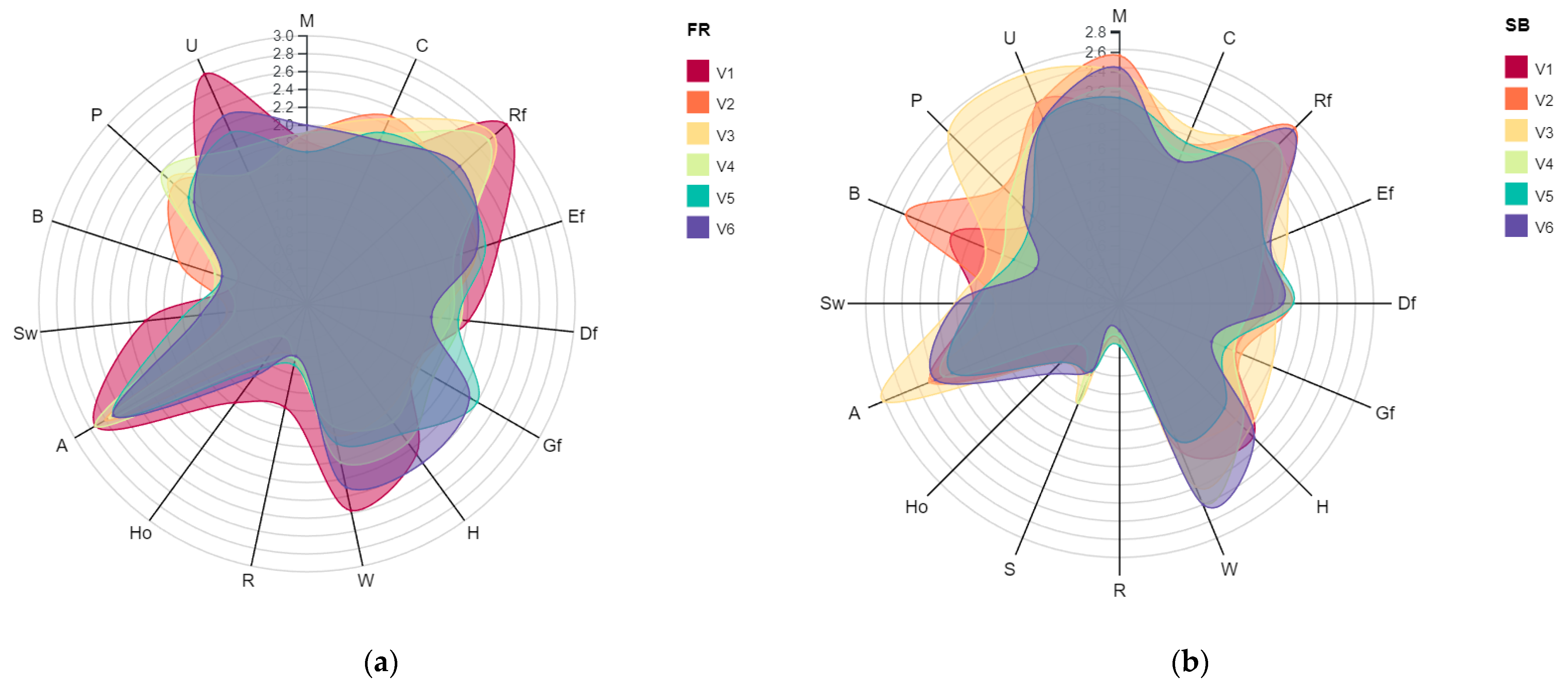
3.2. The Influence of Enzymes on Sensory Perception
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Zhang, Y.; Simpson, B. Food enzymes immobilization: Novel carriers, techniques and applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]
- Yushkova, E.D.; Nazarova, E.A.; Matyuhina, A.V.; Noskova, A.O.; Shavronskaya, D.O.; Vinogradov, V.V.; Skvortsova, N.N.; Krivoshapkina, E.F. Application of immobilized enzymes in food industry. J. Agric. Food Chem. 2019, 67, 11553–11567. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortin, A.B.; Martinez-Cutillas, A.; Ros-Garcia, J.M.; Lopez-Roca, J.M.; Gomez-Plaza, E. Improving colour extraction and stability in red wines: The use of maceration enzymes and enological tannins. Int. J. Food Sci. 2005, 40, 867–878. [Google Scholar] [CrossRef]
- Masino, F.; Montevecchi, G.; Arfelli, G.; Antonelli, A. Evaluation of the combined effects of enzymatic treatment and aging on lees on the aroma of wine from Bombino bianco grapes. J. Agric. Food Chem. 2008, 56, 9495–9501. [Google Scholar] [CrossRef]
- Cosme, F.; Andrea-Silva, J.; Ribeiro, L.F.; Moreira, A.; Malheiro, A.; Coimbra, M.; Domingues, R.; Nunes, F. The origin of pinking phenomena in white wines: An update. BIO Web. Conf. 2018, 12, 02013. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Rozes, N.; Mas, A.; Guillamon, J. Nitrogen catabolite repression in during wine fermentations. FEMS Yeast Res. 2004, 4, 625–632. [Google Scholar] [CrossRef]
- Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Nitrogen compounds in must and volatile profile of white wine: Influence of clarification process before alcoholic fermentation. Food Chem. 2016, 202, 417–425. [Google Scholar] [CrossRef]
- Pinu, F.R.; Edwards, P.J.; Gardner, R.C.; Villas-Boas, S.G. Nitrogen and carbon assimilation by Saccharomyces cerevisiae during Sauvignon blanc juice fermentation. FEMS Yeast Res. 2014, 14, 1206–1222. [Google Scholar] [CrossRef]
- Rusjan, D.; Srlič, M.; Košmer, T.; Prosen, H. The response of monoterpenes to different enzyme preparations in Gewürztraminer (Vitis vinifera L.) wines. S. Afr. J. Enol. 2009, 30, 56–64. [Google Scholar] [CrossRef][Green Version]
- Armada, L.; Fernandez, E.; Falque, E. Influence of several enzymatic treatments on aromatic composition of white wines. LWT-Food Sci. Technol. 2010, 43, 1517–1525. [Google Scholar] [CrossRef]
- Rocha, S.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Enrique, M.; Ibanez, A.; Marcos, J.; Yuste, M.; Martínez, M.; Vallés, S.; Manzanares, P. Î2-Glucanases as a tool for the control of wine spoilage yeasts. J. Food Sci. 2010, 75, M41–M45. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Hu, K.; Zhang, J.X.; Zhu, X.L.; Tao, Y.S. Aroma modulation of Cabernet Gernischt dry red wine by optimal enzyme treatment strategy in winemaking. Food Chem. 2018, 245, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Politowicz, J.; Antoni, S. The effects of enzymatic pre-treatment and type of yeast on chemical properties of white wine. LWT-Food Sci. Technol. 2017, 79, 445–453. [Google Scholar] [CrossRef]
- Ducasse, M.A.; Canal-Llauberes, R.M.; de Lumley, M.; Williams, P.; Souquet, J.M.; Fulcrand, H.; Doco, T.; Cheynier, V. Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chem. 2010, 118, 369–376. [Google Scholar] [CrossRef]
- Guerin, P.L.; Béguin, J.; Cayla, L. Les enzymes en oenologie. Rev. Fr. Oenol. 2010, 289, 29–34. [Google Scholar]
- Borazan, A.A.; Bozan, B. The influence of pectolytic enzyme addition and prefermentative mash heating during the winemaking process on the phenolic composition of Okuzgozu red wine. Food Chem. 2013, 138, 389–395. [Google Scholar] [CrossRef]
- Scutarașu, E.C. Studies on Enzymes Impact on White Wines Technology from Iași Vineyard. Ph.D. Thesis, Iasi University of Life Sciences, Iasi, Romania, 2021. [Google Scholar]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2020, 58, 9–21. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Porretti, M.; Albergamo, A.; Mucari, C.; Tropea, A.; Rando, R.; Nava, V.; Lo Turco, V.; Potortì, A.G. Valorization of traditional alcoholic beverages: The study of the Sicilian Amarena wine during bottle aging. Foods 2022, 11, 2152. [Google Scholar] [CrossRef]
- Marques, C.; Correia, E.; Dinis, L.T.; Vilela, A. An overview of sensory characterization techniques: From classical descriptive analysis to the emergence of novel profiling methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Mezzapesa, G.N.; Stellacci, A.M.; Ferrara, G.; Occhiogrosso, G.; Petrelli, G.; Castellini, M.; Spagnuolo, M. Cover crop for a sustainable viticulture: Effects on soil properties and table grape production. Agronomy 2020, 10, 1334. [Google Scholar] [CrossRef]
- Torres, R.; Ferrara, G.; Soto, F.; López, J.A.; Sanchez, F.; Mazzeo, A.; Pérez-Pastor, A.; Domingo, R. Effects of soil and climate in a table grape vineyard with cover crops. Irrigation management using sensors networks. Cienc. Tec. Vitivinic. 2017, 32, 72–81. [Google Scholar] [CrossRef]
- Gervasi, T.; Oliveri, F.; Gottuso, V.; Squadrito, M.; Bartolomeo, G.; Cicero, N.; Dugo, G. Nero d’Avola and Perricone cultivars: Determination of polyphenols, flavonoids and anthocyanins in grapes and wines. Nat. Prod. Res. 2016, 30, 2329–2337. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Enzyme contribution of non-Saccharomyces yeasts to wine production. Univers. J. Microbiol. Res. 2015, 3, 17–25. [Google Scholar] [CrossRef]
- Cabaroglu, T.; Selli, S.; Canbas, A.; Lepoutre, J.P.; Günata, Z. Wine flavor enhancement through the use of exogenous fungal glycosidases. Enzyme Microb. Technol. 2003, 33, 581–587. [Google Scholar] [CrossRef]
- Crucello, J.; Miron, L.F.O.; Ferreira, V.H.C.; Nan, H.; Marques, M.O.M.; Ritschel, P.S.; Zanus, M.C.; Anderson, J.L.; Poppi, R.J.; Hantao, L.W. Characterization of the aroma profile of novel Brazilian wines by solid-phase microextraction using polymeric ionic liquid sorbent coatings. Anal. Bioanal. Chem. 2018, 410, 4749–4762. [Google Scholar] [CrossRef]
- Ferreira, V.; Fernandez, P.; Gracia, J.P.; Cacho, J.F. Identification of volatile constituents in wines from Vitis vinifera var vidadillo and sensory contribution of the different wine flavour fractions. J. Sci. Food Agric. 1995, 69, 299–310. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, J.; Qian, M.; Li, H. Characterization of the key aroma compounds in Chinese Syrah wine by gas chromatography-olfactometry-mass spectrometry and aroma reconstitution studies. Molecules 2017, 22, 1045. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Vlase, L.; Colibaba, L.C.; Gheldiu, A.M.; Cotea, V.V. Evolution of phenolic profile of white wines treated with enzymes. Food Chem. 2020, 340, 127910. [Google Scholar] [CrossRef] [PubMed]
- International Organization of Wine and Vine. International Code of Oenological Practices, Paris, France, 2020. Available online: https://www.oiv.int/standards/international-code-of-oenological-practices (accessed on 6 July 2022).
- Tiuca, I.D.; Nagy, K.; Oprean, R. Development and optimization of a gas-chromatografic separation method of fatty acids in human serum. World J. Pharm. Res. 2015, 3, 1713–1719. [Google Scholar]
- ISO 3591: 1977; Sensory Analysis. Apparatus. Wine-Tasting Glass. ISO: Geneva, Switzerland, 1977.
- ISO 8589: 2007; Sensory Analysis. General Guidance for the Design of Test Room. ISO: Geneva, Switzerland, 2007.
- 37. International Organization of Wine and Vine. Review document on sensory analysis of wine, Paris, France, 2015. Available online: https://www.oiv.int/public/medias/3307/review-on-sensory-analysis-of-wine.pdf (accessed on 21 September 2022).
- Swiegers, J.H.; Capone, D.L.; Pardon, K.H.; Gordon, M.E.; Sefton, M.A.; Francis, L.I.; Pretorius, I.S. Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast 2007, 24, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Vararu, F.; Moreno-Garcia, J.; Cotea, V.; Moreno, J. Grape musts differentiation based on selected aroma compounds using SBSE-GC-MS and statistical analysis. Vitis 2015, 54, 97–105. [Google Scholar]
- Mojsov, K. Use of enzymes in wine making. Int. J. Technol. Manag. 2013, 3, 112–127. Available online: http://www.ijmra.us (accessed on 3 September 2022).
- Kumar, V.A.; Prakash, S.P. Science and Technology of Aroma Flavor, and Fragrance in Rice; Apple Academic Press: Palm Bay, FL, USA, 2018. [Google Scholar]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com (accessed on 6 September 2022).
- Lytra, G.; Tempere, S.; de Revel, G.; Barbe, J.C. Distribution and organoleptic impact of ethyl 2-methylbutanoate enantiomers in wine. J. Agric. Food Chem. 2014, 62, 5005–5010. [Google Scholar] [CrossRef]
- Hui, Y.H. Handbook of Fruit and Vegetable Flavors; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile profile characterization of Croatian commercial sparkling wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef]
- Moroșanu, A.M.; Luchian, C.E.; Niculaua, M.; Colibaba, C.L.; Tarțian, A.C.; Cotea, V.V. Assessment of major volatile and phenolic compounds from ‘Fetească regală’ wine samples after pre-fermentative treatments using GC-MS Analysis and HPLC analysis. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 247–259. [Google Scholar] [CrossRef]
- McKinnon, A. The impact of amino acids on growth performance and major volatile compound formation by industrial wine yeast. 2013. Available online: http://scholar.sun.ac.za (accessed on 26 September 2022).
- Cotea, D.V.; Zănoagă, C.; Cotea, V.V. Tratat de Oenochimie; Romanian Academy Publishing: Bucharest, Romania, 2009. [Google Scholar]
- Satyanarayana, T.; Kunze, G. Yeast Biotechnology: Diversity and Applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Romano, P.; Brandolini, V.; Ansaloni, C.; Menziani, E. The production of 2,3-butandiol as a differentiating character in wine yeasts. World J. Microbiol. Biotechnol. 1998, 14, 649–653. [Google Scholar] [CrossRef]
- Velisek, J.; Koplik, R.; Cejpek, K. The Chemistry of Food, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Buttner, A. Springer Handbook of Odor; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Pogorzelski, E.; Wilkowska, A. Flavour enhancement through the enzymatic hydrolysis of glycosidic aroma precursors in juices and wine beverages: A review. Flavor Fragr. J. 2007, 22, 251–254. [Google Scholar] [CrossRef]
- de Souza Nascimento, A.; de Souza, J.; dos Santos Lima, M.; Pereira, G. Volatile profiles of sparkling wines produced by the traditional method from a semi-arid region. Beverages 2018, 4, 103. [Google Scholar] [CrossRef]
- Caliari, V.; Panceri, C.P.; Rosier, J.P.; Bordignon-Luiz, M.T. Effect of the Traditional, Charmat and Asti method production on the volatile composition of Moscato Giallo sparkling wines. LWT-Food Sci. Technol. 2015, 61, 393–400. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubordieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ferreira, D.C.; Nicolli, K.P.; Souza-Silva, E.A.; Zini, C.A.; Welke, J.E. Carbonyl compounds in different stages of vinification and exposure risk assessment through Merlot wine consumption. Food Addit. Contam. 2018, 35, 2315–2331. [Google Scholar] [CrossRef]
- Linskens, H.F.; Jackson, J.F. Wine Analysis; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Pérez-Olivero, S.J.; Pérez-Pont, M.L.; Conde, J.E.; Pérez-Trujillo, J.P. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J. Anal. Chem. 2014, 2014, 863019. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, D.; Srlič, M.; Košmer, T.; Prosen, H. Contribution of enzyme preparations to the linalool content of wines made from the non-aromatic grapevine variety Furmint (Vitis vinifera L.). J. Int. Sci. Vigne Vin 2012, 46, 139–143. [Google Scholar] [CrossRef]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Dziadas, M.; Jeleń, H. Influence of glycosidases addition on selected monoterpenes contents in musts and white wines from two grape varieties grown in Poland. Acta Sci. Pol. Technol. Aliment. 2011, 10, 7–17. [Google Scholar]
- Csutoras, C.; Bakos-Barczi, N.; Burkus, B. Medium chain fatty acids and fatty acid esters as potential markers of alcoholic fermentation of white wines. Acta Aliment. 2022, 51, 33–42. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Trincă, L.C.; Cotea, V.V. Increasing amino acids content of white wines with enzymes treatments. Agronomy 2022, 12, 1406. [Google Scholar] [CrossRef]
- Bakker, J.; Bellworthy, S.J.; Reader, H.P.; Watkins, S.J. Effects of enzymes during vinification on color and sensory properties of port wines. Am. J. Enol. Vitic. 1999, 50, 271–276. [Google Scholar]
| FR | SB | Volatile Compounds | Clasiff. | CAS | RT (Min) | OT (ppb) | FT (ppb) | AD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | propan-1-ol | alcohols | 71-23-8 | 11.46 | 9000 | - | ripe fruits | [38] |
| 2 | 2 | ethyl butanoate | esters | 105-54-4 | 11.56 | 1 | 450 | - | - |
| 3 | 2,6-dimethyldecane | hydrocarbons | 13150-81-7 | 11.64 | - | - | - | - | |
| 3 | 4 | 3-methylpropan-1-ol | alcohols | 78-83-1 | 13.44 | - | 50,000 | alcoholic, nail polish, pungent smell | [39] |
| 4 | 5 | 3-methylbutyl acetate | esters | 123-92-2 | 14.91 | 2 | - | fruity, bananas, pears, acetone | [40] |
| 5 | 6 | butan-1-ol | alcohols | 71-36-3 | 15.64 | 500 | - | alcoholic, spirtuous | [38] |
| 6 | 7 | 3-penten-2-ol | alcohols | 1569-50-2 | 16.83 | 1.5 | - | kiwi, fruity | [39] |
| 34 | 8 | undecane | hydrocarbons | 1120-21-4 | 39.15 | - | - | - | - |
| 7 | dodecane | hydrocarbons | 112-40-3 | 18.16 | - | - | petrol | [41] | |
| 8 | 9 | 3-methylbutan-1-ol | alcohols | 123-51-3 | 18.54 | 250–300 | 170 | alcoholic, nail polish, bananas | [38] |
| 10 | decane | hydrocarbons | 124-18-5 | 19.28 | - | - | - | - | |
| 11 | 5-ethyl-5-methyldecane | hydrocarbons | 17312-74-2 | 19.60 | - | - | - | - | |
| 9 | 12 | ethyl hexanoate | esters | 123-66-0 | 19.72 | 1 | - | fruity, bananas, pineapple | [38] |
| 13 | 2,4,6-trimethylheptane | hydrocarbons | 2613-61-8 | 20.07 | - | - | - | - | |
| 14 | 2-methyldecane | hydrocarbons | 6975-98-0 | 20.20 | - | - | - | - | |
| 10 | dotriacontan | hydrocarbons | 544-85-4 | 20.21 | - | - | - | [38] | |
| 11 | hexyl acetate | esters | 142-92-7 | 21.46 | 2 | - | fruity, pear | [18] | |
| 12 | 15 | 3-hydroxy-2-butanone | carbonyl compounds | 51555-24-9 | 22.19 | 800 | - | unctuous, milky | [39] |
| 16 | 3,9-dimethylundecane | hydrocarbons | 17301-31-4 | 22.39 | - | - | - | - | |
| 17 | 3,3-dimethylhexane | hydrocarbons | 563-16-6 | 22.53 | - | - | - | - | |
| 18 | 3-methylpentan-2-ol | alcohols | 565-60-6 | 23.13 | - | - | - | - | |
| 13 | 4-methyltetradecane | hydrocarbons | 25117-24-2 | 22.39 | - | - | pungent smell | [41] | |
| 14 | 2,6,10,14-tetramethylhexadecane | hydrocarbons | 638-36-8 | 22.55 | - | - | - | - | |
| 15 | 19 | ethyl 2-hydroxypropanoate | esters | 97-64-3 | 24.70 | 110,000 | - | unctuous, ethereal | [39] |
| 16 | 20 | hexan-1-ol | alcohols | 111-27-3 | 24.98 | 2500 | - | vegetal, fruity, green apple peel | [40] |
| 17 | 21 | 3-ethoxypropan-1-ol | hydroxyethers | 1589-49-7 | 26.03 | - | - | fruity | [41] |
| 22 | nonadecane | hydrocarbons | 629-92-5 | 28.27 | - | - | - | - | |
| 18 | isotetradecane | hydrocarbons | 1560-96-9 | 26.97 | - | - | - | - | |
| 19 | 23 | ethyl octanoate | esters | 106-32-1 | 28.59 | - | 15 | fruity, banana, apple, pineapple, pear, floral, soapy | [42] |
| 24 | 2,6,10-trimethyldodecane | - | 3891-98-3 | - | - | - | - | - | |
| 20 | 25 | eicosan | hydrocarbons | 112-95-8 | 28.98 | - | - | waxy, floral | [43] |
| 26 | octadecane | hydrocarbons | 593-45-3 | 29.46 | - | - | - | - | |
| 21 | 1-propylaziridine | nitrogen compounds | 104549-74-8 | 30.43 | - | - | - | - | |
| 22 | 27 | acetic acid | acids | 64-19-7 | 30.44 | - | 22,000 | vegetable, rancid, sour | [39] |
| 28 | pentacosan | hydrocarbons | 629-99-2 | - | - | - | |||
| 23 | 29 | ethyl 3-hydroxybutanoate | esters | 5405-41-4 | 32.24 | 14,000 | - | fruity | [44] |
| 30 | benzaldehyde | carbonyl compounds | 100-52-7 | 32.53 | 350–3500 | 1500 | almonds, nuts, fruity | [43] | |
| 24 | 31 | 2,3-butanediol | alcohols | 107-88-0 | 33.14 | 150,000 | - | fruity, fresh | [45] |
| 25 | 32 | 1,3-butanediol | alcohols | 107-88-0 | 34.63 | - | - | - | - |
| 26 | 33 | 2-methylpropanoic acid | acids | 79-31-2 | 35.12 | 8100 | - | fruity, tangy, ethereal, hint of rum | [40] |
| 34 | ethyl acetamide | nitrogen compounds | 625-50-3 | 36.68 | - | - | - | - | |
| 27 | hexadecanoic acid | acids | 57-10-3 | 35.3 | 10,000 | - | fatty, waxy | [44] | |
| 28 | 35 | ethyl decanoate | esters | 110-38-3 | 36.92 | - | 510 | fruity, grape, pear, apple, waxy, oily | [18] |
| 36 | 1,2-hydrazinedicarboxaldehyde | carbonyl compounds | 628-36-4 | 37.13 | - | - | - | - | |
| 37 | 10-methylnonadecane | hydrocarbons | 56862-62-5 | 37.71 | - | - | - | - | |
| 29 | butanoic acid | acids | 107-92-6 | 37.50 | 240 | 6200-6800 | cheese, rancid, sweet, animal | [44] | |
| 30 | 38 | diethyl butanedioate | esters | 123-25-1 | 38.48 | - | - | fruity, floral, waxy, dusty | [40] |
| 31 | docosan | hydrocarbons | 629-97-0 | 38.78 | - | - | wax | [44] | |
| 32 | ethyl 9-decenoate | esters | 67233-91-4 | 38.95 | - | - | fruity, buttery | - | |
| 33 | 39 | 3-methylbutanoic acid | acids | 503-74-2 | 39.02 | 120–170 | - | rancid, cheese, fermented fruit | - |
| 40 | 3-methylsulfanylpropan-1-ol | sulfur compounds | 505-10-2 | 40.09 | - | - | sulphurous, onion, garlic, raw potato | [44] | |
| 35 | 1,3-dithiolane | hydrocarbons | 4829-04-3 | 40.13 | - | - | sweet, sulphurous, fried onions | [44] | |
| 36 | 41 | 2-propanyl acetate | esters | 108-21-4 | 40.92 | 180–670 | - | ethereal, bananas, sweet, ripe apples, fresh fruit | [18] |
| 37 | 42 | ethyl 4-hydroxybutanoate | esters | 999-10-0 | 43.3 | - | - | pineapple, roses, tropical fruits, honey, coconut, nectar | [44] |
| 38 | 43 | 2-phenylethyl acetate | esters | 103-45-7 | 43.69 | 250 | - | fruity, rose, honey, vegetable, pollen, nectar | [39] |
| 39 | heneicosan | hydrocarbons | 629-94-7 | 44.03 | - | - | wax | [44] | |
| 40 | 44 | ethyl dodecanoate | esters | 106-33-2 | 44.52 | - | 200 | floral, fruity, grassy, woody | [18] |
| 45 | 1-octadecene | hydrocarbons | 112-88-9 | 44.63 | - | - | - | - | |
| 41 | 3-methylbutyl decanoate | esters | 2306-91-4 | 45.17 | - | - | bananas, waxy, fruity, cognac, vegetal, unctuous | [44] | |
| 42 | 47 | n-(3-methylbutyl)acetamide | nitrogen compounds | 13434-12-3 | 45.23 | - | - | - | - |
| 43 | 46 | hexanoic acid | acids | 142-62-1 | 45.40 | 3000 | 5400 | cheese, phenolic, unctuous, ripe fruit, tropical fruit | [44] |
| 48 | hydroxybutyric acid | acids | 300-85-6 | 45.79 | - | - | - | ||
| 44 | butyl acetate | esters | 123-86-4 | 45.81 | 66 | - | fruity, pineapple | [18] | |
| 49 | 1-phenyl methanol | alcohols | 100-51-6 | 45.95 | - | - | honey, bubble gum, fruity | [18] | |
| 45 | 51 | 1-phenylethanol | alcohols | 60-12-8 | 47.19 | 10,000 | - | rose, floral, honey | [39] |
| 50 | ethyl 3-methylbutyl butanedioate | esters | 28024-16-0 | 46.66 | - | - | caramel, dried fruit, mineral, medicinal, burnt | [18] | |
| 52 | n-ethyl acetamide | nitrogen compounds | 625-50-3 | 51.38 | - | - | - | - | |
| 53 | diethyl hydroxybutanedioate | esters | 141-05-9 | 51.46 | - | - | caramel, fruity, vegetal | [44] | |
| 46 | 2,6-dimetil-3-7-octadiene-2,6-diol | terpene | 13741-21-4 | 48.12 | - | - | citrus | [18] | |
| 47 | diethyl 2,3-dihydroxybutanedioate | esters | 57968-71-5 | 51.44 | - | - | fruity, caramel, red fruits | [44] | |
| 48 | 54 | octanoic acid | acids | 124-07-2 | 52.51 | 3000 | 5300 | cheese, rancid, fatty, vegetable, sweet | [39] |
| 55 | 2-[ethyl(methyl)amino]ethanol | alcohols | 2893-43-8 | 54.98 | - | - | - | - | |
| 49 | 4-ethenyl-2-methoxyphenol | phenolic compounds | 7786-61-0 | 56.46 | - | - | dry wood, roasted hazelnuts, amber | [44] | |
| 50 | 5-propyl-2-oxolanone | lactones | 105-21-5 | 57.80 | - | - | fruity, grapey | [18] | |
| 56 | 1-docosene | hydrocarbons | 1599-67-3 | 57.70 | - | - | - | - | |
| 57 | ethyl hexadecanoate | esters | 628-97-7 | 57.83 | 2000 | - | fruity | [44] | |
| 51 | 58 | 9-ethyl hexadecanoate | esters | 54546-22-4 | 58.6 | - | - | - | - |
| 52 | 59 | decanoic acid | acids | 334-48-5 | 58.99 | 10,000 | 3500 | rancid, sour, greasy, nasty, woody | [39] |
| 53 | 60 | 2,4-di-tert-butylphenol | phenols | 96-76-4 | 59.77 | - | - | - | - |
| 61 | hexaoxacyclooctadecane | hydrocarbons | 17455-13-9 | 60.36 | - | - | - | - | |
| 62 | 11-octadecenal | carbonyl compounds | 56554-95-1 | 60.56 | - | - | - | - | |
| 54 | 3,7,11-trimethyl-2,6,10-dodecatrienol | terpenes | 4602-84-0 | 60.82 | - | - | lime blossoms, grapefruit, peach, anise, citrus, pear | [44] | |
| 63 | 1,6-anhydro-2,3,4-trimethylgalactose | anhydrous sugars | - | 62.50 | - | - | - | - | |
| 55 | 5-(1-hydroxyethyl)-furan-2-one | lactones | 27610-27-1 | 61.98 | - | - | - | - | |
| 56 | 4-ethenylphenol | phenolic compounds | 2628-17-3 | 62.35 | - | - | pungent smell, medicinal, gouache, medicinal, phenolic | [18] | |
| 57 | 2,3-dimethyl-1-pentene | hydrocarbons | 3404-72-6 | 62.73 | - | - | - | - | |
| 58 | dimethyl butanedioate | esters | 106-65-0 | 62.98 | - | - | fruity, sweet, green fruit, floral, waxy, soapy | [44] | |
| 59 | 64 | ethyl octadecanoate | esters | 111-61-5 | 63.64 | - | - | waxy | [44] |
| 65 | 4-hydroxy benzeneethanol | phenolic compounds | 501-94-0 | 64.01 | - | - | chemical, bitter, honey, wax, toast, smoke, cloves | [46] | |
| 60 | 66 | ethyl 9-octadecenoate | esters | 111-62-6 | 64.21 | - | - | fresh, woody | [47] |
| 61 | methyl-10-octadecenoate | esters | 13038-45-4 | 64.43 | - | - | - | - | |
| 62 | dodecanoic acid | acids | 143-07-7 | 64.92 | 10,000 | - | coconut, waxy, buttery | [44] | |
| 63 | 67 | ethyl octadeca-9,12-dienoate | esters | 544-35-4 | 65.55 | - | - | sweet, freshness | [47] |
| 64 | 2,3,5,8-tetramethyl-1,5,9-decatriene | hydrocarbons | 230646-72-7 | 66.55 | - | - | - | - | |
| 65 | 3,7,11,15-tetramethylhexadeca-1,6,10,14-tetraen-3-ol | esters | 1113-21-9 | 66.55 | - | - | fruity, floral, roses | [39] | |
| 66 | 9-octadecenamide | nitrogen compounds | 3322-62-1 | 66.78 | - | - | - | - | |
| 67 | ethyl octadeca-9,12,15-trienoate | esters | 1191-41-9 | 67.37 | - | - | - | - | |
| 68 | 2-methyl-4-octanol | alcohols | 40575-41-5 | 70.17 | - | - | - | - | |
| 69 | 1-tetradecanoic acid | acids | 544-63-8 | 71,01 | 10,000 | - | waxy, buttery, pineapple, citrus peel | [44] | |
| 70 | octadecanoic acid | acids | 57-11-4 | 72.09 | 20,000 | - | waxy, buttery | [44] | |
| 68 | n-(2-phenylethyl)acetamide | nitrogen compounds | 877-95-2 | 67.30 | - | - | - | - |
| C | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|
| 1 | 0.51 0.02 * | 1.98 0.01 c | 1.63 0.02 a | 2.12 0.03 * | 2.24 0.01 * | 1.96 0.01 c |
| 2 | 0.19 0.00 * | 0.34 0.01 a | 0.42 0.01 d | 0.44 0.01 * | 0.35 0.01 ab | 0.42 0.01 d |
| 3 | 3.83 0.02 * | 4.35 0.02 a | 3.96 0.00 * | 4.46 0.01 * | 4.27 0.00 * | 4.36 0.01 a |
| 4 | 3.60 0.02 d | 1.25 0.02 * | 1.11 0.00 b | 1.40 0.02 * | 1.07 0.00 a | 1.33 0.02 c |
| 5 | 0.18 0.00 bc | 0.12 0.02 a | 0.15 0.00 abc | 0.15 0.02 abc | 0.12 0.01 a | 0.16 0.02 abc |
| 6 | 0.09 0.01 a | 0.12 0.01 bc | 0.09 0.00 a | 0.11 0.00 abd | 0.11 0.01 ab | 0.14 0.01 c |
| 7 | 0.13 0.02 a | 0.19 0.02 c | 0.18 0.00 c | 0.19 0.01 c | 0.14 0.00 ab | 0.14 0.00 ab |
| 8 | 37.19 0.01 * | 41.82 0.03 * | 42.39 0.03 * | 45.67 0.02 * | 45.51 0.02 * | 49.89 0.00 * |
| 9 | 1.15 0.00 * | 0.71 0.03 d | 0.70 0.03 d | 0.78 0.00 * | 0.64 0.00 b | 0.92 0.02 * |
| 10 | 0.25 0.02 b | 0.37 . 0.02* | 0.32 0.00 cd | 0.33 0.01 d | 0.25 0.01 b | 0.26 0.01 b |
| 11 | 0.15 0.07 * | nd | nd | nd | nd | nd |
| 12 | 0.13 0.00 * | nd | nd | nd | nd | nd |
| 13 | nd | nd | nd | nd | nd | nd |
| 14 | nd | nd | nd | nd | nd | nd |
| 15 | 0.95 0.00 * | 1.86 0.01 * | 1.92 0.01 * | 2.07 0.00 a | 2.15 0.00 * | 1.57 0.00 * |
| 16 | 1.22 0.04 * | 0.85 0.01 c | 0.81 0.04 ab | 0.96 0.00 de | 0.78 0.04 a | 0.64 0.00 * |
| 17 | 0.69 0.00 a | 1.09 0.00 bc | 1.13 0.00 d | 1.22 0.00 f | 0.90 0.00 * | 1.21 0.00 ef |
| 18 | 0.19 0.00 * | 0.14 0.01 c | 0.15 0.00 c | 0.17 0.00 b | 0.15 0.00 c | 0.12 0.00 b |
| 19 | 2.33 0.04 de | 1.67 0.3 b | 1.80 0.06 bc | 1.90 0.01 c | 1.66 0.00 bd | 2.22 0.00 * |
| 20 | 0.50 0.02 b | 0.49 0.10 b | 0.05 0.00 * | 0.48 0.00 b | 0.38 0.00 * | 0.49 0.00 b |
| 21 | nd | 0.06 0.00 ac | 0.05 0.00* | 0.06 0.00 ac | 0.03 0.00 b | 0.07 0.00 * |
| 22 | 2.59 0.02 * | 0.57 0.01 bc | 0.56 0.02 b | 0.60 0.00 * | 0.59 0.02 c | 0.69 0.03 * |
| 23 | 0.15 0.00 a | 0.34 0.00 c | 0.34 0.01 c | 0.32 0.02 bc | 0.39 0.02 d | 0.39 0.00 d |
| 24 | 1.64 0.02 * | 1.04 0.02 a | 1.13 0.02 b | 1.00 0.02 * | 1.14 0.01 b | 1.05 0.00 a |
| 25 | 0.41 0.08 bc | 0.41 0.08 abc | 0.36 0.08 ab | 0.48 0.04 cd | 0.34 0.06 a | 0.42 0.04 abc |
| 26 | 0.15 0.01 bcd | 0.13 0.01 ab | 0.14 0.01 ab | 0.15 0.03 bcd | 0.11 0.02 a | 0.18 0.04 d |
| 27 | 0.14 0.00 bcd | 0.12 0.00 abc | 0.11 0.04 ab | 0.12 0.04 abc | 0.11 0.03 ab | 0.11 0.02 ab |
| 28 | 1.85 0.04 * | 1.15 0.02 * | 1.44 0.03 * | 1.26 0.03 * | 1.07 0.03 * | 1.38 0.02 * |
| 29 | 0.32 0.01 d | 0.29 0.01 bc | 0.27 0.03 abc | 0.29 0.02 bc | nd | 0.33 0.03 d |
| 30 | 0.31 0.00 abc | 0.43 0.02 d | 0.42 0.04 d | 0.44 0.03 d | 0.46 0.03 d | 0.51 0.02 * |
| 31 | 0.13 0.02 bc | 0.14 0.01 b | 0.16 0.03 c | 0.13 0.04 bc | 0.13 0.00 b | 0.16 0.00 c |
| 32 | nd | nd | nd | nd | nd | nd |
| 33 | 0.18 0.01 * | 0.31 0.02 a | 0.31 0.03 a | 0.31 0.00 a | 0.28 0.04 a | 0.36 0.02 b |
| 34 | nd | 0.10 0.00 * | 0.00 0.00 a | nd | nd | nd |
| 35 | 0.20 0.04 d | 0.15 0.03 abc | 0.15 0.03 abc | 0.12 0.04 a | 0.12 0.00 a | 0.18 0.00 cd |
| 36 | 1.08 0.03 a | 1.87 0.03 bc | 1.90 0.02 c | 1.53 0.00 * | 1.60 0.00 * | 2.16 0.04 * |
| 37 | 2.18 0.02 * | 0.92 0.03 g | 0.90 0.02 fg | 0.80 0.02 bc | 0.89 0.00 ef | 0.78 0.00 ab |
| 38 | 0.33 0.03 * | nd | nd | nd | 0.00 0.00 a | nd |
| 39 | 0.18 0.01 bcd | 0.18 0.03 bcd | 0.19 0.03 cd | 0.17 0.00 bc | 0.12 0.00 a | 0.12 0.00 a |
| 40 | nd | nd | nd | nd | nd | nd |
| 41 | nd | nd | nd | nd | nd | nd |
| 42 | nd | 0.18 0.02 cd | 0.21 0.00 d | 0.13 0.00 ab | 0.15 0.02 abc | 0.16 0.00 bc |
| 43 | 4.28 0.05 * | 2.42 0.00 b | 2.45 0.00 b | 2.09 0.00 * | 1.93 0.00 * | 2.31 0.00 a |
| 44 | 0.34 0.04 | 0.41 0.02 | 0.46 0.00 | 0.42 0.00 | 0.48 0.04 | 0.38 0.06 |
| 45 | 13.51 0.02 * | 12.85 0.01 * | 12.79 0.00 * | 12.65 0.00 * | 15.26 0.00 * | 10.80 0.02 * |
| 46 | 0.17 0.07 b | 0.09 0.03 a | 0.11 0.00 a | 0.11 0.01 a | 0.13 0.00 * | 0.09 0.01 a |
| 47 | 0.24 0.03 a | 0.46 0.00 * | 0.44 0.02 de | 0.53 0.00 g | 0.54 0.02 g | 0.27 0.00 b |
| 48 | 8.18 0.00 * | 4.44 0.03 a | 4.29 0.02 * | 5.19 0.00 * | 4.74 0.00 b | 3.63 0.01 * |
| 49 | 0.87 0.05 g | 0.74 0.02 * | 0.64 0.02 ab | 0.89 0.00 g | 0.73 0.03 * | 0.50 0.03 * |
| 50 | 0.19 0.06 a | 0.44 0.02 ab | 0.44 0.50 a | 0.49 0.03 b | 0.51 0.02 b | 0.37 0.02 ab |
| 51 | nd | nd | nd | nd | nd | nd |
| 52 | 2.19 0.01 * | 0.89 0.01 * | 0.93 0.00 b | 1.05 0.01 d | 1.06 0.01 d | 0.73 0.00* |
| 53 | 0.49 0.05 fg | 0.44 0.03 de | 0.42 0.02 cd | 0.53 0.02 g | 0.41 0.01 cd | 0.48 0.04 ef |
| 54 | nd | nd | nd | nd | nd | nd |
| 55 | 0.28 0.00 * | 0.14 0.00 * | 0.18 0.00 * | 0.11 0.00 a | 0.15 0.00 * | 0.16 0.00 * |
| 56 | 1.78 0.04 * | 0.61 0.04 d | 0.52 0.03 c | 0.53 0.03 bc | 0.62 0.00 d | 0.43 0.03 a |
| 57 | nd | nd | nd | nd | nd | nd |
| 58 | 2.41 0.06 a | 2.41 0.04 a | 2.47 0.00 * | 2.88 0.00 * | 3.04 0.00 * | 3.31 0.00 * |
| 59 | nd | nd | nd | nd | nd | nd |
| 60 | nd | nd | nd | nd | nd | nd |
| 61 | nd | nd | nd | nd | nd | nd |
| 62 | nd | nd | nd | nd | nd | nd |
| 63 | nd | nd | nd | nd | nd | nd |
| 64 | nd | nd | nd | nd | nd | nd |
| 65 | nd | nd | nd | nd | nd | nd |
| 66 | nd | 5.92 0.01 * | 6.04 0.04 * | nd | nd | nd |
| 67 | nd | nd | nd | nd | nd | nd |
| 68 | nd | nd | nd | nd | nd | nd |
| 69 | nd | nd | nd | nd | nd | nd |
| 70 | nd | 2.03 0.03 * | 1.97 0.02 * | 2.17 0.01 c | 2.21 0.03 c | 1.66 0.02 b |
| C | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|
| 1 | 0.34 0.01 bc | 0.31 0.02 b | 0.65 0.01 * | 0.52 0.02 * | 0.47 0.05 e | 0.40 0.00 d |
| 2 | 0.26 0.00 c | 0.26 0.00 c | 0.07 0.01 * | 0.20 0.01 b | 0.15 0.01 * | 0.20 0.01 b |
| 3 | 0.22 0.01 * | 0.15 0.01 ef | 0.15 0.01 ef | 0.12 0.01 de | 0.17 0.01 f | 0.12 0.01 de |
| 4 | 4.34 0.02 * | 4.38 0.01 * | 4.51 0.02 * | 5.15 0.01 * | 4.10 0.00 * | 4.20 0.01 * |
| 5 | 0.16 0.01 a | 0.19 0.02 * | 0.16 0.00 a | 0.30 0.00 c | 0.10 0.02 * | 0.56 0.00 * |
| 6 | 0.11 0.01 e | 0.06 0.01 bc | 0.05 0.02 b | 0.06 0.00 bc | 0.09 0.00 de | 0.08 0.01 cd |
| 7 | 0.16 0.00 * | 0.12 0.00 abc | 0.13 0.00 abc | 0.21 0.01 * | 0.09 0.05 a | 0.14 0.02 bc |
| 8 | nd | 0.17 0.01 b | 0.18 0.01 b | 0.09 0.00 * | nd | 0.05 0.00 * |
| 9 | 39.21 0.00 * | 33.11 0.00 * | 38.60 0.02 * | 32.76 0.00* | 39.12 0.02 * | 42.15 0.00* |
| 10 | 0.26 0.00 | nd | nd | nd | nd | nd |
| 11 | 0.05 0.02 b | 0.20 0.02 * | 0.25 0.02 * | 0.03 0.05 ab | 0.01 0.05 a | 0.28 0.02 * |
| 12 | nd | nd | nd | nd | nd | nd |
| 13 | 0.28 0.00 * | 0.05 0.00 b | 0.05 0.00 b | 0.02 0.05 a | 0.03 0.00 ab | 0.04 0.00 ab |
| 14 | 0.16 0.01 c | 0.25 0.01 de | 0.29 0.01 e | 0.15 0.05 bc | 0.14 0.00 abc | 0.23 0.05 d |
| 15 | 0.37 0.01 * | 0.18 0.00 b | 0.15 0.01 b | 0.10 0.05 a | 0.11 0.00 a | 0.10 0.00 a |
| 16 | nd | 0.39 0.01 * | 0.35 0.02 * | 0.02 0.02 b | 0.03 0.00 bc | 0.04 0.01 c |
| 17 | 0.13 0.01 | nd | nd | 0.10 0.01 | 0.11 0.00 | 0.12 0.00 |
| 18 | 4.98 0.00 * | 0.10 0.02 a | 0.13 0.01 bc | 0.15 0.00 c | 0.18 0.02 * | 0.15 0.02 c |
| 19 | 0.33 0.01 * | 6.41 0.00 * | 5.20 0.00 * | 5.40 0.00 * | 4.12 0.00 * | 4.00 0.01 * |
| 20 | 0.14 0.05 ab | 0.36 0.00 * | 0.31 0.02 * | 0.20 0.00 * | 0.15 0.02 b | 0.10 0.05 a |
| 21 | nd | 0.16 0.02 cd | 0.12 0.00 ab | 0.13 0.05 abc | 0.10 0.00 a | 0.15 0.00 bc |
| 22 | 0.52 0.05 * | 0.63 0.05 * | nd | 0.05 0.00 * | nd | nd |
| 23 | nd | nd | 0.51 0.05 * | 0.05 0.00 * | 0.10 0.00 b | 0.12 0.05 b |
| 24 | nd | nd | nd | nd | nd | nd |
| 25 | nd | 0.10 0.00 c | 0.06 0.00 * | 0.02 0.00 * | nd | nd |
| 26 | nd | nd | nd | nd | nd | nd |
| 27 | 4.05 0.00 * | 3.88 0.00 * | 3.20 0.01 * | 3.10 0.01 * | 3.33 0.02 * | 0.78 0.00 * |
| 28 | 0.11 0.00 * | 0.10 0.00 * | 0.08 0.00 b | 0.05 0.00 * | 0.06 0.00 * | 0.08 0.00 b |
| 29 | 0.08 0.00 c | 0.07 0.00 bc | 0.06 0.02 ab | 0.05 0.00 a | 0.07 0.00 bc | 0.05 0.00 a |
| 30 | 0.16 0.00 * | 0.13 0.00 * | 0.08 0.00 a | 0.08 0.00 a | 0.10 0.00 * | 0.09 0.02 * |
| 31 | 1.04 0.00 * | 1.02 0.00 * | 0.68 0.00 b | 0.56 0.00 * | 0.70 0.00 * | 0.68 0.00 b |
| 32 | 0.13 0.00 c | 0.48 0.02 * | 0.12 0.00 * | 0.11 0.00 b | 0.11 0.00 b | 0.13 0.00 c |
| 33 | 0.28 0.00a | 0.32 0.00 * | 0.19 0.00 * | 0.30 0.00 * | 0.35 0.00 * | 0.29 0.00 * |
| 34 | 0.83 0.00 * | 0.96 0.00 * | 0.80 0.02 * | 0.75 0.02 * | 0.89 0.02 * | 0.78 0.00 * |
| 35 | 0.04 0.00 a | 0.07 0.04 b | 0.07 0.00 b | 0.07 0.00 b | 0.05 0.00 ab | 0.05 0.00 ab |
| 36 | nd | nd | nd | nd | nd | nd |
| 37 | nd | nd | nd | nd | nd | nd |
| 38 | 2.77 0.00 * | 3.81 0.00 * | 3.36 0.02 * | 3.27 0.05 * | 3.00 0.02 * | 3.15 0.00 * |
| 39 | 0.23 0.03 * | 0.26 0.00 * | 0.18 0.02 a | 0.20 0.00 a | 0.29 0.00 * | 0.19 0.02 a |
| 40 | 0.26 0.00 c | 0.23 0.02 b | 0.19 0.00 a | 0.25 0.01 c | 0.20 0.02 a | 0.22 0.01 b |
| 41 | 0.40 0.00 b | 0.44 0.00 * | 0.35 0.02 a | 0.35 0.00 a | 0.38 0.02 ab | 0.30 0.02 * |
| 42 | 2.67 0.00 * | 2.43 0.02 * | 2.19 0.00 * | 2.32 0.01 * | 2.40 0.05 * | 2.22 0.00 * |
| 43 | 0.08 0.00 cd | 0.07 0.00 bc | 0.07 0.02 bc | 0.06 0.00 ab | 0.07 0.01 bc | 0.05 0.00 a |
| 44 | nd | 0.04 0.00 * | 0.03 0.00 a | 0.02 0.00 * | 0.01 0.02 * | 0.03 0.00 a |
| 45 | 0.40 0.00 * | 0.42 0.02 * | 0.04 0.02 * | 0.09 0.00 a | 0.20 0.00 * | 0.10 0.00 a |
| 46 | 0.52 0.02 b | 0.34 0.05 * | nd | 0.05 0.00 a | 0.17 0.02 * | 0.07 0.00 a |
| 47 | 0.55 0.01 b | nd | 0.55 0.01 b | 0.44 0.02 a | 0.09 0.02 * | 0.45 0.00 a |
| 48 | 0.07 0.00 * | 0.06 0.00 * | 0.10 0.00 a | 0.15 0.01 * | 0.18 0.00* | 0.14 0.01 c |
| 49 | 0.07 0.01 a | 0.07 0.02 a | 0.08 0.02 ab | 0.14 0.00 d | 0.12 0.00* | 0.09 0.00 b |
| 50 | nd | 0.08 0.01 cd | 0.10 0.00 de | 0.05 0.01 ab | 0.07 0.02 bc | 0.04 0.01 a |
| 51 | 16.00 0.01 * | 17.08 0.05 * | 17.17 0.01 * | 14.26 0.05 * | 13.67 0.05 * | 13.26 0.00 * |
| 52 | nd | 0.73 . 0.00 * | 0.52 0.02 * | 0.15 0.00 c | 0.17 0.00 c | 0.12 0.00 b |
| 53 | nd | nd | nd | nd | nd | nd |
| 54 | 0.76 0.03 * | nd | nd | nd | nd | nd |
| 55 | nd | nd | nd | 0.05 0.01 b | nd | nd |
| 56 | 0.14 0.02 c | 0.10 0.01 b | 0.11 0.00 b | 0.08 0.00 a | 0.05 0.00 * | 0.08 0.00 a |
| 57 | 0.04 0.00 c | 0.03 0.00 bc | 0.02 0.00 b | 0.01 0.01 a | 0.02 0.00 b | nd |
| 58 | 0.19 0.01 d | 0.17 0.05 cd | 0.19 0.00 d | 0.07 0.01 b | 0.08 0.00 b | 0.15 0.00 c |
| 59 | 0.12 0.01 * | 0.06 0.02 * | nd | 0.28 0.00 a | 0.36 0.00 * | 0.30 0.02 a |
| 60 | 0.06 0.00 cd | 0.05 0.01 bc | nd | 0.04 0.00 b | 0.05 0.02 bc | 0.09 0.00 f |
| 61 | nd | 0.07 0.02 * | nd | 0.11 0.02 c | 0.15 0.00 * | 0.23 0.00* |
| 62 | nd | nd | nd | 0.10 0.01 b | 0.15 0.05 * | 0.07 0.01 * |
| 63 | 16.17 0.05 * | 18.73 0.05 * | 16.92 0.02 * | 14.00 0.00 * | 12.32 0.05 * | 10.14 0.00 * |
| 64 | nd | nd | nd | nd | nd | nd |
| 65 | nd | nd | nd | nd | nd | nd |
| 66 | nd | nd | nd | nd | nd | nd |
| 67 | nd | nd | nd | nd | nd | nd |
| 68 | 0.33 0.00 * | 0.31 0.01 * | 0.29 0.01 * | 0.15 0.00 * | 0.09 0.00 * | 0.05 0.00 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutarașu, E.C.; Luchian, C.E.; Vlase, L.; Nagy, K.; Colibaba, L.C.; Trinca, L.C.; Cotea, V.V. Influence Evaluation of Enzyme Treatments on Aroma Profile of White Wines. Agronomy 2022, 12, 2897. https://doi.org/10.3390/agronomy12112897
Scutarașu EC, Luchian CE, Vlase L, Nagy K, Colibaba LC, Trinca LC, Cotea VV. Influence Evaluation of Enzyme Treatments on Aroma Profile of White Wines. Agronomy. 2022; 12(11):2897. https://doi.org/10.3390/agronomy12112897
Chicago/Turabian StyleScutarașu, Elena Cristina, Camelia Elena Luchian, Laurian Vlase, Katalin Nagy, Lucia Cintia Colibaba, Lucia Carmen Trinca, and Valeriu V. Cotea. 2022. "Influence Evaluation of Enzyme Treatments on Aroma Profile of White Wines" Agronomy 12, no. 11: 2897. https://doi.org/10.3390/agronomy12112897
APA StyleScutarașu, E. C., Luchian, C. E., Vlase, L., Nagy, K., Colibaba, L. C., Trinca, L. C., & Cotea, V. V. (2022). Influence Evaluation of Enzyme Treatments on Aroma Profile of White Wines. Agronomy, 12(11), 2897. https://doi.org/10.3390/agronomy12112897










