Biochar-Derived Smoke Waters Affect Bactrocera oleae Behavior and Control the Olive Fruit Fly under Field Conditions
Abstract
1. Introduction
- (i)
- ingestion of SWs can increase mortality and reduce the fitness of OLF;
- (ii)
- SWs alter the OLF microbiome reducing the “Ca. Erwinia dacicola” titer;
- (iii)
- exposure to SWs can hamper the ability of adult OLF to locate the position of fresh green olives in the lab; and
- (iv)
- SW application can reduce the damage to olive production caused by the female OLF.
2. Materials and Methods
2.1. SWs Production
2.2. SWs Chemical Characterization
2.3. OLF Collection
2.4. OLF Ingestion Bioassay and Effect on Microbiome
2.5. SWs Topic Bioassay
2.6. SWs Olfactometry Trials
2.7. OLF Electroantennography
2.8. SWs Effect on OLF under Field Conditions
2.9. Statistics and Data Analysis
3. Results
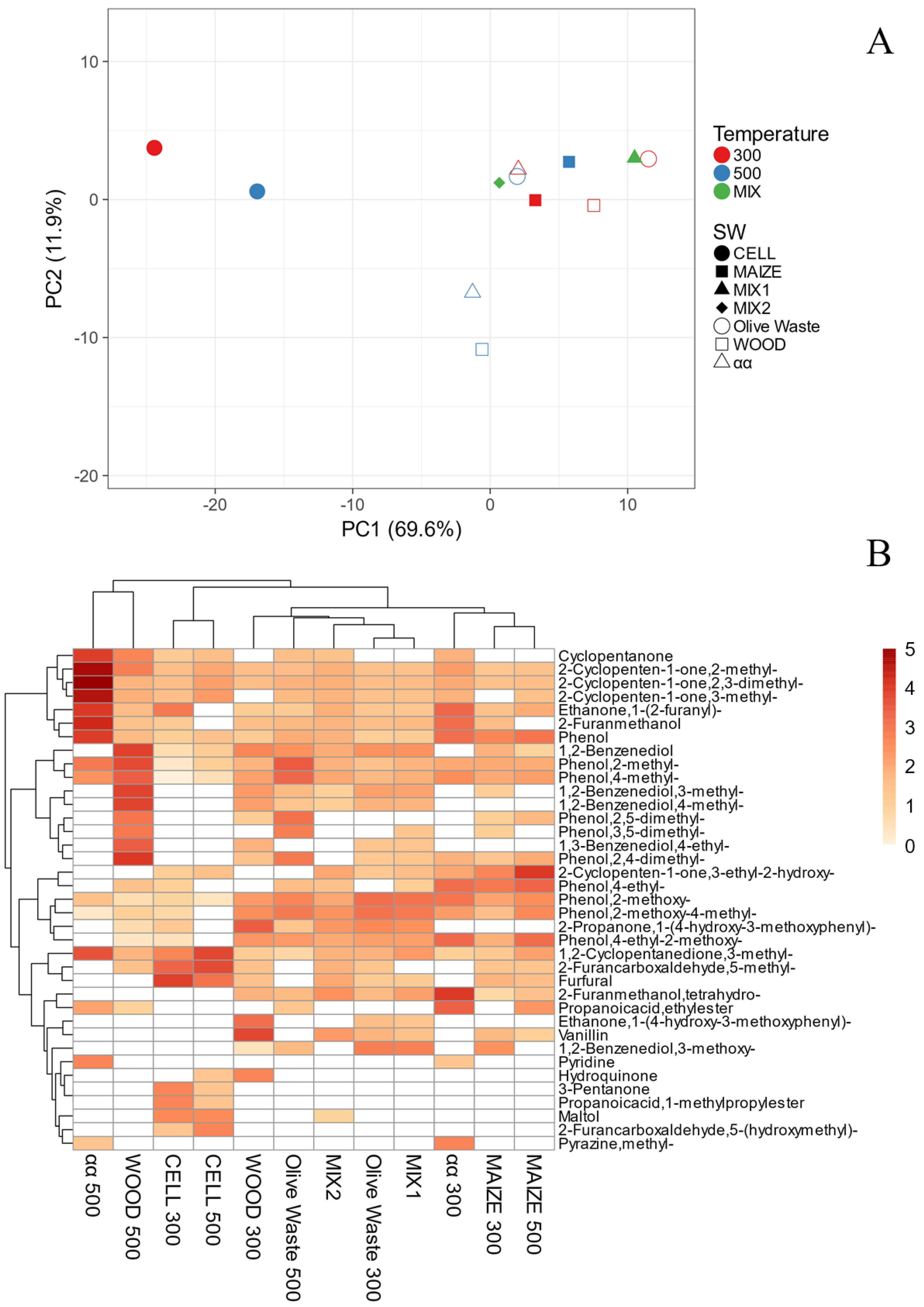
3.1. SWs Chemical Characterization
3.2. OLF Ingestion Bioassay and Effect on Microbiome
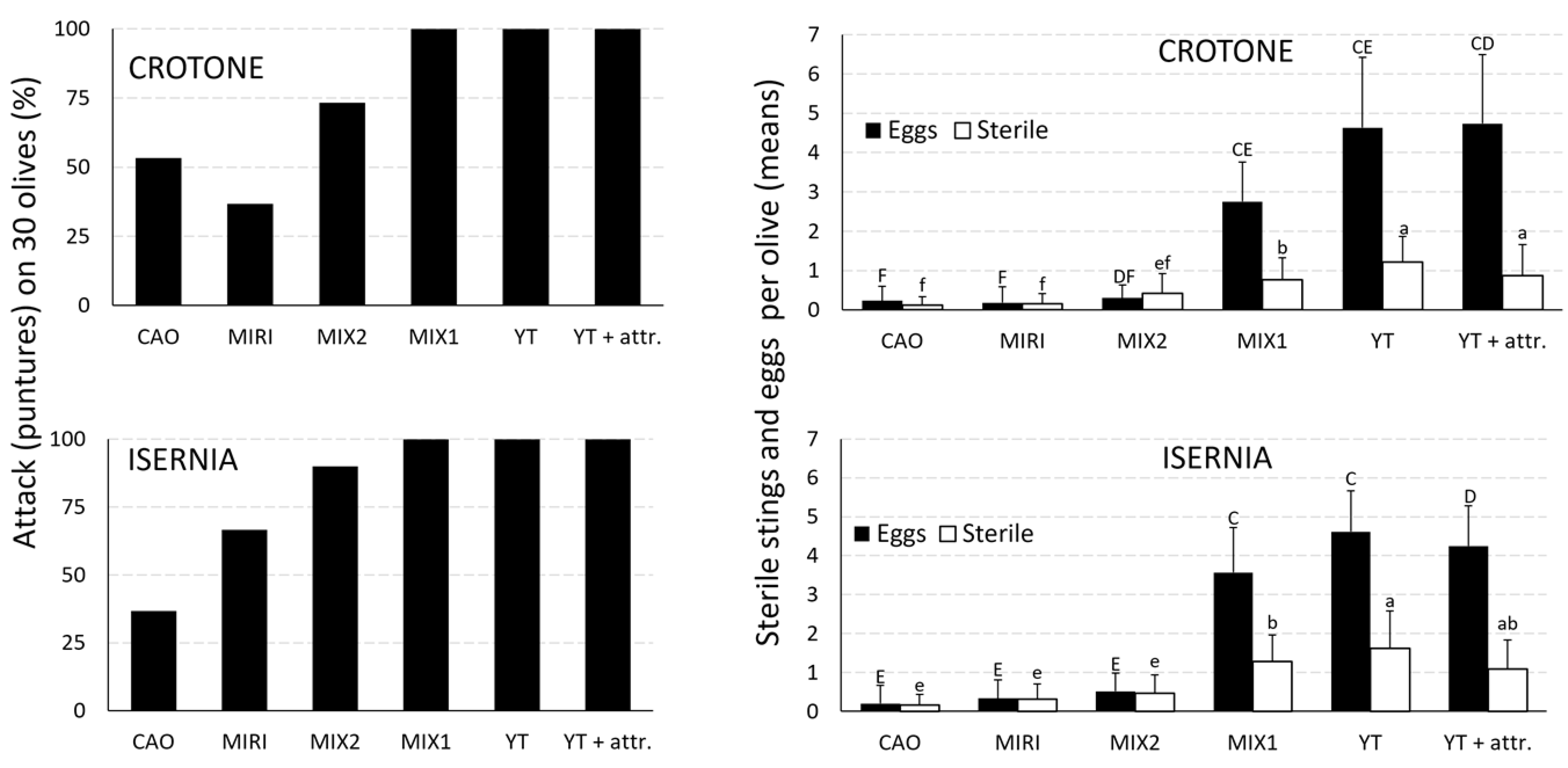
3.3. SWs Topic Bioassays
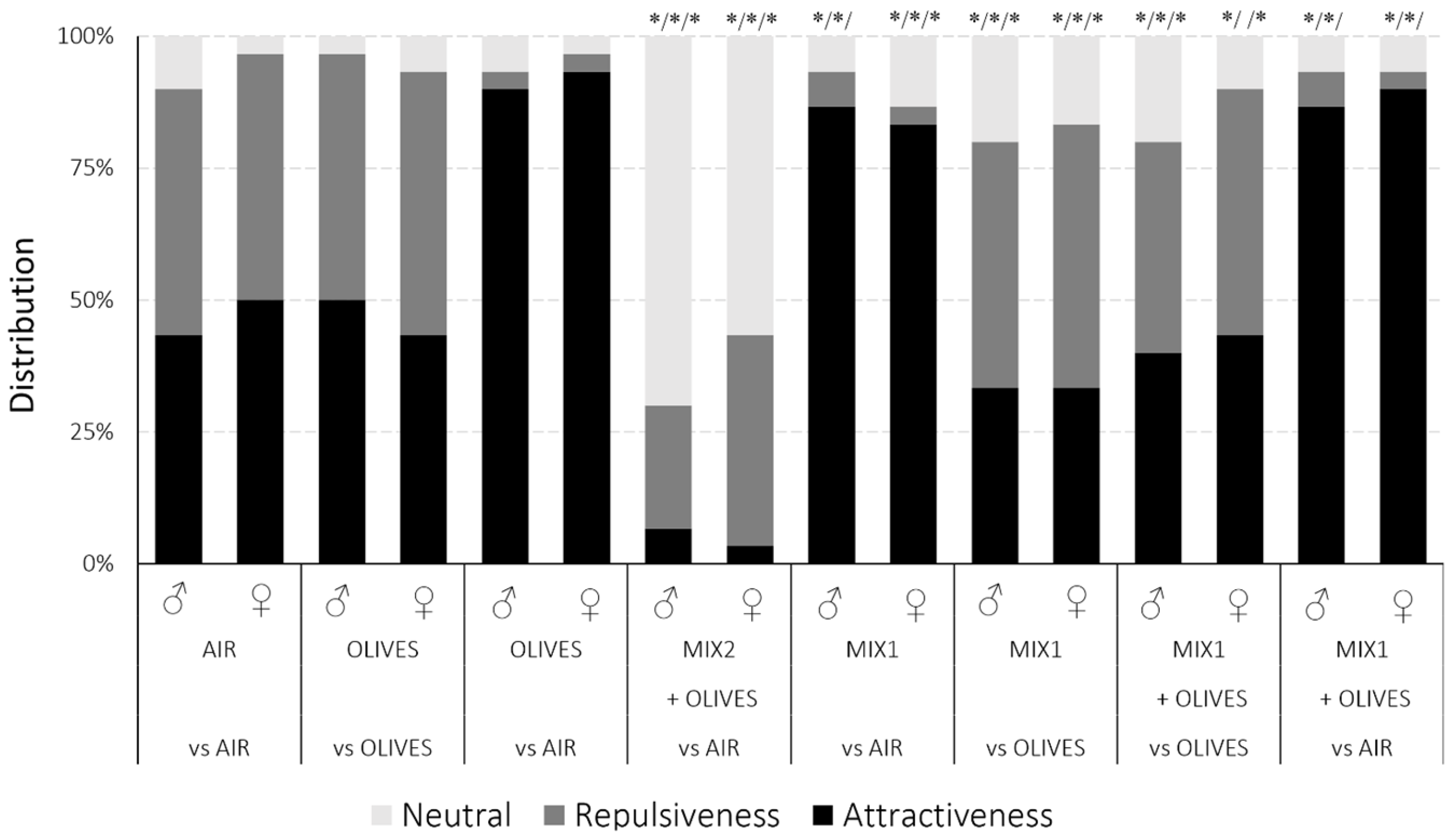
3.4. SWs Olfactometry Trials
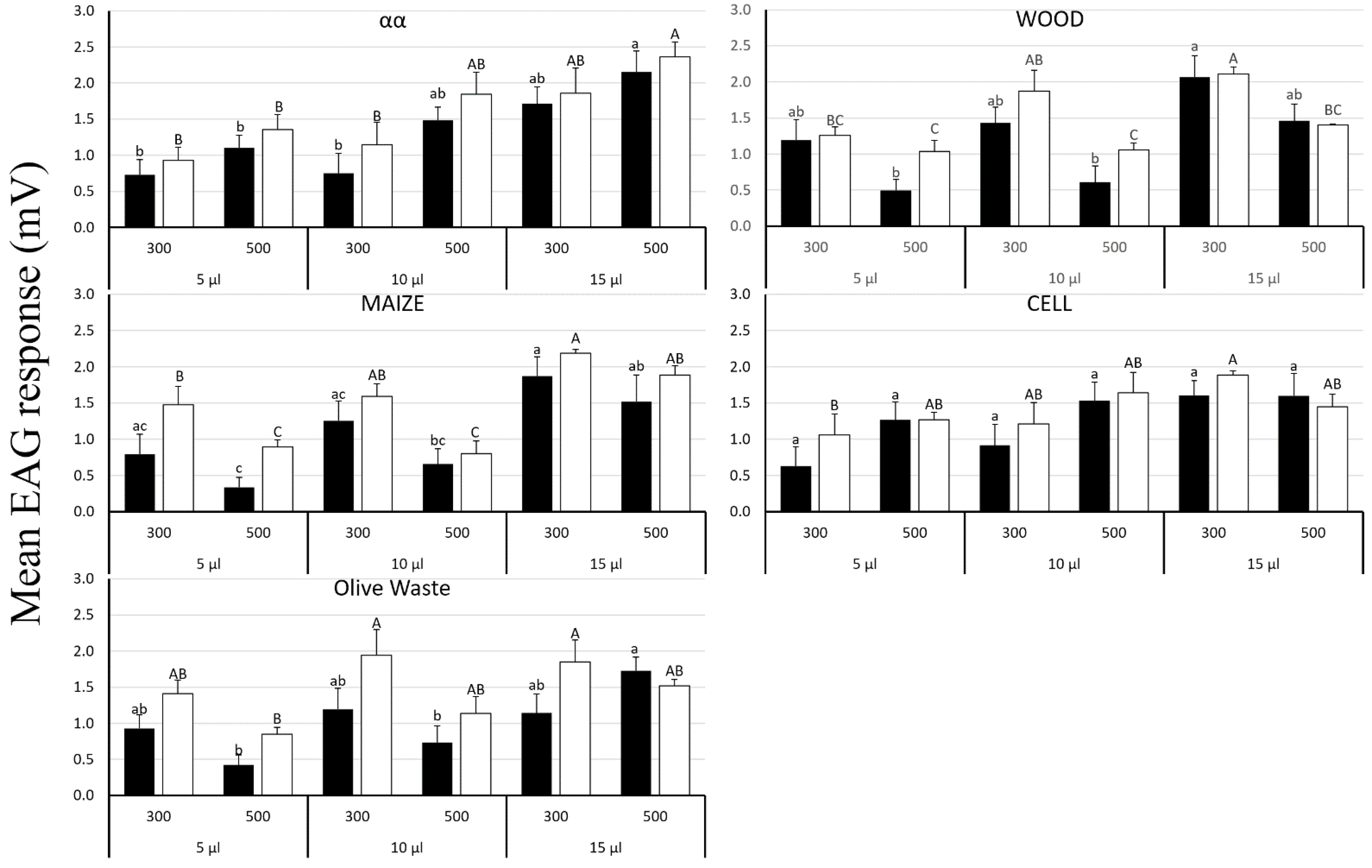
3.5. OLF Electroantennography
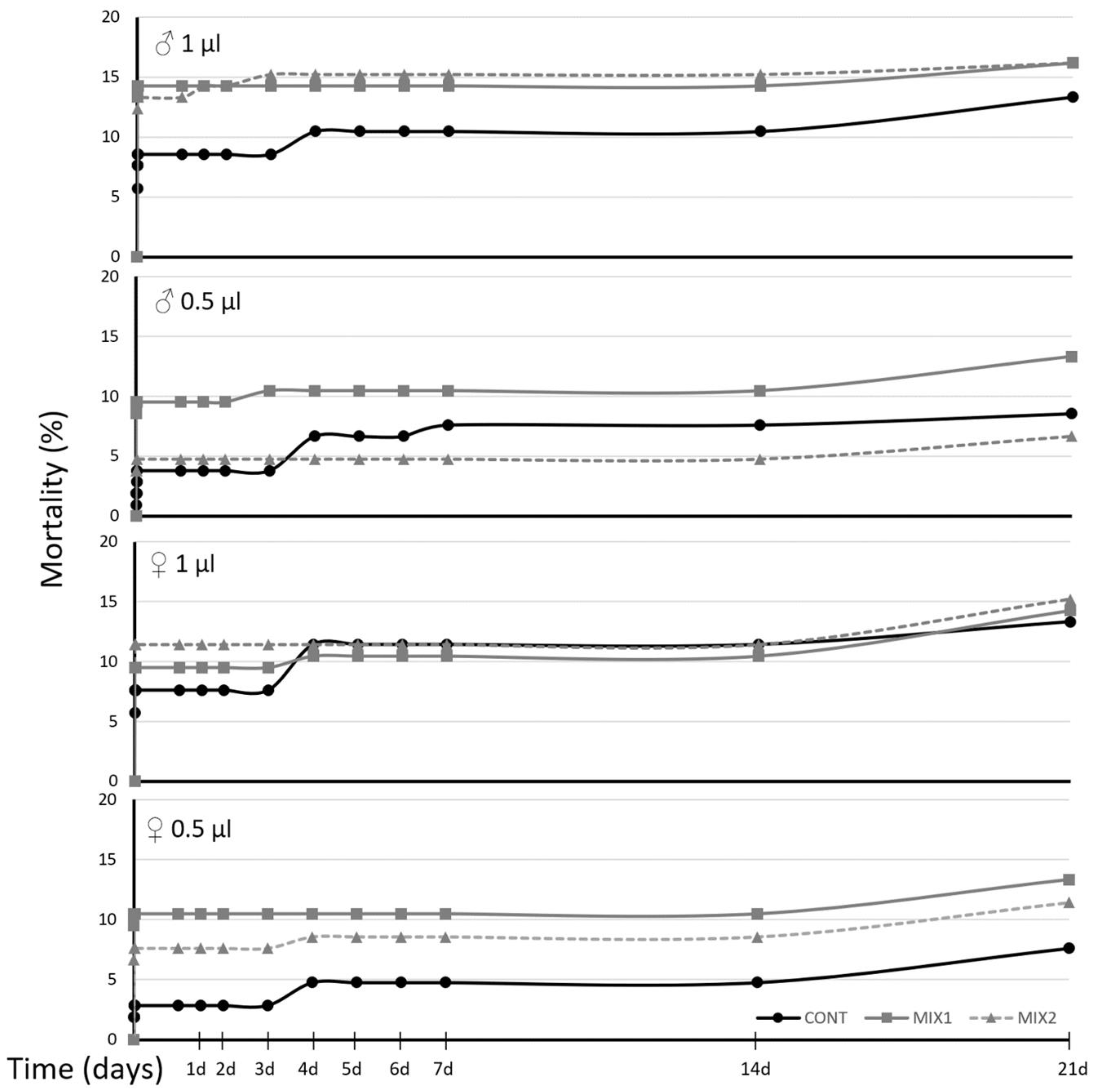
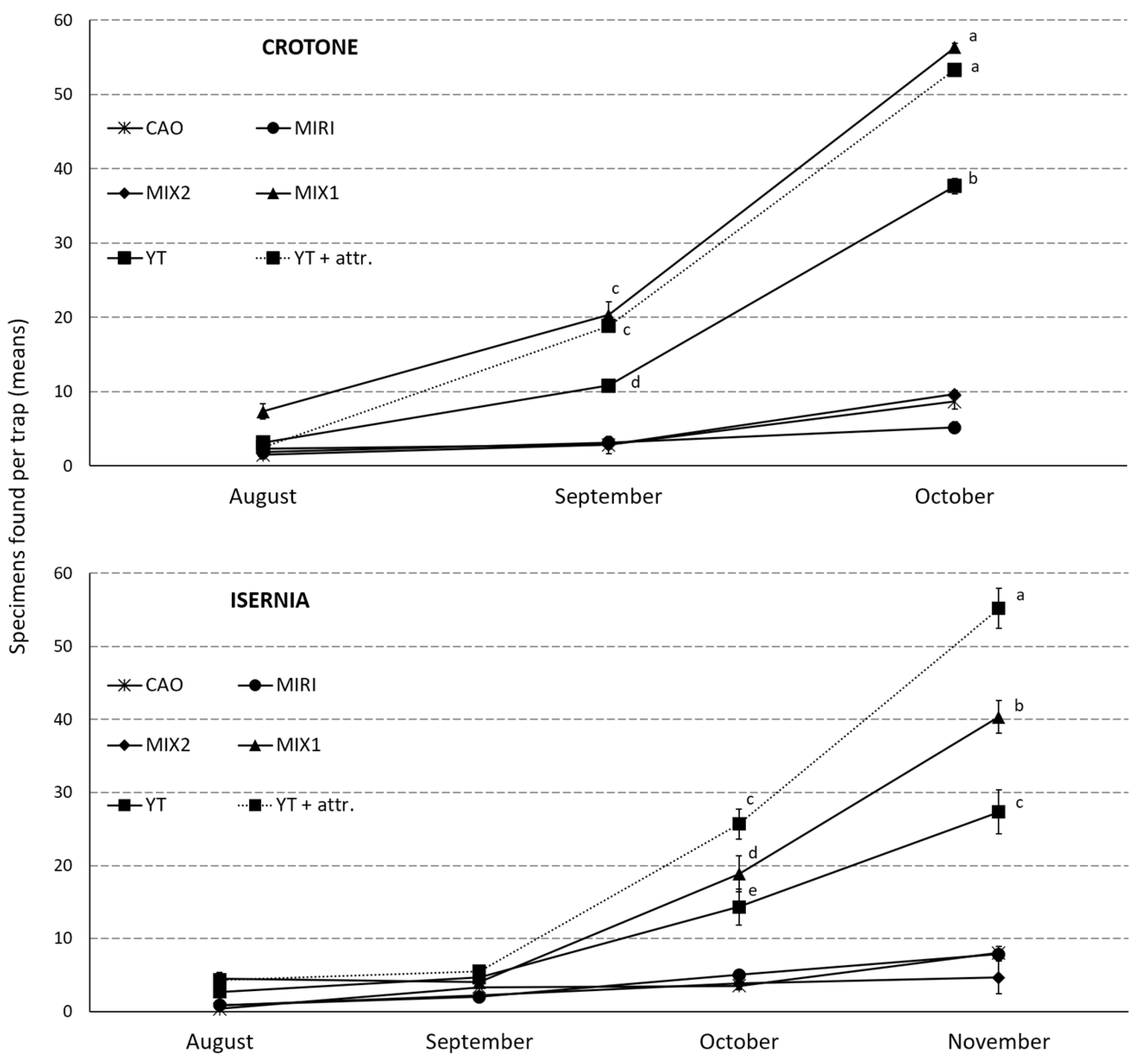
3.6. SWs Effect on OLF under Field Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montiel Bueno, A.; Jones, O.T. Alternative Methods for Controlling the Olive Fly Bactrocera oleae Involving Semiochemicals. IOBC WPRS Bull. 2002, 25, 1–11. [Google Scholar]
- Ordano, M.; Engelhard, I.; Rempoulakis, P.; Nemny-Lavy, E.; Blum, M.; Yasin, S.; Lensky, I.M.; Papadopoulos, N.T.; Nestel, D. Olive Fruit Fly (Bactrocera oleae) Population Dynamics in the Eastern Mediterranean: Influence of Exogenous Uncertainty on a Monophagous Frugivorous Insect. PLoS ONE 2015, 10, e0127798. [Google Scholar] [CrossRef] [PubMed]
- Daane, K.M.; Johnson, M.W. Olive Fruit Fly: Managing an Ancient Pest in Modern Times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Del Carlo, M.; Compagnone, D.; Cichelli, A. Effects of Fly Attack (Bactrocera oleae) on the Phenolic Profile and Selected Chemical Parameters of Olive Oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef]
- Pereira, J.A.; Rui Alves, M.; Casal, S.; Oliveira, M. Effect of Olive Fruit Fly Infestation on the Quality of Olive Oil from Chemlali Cultivar during Ripening. Ital. J. Food Sci. 2004, 3, 355–365. [Google Scholar] [CrossRef]
- Notario, A.; Sánchez, R.; Luaces, P.; Sanz, C.; Pérez, A.G. The Infestation of Olive Fruits by Bactrocera oleae (Rossi) Modifies the Expression of Key Genes in the Biosynthesis of Volatile and Phenolic Compounds and Alters the Composition of Virgin Olive Oil. Molecules 2022, 27, 1650. [Google Scholar] [CrossRef]
- Burrack, H.J.; Zalom, F.G. Olive Fruit Fly, Bactrocera Oleae (Gmel.) Ovipositional Preference and Larval Performance in Several Commercially Important Olive Varieties in California. J. Econ. Entomol. 2008, 101, 750–758. [Google Scholar] [CrossRef]
- Iannotta, N.; Scalercio, S. Susceptibility of Cultivars to Biotic Stresses. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; INTECH Open Access Publisher: London, UK, 2012; pp. 81–106. [Google Scholar]
- Ortega, M.; Moreno, N.; Fernández, C.E.; Pascual, S. Olive Landscape Affects Bactrocera oleae Abundance, Movement and Infestation. Agronomy 2022, 12, 4. [Google Scholar] [CrossRef]
- Caleca, V.; Lo Verde, G.; Lo Verde, V.; Palumbo Piccionello, M.; Rizzo, R. Control of Bactrocera oleae and Ceratitis Capitata in Organic Orchards: Use of Clays and Copper Products. Acta Hortic. 2010, 873, 227–234. [Google Scholar] [CrossRef]
- Saour, G.; Makee, H. A Kaolin-Based Particle Film for Suppression of the Olive Fruit Fly Bactrocera oleae Gmelin (Dip., Tephritidae) in Olive Groves. J. Appl. Entomol. 2004, 128, 28–31. [Google Scholar] [CrossRef]
- Rosi, M.C.; Sacchetti, P.; Librandi, M.; Belcari, A. Effectiveness of Different Copper Products against the Olive Fly in Organic Olive Groves. Integr. Prot. Olive Crop. IOBC WPRS Bull. 2007, 30, 277–281. [Google Scholar]
- Skouras, P.J.; Margaritopoulos, J.T.; Seraphides, N.A.; Ioannides, I.M.; Kakani, E.G.; Mathiopoulos, K.D.; Atsitsipis, J. Organophosphate Resistance in Olive Fruit Fly, Bactrocera oleae, Populations in Greece and Cyprus. Pest Manag. Sci. 2006, 63, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Margaritopoulos, J.T.; Skavdis, G.; Kalogiannis, N.; Nikou, D.; Morou, E.; Skouras, P.J.; Tsitsipis, J.A.; Vontas, J. Efficacy of the Pyrethroid Alpha-Cypermethrin against Bactrocera oleae Populations from Greece, and Improved Diagnostic for an IAChE Mutation. Pest Manag. Sci. 2008, 64, 900–908. [Google Scholar] [CrossRef]
- Broumas, T. Application Trials of Trapping Systems for Control of Dacus oleae. Ann. Inst. Phytopathol. Benaki 1985, 14, 157–166. [Google Scholar]
- Prokopy, R.J.; Economopoulos, A.P.; McFadden, M.W. Attraction of Wild and Laboratory-Cultured Dacus oleae Flies to Small Rectangles of Different Hues, Shades and Tints. Entomol. Exper. Appl. 1975, 18, 141–152. [Google Scholar] [CrossRef]
- Ahmadi, M.; Salehi, B.; Abd-Alla, A.M.M.; Babaie, M. Feasibility of Using the Radiation-Based Sterile Insect Technique (SIT) to Control the Olive Fruit Fly, Bactrocera oleae Gmelin (Diptera: Tephritidae) in Iran. Appl. Radiat. Isot. 2018, 139, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.M.; Hearn, D.J.; Burrack, H.J.; Rempoulakis, P.; Pierson, E.A. Prevalence of Candidatus Erwinia dacicola in Wild and Laboratory Olive Fruit Fly Populations and across Developmental Stages. Environ. Entomol. 2012, 41, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hoelmer, K.A.; Kirk, A.A.; Pickett, C.H.; Daane, K.M.; Johnson, M.W. Prospects for Improving Biological Control of Olive Fruit Fly, Bactrocera oleae (Diptera: Tephritidae), with Introduced Parasitoids (Hymenoptera). Biocontrol. Sci. Technol. 2011, 21, 1005–1025. [Google Scholar] [CrossRef]
- Silva, F.J.; Moret, A.; Neef, A.; Belda, E. Bacterial Microbiota Associated with a Bactrocera oleae Population from Eastern Spain. In Abstract Book, Proceedings of the First Meeting of TEAM, Palma de Mallorca, Spain, 7–8 April 2008; Miranda-Chueca, M.A., Ed.; Universitat de les Illes Balears: Mallorca, Spain, 2008; Volume 15, p. 15. [Google Scholar]
- Yokoyama, V.Y.; Rendon, P.A.; Sivinsiki, J.M. Psyttalia Cf. Concolor (Hymenoptera: Braconidae) for Biological Control of Olive Fruit Fly (Diptera: Tephritidae) in California. Environ. Entomol. 2008, 37, 764–773. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Aharon, Y.; Jurkevitch, E.; Yuval, B. Give Us the Tools and We Will Do the Job: Symbiotic Bacteria Affect Olive Fly Fitness in a Diet-Dependent Fashion. Proc. R. Soc. B Biol. Sci. 2010, 277, 1545–1552. [Google Scholar] [CrossRef]
- Savio, C.; Mazzon, L.; Martinez-Sañudo, I.; Simonato, M.; Squartini, A.; Girolami, V. Evidence of Two Lineages of the Symbiont “Candidatus Erwinia Dacicola” in Italian Populations of Bactrocera oleae (Rossi) Based on 16S RRNA Gene Sequences. Int. J. Syst. Evol. Microbiol. 2012, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.M.; Hearn, D.J.; Bronstein, J.L.; Pierson, E.A. The Olive Fly Endosymbiont, “Candidatus Erwinia dacicola,” Switches from an Intracellular Existence to an Extracellular Existence during Host Insect Development. Appl. Environ. Microbiol. 2009, 75, 7097–7106. [Google Scholar] [CrossRef] [PubMed]
- Caleca, V.; Belcari, A.; Sacchetti, P. Lotta Alla Mosca Delle Olive in Olivicoltura Integrata e Biologica. Protezione Colture 2012, 3, 27–33. [Google Scholar]
- Sacchetti, P.; Granchietti, A.; Landini, S.; Viti, C.; Giovannetti, L.; Belcari, A. Relationships between the Olive Fly and Bacteria. J. Appl. Entomol. 2008, 132, 682–689. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic Bacteria Enable Olive Fly Larvae to Overcome Host Defences. R. Soc. Open Sci. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Ras, E.; Beukeboom, L.W.; Cáceres, C.; Bourtzis, K. Review of the Role of Gut Microbiota in Mass Rearing of the Olive Fruit Fly, Bactrocera oleae, and Its Parasitoids. Entomol. Exp. Appl. 2017, 164, 237–256. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. 2015. Available online: https://biochar-international.org/characterizationstandard/ (accessed on 11 October 2022).
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge, Ed.; Earthscan Publications Ltd.: London, UK, 2015; ISBN 9780415704151. [Google Scholar]
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and Char Quality of Flame-Curtain “Kon Tiki” Kilns for Farmer-Scale Charcoal/Biochar Production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef]
- Pennise, D.M.; Smith, K.R.; Kithinji, J.P.; Rezende, M.E.; Raad, T.J.; Zhang, J.; Fan, C. Emissions of Greenhouse Gases and Other Airborne Pollutants from Charcoal Making in Kenya and Brazil. J. Geophys. Res. Atmos. 2001, 106, 24143–24155. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Light, M.E.; Van Staden, J. Plant-Derived Smoke: Old Technology with Possibilities for Economic Applications in Agriculture and Horticulture. S. Afr. J. Bot. 2011, 77, 972–979. [Google Scholar] [CrossRef]
- De Lange, J.H.; Boucher, C. Autecological Studies on Audouinia capitata (Bruniaceae). I. Plant-Derived Smoke as a Seed Germination Cue. S. Afr. J. Bot. 1990, 56, 700–703. [Google Scholar] [CrossRef]
- Antala, M.; Sytar, O.; Rastogi, A.; Brestic, M. Potential of Karrikins as Novel Plant Growth Regulators in Agriculture. Plants 2020, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Waters, M.T. Perception of Karrikins by Plants: A Continuing Enigma. J. Exp. Bot. 2020, 71, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Bursch, K.; Niemann, E.T.; Nelson, D.C.; Johansson, H. Karrikins Control Seedling Photomorphogenesis and Anthocyanin Biosynthesis through a HY5-BBX Transcriptional Module. Plant J. 2021, 107, 1346–1362. [Google Scholar] [CrossRef] [PubMed]
- Gokdas, Z.; Yildirim, E.; Gupta, S.; Demir, I. Karrikinolide Stimulated Seed Germination of Artificially Aged Marrow, Cabbage and Pepper Seeds through Repair of Cell Structure and Enzyme Activity. S. Afr. J. Bot. 2022, 151, 208–213. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A Compound from Smoke That Promotes Seed Germination. Science 2004, 305, 977. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Jesu, G.; Zotti, M.; Idbella, M.; Abd-elgawad, A.; D’Errico, G.; Laudonia, S.; Vinale, F.; Abd-elgawad, A. Biochar-Derived Smoke-Water Exerts Biological Effects on Nematodes, Insects, and Higher Plants but Not Fungi. Sci. Total Environ. 2021, 750, 142307. [Google Scholar] [CrossRef]
- Boeckh, J.; Kaissling, K.E.; Schneider, D. Insect Olfactory Receptors. Cold Spring Harb. Symp. Quant. Biol. 1965, 30, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, S.G.; Smart, K.F.; Sivakumaran, S.; Lane, G.A. Alkylation or Silylation for Analysis of Amino and Non-Amino Organic Acids by GC-MS? Metabolites 2011, 1, 3–20. [Google Scholar] [CrossRef]
- Zarate, E.; Boyle, V.; Rupprecht, U.; Green, S.; Villas-Boas, S.G.; Baker, P.; Pinu, F.R. Fully Automated Trimethylsilyl (TMS) Derivatisation Protocol for Metabolite Profiling by GC-MS. Metabolites 2017, 7, 1. [Google Scholar] [CrossRef]
- Bertolini, E.; Kistenpfennig, C.; Menegazzi, P.; Keller, A.; Koukidou, M.; Helfrich-Förster, C. The characterization of the circadian clock in the olive fly Bactrocera oleae (Diptera: Tephritidae) reveals a Drosophila-like organization. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Germinara, G.S.; Beleggia, R.; Fragasso, M.; Pistillo, M.O.; De Vita, P. Kernel Volatiles of Some Pigmented Wheats Do Not Elicit a Preferential Orientation in Sitophilus granarius Adults. J. Pest Sci. 2019, 92, 653–664. [Google Scholar] [CrossRef]
- Paventi, G.; de Acutis, L.; De Cristofaro, A.; Pistillo, M.; Germinara, G.S.; Rotundo, G. Biological Activity of Humulus lupulus (L.) Essential Oil and Its Main Components against Sitophilus Granarius (L.). Biomolecules 2020, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Pistillo, M.; Germinara, G.S.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Petrelli, R.; Spinozzi, E.; Cappellacci, L.; Zeni, V.; et al. Bioactivity of Carlina acaulis Essential Oil and Its Main Component towards the Olive Fruit Fly, Bactrocera oleae: Ingestion Toxicity, Electrophysiological and Behavioral Insights. Insects 2021, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, S.; Cascone, P.; Di Domenico, C.; Pistillo, M.; Formisano, G.; Giorgini, M.; Grazioso, P.; Germinara, G.S.; Cristofaro, A.D.; Guerrieri, E. Electrophysiological and Behavioural Response of Philaenus spumarius to Essential Oils and Aromatic Plants. Sci. Rep. 2020, 10, 3114. [Google Scholar] [CrossRef] [PubMed]
- Canale, A.; Benelli, G.; Germinara, G.S.; Carpita, A.; Raspi, A.; Rotundo, G. Shedding Light on the Sexual Chemoecology of Olive Fruit Fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae). In Proceedings of the Atti Accademia Nazionale Italiana di Entomologia, Anno LXIV, Firenze, Italy; 2016; pp. 63–66. [Google Scholar]
- Rizzo, R.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Reza Morshedloo, M.; Yvette Fofie, N.G.B.; Benelli, G. Developing Green Insecticides to Manage Olive Fruit Flies? Ingestion Toxicity of Four Essential Oils in Protein Baits on Bactrocera oleae. Ind. Crops Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Estes, A.M.; Hearn, D.J.; Agrawal, S.; Pierson, E.A.; Dunning Hotopp, J.C. Comparative Genomics of the Erwinia and Enterobacter Olive Fly Endosymbionts. Sci. Rep. 2018, 8, 15936. [Google Scholar] [CrossRef]
- Jurkevitch, E. Riding the Trojan Horse: Combating Pest Insects with Their Own Symbionts. Microb. Biotechnol. 2011, 4, 620–627. [Google Scholar] [CrossRef]
- Salima, B.; Hachemi, B.; Zineb, B. Insecticidal Activity of Inula Viscosa L. (Asteraceae) Essential Oils on Adults of Bactrocera oleae Gmel (Diptera Tephritidae). J. Glob. Agric. Ecol. 2016, 5, 225–230. [Google Scholar]
- Yousef, M.; Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E. Biocontrol of Bactrocera oleae (Diptera: Tephritidae) with Metarhizium brunneum and Its Extracts. J. Econ. Entomol. 2013, 106, 1118–1125. [Google Scholar] [CrossRef]
- Benelli, G.; Bonsignori, G.; Stefanini, C.; Raspi, A.; Canale, A. The Production of Female Sex Pheromone in Bactrocera oleae (Rossi) Young Males Does Not Influence Their Mating Chances. Entomol. Sci. 2013, 16, 47–53. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Pantazi-Mazomenou, A.; Stefanou, D. Attract and Kill of the Olive Fruit Fly Bactrocera oleae in Greece as a Part of an Integrated Control System. IOBC WPRS Bull. 2002, 25, 137–146. [Google Scholar]
- Petacchi, R.; Rizzi, I.; Guidotti, D. The “lure and Kill” Technique in Bactrocera oleae (Gmel.) Control: Effectiveness Indices and Suitability of the Technique in Area-Wide Experimental Trials. Int. J. Pest Manag. 2003, 49, 305–311. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Germinara, G.S.; Fusini, G.; Romano, D.; Rapalini, F.; Desneux, N.; Rotundo, G.; Raspi, A.; Carpita, A. Behavioural and Electrophysiological Responses to Overlooked Female Pheromone Components in the Olive Fruit Fly, Bactrocera oleae (Diptera: Tephritidae). Chemoecology 2015, 25, 147–157. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Conte, G.; Mele, M.; Caruso, G.; Gucci, R.; Flamini, G.; Canale, A. VOCs-Mediated Location of Olive Fly Larvae by the Braconid Parasitoid Psyttalia Concolor: A Multivariate Comparison among VOC Bouquets from Three Olive Cultivars. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Liscia, A.M.; Sacchetti, P.; Setzu, M.D.; Poddeghe, S.; De Rose, F.; Belcari, A. Palpal Receptors of the Olive Fly Bactrocera oleae Play a Role in Foraging Behavior and Host Finding. Eur. J. Histochem. 2013, 57, 5. [Google Scholar]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Identification of Leaf Volatiles from Olive (Olea Europaea) and Their Possible Role in the Ovipositional Preferences of Olive Fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae). Phytochemistry 2016, 121, 11–19. [Google Scholar] [CrossRef]
- Nor Qhairul Izzreen, M.N.; Hansen, Å.S.; Petersen, M.A. Volatile Compounds in Whole Meal Bread Crust: The Effects of Yeast Level and Fermentation Temperature. Food Chem. 2016, 210, 566–576. [Google Scholar] [CrossRef]
- Yin, W.; Washington, M.; Yang, X.; Lu, A.; Ma, X.; Rui, S.; Wang, X.; Zhao, R. Consumer Acceptability and Sensory Profiling of Sesame Oils Obtained from Different Processes. Grain Oil Sci. Technol. 2020, 3, 39–48. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Yoshimura, T.; Rusmiyanto, E.; Wardoyo, P. Antifungal and Antitermitic Activities of Vinegars From Two Biomass Resources At Different Pyrolytic Temperatures. J. Appl. Biol. Sci. E 2020, 14, 26–38. [Google Scholar]
- Balasubramanian, S.; Ganesh, D.; Panchal, P.; Teimouri, M.; Surya Narayana, V.V.S. GC-MS Analysis of Phytocomponents in the Methanolic Extract of Emblica officinalis Gaertn (Indian Gooseberry). J. Chem. Pharm. Res. 2014, 6, 843–845. [Google Scholar]
- Sung, W.C. Volatile Constituents Detected in Smoke Condensates from the Combination of the Smoking Ingredients Sucrose, Black Tea Leaves, and Bread Flour. J. Food Drug Anal. 2013, 21, 292–300. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B.; Jones, J.M. Pyrolysis Behaviour of the Main Carbohydrates of Brown Macro-Algae. Fuel 2011, 90, 598–607. [Google Scholar] [CrossRef]
- Mahadevan, K.; Farmer, L. Key Odor Impact Compounds in Three Yeast Extract Pastes. J. Agric. Food Chem. 2006, 54, 7242–7250. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V.; Krishnaveni, S. Larvicidal Efficacy of Leaf Extracts of Heliotropium indicum and Mukia maderaspatana against the Dengue Fever Mosquito Vector Aedes aegypti. J. Entomol. Zool. Stud. 2014, 2, 40–45. [Google Scholar]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. A Note: Gut Bacteria Produce Components of a Locust Cohesion Pheromone. J. Appl. Microbiol. 2002, 92, 759–763. [Google Scholar] [CrossRef]
- Revadi, S.V.; Giannuzzi, V.A.; Vetukuri, R.R.; Walker, W.B.; Becher, P.G. Larval Response to Frass and Guaiacol: Detection of an Attractant Produced by Bacteria from Spodoptera littoralis Frass. J. Pest Sci. 2021, 94, 1105–1118. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007; pp. 685–688. [Google Scholar]
- Chai, W.M.; Liu, X.; Hu, Y.H.; Feng, H.L.; Jia, Y.L.; Guo, Y.J.; Zhou, H.T.; Chen, Q.X. Antityrosinase and Antimicrobial Activities of Furfuryl Alcohol, Furfural and Furoic Acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef]
- Paczkowski, S. Insect Olfaction as an Information Filter for Chemo-Analytical Applications; Georg-August-Universit: Göttingen, Germany, 2013. [Google Scholar]
- Attygalle, A.B.; Xu, S.; Moore, W.; McManus, R.; Gill, A.; Will, K. Biosynthetic Origin of Benzoquinones in the Explosive Discharge of the Bombardier Beetle Brachinus elongatulus. Sci. Nat. 2020, 107, 26. [Google Scholar] [CrossRef]
- Williams, M.; Eveleigh, E.; Forbes, G.; Lamb, R.; Roscoe, L.; Silk, P. Evidence of a Direct Chemical Plant Defense Role for Maltol against Spruce Budworm. Entomol. Exp. Appl. 2019, 167, 755–762. [Google Scholar] [CrossRef]
- Noce, M.E.; Belfiore, T.; Scalercio, S.; Vizzarri, V.; Iannotta, N. Efficacy of New Mass-Trapping Devices against Bactrocera oleae (Diptera Tephritidae) for Minimizing Pesticide Input in Agroecosystems. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2009, 44, 442–448. [Google Scholar] [CrossRef]
- Caleca, V.; Giacalone, C.; Maltese, M.; Tortorici, F. Contenimento Naturale Di Bactrocera oleae (Rossi): Clima o Parassitoidi? Confronto Tra Western Cape (Sud Africa) e Sicilia. Atti Accad. Naz. Ital. Entomol. 2016, 14, 99–105. [Google Scholar]
- Castrignanò, A.; Boccaccio, L.; Cohen, Y.; Nestel, D.; Kounatidis, I.; Papadopoulos, N.T.; de Benedetto, D.; Mavragani-Tsipidou, P. Spatio-Temporal Population Dynamics and Area-Wide Delineation of Bactrocera oleae Monitoring Zones Using Multi-Variate Geostatistics. Precis. Agric. 2012, 13, 421–441. [Google Scholar] [CrossRef]
- Broumas, T.; Haniotakis, G.E.; Liaropoulos, C.; Tomazou, T.; Ragoussis, N. The Efficacy of an Improved Form of the Mass-Trapping Method, for the Control of the Olive Fruit Fly, Bactrocera oleae (Gmelin) (Dipt., Tephritidae): Pilot-Scale Feasibility Studies. J. Appl. Entomol. 2002, 126, 217–223. [Google Scholar] [CrossRef]
- Caleca, V.; Rizzo, R. Effectiveness of Clays and Copper Products in the Control of Bactrocera oleae (Gmelin). IOBC WPRS Bull. 2007, 39, 111–117. [Google Scholar]
- Rotundo, G.; Germinara, G.S.; De Cristofaro, A.; Rama, F. Identificazione Di Composti Volatili in Estratti Da Diverse Cultivar Di Olea europaea L. Biologicamente Attivi Su Bactrocera oleae (Gmelin) (Diptera: Tephritidae). Boll. Lab. Entomol. Agrar. Filippo Silvestri 2001, 57, 25–34. [Google Scholar]
- Pascual, S.; Sánchez-Ramos, I.; González-Núñez, M. Repellent/Deterrent Effect of Kaolin and Copper on Bactrocera oleae Oviposition in the Laboratory. In Integrated Protection of Olive Crops, Proceedings of the Meeting at Cordoba, Spain, 1–4 June 2009; Kalaitzaki, A., Minachilis, K., Eds.; International Organization for Biological and Integrated Control of Noxious Animals and Plants (OIBC/OILB), West Palaearctic Regional Section (WPRS/SROP): Dijon, France, 2010; Volume 59, pp. 83–88. [Google Scholar]
- Reza Abbasi Mojdehi, M.; Akbar Keyhanian, A.; Rafiei, B. Application of Oviposition Deterrent Compounds for the Control of Olive Fruit Fly, Bactrocera oleae Rossi (Dip. Tephritidae) Control. Int. J. Trop. Insect Sci. 2021, 42, 63–70. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesu, G.; Laudonia, S.; Bonanomi, G.; Flematti, G.; Germinara, S.G.; Pistillo, M.; Giron, D.; Bézier, A.; Vinale, F. Biochar-Derived Smoke Waters Affect Bactrocera oleae Behavior and Control the Olive Fruit Fly under Field Conditions. Agronomy 2022, 12, 2834. https://doi.org/10.3390/agronomy12112834
Jesu G, Laudonia S, Bonanomi G, Flematti G, Germinara SG, Pistillo M, Giron D, Bézier A, Vinale F. Biochar-Derived Smoke Waters Affect Bactrocera oleae Behavior and Control the Olive Fruit Fly under Field Conditions. Agronomy. 2022; 12(11):2834. https://doi.org/10.3390/agronomy12112834
Chicago/Turabian StyleJesu, Giovanni, Stefania Laudonia, Giuliano Bonanomi, Gavin Flematti, Salvatore Giacinto Germinara, Marco Pistillo, David Giron, Annie Bézier, and Francesco Vinale. 2022. "Biochar-Derived Smoke Waters Affect Bactrocera oleae Behavior and Control the Olive Fruit Fly under Field Conditions" Agronomy 12, no. 11: 2834. https://doi.org/10.3390/agronomy12112834
APA StyleJesu, G., Laudonia, S., Bonanomi, G., Flematti, G., Germinara, S. G., Pistillo, M., Giron, D., Bézier, A., & Vinale, F. (2022). Biochar-Derived Smoke Waters Affect Bactrocera oleae Behavior and Control the Olive Fruit Fly under Field Conditions. Agronomy, 12(11), 2834. https://doi.org/10.3390/agronomy12112834


_Asaduzzaman.jpg)







