Mycorrhizal Effects on Active Components and Associated Gene Expressions in Leaves of Polygonum cuspidatum under P Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Set-Up
2.3. Variable Measurement
2.4. Statistical Analysis
3. Results
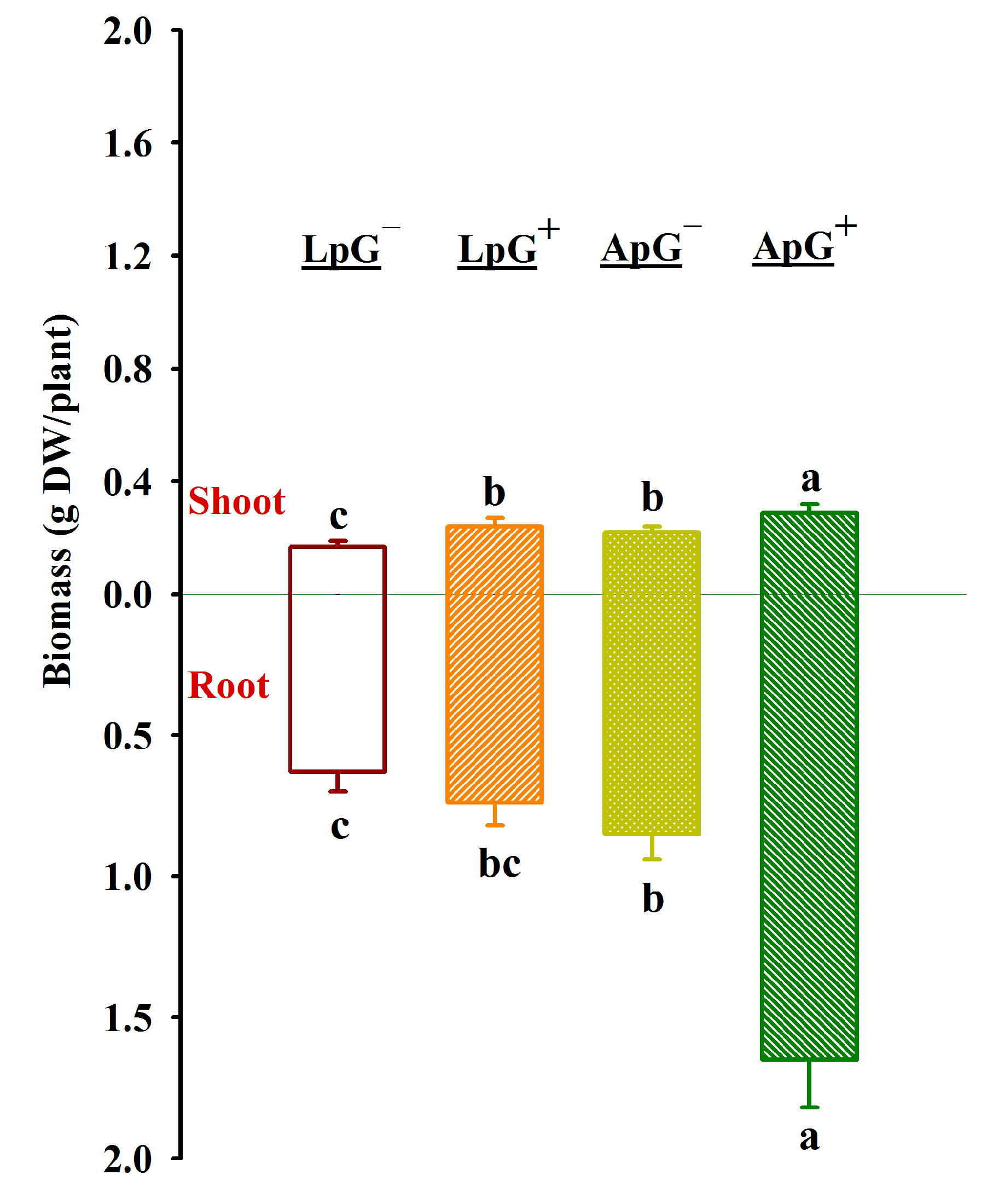
3.1. Changes in Root Mycorrhizal Colonization and Biomass Production
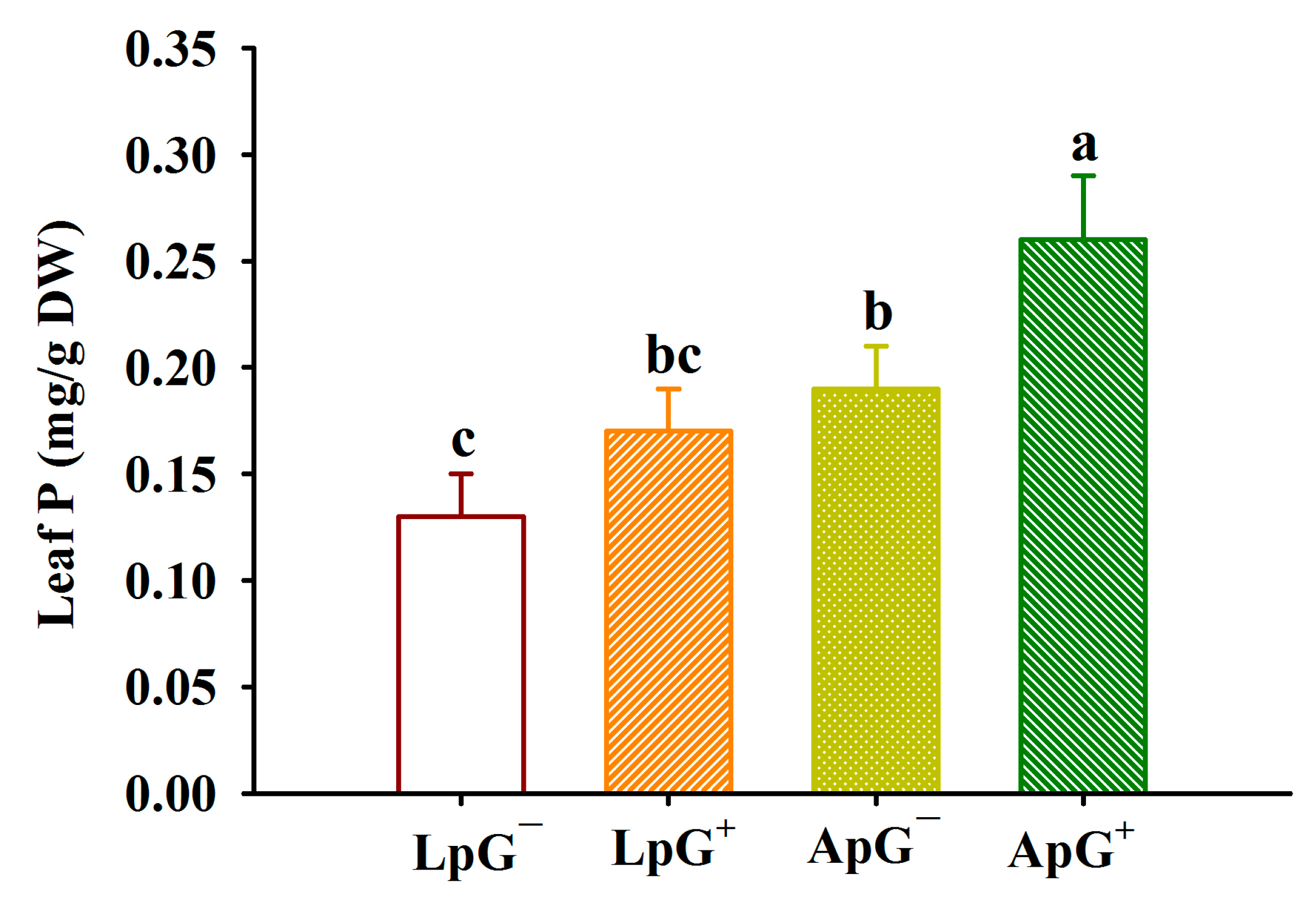
3.2. Changes in Leaf P Concentrations
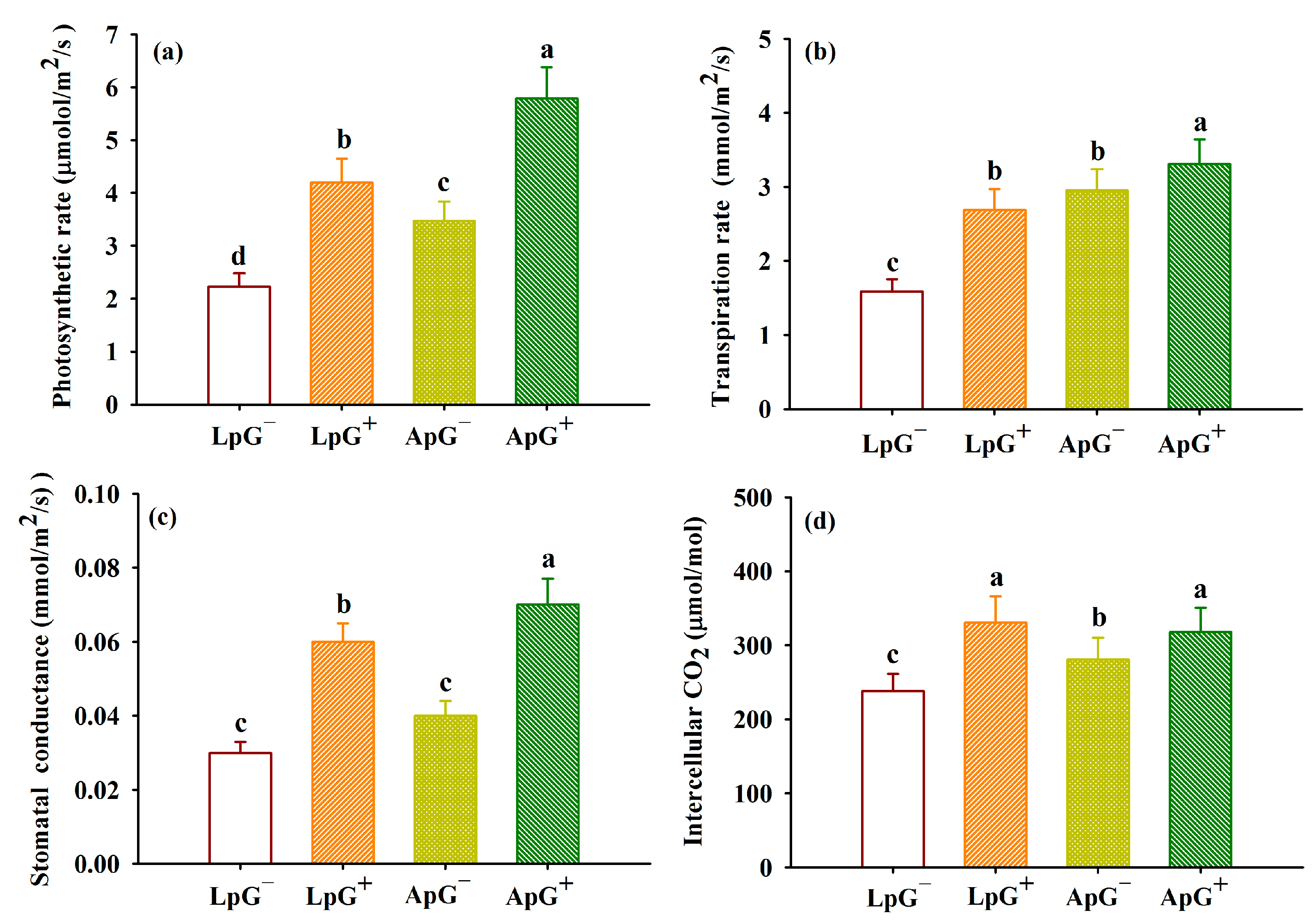
3.3. Changes in Leaf Gas Exchange
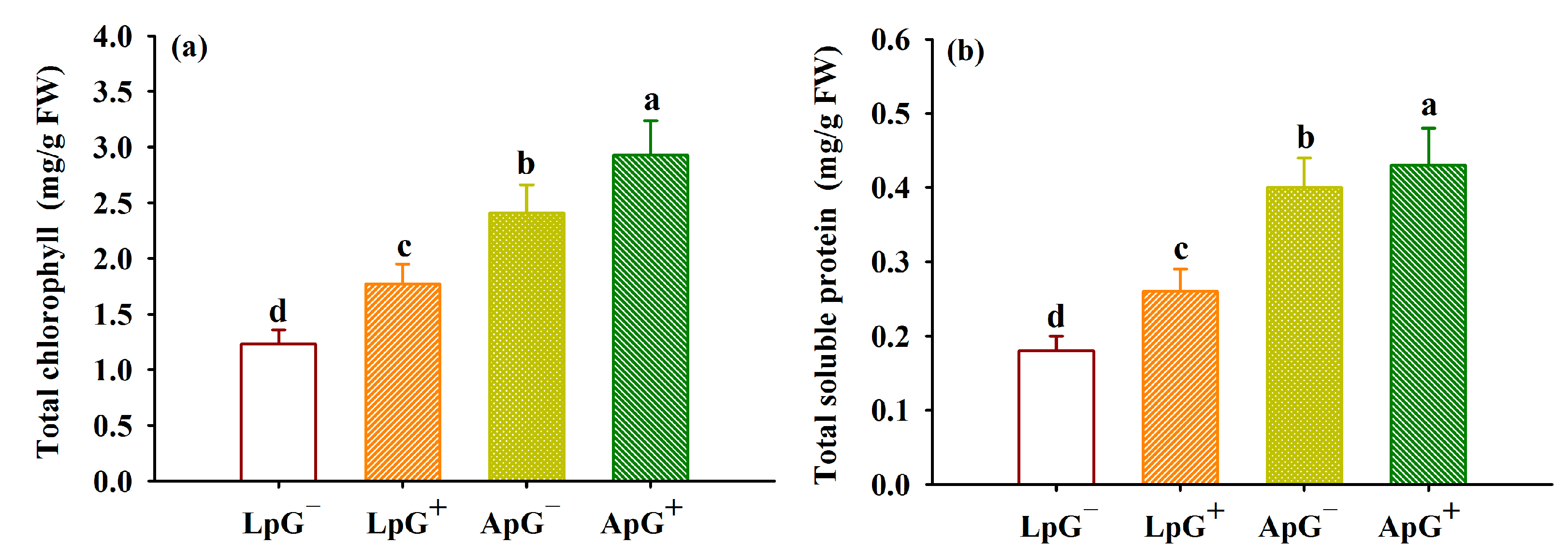
3.4. Changes in Total Chlorophyll and Total Soluble Protein in Leaves
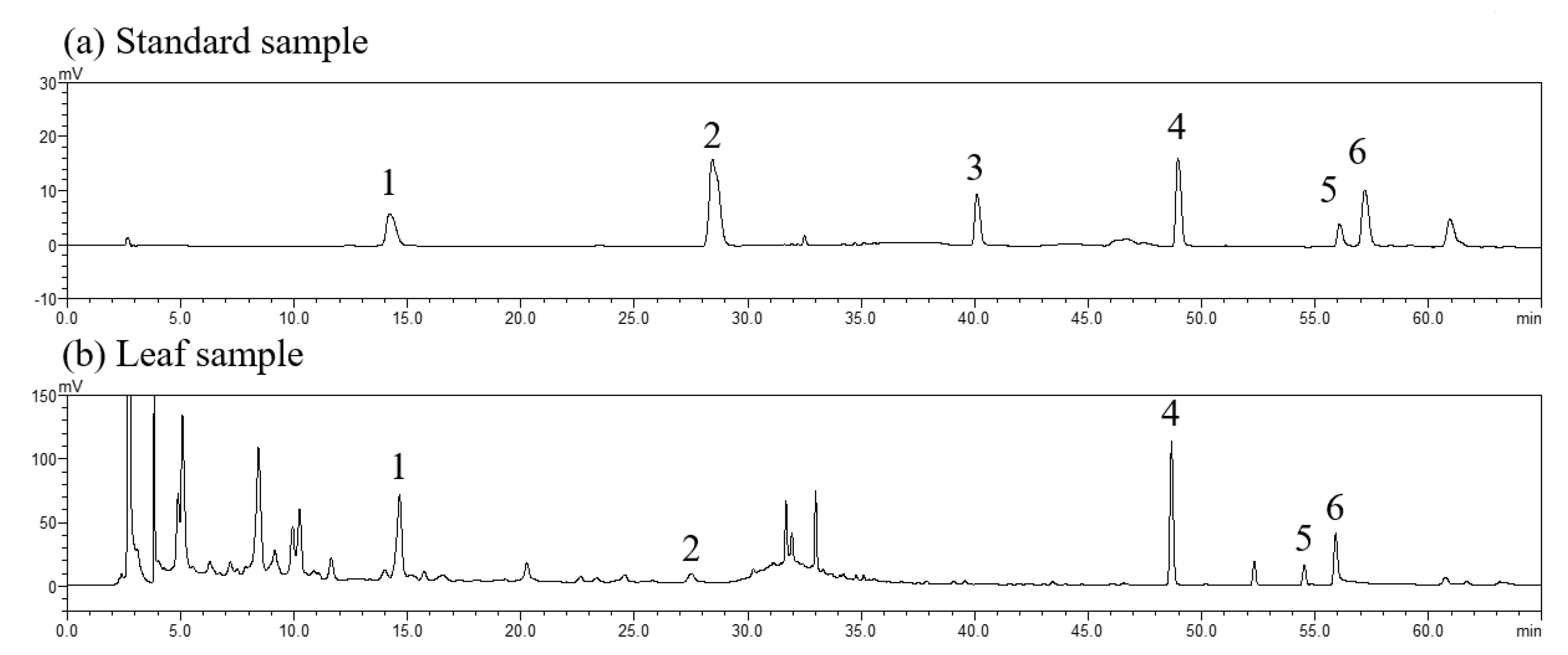
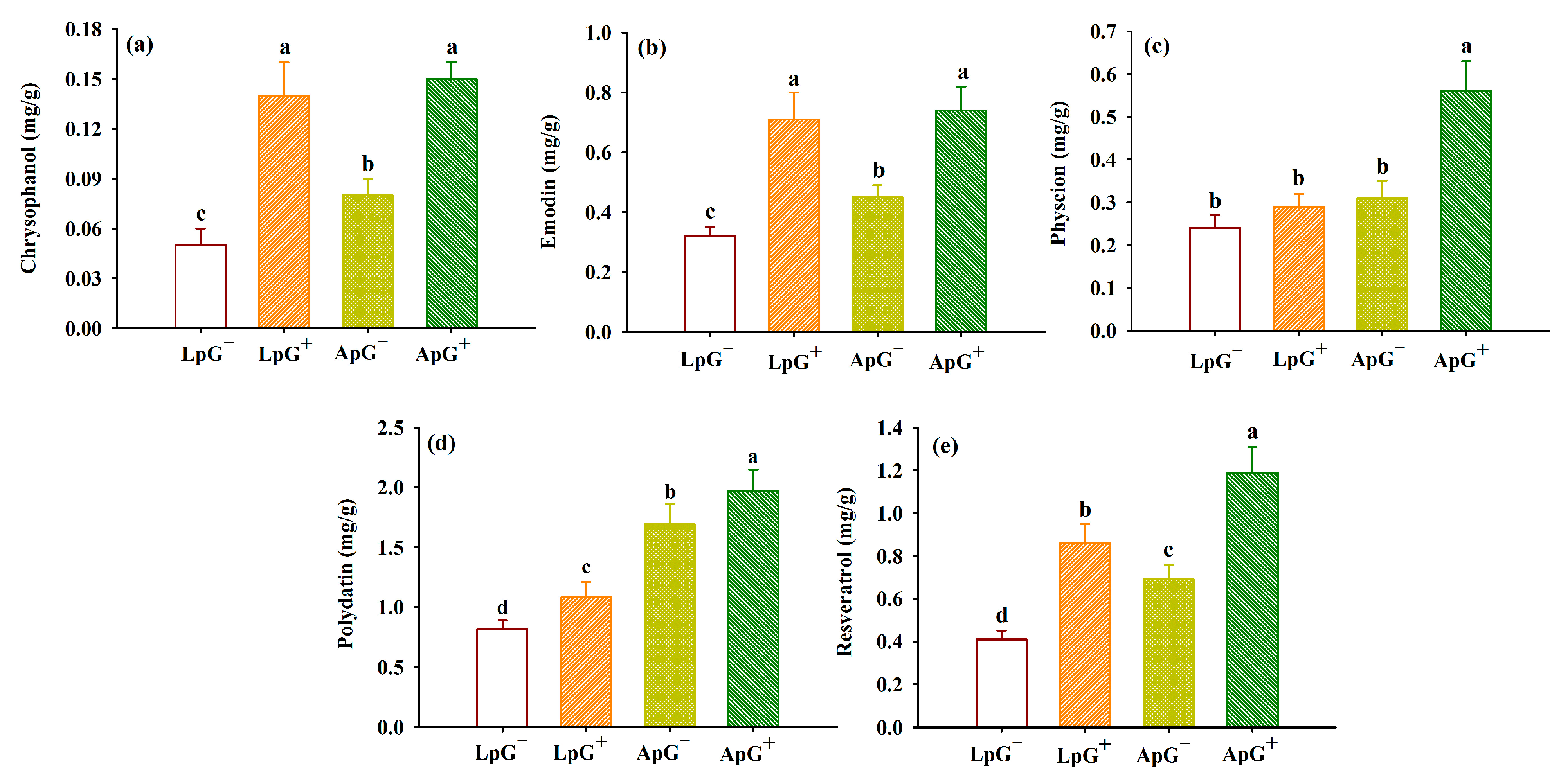
3.5. Changes in Active Components in Leaves
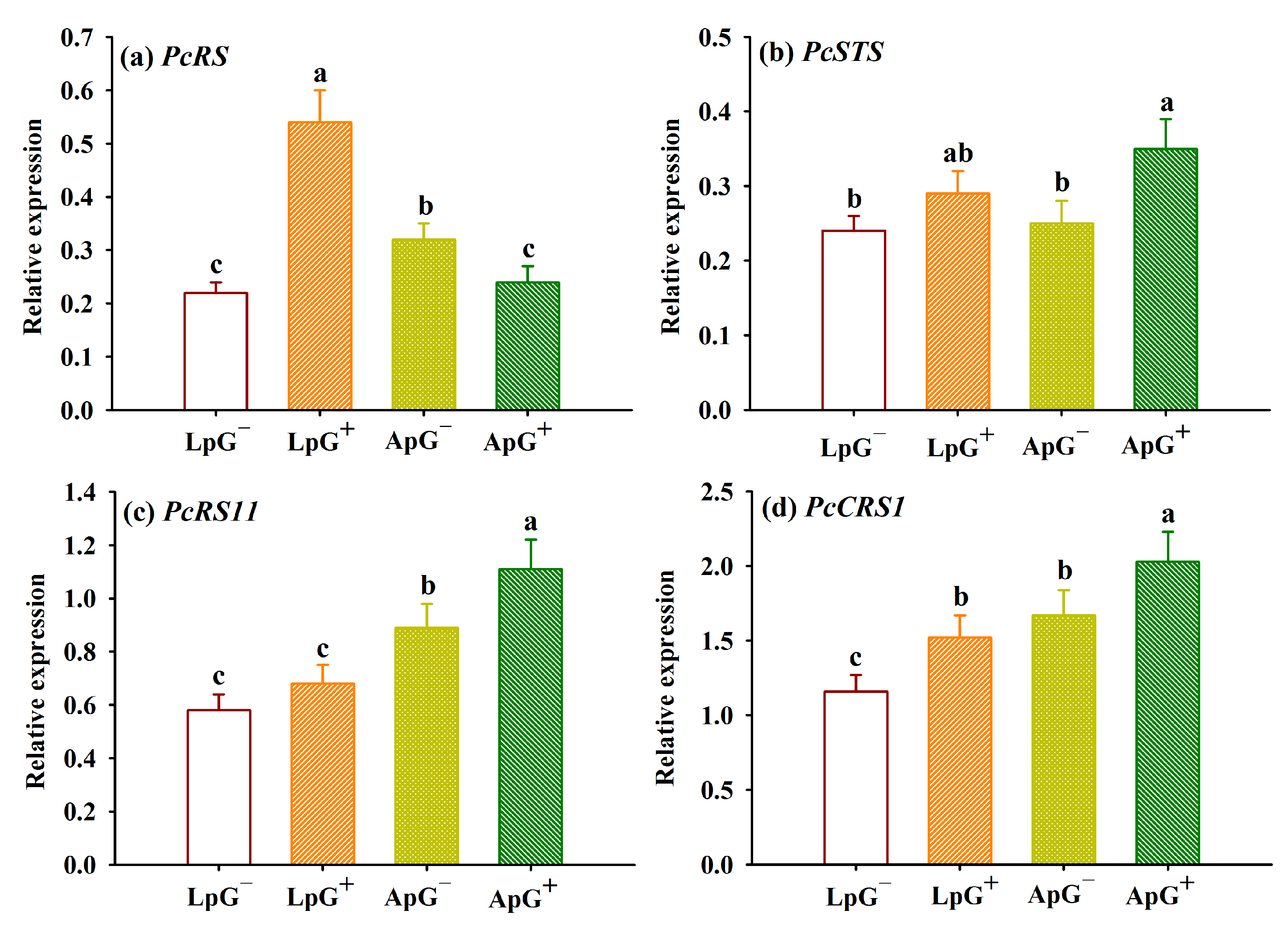
3.6. Changes in Expressions of Resveratrol-Associated Enzyme Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, D.; Wu, Y. Effect of phosphorus deficiency on photosynthetic inorganic carbon assimilation of three climber plant species. Bot. Stud. 2014, 55, 60. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Amarh, F.; Voegborlo, R.B.; Essuman, E.K.; Agorku, E.S.; Kortei, N.K. Effects of soil depth and characteristics on phosphorus adsorption isotherms of different land utilization types phosphorus adsorption isotherms of soil. Soil Till. Res. 2021, 213, 105139. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Xie, M.-M.; Li, Y.; Liu, B.-Y.; Liu, C.-Y.; Wu, Q.-S.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2021, 170, 104308. [Google Scholar] [CrossRef]
- He, H.; Peng, Q.; Wang, X.; Fan, C.; Pang, J.; Lambers, H.; Zhang, X. Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil 2017, 416, 565–584. [Google Scholar] [CrossRef]
- Turrión, M.-B.; Bueis, T.; Lafuente, F.; López, O.; José, E.S.; Eleftheriadis, A.; Mulas, R. Effects on soil phosphorus dynamics of municipal solid waste compost addition to a burnt and unburnt forest soil. Sci. Total Environ. 2018, 642, 374–382. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, I.; Hameed, A.; Ullah, I.; Chaudhry, M.S.; Naveed-Ul-Haq, A.; Kaleem, S. Arbuscular mycorrhizal fungi (AMF) and soil chemical heterogeneity significantly alters nutritional value of tomato fruit. Pak. J. Bot. 2021, 54. [Google Scholar] [CrossRef]
- He, J.D.; Dong, T.; Wu, H.H.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar] [CrossRef]
- Meena, M.; Narjary, B.; Sheoran, P.; Jat, H.; Joshi, P.; Chinchmalatpure, A.; Yadav, G.; Yadav, R. Changes of phosphorus fractions in saline soil amended with municipal solid waste compost and mineral fertilizers in a mustard-pearl millet cropping system. Catena 2018, 160, 32–40. [Google Scholar] [CrossRef]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour. Technol. 2004, 93, 307–311. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wang, Y.S.; Yin, X.F.; Liu, J.B.; Wu, F.X. Development of arbuscular mycorrhizal (AM) fungi and their influences on the absorption of N and P of maize at different soil phosphorus application levels. J. Plant Nutr. Fert. 2017, 23, 649–657. [Google Scholar] [CrossRef]
- Khaosaad, T.; Vierheilig, H.; Nell, M.; Zitterl-Eglseer, K.; Novák, J. Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 2006, 16, 443–446. [Google Scholar] [CrossRef]
- Tan, W.D.; Shen, M.J.; Qiu, H.J.; Zeng, F.L.; Huang, J.H.; Huang, R.S.; Luo, W.G.; Liu, Y.X. Effects of different phosphorus treatments on arbuscular mycorrhizal formation, growth and artemisinin content of Artemisia annua. J. South. Agric. 2013, 44, 1303–1307. [Google Scholar]
- Pedone-Bonfim, M.V.; A Lins, M.; Coelho, I.R.; Santana, A.S.; Silva, F.S.; Maia, L.C. Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J. Sci. Food Agric. 2012, 93, 1479–1484. [Google Scholar] [CrossRef]
- Kurita, S.; Kashiwagi, T.; Ebisu, T.; Shimamura, T.; Ukeda, H. Content of resveratrol and glycoside and its contribution to the antioxidative capacity of Polygonum cuspidatum (Itadori) harvested in Kochi. Biosci. Biotechnol. Biochem. 2014, 78, 499–502. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Shaheen, H.M.; Alsenosy, A.A. Nuclear factorkappa B inhibition as a therapeutic target of nutraceuticals in arthritis, osteoarthritis, and related inflammation. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, section editionl; Watson, R.R., Preedy, V.R., Eds.; Academic Press: London, UK, 2019; pp. 437–453. [Google Scholar]
- Hao, D.; Ma, P.; Mu, J.; Chen, S.; Xiao, P.; Peng, Y.; Huo, L.; Xu, L.; Sun, C. De novo characterization of the root transcriptome of a traditional Chinese medicinal plant Polygonum cuspidatum. Sci. China Life Sci. 2012, 55, 452–466. [Google Scholar] [CrossRef]
- Jiang, J.; Xi, H.; Dai, Z.; Lecourieux, F.; Yuan, L.; Liu, X.; Patra, B.; Wei, Y.; Li, S.; Wang, L. VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. J. Exp. Bot. 2018, 70, 715–729. [Google Scholar] [CrossRef]
- Kiselev, K.V. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liu, W.-Y.; Chen, G.-T. A simple method for the isolation and purification of resveratrol from Polygonum cuspidatum. J. Pharm. Anal. 2013, 3, 241–247. [Google Scholar] [CrossRef]
- Ren, L.F.; Zhang, W.H.; Li, Y.S. Effect of phosphorus deficiency on physiological properties of Medicago falcata. Acta Pratacult. Sin. 2012, 21, 242–249. [Google Scholar]
- Sun, R.-T.; Zhang, Z.-Z.; Feng, X.-C.; Zhou, N.; Feng, H.-D.; Liu, Y.-M.; Harsonowati, W.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Endophytic fungi accelerate leaf physiological activity and resveratrol accumulation in Polygonum cuspidatum by up-regulating expression of associated genes. Agronomy 2022, 12, 1220. [Google Scholar] [CrossRef]
- Sun, R.-T.; Feng, X.-C.; Zhang, Z.-Z.; Zhou, N.; Feng, H.-D.; Liu, Y.-M.; Hashem, A.; Al-Arjani, A.-B.F.; Abd_Allah, E.F.; Wu, Q.-S. Root endophytic fungi regulate changes in sugar and medicinal compositions of Polygonum cuspidatum. Front. Plant Sci. 2022, 13, 818909. [Google Scholar] [CrossRef] [PubMed]
- Song, A.Q.; Tong, F.P.; Yi, A.Q.; Ding, J.; Li, G.; Huang, Z. Rooting out of bottles and fertilization on leaves experiment on tissue culture of Ploygonum cuspidatum sieb et zucc. Hunan For. Sci. Technol. 2006, 33, 27–30. [Google Scholar] [CrossRef]
- AbdElgawad, H.; El-Sawah, A.M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.S.; Sheteiwy, M.S. Increasing atomspheric CO2 differentially supports arsenite stress mitigating impact of arbuscular mycorrhizal fungi in wheat. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Sawah, A.M. The mode of integration between Azotobacter and Rhizobium affect plant growth, yield, and physiological responses of pea (Pisum sativum L.). J. Soil Sci. Plant Nutr. 2022, 22, 1238–1251. [Google Scholar] [CrossRef]
- Cavell, A.J. The colorimetric determination of phosphorus in plant materials. J. Sci. Food Agric. 1955, 6, 479–480. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Li, Y.; Zou, Y.-N.; He, X.-H. Arbuscular mycorrhiza mediates glomalin-related soil protein production and soil enzyme activities in the rhizosphere of trifoliate orange grown under different P levels. Mycorrhiza 2014, 25, 121–130. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Liu, C.-Y.; Zhang, D.-J.; Zou, Y.-N.; He, X.-H.; Wu, Q.-H. Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza 2015, 26, 237–247. [Google Scholar] [CrossRef]
- Sun, R.-T.; Zhang, Z.-Z.; Liu, M.-Y.; Feng, X.-C.; Zhou, N.; Feng, H.-D.; Hashem, A.; Abd_Allah, E.F.; Harsonowati, W.; Wu, Q.-S. Arbuscular mycorrhizal fungi and phosphorus supply accelerate main medicinal component production of Polygonum cuspidatum. Front. Microbiol. 2022, 13, 1006140. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018, 12, 2339–2351. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Yu, M.; Wu, Z.; Zhao, A.; Li, J.; Zhang, X.; Chen, B. Improved phosphorus nutrition by arbuscular mycorrhizal symbiosis as a key factor facilitating glycyrrhizin and liquiritin accumulation in Glycyrrhiza uralensis. Plant Soil 2018, 439, 243–257. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Zhou, X.; Jiang, X.; Xu, L.; Wu, S.; Zhao, A.; Zhang, X.; Chen, B. Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza 2018, 28, 285–300. [Google Scholar] [CrossRef]
- Shao, Y.-D.; Hu, X.-C.; Wu, Q.-S.; Yang, T.-Y.; Srivastava, A.; Zhang, D.-J.; Gao, X.-B.; Kuča, K. Mycorrhizas promote P acquisition of tea plants through changes in root morphology and P transporter gene expression. South Afr. J. Bot. 2020, 137, 455–462. [Google Scholar] [CrossRef]
- Cao, M.A.; Liu, R.C.; Xiao, Z.Y.; Hashem, A.; Abd_Allah, E.F.; Alsayed, M.F.; Harsonowati, W.; Wu, Q.S. Symbiotic fungi alter the acquisition of phosphorus in Camellia oleifera through regulating root architecture, plant phosphate transporter gene expressions and soil phosphatase activities. J. Fungi 2022, 8, 800. [Google Scholar] [CrossRef]
- Zou, H.; Wang, C.S.; Zeng, J. Effect of native mycorrhizal fungi on photosynthetic physiology of Betula alnoides clones seedlings. J. Centr. South Univ. For. Technol. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Kapoor, R.; Sharma, D.; Bhatnagar, A. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- He, C.; He, X.L.; Ma, L.; Chen, W.Y. Effects of inoculation AM fungi and phosphate application on growth and photosynthetic characteristics of Scuteliaria baiealensis. Acta Agric. Bor. Occid. Sin. 2014, 23, 180–184. [Google Scholar] [CrossRef]
- Nong, Z.; Ding, B.; Feng, Y.; Qi, W.-H.; Zhang, H.; Guo, D.-Q.; Xiang, J. Effects of mycorrhizal colonization and medicine quality of Paris polyphylla var. yunnanensis inoculated by different foreign AM fungi species. China J. Chin. Mater. Med. 2015, 40, 3158–3167. [Google Scholar] [CrossRef]
- Sun, R.-T.; Zhang, Z.-Z.; Zhou, N.; Srivastava, A.; Kuča, K.; Abd_Allah, E.F.; Hashem, A.; Wu, Q.-S. A review of the interaction of medicinal plants and arbuscular mycorrhizal fungi in the rhizosphere. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12454. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, X.; Zhao, R.H.; Yu, J.; Gu, W.; Li, R.; Cao, G.H.; He, S. Effect and mechanism of arbuscular mycorrhizal fungi in herbs. Chin. J. Exp. Tradit. Med. Form. 2020, 26, 217–226. [Google Scholar] [CrossRef]
| Genes | Gene ID | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) |
|---|---|---|---|
| PcCRS1 | DQ459350.1 | TGAGCGAGTACGGGAATTTG | CCTTCTCCAGTCGTCTTCTTAC |
| PcRS11 | EF117977.1 | GATGAGATGATGAAGGCACAAAC | GGAAGTAGAAGTCGGGAAAGTC |
| PcRS | DQ900615.1 | GAGATGACGAAGGCACTAACA | GGAAGTAGAAGTCGGGAAAGTC |
| PcSTS | EU647245.1 | GAAGAGATGATGAAGGCACAAAC | GGAAGTAGAAGTCGGGAAAGTC |
| PcActin | MK288156.1 | TACAATGAGCTTCGGGTTGC | GCTCTTTGCAGTTTCCAGCT |
| Variables | P Treatments | AMF Inoculation | Interaction | Variables | P Treatments | AMF Inoculation | Interaction |
|---|---|---|---|---|---|---|---|
| Shoot biomass | 0.0036 | 0.0010 | 1.0000 | Chrysophanol | 0.0323 | <0.0001 | 0.2126 |
| Root biomass | <0.0001 | <0.0001 | 0.0005 | Emodin | 0.0303 | <0.0001 | 0.1846 |
| P concentrations | 0.0004 | 0.0032 | 0.3621 | Physcion | <0.0001 | 0.0001 | 0.0030 |
| Photosynthetic rate | <0.0001 | <0.0001 | 0.2598 | Polydatin | <0.0001 | 0.0030 | 0.8879 |
| Transpiration rate | <0.0001 | <0.0001 | 0.0007 | Resveratrol | 0.5037 | <0.0001 | <0.0001 |
| Stomatal conductance | <0.0001 | <0.0001 | 0.0301 | PcRS | 0.0015 | 0.0004 | <0.0001 |
| Intercellular CO2 level | 0.1799 | <0.0001 | 0.0170 | PcSTS | 0.0752 | 0.0020 | 0.2602 |
| Total chlorophyll | <0.0001 | 0.0006 | 0.9461 | PcRS11 | <0.0001 | 0.0121 | 0.2445 |
| Total soluble protein | <0.0001 | 0.0070 | 0.1698 | PcCRS1 | 0.0006 | 0.0049 | 0.9583 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Sun, R.-T.; Ma, Q.; Yang, Q.-H.; Zhou, N.; Hashem, A.; Al-Arjani, A.-B.F.; Abd_Allah, E.F.; Wu, Q.-S. Mycorrhizal Effects on Active Components and Associated Gene Expressions in Leaves of Polygonum cuspidatum under P Stress. Agronomy 2022, 12, 2970. https://doi.org/10.3390/agronomy12122970
Deng C, Sun R-T, Ma Q, Yang Q-H, Zhou N, Hashem A, Al-Arjani A-BF, Abd_Allah EF, Wu Q-S. Mycorrhizal Effects on Active Components and Associated Gene Expressions in Leaves of Polygonum cuspidatum under P Stress. Agronomy. 2022; 12(12):2970. https://doi.org/10.3390/agronomy12122970
Chicago/Turabian StyleDeng, Ci, Rui-Ting Sun, Qiang Ma, Qing-He Yang, Nong Zhou, Abeer Hashem, Al-Bandari Fahad Al-Arjani, Elsayed Fathi Abd_Allah, and Qiang-Sheng Wu. 2022. "Mycorrhizal Effects on Active Components and Associated Gene Expressions in Leaves of Polygonum cuspidatum under P Stress" Agronomy 12, no. 12: 2970. https://doi.org/10.3390/agronomy12122970
APA StyleDeng, C., Sun, R.-T., Ma, Q., Yang, Q.-H., Zhou, N., Hashem, A., Al-Arjani, A.-B. F., Abd_Allah, E. F., & Wu, Q.-S. (2022). Mycorrhizal Effects on Active Components and Associated Gene Expressions in Leaves of Polygonum cuspidatum under P Stress. Agronomy, 12(12), 2970. https://doi.org/10.3390/agronomy12122970








