Mycorrhizal Inoculation Improves the Quality and Productivity of Essential Oil Distilled from Three Aromatic and Medicinal Plants: Thymus satureioides, Thymus pallidus, and Lavandula dentata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Fungal Materials
2.2. Experimental Setup
2.3. Determination of Arbuscular Mycorrhizal Colonization and Plant Growth
2.4. Determination of Shoot Dry Matter
2.5. Total Chlorophyll, Soluble Sugar, and Protein Content
2.6. Nitrogen Content
2.7. Phosphorus and Potassium Content
2.8. Extraction of Essential Oils
2.9. Determination of the Essential Oil Chemical Composition
2.10. Biological Properties of Essential Oil
2.10.1. Antifungal Activity
2.10.2. Anti-Germinative Activity
2.11. Statistical Analysis
3. Results
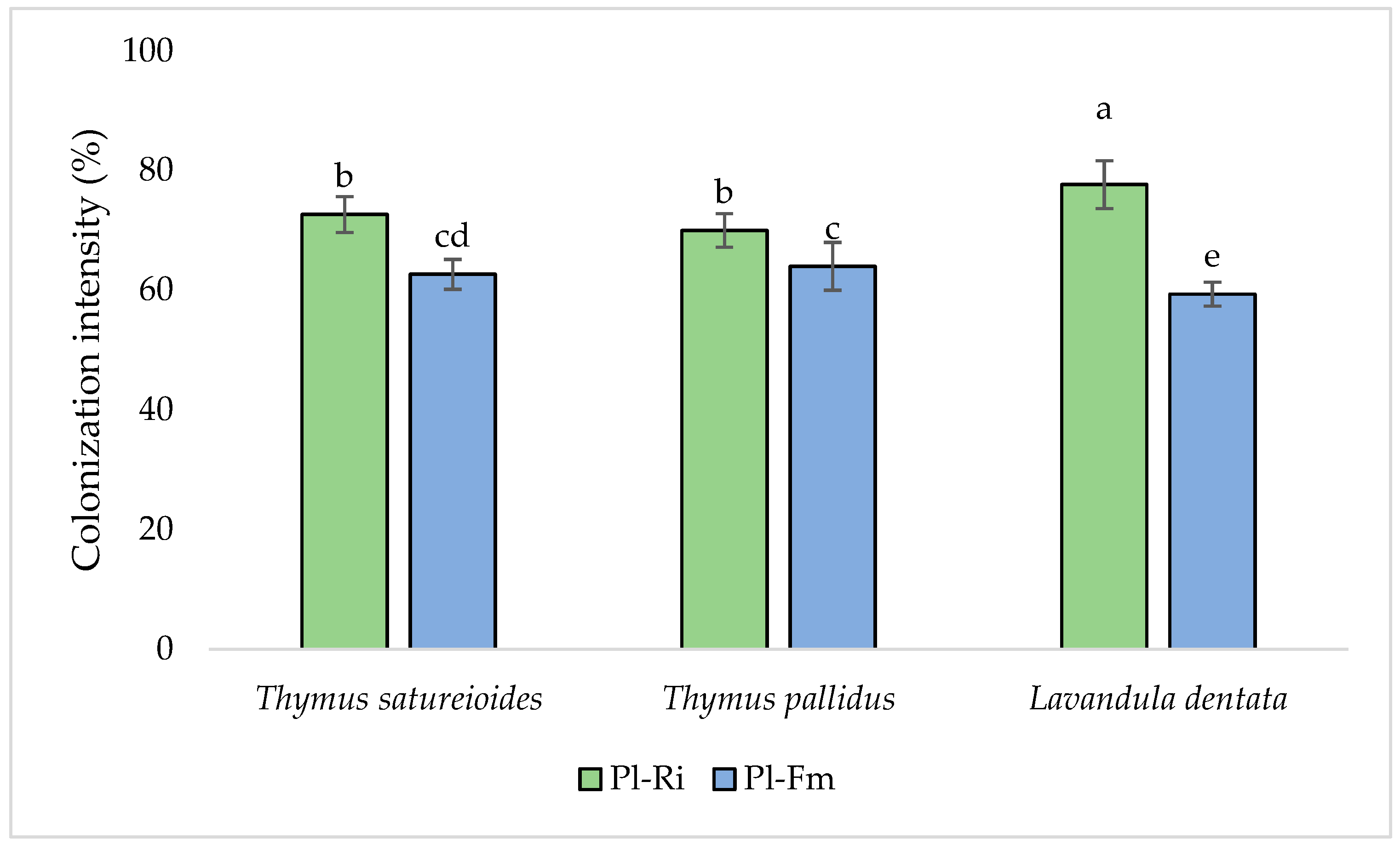
3.1. Mycorrhizal Colonization
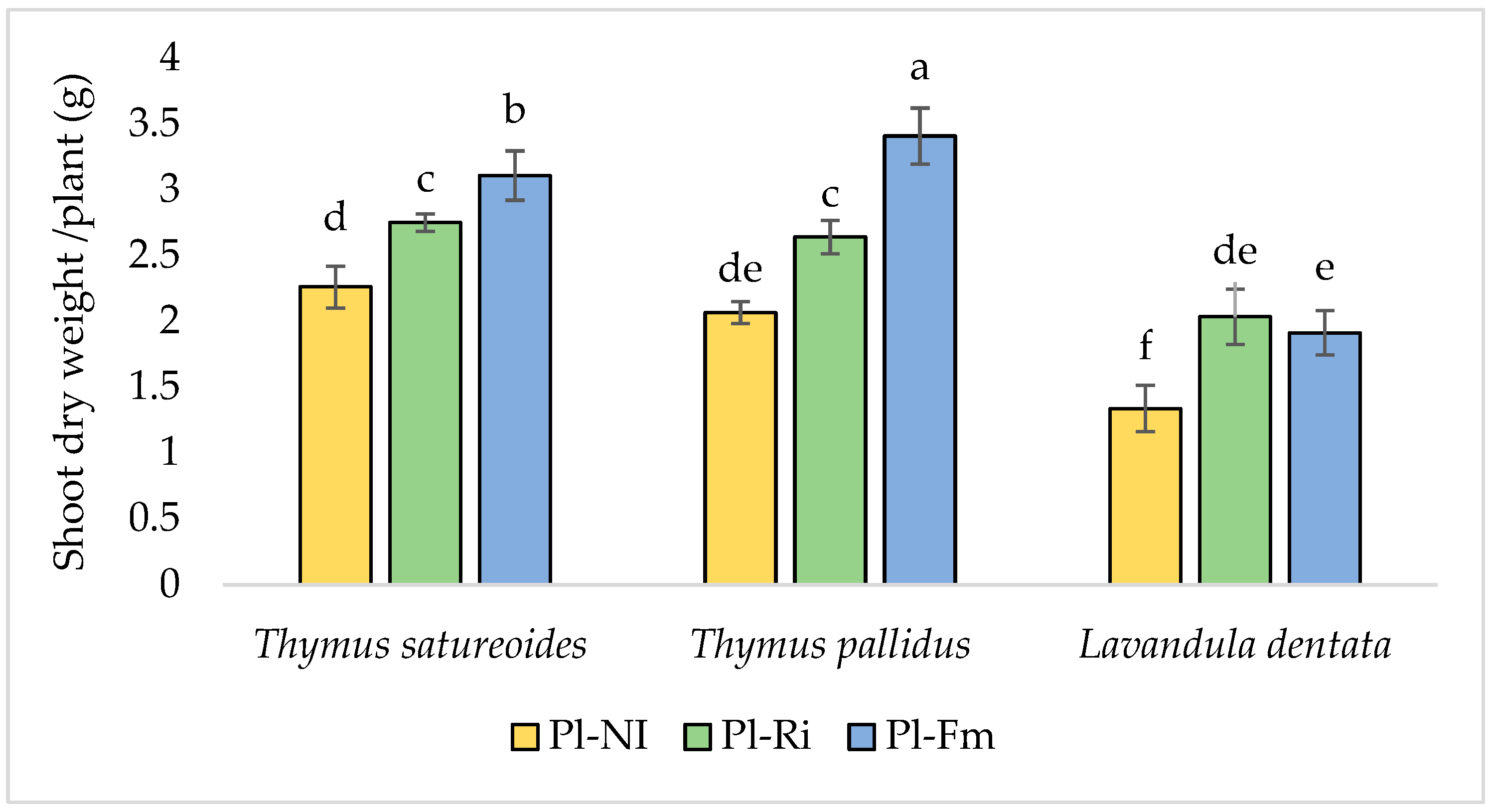
3.2. Shoot Biomass
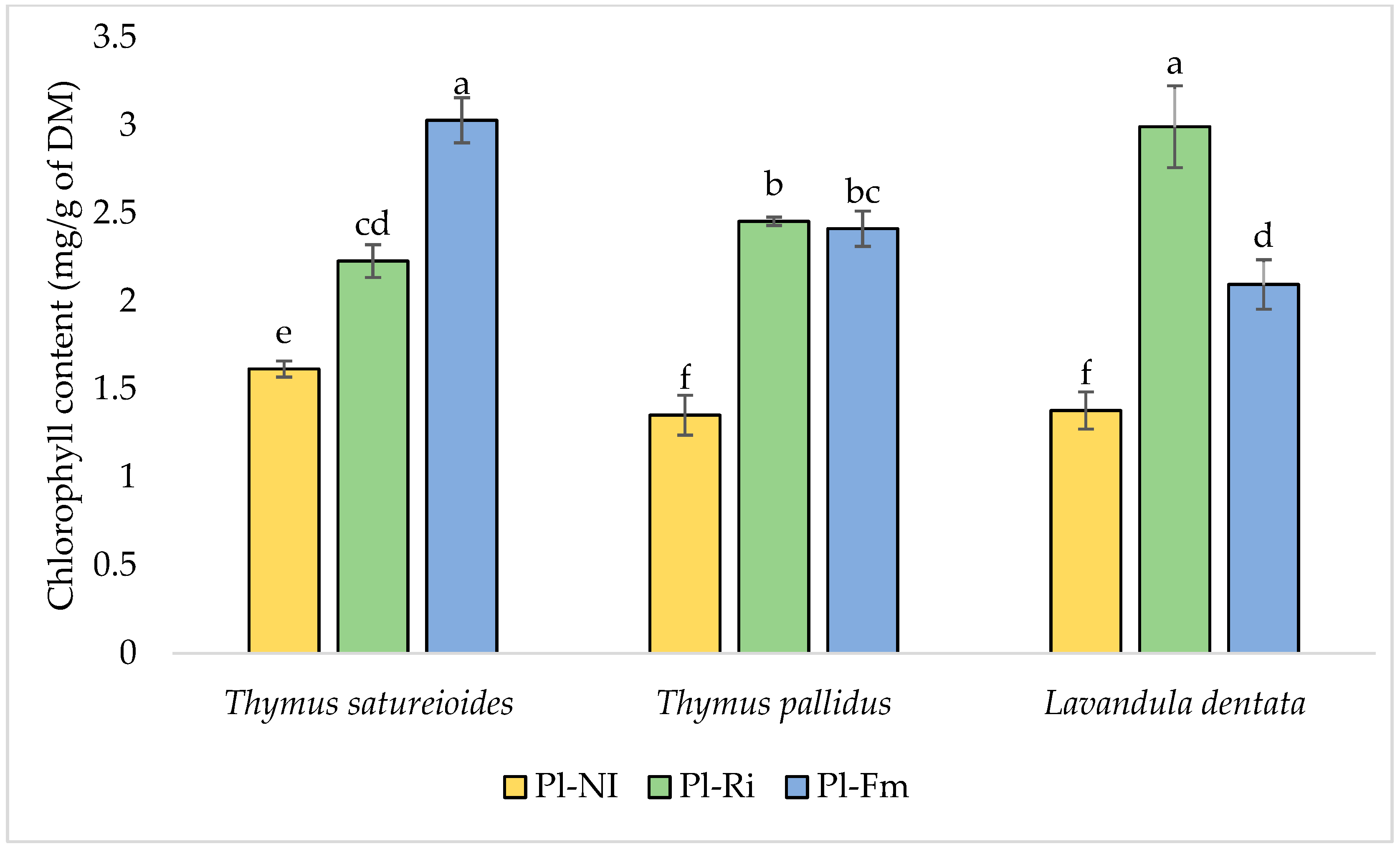
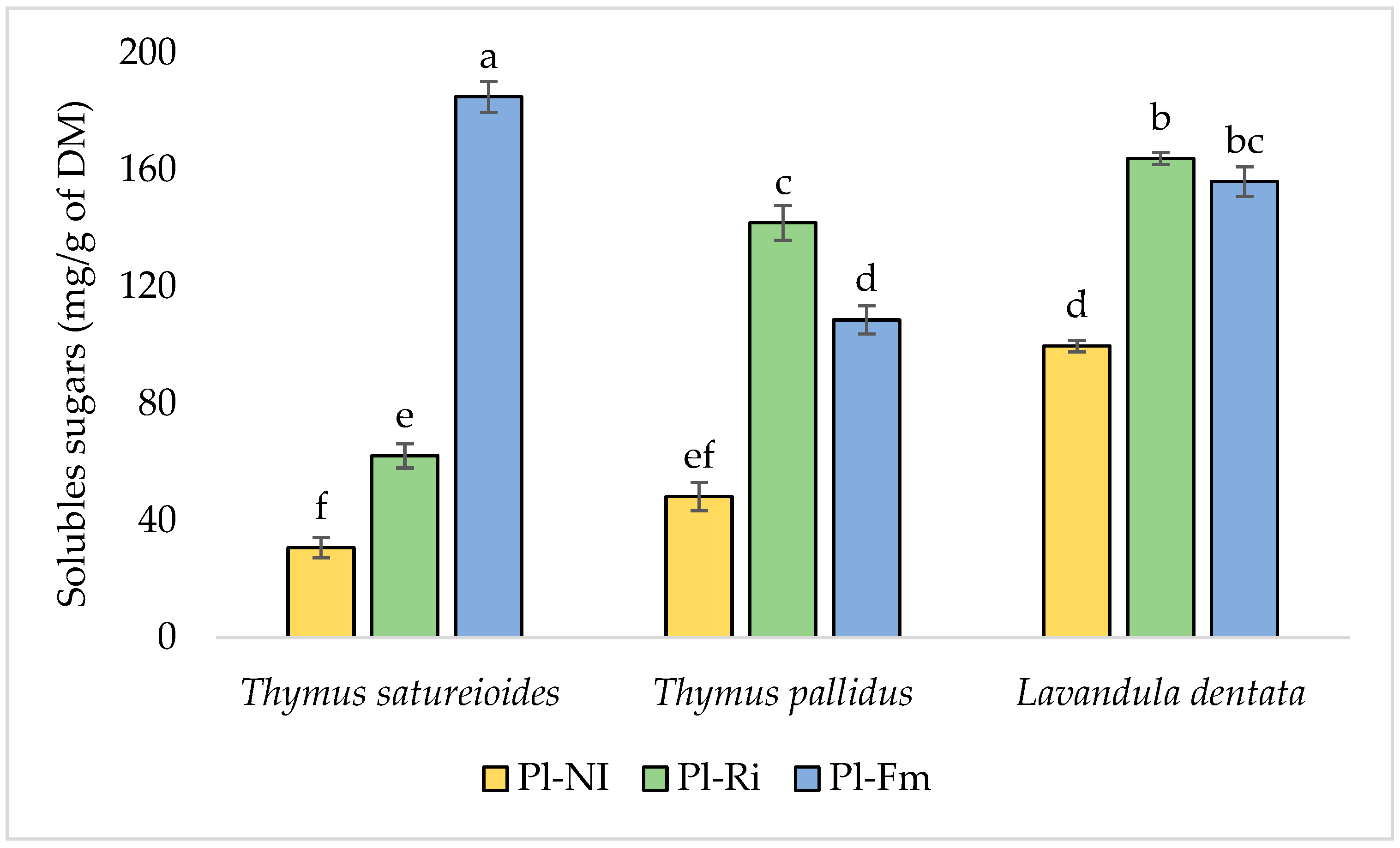
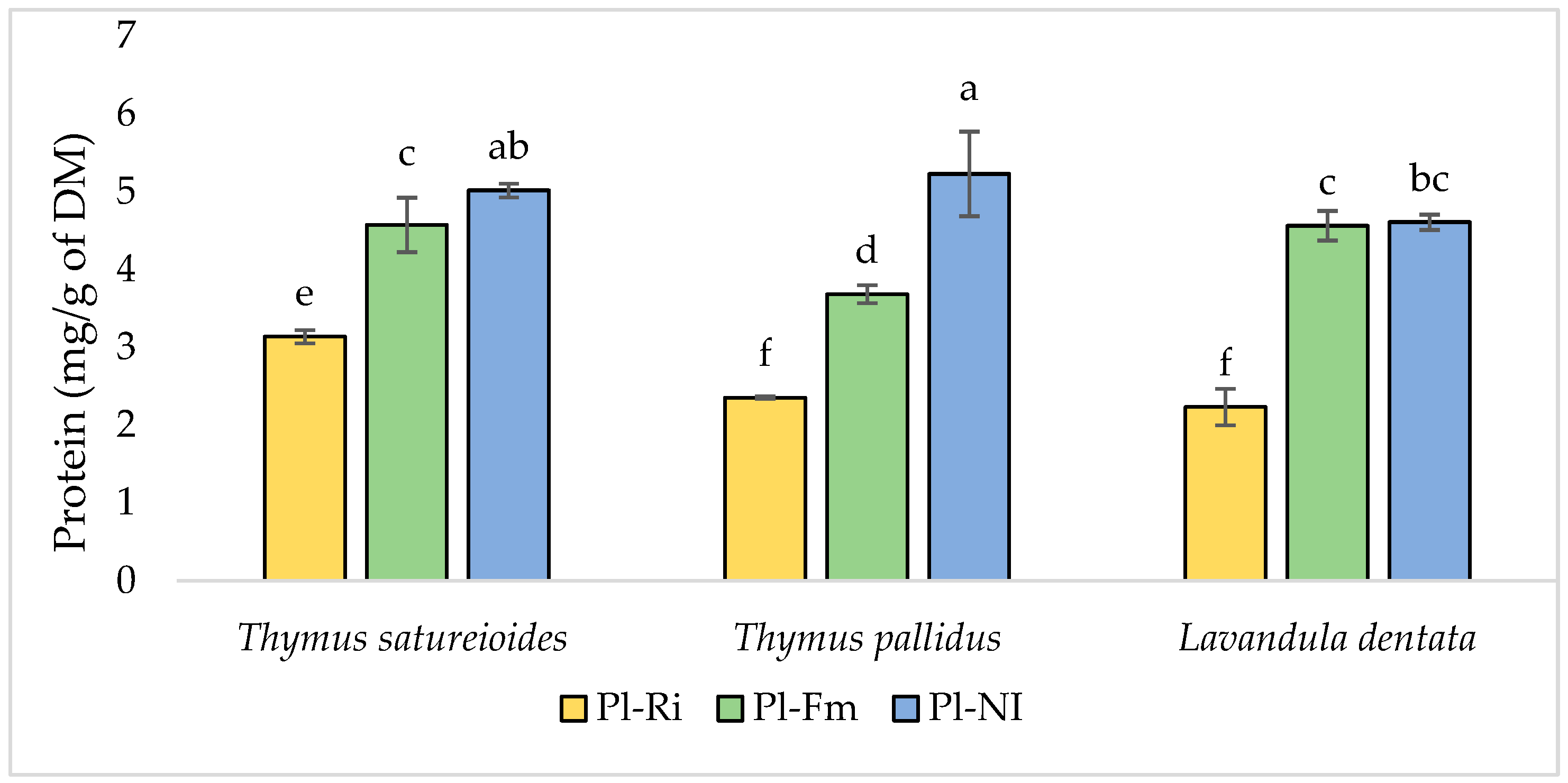
3.3. Total Chlorophyll, Soluble Sugar, and Protein Content
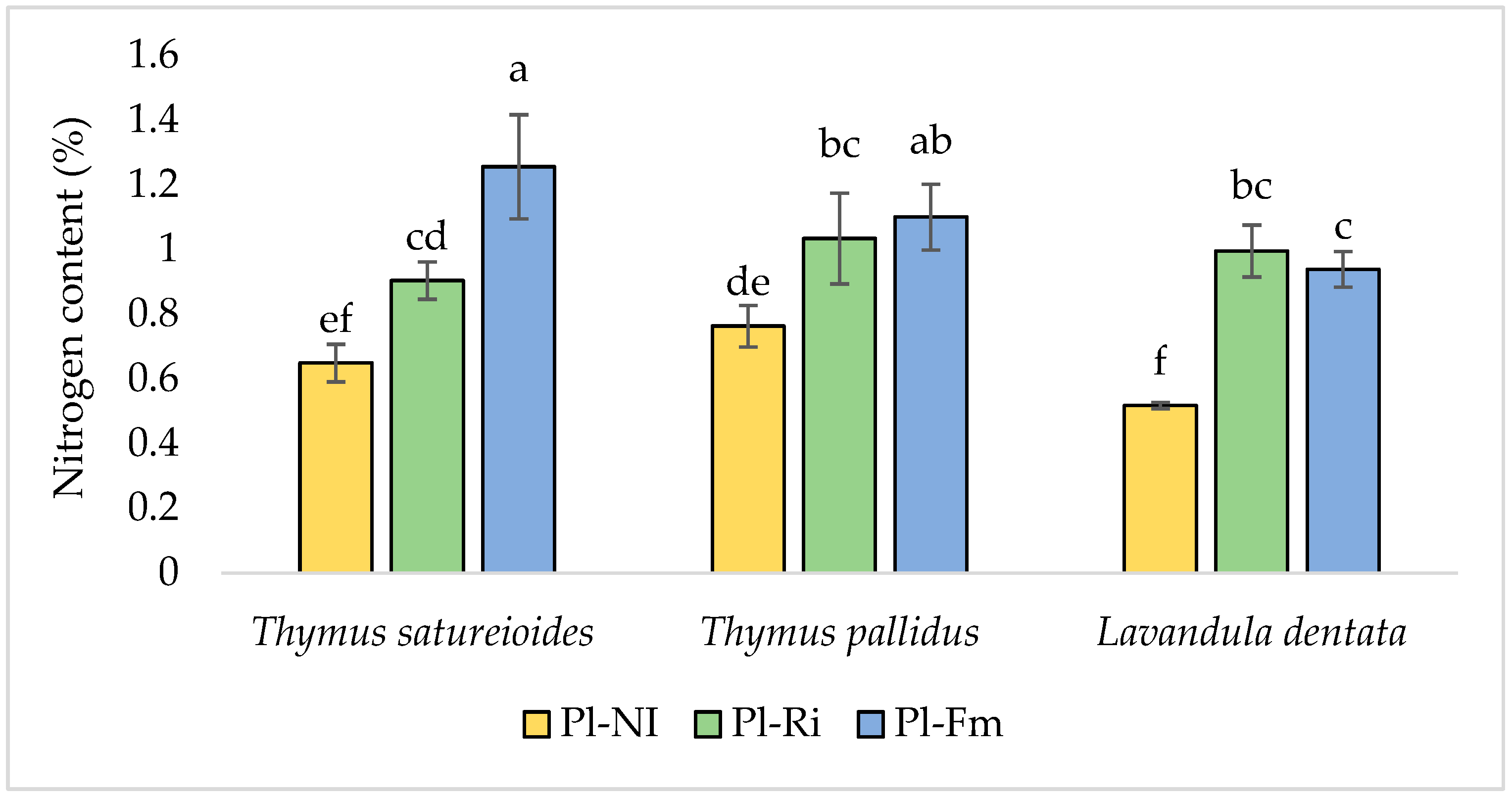
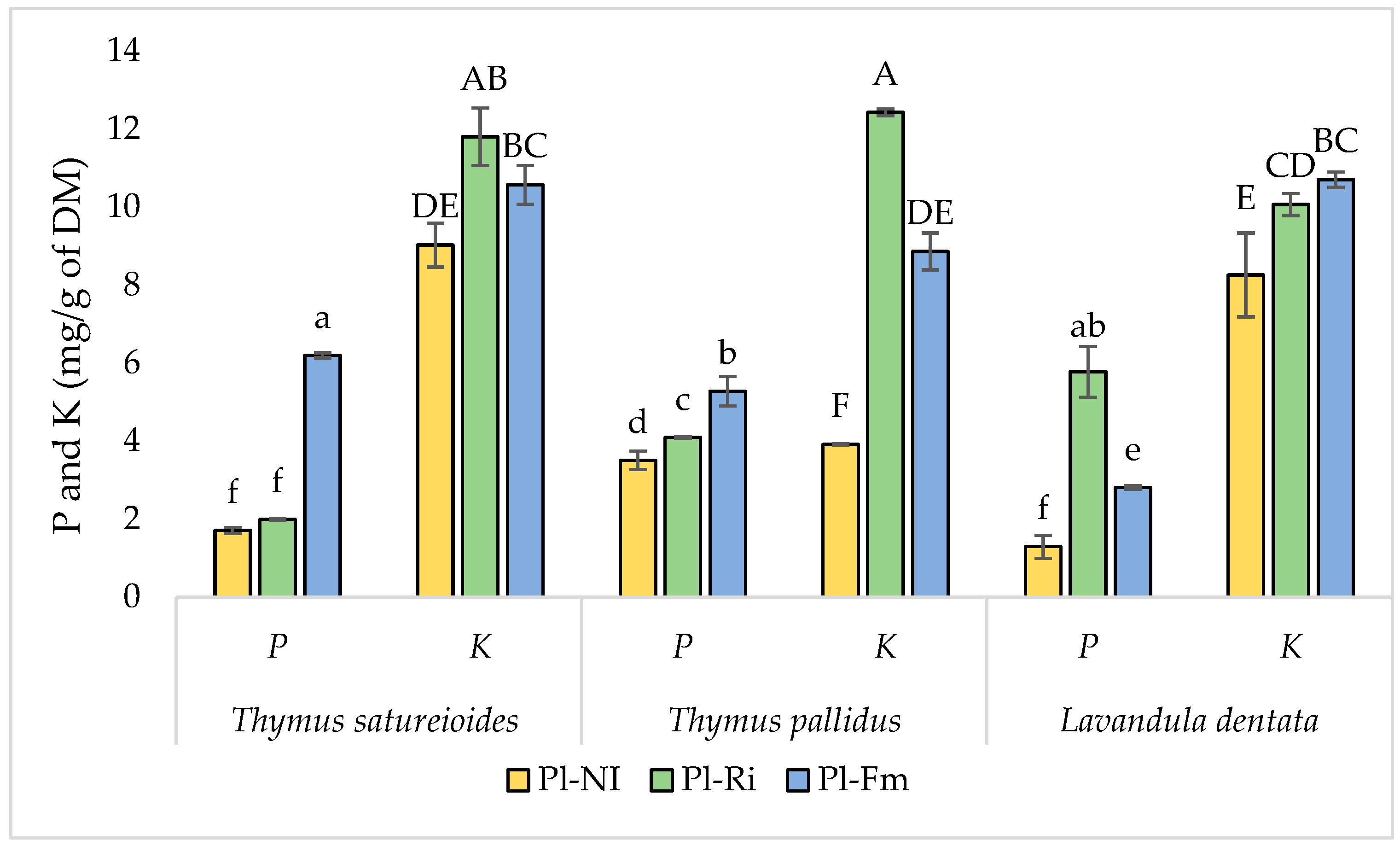
3.4. Nitrogen, Phosphorus, and Potassium Content
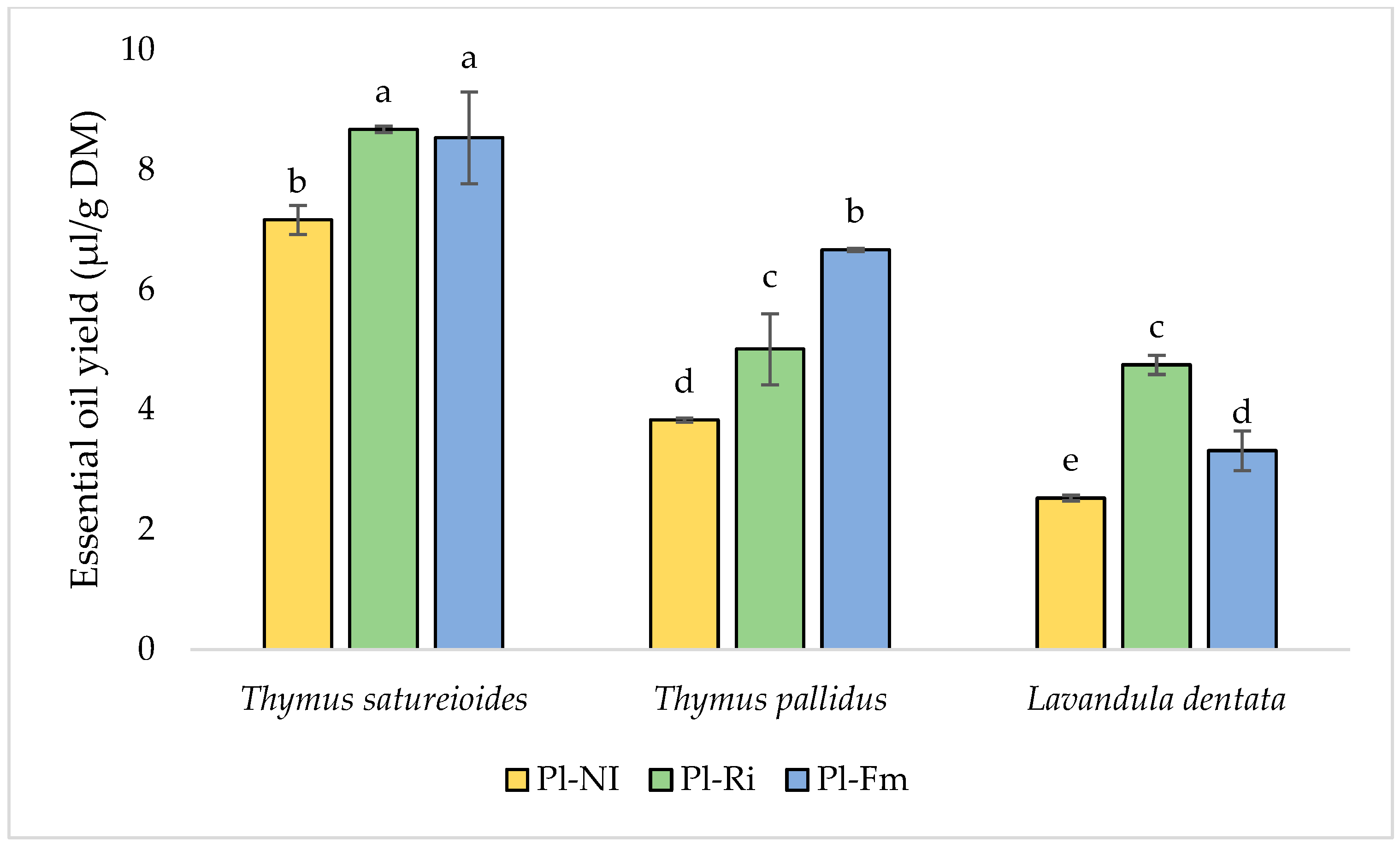
3.5. Essential Oil Yields
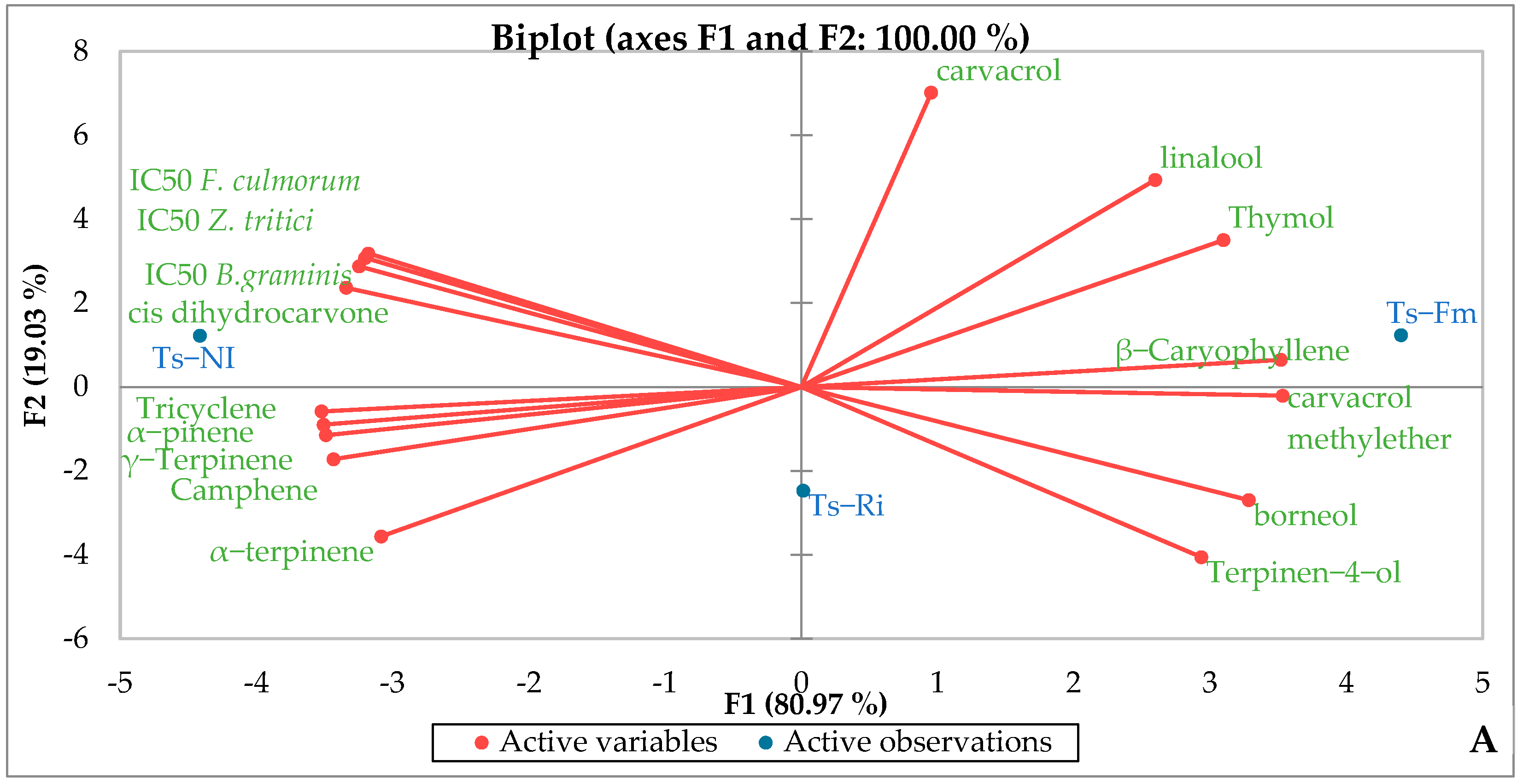
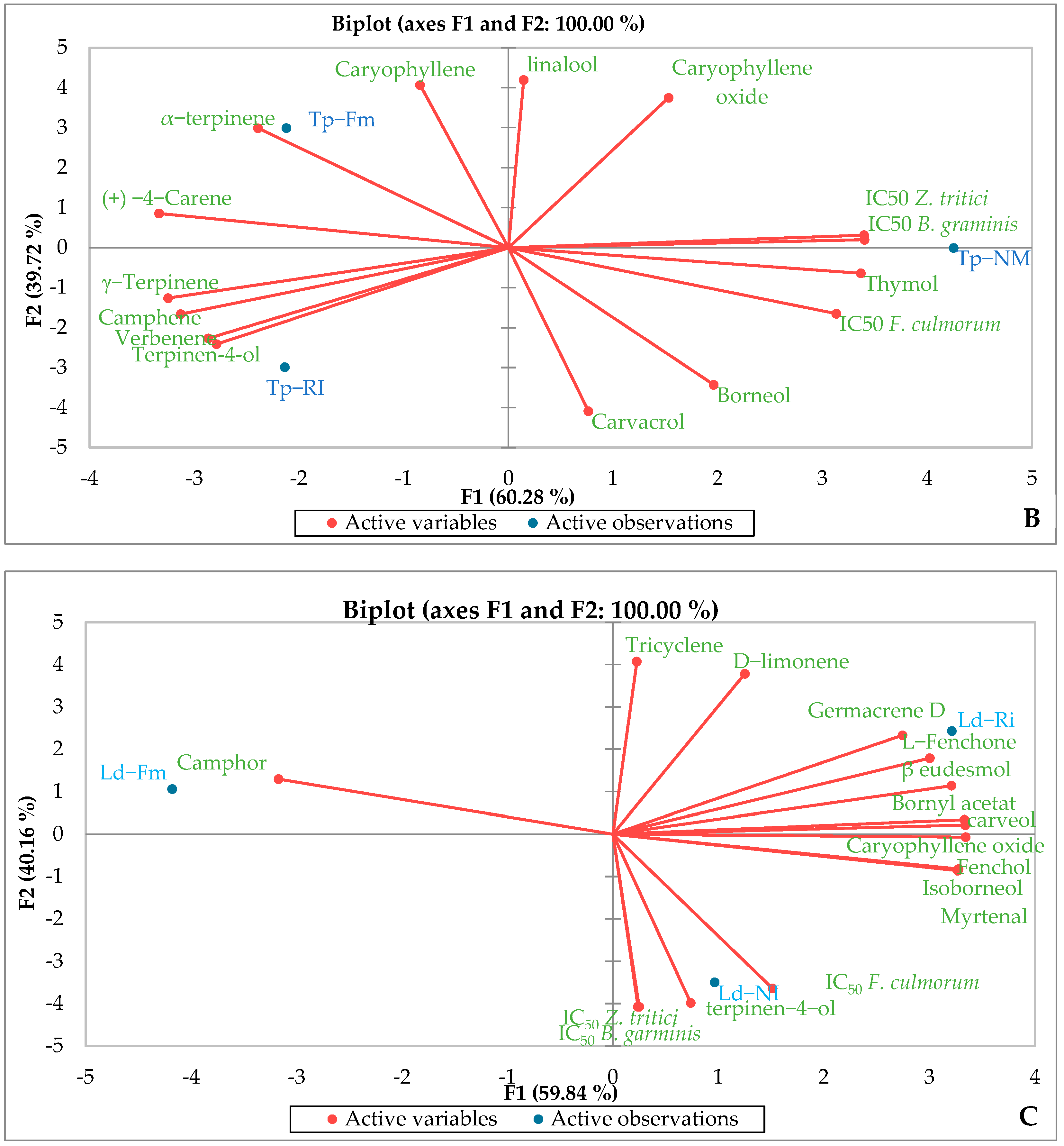
3.6. Essential Oil Chemical Composition
3.7. Biological Activities of Essential Oil
3.7.1. Anti-Germinative Activity
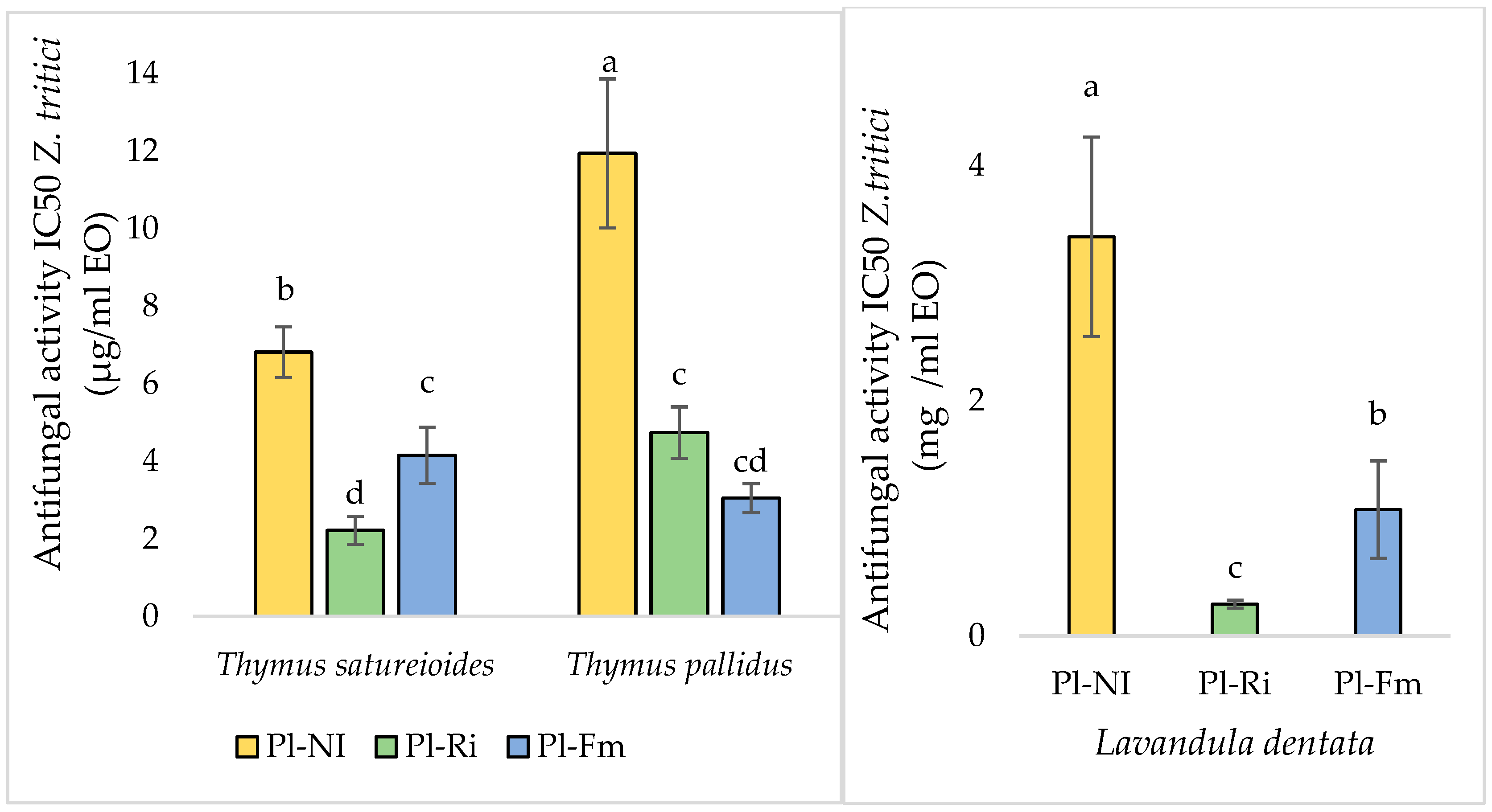
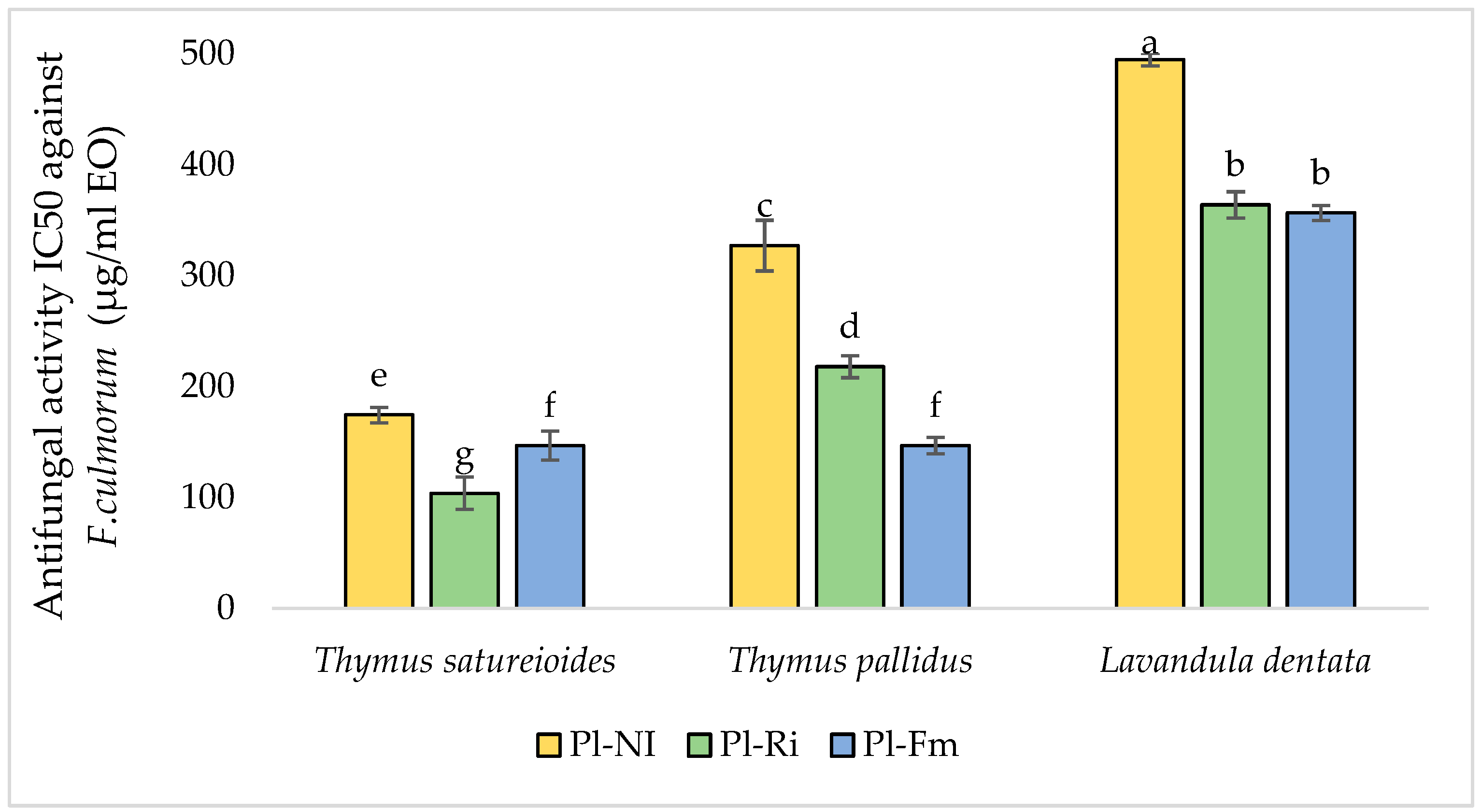
3.7.2. Antifungal Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chabrier, J.-Y. Plantes Médicinales et Formes D’utilisation en Phytothérapie. 2010, p. 184. Available online: https://hal.univ-lorraine.fr/hal-01739123 (accessed on 30 August 2022).
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rankou, H.; Culham, A.; Jury, S.L.; Christenhusz, M.J.M. The endemic flora of Morocco. Phytotaxa 2013, 78, 1–69. [Google Scholar] [CrossRef]
- Zrira, S. Some Important Aromatic and Medicinal Plants of Morocco. In Medicinal and Aromatic Plants of the World—Africa Volume 3; Neffati, M., Najjaa, H., Máthé, Á., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 91–125. [Google Scholar] [CrossRef]
- HCEFLCD (Haut-Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification). Stratégie Nationale de Développement du Secteur des Plantes Aromatiques et Médicinales; Mission USAID/Maroc; HCEFLCD: Rabat, Morocco, 2016; 72p. [Google Scholar]
- Jamaleddine, M.; El Oualidi, J.; Taleb, M.S.; Thévenin, T.; El Alaoui-Faris, F.E. Inventaire et état de conservation des plantes aromatiques et médicinales (PAM) au Maroc. Phytothér Apie 2017, 15, 114–122. [Google Scholar] [CrossRef]
- Lamrani-Alaoui, M.; Hassikou, R. Rapid risk assessment to harvesting of wild medicinal and aromatic plant species in Morocco for conservation and sustainable management purposes. Biodivers Conserv. 2018, 27, 2729–2745. [Google Scholar] [CrossRef]
- Jamaleddine, M.; Oualidi, J.E.; Sghir, M.; Alaoui-Faris, F.E.E.; Benzine, A. Les plantes aromatiques et médicinales au Maroc: Statut, endémisme, chorologie et bioclimat. Méd. Thér. 2019, 25, 7. [Google Scholar]
- El Yaagoubi, M.; Mechqoq, H.; El Hamdaoui, A.; Mukku, V.J.; El Mousadik, A.; Msanda, F.; El Aouad, N. A review on Moroccan Thymus species: Traditional uses, essential oils chemical composition and biological effects. J. Ethnopharmacol. 2021, 278, 114205. [Google Scholar] [CrossRef] [PubMed]
- Soulaimani, B.; Hidar, N.E.; Ben El Fakir, S.; Mezrioui, N.; Hassani, L.; Abbad, A. Combined antibacterial activity of essential oils extracted from Lavandula maroccana (Murb.), Thymus pallidus Batt. and Rosmarinus officinalis L. against antibiotic-resistant Gram-negative bacteria. Eur. J. Integr. Med. 2021, 43, 101312. [Google Scholar] [CrossRef]
- El-Bakkal, S.E.; Zeroual, S.; Elouazkiti, M.; Mansori, M.; Bouamama, H.; Zehhar, N.; El-Kaoua, M. Comparison of Yield Chemical Composition and Biological Activities of Essential Oils Obtained from Thymus pallidus and Thymus satureioides Coss. Grown in Wild and Cultivated Conditions in Morocco. J. Essent. Oil Bear. Plants 2020, 23, 1–14. [Google Scholar] [CrossRef]
- Wagner, L.S.; Sequin, C.J.; Foti, N.; Campos-Soldini, M.P. Insecticidal, fungicidal, phytotoxic activity and chemical composition of Lavandula dentata essential oil. Biocatal. Agric. Biotechnol. 2021, 35, 102092. [Google Scholar] [CrossRef]
- Rathore, S.; Kumar, R. Essential Oil Content and Compositional Variability of Lavandula Species Cultivated in the Mid Hill Conditions of the Western Himalaya. Molecules 2022, 27, 3391. [Google Scholar] [CrossRef]
- El Bakkal, S.E. Impact de la Domestication sur la Variation Qualitative et Quantitative des Métabolites de Thymus pallidus batt., Thymus satureioides Coss. et Lavandula dentata L.: Influence du Déficit Hydrique et de la Fertilisation Algale. Ph.D. Thesis, Université Cadi Ayyad, Marrakech, Maroc, 2022. [Google Scholar]
- Montanari, B. Moroccan Thyme (Thymus satureioides Coss.): Modern and Traditional Applications. Int. J. Prof. Holist. Aromather. 2019, 8, 31–35. [Google Scholar]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen fertilisation decreases the yield of bioactive compounds in Carlina acaulis L. grown in the field. Ind. Crops Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Habibi Machiani, R.; Sadeghpour, A. Arbuscular mycorrhizal Fungi and Changes in Primary and Secondary Metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Benhiba, L.; Fouad, M.O.; Essahibi, A.; Ghoulam, C.; Qaddoury, A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 2015, 29, 1725–1733. [Google Scholar] [CrossRef]
- Essahibi, A.; Benhiba, L.; Oussouf, F.; Babram, M.; Ghoulam, C.; Qaddoury, A. Improved rooting capacity and hardening efficiency of carob (Ceratonia siliqua L.) cuttings using arbuscular mycorrhizal fungi. Arch. Biol. Sci. 2017, 69, 291–298. [Google Scholar] [CrossRef]
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological Interactions Between Symbionts in Vesicular-Arbuscular Mycorrhizal Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 221–244. [Google Scholar] [CrossRef]
- Qaddoury, A. Arbuscular Mycorrhizal Fungi Provide Complementary Characteristics that Improve Plant Tolerance to Drought and Salinity: Date Palm as Model. In Mycoremediation and Environmental Sustainability; Prasad, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 189–215, (Fungal Biology); Available online: http://link.springer.com/10.1007/978-3-319-68957-9_11 (accessed on 30 August 2022).
- Shrivastava, N.; Mahajan, S.; Varma, A. (Eds.) Symbiotic Soil Microorganisms: Biology and Applications; Springer International Publishing: Cham, Switzerland, 2021; Volume 60, (Soil Biology); Available online: http://link.springer.com/10.1007/978-3-030-51916-2 (accessed on 30 August 2022).
- Freitas, M.; Martins, M.; Vieira, I. Yield and quality of essential oils of Mentha arvensis in response to inoculation with arbuscular mycorrhizal fungi. Pesqui. Agropecu. Bras. 2004, 39, 887–894. [Google Scholar] [CrossRef]
- Cabello, M.; Irrazabal, G.; Bucsinszky, A.M.; Saparrat, M.; Schalamuk, S. Effect of an arbuscular mycorrhizal fungus, Glomus mosseae, and a rock-phosphate-solubilizing fungus, Penicillium thomii, on Mentha piperita growth in a soilless medium. J. Basic Microbiol. 2005, 45, 182–189. [Google Scholar] [CrossRef]
- Khaosaad, T.; Vierheilig, H.; Nell, M.; Zitterl-Eglseer, K.; Novak, J. Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 2006, 16, 443–446. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Nazeri, V.; Fatemeh, S.; Farhad, R. Effect of Arbuscular Mycorrhizal Fungi on Plant Growth and Essential Oil Content and Composition of Ocimum basilicum L. Iran. J. Plant Physiol. 2013, 3, 643–650. [Google Scholar]
- Tarraf, W.; Ruta, C.; De Cillis, F.; Tagarelli, A.; Tedone, L.; De Mastro, G. Effects of mycorrhiza on growth and essential oil production in selected aromatic plants. Italy J. Agron. 2015, 10, 160–162. [Google Scholar] [CrossRef]
- Arpanahi, A.A.; Feizian, M.; Mehdipourian, G.; Khojasteh, D.N. Arbuscular mycorrhizal fungi inoculation improve essential oil and physiological parameters and nutritional values of Thymus daenensis Celak and Thymus vulgaris L. under normal and drought stress conditions. Eur. J. Soil Biol. 2020, 100, 103217. [Google Scholar] [CrossRef]
- Rankou, H.; Culham, A.; Sghir Taleb, M.; Ouhammou, A.; Martin, G.; Jury, S.L. Conservation assessments and Red Listing of the endemic Moroccan flora (monocotyledons): Red Listing of the Moroccan Flora. Bot. J. Linn. Soc. 2015, 177, 504–575. [Google Scholar] [CrossRef]
- Fennane, M.; Rejdali, M. Aromatic and medicinal plants of Morocco: Richness, diversity and threats. Bull. De L’institut Sci. Sect. Sci. De La Vie 2016, 38, 27–42. [Google Scholar]
- Baslam, M.; Qaddoury, A.; Goicoechea, N. Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 2014, 28, 161–172. [Google Scholar] [CrossRef]
- Essahibi, A.; Benhiba, L.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.). Trees 2018, 32, 87–97. [Google Scholar] [CrossRef]
- Essahibi, A.; Benhiba, L.; Fouad, M.O.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Responsiveness of Carob (Ceratonia siliqua L.) Plants to Arbuscular Mycorrhizal Symbiosis Under Different Phosphate Fertilization Levels. J. Plant Growth Regul. 2019, 38, 1243–1254. [Google Scholar] [CrossRef]
- Fouad, M.O.; Essahibi, A.; Benhiba, L.; Qaddoury, A. Effectiveness of arbuscular mycorrhizal fungi in the protection of olive plants against oxidative stress induced by drought. Span. J. Agric. Res. 2014, 12, 763–771. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire: Recherche des méthodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kjeldahl, J. New method for the determination of nitrogen in organic matter. Z. Anal. Chem. News 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31. [Google Scholar] [CrossRef]
- Brown, J.G.; Lilleland, O. Rapid determination of potassium and sodium in plant materials and soil extracts by flame photometry. Proc. Am. Soc. Hort. Sci. 1946, 48, 341–345. [Google Scholar]
- Znini, M.; Cristofari, G.; Majidi, L.; El Harrak, A.; Paolini, J.; Costa, J. In vitro antifungal activity and chemical composition of Warionia saharae essential oil against 3 apple phytopathogenic fungi. Food Sci. Biotechnol. 2013, 22, 113–119. [Google Scholar] [CrossRef]
- El-Alam, I.; Raveau, R.; Fontaine, J.; Verdin, A.; Laruelle, F.; Fourmentin, S.; Lounès-Hadj Sahraoui, A. Antifungal and Phytotoxic Activities of Essential Oils: In Vitro Assays and Their Potential Use in Crop Protection. Agronomy 2020, 10, 825. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance: The Assessment of Risk, 2nd ed.; Imprint Academic: Exeter, UK, 2007; Available online: https://www.frac.info/docs/default-source/publications/monographs/monograph-2.pdf (accessed on 30 August 2022).
- Russell, P.E. Fungicide Resistance Action Committee (FRAC). Outlook Pest Man. 2006, 17, 119–121. [Google Scholar] [CrossRef]
- Pherobase: Semiochemicals—Index. Available online: https://www.pherobase.com/database/compound/compounds-index.php (accessed on 30 August 2022).
- Geneva, M.P.; Stancheva, I.V.; Boychinova, M.M.; Mincheva, N.H.; Yonova, P.A. Effects of foliar fertilization and arbuscular mycorrhizal colonization on Salvia officinalis L. growth, antioxidant capacity, and essential oil composition. J. Sci. Food Agric. 2010, 90, 696–702. [Google Scholar] [CrossRef]
- Lahdachi, F.Z.; Bouamri, R.; Nassiri, L.; Ibijbijen, J. Rock phosphate and arbuscular mycorrhiza effects on growth and mineral nutrition of Acacia gummifera Wild. Int. J. Innov. Appl. Stud. 2018, 23, 384–389. [Google Scholar]
- Oliveira, R.S.; Ma, Y.; Rocha, I.; Carvalho, M.F.; Vosátka, M.; Freitas, H. Arbuscular mycorrhizal fungi are an alternative to the application of chemical fertilizer in the production of the medicinal and aromatic plant Coriandrum sativum L. J. Toxicol. Environ. Health Part A 2016, 79, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; Available online: https://www.elsevier.com/books/mycorrhizal-symbiosis/smith/978-0-12-370526-6 (accessed on 30 August 2022).
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin, Germany, 1995; Available online: https://archive.org/details/physiologicalpla0000larc_l1z7 (accessed on 30 August 2022).
- Barea, J.M.; Palenzuela, J.; Cornejo, P.; Sánchez-Castro, I.; Navarro-Fernández, C.; Lopéz-García, A.; Azcón-Aguilar, C. Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J. Arid Environ. 2011, 75, 1292–1301. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2019.01068 (accessed on 30 August 2022). [CrossRef] [PubMed]
- Yadav, K.; Aggarwal, A.; Singh, N. Arbuscular mycorrhizal fungi (AMF) induced acclimatization, growth enhancement and colchicine content of micropropagated Gloriosa superba L. plantlets. Ind. Crops Prod. 2013, 45, 88–93. [Google Scholar] [CrossRef]
- Alipour, A.; Rahimi, M.M.; Hosseini, S.M.A.; Bahrani, A. Mycorrhizal fungi and growth-promoting bacteria improves fennel essential oil yield under water stress. Ind. Crops Prod. 2021, 170, 113792. [Google Scholar] [CrossRef]
- Ercoli, L.; Mariotti, M.; Masoni, A.; Massantini, F. Relationship between nitrogen and chlorophyll content and spectral properties in maize leaves. Eur. J. Agron. 1993, 2, 113–117. [Google Scholar] [CrossRef]
- Ghasemi, H.; Esmaeili, M.A.; Mohammadian, R. Effects of nitrogen on chlorophyll fluorescence and the relationship between chlorophyll content and spad values in sugar beet (Beta vulgaris L.) Under drip-tape system. J. Agric. Biol. Sci. 2017, 12, 6. [Google Scholar]
- Adolfsson, L.; Solymosi, K.; Andersson, M.X.; Keresztes, A.; Uddling, J.; Schoefs, B.; Spetea, C. Mycorrhiza Symbiosis Increases the Surface for Sunlight Capture in Medicago truncatula for Better Photosynthetic Production. PLoS ONE 2015, 18, e0115314. [Google Scholar] [CrossRef]
- Everson, R.G.; Cockburn, W.; Gibbs, M. Sucrose as a Product of Photosynthesis in Isolated Spinach Chloroplasts. Plant Physiol. 1967, 42, 840–844. [Google Scholar] [CrossRef][Green Version]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited Review: Essential Oils as Modifiers of Rumen Microbial Fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Furelaud, G. Glucides et Lipides, des Sources D’énergie Pour L’organisme. Planet-Vie. Available online: https://planet-vie.ens.fr/thematiques/cellules-et-molecules/metabolisme-cellulaire/glucides-et-lipides-des-sources-d-energie (accessed on 30 August 2022).
- dos Santos, E.L.; Falcão, E.L.; da Silva, F.S.B. Tecnologia Micorrízica Como Bioinsumo Para Produção De Compostos Fenólicos de Importância À Indústria de Fitomedicamentos. Res. Soc. Dev. 2021, 10, e54810212856. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants; Springer Nature: Cham Switzerland, 2019; pp. 333–359. [Google Scholar] [CrossRef]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Rostaei, M.; Fallah, S.; Lorigooini, Z.; Abbasi Surki, A. The effect of organic manure and chemical fertilizer on essential oil, chemical compositions and antioxidant activity of dill (Anethum graveolens) in sole and intercropped with soybean (Glycine max). J. Clean. Prod. 2018, 199, 18–26. [Google Scholar] [CrossRef]
- Kapoor, R.; Anand, G.; Gupta, P.; Mandal, S. Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem. Rev. 2017, 16, 677–692. [Google Scholar] [CrossRef]
- Chandel, N.S. Glycolysis. Cold Spring Harb Perspect Biol. 2021, 13, a040535. [Google Scholar] [CrossRef]
- Maes, L.; Van Nieuwerburgh, F.C.; Zhang, Y.; Reed, D.W.; Pollier, J.; Vande Casteele, S.R.; Inzé, D.; Covello, P.S.; Deforce, D.L.D.; Goossens, A. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytologist. 2011, 189, 176–189. [Google Scholar] [CrossRef]
- Ringer, K.L.; Davis, E.M.; Croteau, R. Monoterpene Metabolism. Cloning, Expression, and Characterization of (−)-Isopiperitenol/(−)-Carveol Dehydrogenase of Peppermint and Spearmint. Plant Physiol. 2005, 137, 863–872. [Google Scholar] [CrossRef]
- Bartram, S.; Jux, A.; Gleixner, G.; Boland, W. Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 2006, 67, 1661–1672. [Google Scholar] [CrossRef]
- Behnam, S.; Farzaneh, M.; Ahmadzadeh, M.; Tehrani, A.S. Composition and antifungal activity of essential oils of Mentha piperita and Lavendula angustifolia on post-harvest phytopathogens. Commun. Agric. Appl. Biol. Sci. 2006, 71 Pt B, 1321–1326. [Google Scholar]
- Allen, M.F.; Allen, M.F.; Allen, M.F. The Ecology of Mycorrhizae; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Siqueira, J.O.; Saggin-Júnior, O.J. Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza 2001, 11, 245–255. [Google Scholar] [CrossRef]
- Urcoviche, R.C.; Castelli, M.; Gimenes, R.M.T.; Alberton, O. Spore density and diversity of arbuscular mycorrhizal fungi in medicinal and seasoning plant. Afr. J. Agric. Res. 2014, 9, 1244–1251. [Google Scholar]
- Fokom, R.; Adamou, S.; Essono, D.; Ngwasiri, D.P.; Eke, P.; Mofor, C.T.; Sharma, A.K. Growth, essential oil content, chemical composition and antioxidant properties of lemongrass as affected by harvest period and arbuscular mycorrhizal fungi in field conditions. Ind. Crops Prod. 2019, 138, 111477. [Google Scholar] [CrossRef]
- Beylier, M.F. Bacteriostatic Activity of Some Australian Essential Oils; Perfumer and Flavorist: Carol Stream, USA, 1979; p. 3. [Google Scholar]
- Morris, J.A.; Khettry, A.; Seitz, E.W. Antimicrobial activity of aroma chemicals and essential oils. J. Am. Oil Chem. Soc. 1979, 56, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and Antifungal Properties of Essential Oil Components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj, S.A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Hector, R.F. Compounds active against cell walls of medically important fungi. Clin. Microbiol. Rev. 1993, 6, 1–21. [Google Scholar] [CrossRef]
- Freires, I.D.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.M.D.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (Coriander) essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. De Mycol. Méd. 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Ben Ghnaya, A.; Hanana, M.; Amri, I.; Balti, H.; Gargouri, S.; Jamoussi, B.; Hamrouni, L. Chemical composition of Eucalyptus erythrocorys essential oils and evaluation of their herbicidal and antifungal activities. J. Pest. Sci. 2013, 86, 571–577. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Cartagena, E.; Bardón, A.; Arena, M.E. Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT Food Sci. Technol. 2016, 68, 373–380. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The Mechanism of Antifungal Action of Essential Oil from Dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Lima, M.I.; Araújo de Medeiros, A.C.; Souza Silva, K.V.; Cardoso, G.N. Investigation of the antifungal potential of linalool against clinical isolates of fluconazole resistant Trichophyton rubrum. J. Mycol. Méd. 2017, 27, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, N.; Ohta, T.; Danno, N.; Taniguchi, S.; Matsumoto, I.; Nishiguchi, M. The role of primary germ tubes in the life cycle of Blumeria graminis: The primary germ tube is responsible for the suppression of resistance induction of a host plant cell. Physiol. Mol. Plant Pathol. 2007, 71, 184–191. [Google Scholar] [CrossRef]


| Name | RT | IK | IK* | Ts-NI | Ts-Ri | Ts-Fm |
|---|---|---|---|---|---|---|
| Tricyclene | 1.26 | 919 | 919 | 2.1 | 1.19 | - |
| α-pinene | 1.34 | 937 | 933 | 2.06 | 1.27 | 0.06 |
| Camphene | 1.49 | 967 | 952 | 0.65 | 0.46 | - |
| β-Pinene | 1.54 | 979 | 981 | 0.47 | 0.28 | - |
| α-terpinene | 1.74 | 1018 | 1018 | 8.47 | 8.37 | 3.11 |
| δ-Terpinene | 1.93 | 1053 | 1059 | 4.18 | 3.4 | 2.04 |
| cis-sabinene hydrate | 2.01 | 1066 | 1069 | 0.14 | 0.07 | 0.07 |
| Linalool | 2.17 | 1097 | 1098 | 1.67 | 1.62 | 1.84 |
| Borneol | 2.61 | 1173 | 1165 | 5.93 | 16.73 | 18.68 |
| Terpinen-4-ol | 2.65 | 1181 | 1179 | 0.97 | 1.1 | 1.09 |
| Cis dihydrocarvone | 2.74 | 1196 | 1198 | 2.71 | 2.29 | 2.23 |
| Carvacrol methylether | 2.98 | 1239 | 1244 | 11.28 | 13.47 | 15.44 |
| Bornyl acetate | 3.23 | 1284 | 1285 | 0.21 | 0.24 | 0.17 |
| Thymol | 3.27 | 1292 | 1290 | 16.55 | 16.75 | 23.77 |
| Carvacrol | 3.33 | 1301 | 1299 | 18.5 | 6.05 | 23.36 |
| α-Copaene | 3.73 | 1377 | 1376 | 0.13 | 0.18 | 0.17 |
| β- Caryophyllene | 3.97 | 1424 | 1428 | 2.26 | 2.57 | 2.99 |
| α- Humulene | 4.15 | 1458 | 1460 | 0.34 | 0.41 | 0.54 |
| δ-Muurolene | 4.44 | 1515 | 0.15 | 0.17 | 0.21 | |
| δ-Cadinene | 4.46 | 1519 | 0.34 | 0.42 | 0.51 | |
| Caryophyllene oxide | 4.77 | 1585 | 1573 | 0.25 | 0.28 | 0.43 |
| Tau-Cadinol | 5.05 | 1644 | 1640 | 0.34 | 0.36 | 0.46 |
| Oxygenated monoterpene | 57.96 | 58.32 | 86.65 | |||
| Hydrocarbon monoterpene | 17.93 | 14.97 | 5.21 | |||
| Oxygenated sesquiterpene | 0.59 | 0.64 | 0.89 | |||
| Hydrocarbon sesquiterpene | 3.22 | 3.75 | 4.42 | |||
| Nom | RT | IK | IK* | Tp-NI | Tp-RI | Tp-Fm |
|---|---|---|---|---|---|---|
| Tricyclene | 1.26 | 919 | 919 | 0.09 | 1.46 | 0.58 |
| Camphene | 1.34 | 936 | 952 | 0.04 | 1.44 | 0.88 |
| Verbenene | 1.49 | 967 | 967 | 0.02 | 0.37 | 0.18 |
| (+)-4-Carene | 1.71 | 1010 | 1011 | 0.01 | 0.49 | 0.62 |
| α-terpinene | 1.76 | 1020 | 1018 | 18.63 | 22.3 | 32.6 |
| δ-Terpinene | 1.93 | 1053 | 1059 | 7.05 | 8.6 | 8.12 |
| Linalool | 2.18 | 1097 | 1098 | 6.35 | 5.15 | 7.39 |
| Borneol | 2.61 | 1172 | 1165 | 16.09 | 15.07 | 6.03 |
| Terpinen-4-ol | 2.65 | 1180 | 1178 | 0.79 | 0.98 | 0.87 |
| Isobornyl acetate | 3.24 | 1285 | 1285 | 0.1 | 0.08 | 0.02 |
| Thymol | 3.29 | 1294 | 1290 | 37.14 | 30.14 | 28.79 |
| Carvacrol | 3.32 | 1300 | 1298 | 6.88 | 7.74 | 4.86 |
| Caryophyllene | 3.97 | 1423 | 1418 | 2.57 | 2.16 | 3.64 |
| Humulene | 4.15 | 1458 | 1460 | 0.18 | 0.16 | 0.09 |
| Caryophyllene oxide | 4.78 | 1584 | 1581 | 0.78 | 0.5 | 0.8 |
| α-cadinol | 5.05 | 1643 | 1652 | 0.02 | 0.09 | - |
| Oxygenated monoterpene | 67.35 | 59.16 | 47.96 | |||
| Hydrocarbon monoterpene | 25.84 | 34.66 | 42.98 | |||
| Oxygenated sesquitrepene | 0.78 | 0.59 | 0,8 | |||
| Hydrocarbon sesquiterpene | 2.75 | 2.32 | 3.73 | |||
| Name | RT | IK | IK* | Ld-NI | Ld-Ri | Ld-Fm |
|---|---|---|---|---|---|---|
| Tricyclene | 1.26 | 918 | 919 | - | 1.25 | 0.88 |
| α- pinene | 1.34 | 936 | 937 | - | 1.04 | 1.46 |
| Camphene | 1.49 | 966 | 953 | 0.54 | 2.89 | 1.89 |
| D-limonene | 1.77 | 1022 | 1031 | - | 1.61 | 0.69 |
| Eucalyptol | 1.79 | 1025 | 1033 | - | - | 0.79 |
| L-fenchone | 2.1 | 1083 | 1062 | 1.78 | 3.19 | 1.19 |
| Linalool | 2.18 | 1097 | 1098 | - | - | 0.68 |
| Fenchol | 2.31 | 1121 | 1123 | 17.84 | 19.5 | 4.14 |
| α-campholenal | 2.34 | 1125 | 1127 | - | 0.85 | - |
| (1r)-(+)-nopiune | 2.43 | 1141 | 1142 | 1.36 | 1.17 | - |
| Camphor | 2.47 | 1148 | 1143 | 6.89 | 8.19 | 58.44 |
| Pinocarvone | 2.55 | 1162 | 1162 | 0.8 | 0.91 | 0.72 |
| Isoborneol | 2.64 | 1177 | 1177 | 25.12 | 27.04 | 8.87 |
| Terpinene-4-ol | 2.7 | 1189 | 1179 | 0.51 | - | - |
| Myrtenal | 2.74 | 1195 | 1195 | 2.61 | 2.7 | 1.8 |
| Carveol | 2.92 | 1228 | 1229 | 2.21 | 3.06 | 0.64 |
| Cuminaldehyde | 3.01 | 1244 | 1239 | 0.89 | - | - |
| Bornyl acetat | 3.24 | 1284 | 1285 | 0.43 | 0.51 | 0.28 |
| Germacrene D | 4.32 | 1490 | 1499 | 4.11 | 6.76 | 3.65 |
| Geranyl-, α-terpinene | 4.71 | 1570 | - | - | - | 0.83 |
| Caryophyllene oxide | 4.78 | 1586 | 1581 | 3.61 | 4.3 | 1.92 |
| Valencen | 5.01 | 1634 | - | 0.42 | 0.3 | - |
| β-eudesmol | 5.12 | 1657 | 1654 | 7.98 | 10.37 | 6.05 |
| Cadalene | 5.19 | 1673 | 1674 | 0.62 | 0.39 | - |
| Oxygenated monoterpene | 58.23 | 63.42 | 75.29 | |||
| Hydrocarbon monoterpene | 3.11 | 10.46 | 8.04 | |||
| Oxygenated sesquiterpene | 14.04 | 17.48 | 11.41 | |||
| Hydrocarbon sesquiterpene | 5.15 | 7.45 | 3.65 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akachoud, O.; Bouamama, H.; Facon, N.; Laruelle, F.; Zoubi, B.; Benkebboura, A.; Ghoulam, C.; Qaddoury, A.; Lounès-Hadj Sahraoui, A. Mycorrhizal Inoculation Improves the Quality and Productivity of Essential Oil Distilled from Three Aromatic and Medicinal Plants: Thymus satureioides, Thymus pallidus, and Lavandula dentata. Agronomy 2022, 12, 2223. https://doi.org/10.3390/agronomy12092223
Akachoud O, Bouamama H, Facon N, Laruelle F, Zoubi B, Benkebboura A, Ghoulam C, Qaddoury A, Lounès-Hadj Sahraoui A. Mycorrhizal Inoculation Improves the Quality and Productivity of Essential Oil Distilled from Three Aromatic and Medicinal Plants: Thymus satureioides, Thymus pallidus, and Lavandula dentata. Agronomy. 2022; 12(9):2223. https://doi.org/10.3390/agronomy12092223
Chicago/Turabian StyleAkachoud, Oumaima, Hafida Bouamama, Natacha Facon, Frédéric Laruelle, Btissam Zoubi, Abderrazak Benkebboura, Cherki Ghoulam, Ahmed Qaddoury, and Anissa Lounès-Hadj Sahraoui. 2022. "Mycorrhizal Inoculation Improves the Quality and Productivity of Essential Oil Distilled from Three Aromatic and Medicinal Plants: Thymus satureioides, Thymus pallidus, and Lavandula dentata" Agronomy 12, no. 9: 2223. https://doi.org/10.3390/agronomy12092223
APA StyleAkachoud, O., Bouamama, H., Facon, N., Laruelle, F., Zoubi, B., Benkebboura, A., Ghoulam, C., Qaddoury, A., & Lounès-Hadj Sahraoui, A. (2022). Mycorrhizal Inoculation Improves the Quality and Productivity of Essential Oil Distilled from Three Aromatic and Medicinal Plants: Thymus satureioides, Thymus pallidus, and Lavandula dentata. Agronomy, 12(9), 2223. https://doi.org/10.3390/agronomy12092223







