Diurnal Variation in Transport and Use of Intracellular Leaf Water and Related Photosynthesis in Three Karst Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Determination of Electrophysiological Parameters and Leaf Water Potential
2.3. Determination of Photosynthetic Parameters
2.4. Determination of Chlorophyll Fluorescence Parameters
2.5. Statistical Analysis
3. Results
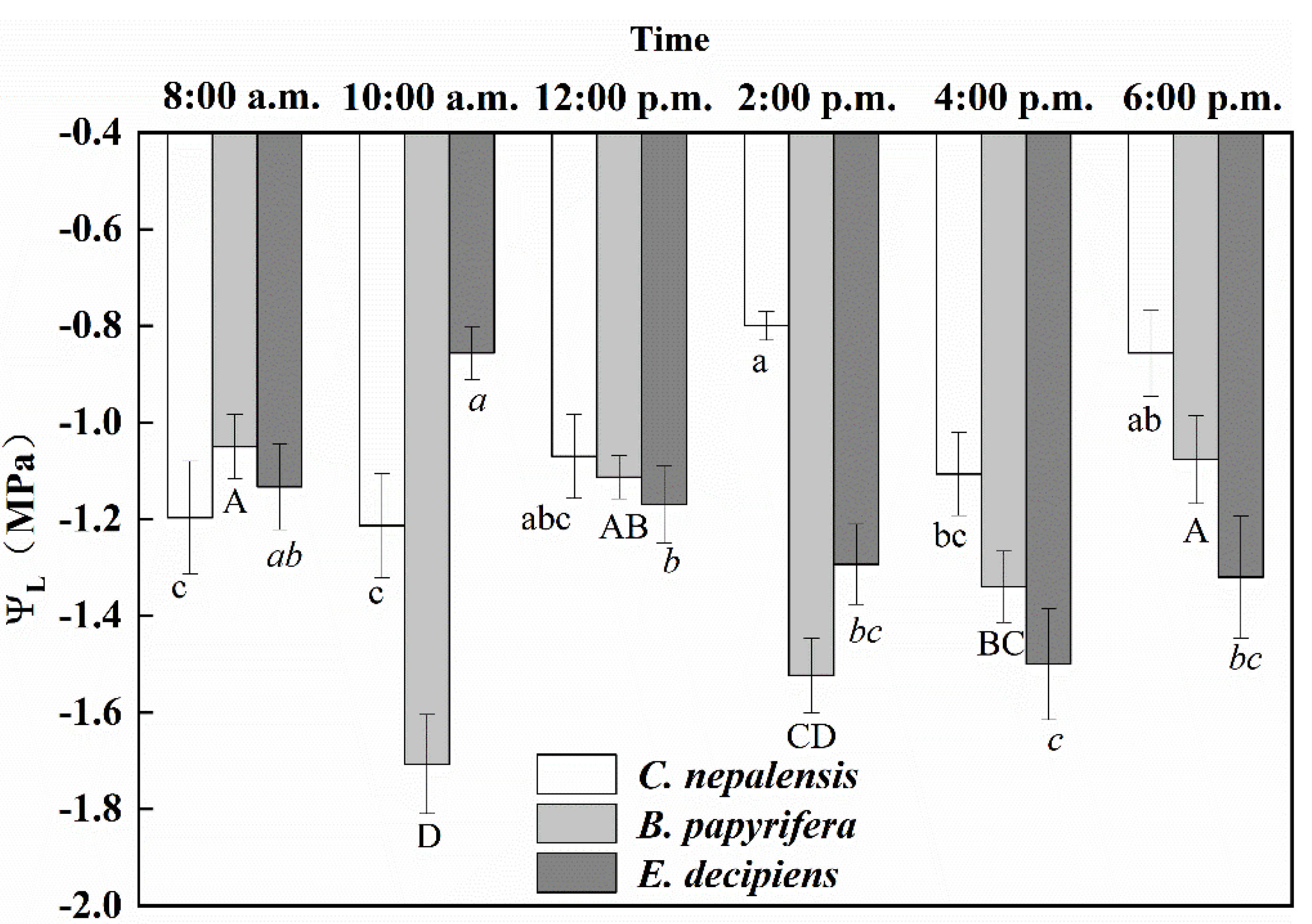
3.1. Diurnal Variations in Leaf Water Potential and Electrophysiological Parameters
3.2. Diurnal Variations in Photosynthetic Parameters and Instantaneous Water-Use Efficiency
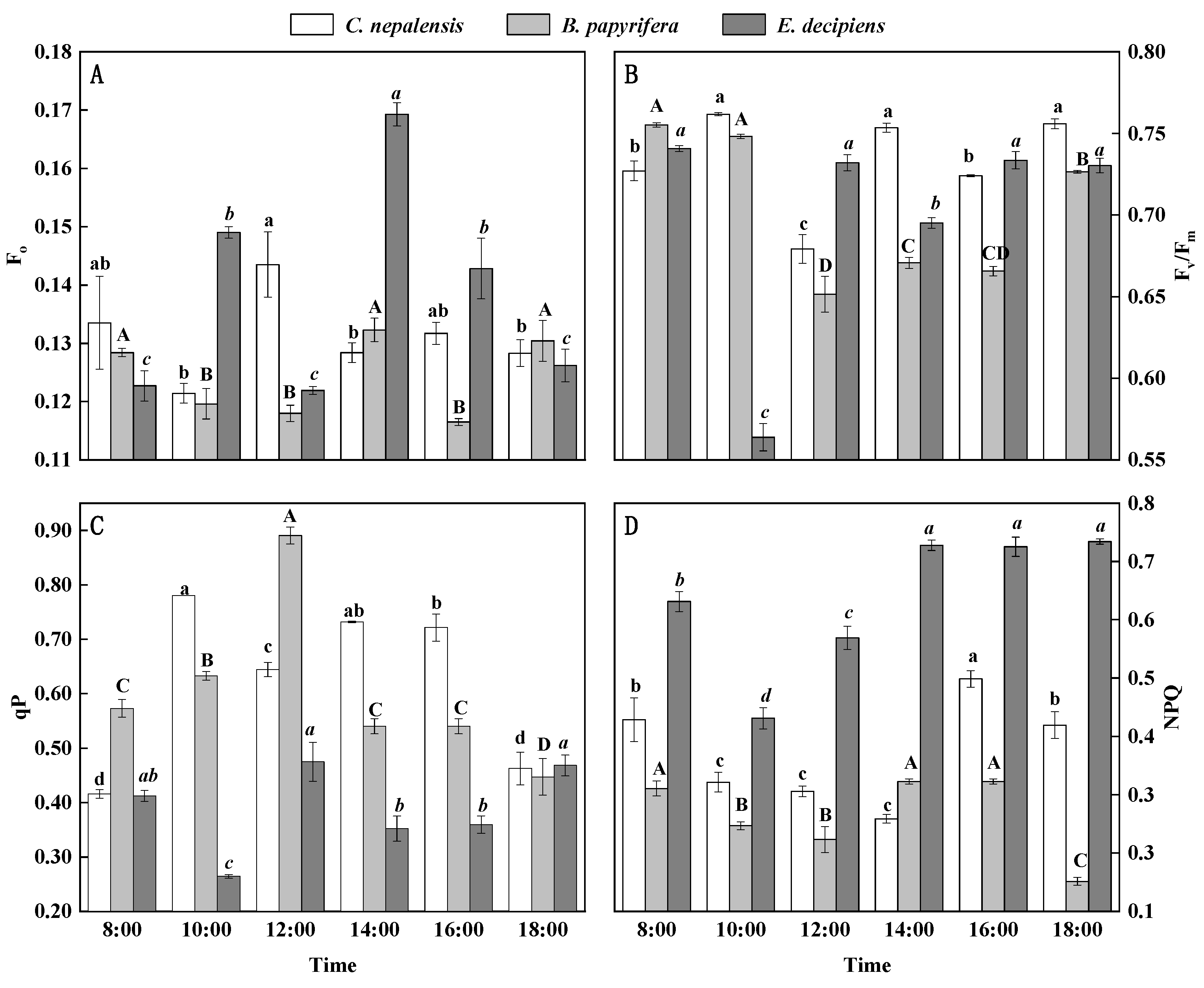
3.3. Diurnal Variations in Chlorophyll Fluorescence Parameters
3.4. Difference among Intracellular Water and Photosynthesis in C. nepalensis, B. papyrifera, and E. decipiens
4. Discussion
4.1. Intracellular Water Use vs. Photosynthesis
4.2. Dynamic Traits of Leaf Intracellular Water
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.R.; Liu, H.Y.; Wu, L.; Liang, B.Y.; Liu, F.; He, W.Q. Impact of Bedrock Geochemistry on Vegetation Productivity Depends on Climate Dryness in the Guizhou Karst of China. Pro. Phys. Geog. 2021, 45, 20–32. [Google Scholar] [CrossRef]
- Peng, T.; Wang, S.J. Effects of Land Use, Land Cover and Rainfall Regimes on the Surface Runoff and Soil Loss on Karst Slopes in Southwest China. Catena 2012, 90, 53–62. [Google Scholar] [CrossRef]
- Wang, Z.H.; Luo, D.; Xiong, K.N.; Gu, X.; Zhu, Z.Z. Studies on Hydrological Processes on Karst Slopes for Control of Soil and Water Loss. Sustainability 2022, 14, 5789. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Tan, W.; Deng, P.Y.; Yang, J. Seasonal Water Use Patterns of Woody Species Growing on the Continuous Dolostone Outcrops and Nearby Thin Soils in Subtropical China. Plant Soil 2011, 341, 399–412. [Google Scholar] [CrossRef]
- Liu, Z.Q.; She, R.; Xiong, K.N.; Li, Y.; Cai, L.L. Effect of Vegetation Restoration on Soil Hydrology in Karst Area of Southwest China: Inspiration from Barrel Planting Experiments. Water 2021, 13, 1719. [Google Scholar] [CrossRef]
- Bonacci, O.; Pipan, T.; Culver, D.C. A Framework for Karst Ecohydrology. Environ. Geol. 2009, 56, 891–900. [Google Scholar] [CrossRef]
- Yue, Y.M.; Wang, K.L.; Xiong, Y. Feasibility of Monitoring Karst Standing Conditions with Vegetation Spectra. Spectrosc. Spectr. Anal. 2012, 32, 1891–1894. [Google Scholar]
- Chen, H.S.; Zhang, W.; Wang, K.L.; Fu, W. Soil Moisture Dynamics under Different Land Uses on Karst Hillslope in Northwest Guangxi, China. Environ. Earth Sci. 2010, 61, 1105–1111. [Google Scholar] [CrossRef]
- Dawson, T.E. Fog in the California Redwood Forest: Ecosystem Inputs and Use by Plants. Oecologia 1998, 117, 476–485. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Phillips, S.L.; Schuster, W.S.F.; Sandquist, D.R. Differential Utilization of Summer Rains by Desert Plants. Oecologia 1991, 88, 430–434. [Google Scholar] [CrossRef]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought Tolerance in Rice: Focus on Recent Mechanisms and Approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; John, G.P.; Pan, R.; Bartlett, M.K.; Fletcher, L.R.; Scoffoni, C.; Sack, L. A Stomatal Safety-Efficiency Trade-off Constrains Responses to Leaf Dehydration. Nat. Commun. 2019, 10, 3398. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wu, Y.Y.; Xing, D.K. The Differential Response of Intracellular Water Metabolism Derived from Intrinsic Electrophysiological Information in Morus Alba L. and Broussonetia Papyrifera (L.) Vent. Subjected to Water Shortage. Horticulturae 2022, 8, 182. [Google Scholar] [CrossRef]
- Ozeki, K.; Miyazawa, Y.; Sugiura, D. Rapid Stomatal Closure Contributes to Higher Water Use Efficiency in Major C4 Compared to C3 Poaceae Crops. Plant Physiol. 2022, 189, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Y.; Ji, R.P.; Feng, R.; Zhao, X.L.; Zhang, Y.S. Response of Water Stress on Photosynthetic Characteristics and Water Use Efficiency of Maize Leaves in Different Growth Stage. Acta Ecol. Sin. 2015, 35, 2902–2909. [Google Scholar]
- Jin, T.T.; Fu, B.J.; Liu, G.H.; Hu, C.J.; Su, C.H.; Liu, Y. Diurnal Changes of Photosynthetic Characteristics of Hippophae Rhamnoides and the Relevant Environment Factors at Different Slope Locations. Acta Ecol. Sin. 2011, 31, 1783–1793. [Google Scholar]
- Hájková, M.; Kummerová, M.; Zezulka, Š.; Babula, P.; Váczi, P. Diclofenac as an Environmental Threat: Impact on the Photosynthetic Processes of Lemna Minor Chloroplasts. Chemosphere 2019, 224, 892–899. [Google Scholar] [CrossRef]
- Nedbal, L.; Soukupová, J.; Kaftan, D.; Whitmarsh, J.; Trtílek, M. Kinetic Imaging of Chlorophyll Fluorescence Using Modulated Light. Photosynth. Res. 2000, 66, 3–12. [Google Scholar] [CrossRef]
- Kooten, O.V.; Snel, J.F.H. The Use of Chlorophyll Fluorescence Nomenclature in Plant Stress Physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. BBA—Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Xing, D.K.; Dai, Y.; Wu, Y.S.; Fang, L. A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism. Plants 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.K.; Chen, L.; Wu, Y.Y.; Zwiazek, J.J. Leaf Physiological Impedance and Elasticity Modulus in Orychophragmus Violaceus Seedlings Subjected to Repeated Osmotic Stress. Sci. Hortic. 2021, 276, 109763. [Google Scholar] [CrossRef]
- Buckley, D.J.; Lefebvre, M.; Meijer, E.G.M.; Brown, D.C.W. A Signal Generator for Electrofusion of Plant Protoplasts. Comput. Electron. Agr. 1990, 5, 179–185. [Google Scholar] [CrossRef]
- Jócsák, I.; Végvári, G.; Vozáry, E. Electrical Impedance Measurement on Plants: A Review with Some Insights to Other Fields. Theor. Exp. Plant Physiol. 2019, 31, 359–375. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wu, Y.Y.; Xing, D.K.; Zhao, K.; Yu, R. Rapid Measurement of Drought Resistance in Plants Based on Electrophysiological Properties. T. ASABE 2015, 58, 1441–1446. [Google Scholar]
- Mourya, N.R.; Bargali, K.; Bargali, S.S. Impacts of Coriaria Nepalensis Colonization on Vegetation Structure and Regeneration Dynamics in a Mixed Conifer Forest of Indian Central Himalaya. J. For. Res. 2019, 30, 305–317. [Google Scholar] [CrossRef]
- Guo, L.; Qiang, T.; Ma, Y.; Wang, K.; Du, K. Optimisation of Tannin Extraction from Coriaria Nepalensis Bark as a Renewable Resource for Use in Tanning. Ind. Crop. Prod. 2020, 149, 112360. [Google Scholar] [CrossRef]
- Guo, L.X.; Qiang, T.T.; Ma, Y.M.; Ren, L.F.; Dai, T.T. Purification and Characterization of Hydrolysable Tannins Extracted from Coriaria Nepalensis Bark Using Macroporous Resin and Their Application in Gallic Acid Production. Ind. Crop. Prod. 2021, 162, 113302. [Google Scholar] [CrossRef]
- Lv, M.; Tan, M.H.; Lu, L.W.; Bao, S.S.; Guo, Z.Y.; Deng, Z.S.; Zou, K. Secondary Metabolites from Endophytes of Elaeocarpus Decipiens Hemsl. with Co-Cultivation Method. J. China Three Gorges Univ. (Nat. Sci.) 2018, 40, 108–112. [Google Scholar]
- Liu, Z.B.; Xiao, D.M.; Zhang, G.L.; Li, X. Cutting Propagation Technology for Elaeocarpus Decipiens Hems1. North. Hortic. 2010, 3, 83–85. [Google Scholar]
- Hang, H.T.; Wang, R.; Xing, D.K.; Wu, Y.Y.; Zhang, K.Y.; Rao, S.; Zhao, L.H. Photosynthetic Capacity and Adaptability of Three Herbaceous Energy Plants in Karst Habitats. Jiangsu Agr. Sci. 2018, 46, 248–254. [Google Scholar]
- Laiming, H.; Wen, Z.; Ming’an, S. Response of Plant Physiological Parameters to Soil Water Availability During Prolonged Drought is Affected by Soil Texture. J. Arid Land 2021, 13, 688–698. [Google Scholar]
- Yang, Z.; Tian, J.; Wang, Z.; Feng, K. Monitoring the Photosynthetic Performance of Grape Leaves Using a Hyperspectral-Based Machine Learning Model. Eur. J. Agron. 2022, 140, 126589. [Google Scholar] [CrossRef]
- Xing, D.K.; Mao, R.L.; Li, Z.Y.; Wu, Y.Y.; Qin, X.J.; Fu, W.G. Leaf Intracellular Water Transport Rate Based on Physiological Impedance: A Possible Role of Leaf Internal Retained Water in Photosynthesis and Growth of Tomatoes. Front. Plant Sci. 2022, 13, 845628. [Google Scholar] [CrossRef]
- Li, M.H. Response of electrophysiological properties of Broussonetia papyrifera (Linn L’Hér. ex Vent.and Morus alba L. to drought. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2018. [Google Scholar]
- Chi, Y.K.; Xiong, K.N.; Zhang, J.H.; Wang, Y.S.; Zhang, Y. Study on the Photosynthetic Rate and Water Use Efficiency of Three Leguminous Grass Species in Karst Rocky Desertification Area. Chin. J. Grassl. 2014, 36, 116–120. [Google Scholar]
- Yan, T.; Wang, Z.; Liao, C.; Xu, W.; Li, W. Effects of the Morphological Characteristics of Plants on Rainfall Interception and Kinetic Energy. J. Hydrol. 2021, 592, 125807. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y. The Responses of Leaf Photosynthesis and Photorespiration to the Simulated Drought by Two Moraceae Plants. Earth Environ. 2019, 47, 141–150. [Google Scholar]
- Bargali, K.; Tewari, A. Growth and Water Relation Parameters in Drought-Stressed Coriaria nepalensis Seedlings. J. Arid Environ. 2004, 58, 505–512. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of Chlorophyll Fluorescence Parameters as Indicators of Photosynthetic Efficiency in Large Scale Plant Ecological Studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.J.; Zhang, S.B. Specific Roles of Cyclic Electron Flow around Photosystem I in Photosynthetic Regulation in Immature and Mature Leaves. J. Plant Physiol. 2017, 209, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Mittelheuser, C.J.; Steveninck, R.F.M.V. Stomatal Closure and Inhibition of Transpiration Induced by (RS)-Abscisic Acid. Nature 1969, 221, 281–282. [Google Scholar] [CrossRef]


| Plant Species | Distribution | Adaptive Habitats | Biological Traits |
|---|---|---|---|
| C. nepalensis | Yunnan, Guizhou, Sichuan, Hubei, Shanxi, Gansu, Xizang provinces in China; India; Nepal | Drought, low nutrition, neutral alkaline, heliophilous, low temperature | Shrub, fast-growing, leathery leaf, shallow root system with many horizontal and oblique roots |
| B. papyrifera | Yellow, Yangtze and Pearl River Basins in China; Vietnam; Japan; India; Malaysia; Thailand; Burma | Drought, low nutrition, waterlog, heliophilous, acidic and neutral soil, chimney, air pollution, limestone | Tree, fast-growing, papery tomentose leaf, shallow root system with wide lateral root distribution |
| E. decipiens | Guangxi, Guangdong, Guizhou, Jiangxi, Fujian, Zhejiang, Yunnan, Hunan, Taiwan provinces in China; Vietnam; Japan | Slightly shade-tolerant, liked warmth and humid, acid soil, strong tolerance to SO2 | Tree, fast-growing, leathery leaf, developed deep root system |
| Plant Species | Time | LIWTR | LIWHC | LIWUE |
|---|---|---|---|---|
| C. nepalensis | 8:00 a.m. | 0.24 ± 0.04 abc | 288.38 ± 31.05 b | 0.05 ± 0.00 bc |
| 10:00 a.m. | 0.41 ± 0.05 a | 235.40 ± 31.40 b | 0.06 ± 0.01 ab | |
| 12:00 p.m. | 0.43 ± 0.17 a | 287.49 ± 101.52 b | 0.09 ± 0.02 a | |
| 2:00 p.m. | 0.35 ± 0.06 ab | 226.67 ± 41.76 b | 0.07 ± 0.00 ab | |
| 4:00 p.m. | 0.04 ± 0.01 c | 3039.45 ± 673.30 a | 0.02 ± 0.00 c | |
| 6:00 p.m. | 0.09 ± 0.03 bc | 739.56 ± 175.68 b | 0.04 ± 0.01 bc | |
| B. papyrifera | 8:00 a.m. | 0.25 ± 0.03 a | 371.46 ± 26.46 a | 0.11 ± 0.02 c |
| 10:00 a.m. | 0.29 ± 0.02 a | 58.01 ± 9.22 c | 0.56 ± 0.12 a | |
| 12:00 p.m. | 0.46 ± 0.04 a | 126.41 ± 18.04 bc | 0.20 ± 0.03 bc | |
| 2:00 p.m. | 0.27 ± 0.08 a | 65.69 ± 9.44 c | 0.34 ± 0.06 b | |
| 4:00 p.m. | 0.46 ± 0.15 a | 49.91 ± 0.00 c | 0.75 ± 0.00 a | |
| 6:00 p.m. | 0.31 ± 0.01 a | 183.39 ± 50.75 b | 0.23 ± 0.09 bc | |
| E. decipiens | 8:00 a.m. | 0.89 ± 0.25 abc | 198.25 ± 25.86 bc | 0.06 ± 0.01 ab |
| 10:00 a.m. | 1.03 ± 0.20 ab | 123.98 ± 39.73 c | 0.13 ± 0.06 a | |
| 12:00 p.m. | 0.52 ± 0.10 bcd | 220.49 ± 24.54 bc | 0.06 ± 0.01 ab | |
| 2:00 p.m. | 1.18 ± 0.19 a | 90.41 ± 15.75 c | 0.12 ± 0.02 ab | |
| 4:00 p.m. | 0.20 ± 0.06 d | 534.29 ± 82.44 a | 0.04 ± 0.00 b | |
| 6:00 p.m. | 0.47 ± 0.11 cd | 341.94 ± 54.26 b | 0.05 ± 0.00 ab |
| Plant Species | Leaf Intracellular Water Traits | Photosynthesis | ||||
|---|---|---|---|---|---|---|
| LIWTR | LIWHC | LIWUE | PN | E | WUEi | |
| C. nepalensis | low | high | low | high | high | low |
| B. papyrifera | low | Low | high | high | low | high |
| E. decipiens | high | middle | low | low | low | low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Xing, D.; Wu, Y.; Wang, W.; Li, M.; Solangi, K. Diurnal Variation in Transport and Use of Intracellular Leaf Water and Related Photosynthesis in Three Karst Plants. Agronomy 2022, 12, 2758. https://doi.org/10.3390/agronomy12112758
Qin X, Xing D, Wu Y, Wang W, Li M, Solangi K. Diurnal Variation in Transport and Use of Intracellular Leaf Water and Related Photosynthesis in Three Karst Plants. Agronomy. 2022; 12(11):2758. https://doi.org/10.3390/agronomy12112758
Chicago/Turabian StyleQin, Xiaojie, Deke Xing, Yanyou Wu, Weixu Wang, Meiqing Li, and Kashif Solangi. 2022. "Diurnal Variation in Transport and Use of Intracellular Leaf Water and Related Photosynthesis in Three Karst Plants" Agronomy 12, no. 11: 2758. https://doi.org/10.3390/agronomy12112758
APA StyleQin, X., Xing, D., Wu, Y., Wang, W., Li, M., & Solangi, K. (2022). Diurnal Variation in Transport and Use of Intracellular Leaf Water and Related Photosynthesis in Three Karst Plants. Agronomy, 12(11), 2758. https://doi.org/10.3390/agronomy12112758








