Abstract
Root-derived carbon sources supporting photosynthesis have been demonstrated to contribute to plant carbon gain in many laboratory experiments. However, it remains largely unknown whether and to what extent soil dissolved inorganic carbon (DIC) influences leaf photosynthesis in karst habitats characterized by alkaline soils with low water content. We explored this relationship by measuring the concentrations and carbon isotope signals (δ13C) of soil DIC, as well as the δ13C of water-soluble organic matter (δWSOM) in leaves of nine woody species across an altitudinal gradient in karst habitats. The δWSOM varied among species by 7.23‰ and deviated from the δ13C of photosynthates solely assimilated from atmospheric CO2 (δA) by 0.44–5.26‰, with a mean value of 2.20‰. This systematical discrepancy (δA − δWSOM) could only be explained by the contribution of soil DIC to leaf total photosynthesis (fDIC_soil). The average values of fDIC_soil considerably varied among the nine species, ranging from 2.48% to 9.99%, and were comparable with or slightly lower than those of previous laboratory experiments. Furthermore, the fDIC_soil of two species significantly increased with altitude, whereas another species exhibited an opposite pattern, suggesting a highly spatial heterogeneity of DIC utilization. The present study improved our understanding of how plants adapt to the alkaline–drought soil conditions of karst habitats and thus acquire additional carbon for growth.
1. Introduction
Karst is a distinctive topography developed in regions with soluble bedrocks, such as limestone, dolomite, and gypsum [1], and covers about 22 × 106 km2 of the earth’s surface [2]. The karst habitats are characterized by thin soil layers, high content of bicarbonate and high pH in soils, and less vegetation cover [3,4,5]. For plants growing in karst habitats, water demand for maintaining the basic functions is often restricted due to the shallow soil [6]. For example, drought stress can lead to the decline of stomata opening and photosynthesis and downregulated metabolic processes [7]. A prolonged drought will result in hydraulic failure and/or carbon starvation, increasing the risk of mortality [8,9]. On the other hand, the alkaline soil condition in karst habitats is unfavorable for plant uptake of nutrients, such as phosphate, ferrous iron, and zinc [10,11]. As a consequence, the vegetation productivity of the karst ecosystem is usually much less than that in nonkarst regions [12]. Although plants can adopt tolerance and/or avoidance strategies to cope with environmental stress [13,14], it does not fully explain why some species grow well in karst habitats. Hence, other physiological processes of karst plants are suspected to play an important role in regulating their growth under dry and alkaline soil conditions.
Recently, root-derived carbon sources supporting photosynthesis have reemerged as a hotspot in plant ecophysiological studies [15,16,17,18]. Research shows that 13C-enriched CO2 or HCO3− labeling in the root zones can be transported upward through the transpiration stream [19] and thus affects the carbon isotope composition (δ13C) of leaf photoassimilates and aboveground tissues [20,21]. Although these results are obtained from the manipulated experiments inside a laboratory, it may be applied to karst plants grown in the field, given the high concentration of bicarbonate contained in karst soils. The additional carbon gain may also compensate for the decrease in the fixation of atmospheric CO2 induced by drought stress [22,23]. However, to the best of our knowledge, no research has been reported to investigate plants’ use of dissolved inorganic carbon (DIC, mainly including dissolved CO2 and HCO3−) in karst habitats. Although many hydroponic experiments were designed to simulate karst soil conditions, for example, bicarbonate stress (usually more than 10 mM) [24,25], osmotic stress (simulating water scarcity) [26], and their interactions [26], their results do not necessarily reflect the utilization of soil DIC by plants due to the differences in the microenvironment of the root zones between soil conditions and hydroponic solutions [27]. Hence, there is a need to fill this knowledge gap between laboratory experiments and field trials.
Appropriate methods and models for quantification are critical to understanding plants’ use of soil DIC. Currently, two methods are available to estimate this utilization. Method (i) applies the high abundance 13C labeling and then calculates the ratio of soil DIC fixed in specific tissues or organs to the total carbon gain. The total carbon gain includes soil DIC fixation, apparent photosynthesis measured by commercial infrared gas analyzers, and respiration [20,28]. However, this calculation is not directly linked to photosynthesis. Method (ii) uses a two-source 13C labeling in combination with isotope mixing models to determine the contribution of root-derived DIC to leaf total photosynthesis [22,23,24,29]. Nevertheless, uncertainties remain associated with the photosynthetic 13C discrimination and post-photosynthesis processes. Additionally, both labeling methods depend on irrigation or hydroponic culture, which will inevitably change the soil conditions, especially in karst environments. The labeling experiment also requires a sealed environment and has a high cost, which does not apply to large-scale field experiments. For these reasons, a new approach needs to be developed to calculate the contribution of soil DIC to leaf total photosynthesis (see Section 2). It has been shown that the natural abundance of 13C has the potential to address a wide range of ecophysiological and biochemical questions [30]. For example, naturally occurring δ13C signals in plant tissues integrate plant–environment interactions over long periods [31]. In C3 plants, δ13C of leaves is mainly controlled by photosynthetic 13C discrimination [32], which can be altered by stomatal control and the activity of the carboxylation enzyme, Rubisco. Furthermore, when other carbon sources are supplying the leaf photosynthesis, for instance, uptake of DIC from the xylem sap of the host by the mistletoe or utilization of root-derived DIC by some plants [26,33], the δ13C of leaves can also be modified.
In karst habitats, altitude plays an important role in affecting the soil conditions and microclimate [34], which determine the distribution of plant species [35]. Species with different life forms may vary in functional traits, such as root uptake and xylem transport of water and DIC, thus influencing the extent of soil DIC used by plants. In addition, the concentration of soil DIC may change with altitudes, which probably acts on the proportion of soil DIC used by leaf photosynthesis. In this study, the primary objective was to investigate whether and to what extent soil DIC influences the leaf photosynthesis of karst plants at different altitudes. Based on our previous observation [26], we expected to find a large discrepancy between the measured δ13C of photosynthates and that predicted by a photosynthetic 13C discrimination model, which indicates the involvement of soil DIC in leaf photosynthesis. We hypothesized that the contribution of soil DIC to leaf total photosynthesis was species-specific due to the different physiological responses (e.g., leaf gas exchange) to the environmental factors. Our third hypothesis is the altitude exerts an influence on plants’ use of soil DIC, given the differences in soil conditions and microclimate induced by altitude.
2. Materials and Methods
2.1. Study Site
The field experiments were conducted in August 2016 on Mt. Sanmao, Nanming District, Guiyang, southwest China (26°33′57″ N, 106°44′56″ E), with an elevation between 1080 and 1315 m above sea level. In this region, the annual mean precipitation and mean air temperature during 2011 and 2015 were 1129.5 mm and 15.3 °C, respectively. The mean minimum temperature of the coolest month (January) was 4.6 °C, whereas the mean maximum temperature of the warmest month (July) ranged from 25 to 28 °C. Rainfall in August was decreased compared with that in July. The study site was a typical karst landform covered with a secondary forest. Soils under the vegetation examined were alkaline (pH 7.09–7.75), rich in organic matter and bicarbonate, and have low soil water content according to our preliminary survey at the same site [34]. Moreover, these soil properties changed along an altitude gradient. The bedrock was mainly carbonatite, which developed shallow calcareous soil. The thickness of the soil layer varied between 27 and 58 cm. The vegetation community was mixed with evergreen and deciduous broad-leaved forest, with the canopy height in the range of 0.8–7.6 m.
2.2. Experimental Design
Six plots were established along three altitudes: S1 and S2 on the lower altitude, S3 and S4 on the medium altitude, and S5 and S6 on the higher altitude (Figure 1). Each plot had an area of 20 × 20 m2 and 60–80 m away from the neighboring plot at each altitude. Common species in each plot were recorded in Table 1. Collectively, four tree species (Ligustrum lucidum ait., Broussonetia papyrifera L., Platycarya longipes Wu., and Zelkova serrata (Thunb.) Makino) and five shrub species (Viburnum dilatatum Thunb., Ampelopsis delavayana Planch., Rosa cymosa Tratt., Zanthoxylum armatum DC., and Rubus biflorus Buch.-Ham. ex Smith) were selected for the subsequent measurement and sampling. Species in each plot included four replicates (individuals).
Figure 1.
Location of sampling sites (S, circles) near Nanming District, Guiyang, southwest China (adapted from Google Map).

Table 1.
Common species in plots of different altitudes. S1–S6 represented six plots established at three altitudes.
2.3. Leaf Gas-Exchange Measurements
Leaf gas exchange was measured in the morning (9:00–11:30 a.m.) of three sunny days (17–19 August). The net photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E), ratio of intercellular to the ambient partial pressure of CO2 (ci/ca), and instantaneous water use efficiency (WUEi) of the fully expanded leaves were measured using an infrared gas analyzer LI-6400 (Li-Cor, Lincoln, NE, USA), equipped with a clear topped 2 × 3 cm cuvette. The block temperature of the leaf chamber was set to be close to the air temperature. Near-ambient air at the height of 5 m above the ground was supplied to the leaf chamber at a rate of 500 μmol s−1 in the sample cell of LI-6400. Leaves were placed inside the cuvette for 2–3 min until steady state, and then the gas exchange parameters were manually logged. In addition, dark respiration of leaves was measured after 30 min of dark adaptation. Three leaves were measured per tree or shrub. At the end of the measurements, leaf samples were immediately collected and stored in a cooler box for further determination.
2.4. Air and Soil Sampling
Ambient air at the height of 5 m above the ground was sampled once in each plot during the measurement of leaf gas exchange. The air sample was pumped into gas sampling bags (Dalian Delin Gas Packing Co., Ltd., Dalian, China) for further analysis. Soil air was sampled in the root zone of each plant. Three days prior to our sampling, two holes of 10–15 cm depth with 2 cm diameter were drilled into the soil. Subsequently, two soft gas-piping tubes with filters were inserted into the holes, and then soil air was sucked out. Finally, the end of the tubes was clamped with clips. These processes allowed a long time for soil air to equilibrate between soil porosity and the internal space of tubes. At the day of sampling, the litter layer was removed, and then soil air was pumped into a gas sampling bag with a volume of 30 mL through one of the tubes, waiting for further determination. Another tube was connected to LI-6400, and, thus, the CO2 concentration of soil air was directly measured when it reached a peak value. Soil samples (approximately 150 g) were collected from the top layer of soil (0 to 20 cm) after removing the litter. They were immediately kept in sealed plastic bags and stored in a cooler box for further analysis.
2.5. Determination of Carbon Isotope Composition
The δ13C of water-soluble organic matter (WSOM) in leaves instead of bulk leaves was determined to represent the carbon isotope signals of photosynthates. Leaf WSOM has a high turnover rate [36,37,38] and mainly includes soluble carbohydrates, the hydrolysates of starch, and amino acids [39,40]. The extraction of WSOM referred to the protocols of [41]. Firstly, dried leaf material was ground, and 50 mg sample was put into a 2.5 mL centrifuge tube. After 1 mL of double-deionized water was added, the tube was agitated and then placed in a freezer for 1 h at 4 °C. Secondly, the samples were heated for 10 min at 95 °C, cooled to room temperature (RT), and centrifuged for 10 min at 12,000 g. Thirdly, an aliquot of 25 μL of the supernatant was transferred to a tin capsule and dried at 60 °C for 24 h. The δ13C of the WSOM was analyzed with an Isotope Ratio Mass Spectrometer (MAT-253, Thermo Fisher Scientific Inc., Waltham, MA, USA). Three leaf samples were measured per plant. The carbon isotopic ratio of the samples was calculated as δ13C (‰) = [(Rsample /Rstandard) − 1] × 1000, where Rsample and Rstandard are the 13C/12C ratio of the sample and Vienna Pee Dee Belemnite (VPDB), respectively. The standard deviation of repeated measurements for MAT253 was 0.1‰ for δ13C.
The δ13C of atmospheric and soil CO2 was determined by TG-IsoPrime (GV. Instruments Ltd., Manchester, UK). The method for soil DIC extraction was slightly modified from that of [42]. Briefly, 100 g of soil was placed into a 500 mL glass bottle and then mixed with 200 mL of CO2-free water. The mixture was homogeneously stirred for 5 min and then incubated at RT for 30 min. Subsequently, the supernatant was centrifuged for 15 min at 3500× g and then filtered through a 0.45 µm water-base membrane. The total alkalinity of the filtrate was detected by an Aquamerck alkalinity kit (MColortest, Merck KGaA, Darmstadt, Germany). At the same time, soil pH was measured using a pH meter (S210-B, Mettler Toledo Group Ltd., Zurich, Switzerland). The remaining filtrate was transferred to a 50 mL bottle and sealed with a rubber plug. The air inside the bottle was drawn by a vacuum pump. Subsequently, the filtrate of DIC was pretreated with an aliquot of 3 mL phosphoric acid to react with DIC and produce CO2. The generated CO2 was then processed with a custom-built vacuum extraction system, through which the CO2 was purified. The δ13C of the CO2 generated from soil DIC was then analyzed by MAT-252 (Finnigan, Bremen, Germany). The measurement precision for TG-IsoPrime and MAT252 were 0.1‰ and 0.01‰ for δ13C, respectively.
2.6. Quantification of the Utilization of Soil DIC by Plants
We accounted for two end members, namely atmospheric CO2 and soil DIC, that might contribute to the constitution of newly formed photosynthates as well as its δ13C [26]. The δ13C of photosynthates (δA) solely assimilated from atmospheric CO2 could be predicted with Equation (1) [43]:
where δa is the carbon isotope composition of atmospheric CO2, whereas Δ13Ccom is the comprehensive discrimination against 13C including the diffusion of CO2 across the boundary layer, intercellular air space, mesophyllcell, as well as the contributions of respiration and photorespiration. Δ13Ccom is calculated using Equation (2) [44]:
where ca, ci, and cc denote the CO2 partial pressure in the ambient air of the cuvette, intercellular air, and chloroplast, respectively; am is the summed 12C/13C fractionation during the dissolution of CO2 and liquid-phase diffusion (am = 1.8‰); b3′ is the 12C/13C fractionation of Rubisco (b3′ = 29‰, [45]); e is the 12C/13C fractionation for day respiration (e = 0, [46]); and f is the 12C/13C fractionation during photorespiration (f = 11‰, [47]); Rd, Vc, and F are nonphotorespiratory CO2 released in the dark, Rubisco carboxylation rate for the fixed atmospheric CO2, and photorespiratory rate, respectively; αb = 1 + b3′, αe = 1 + e, and αf = 1 + f; t and ā are the ternary correction factor and the weighed fractionation for CO2 diffusion across the boundary layer and stomata, respectively, which are calculated as [32]
where αac = 1 + ā, E is the transpiration rate; is the combined boundary layer and stomatal conductance to CO2; ab and as are the 12C/13C fractionation for CO2 diffusion across the boundary layer and through the stomata (ab = 2.9‰, as = 4.4‰), respectively; and cs is the CO2 partial pressure at the leaf surface. cc was not directly measured by LI-6400, but it was calculated with Equation (5) [37]:
where gi is an internal CO2 conductance. In this study, we assumed gi of 0.5 mol m−2 s−1 for woody species according to [48]. Vc and F are calculated as [44]
where
where * is the CO2 compensation point in the absence of Rd, which was fitted using Equation (8) [49]:
where c, ΔH, and R are the scaling constant (13.49), energy of activation (24.46 kJ K−1 mol−1), and molar gas constant (0.008314 kJ J−1 mol−1), respectively.
When accounting for the involvement of soil DIC in leaf photosynthesis, the carbon isotopic composition of photosynthate (δA′) originating from the assimilation of soil DIC is given by
where δDIC is the carbon isotope composition of soil DIC collected from root zones of each plant; Δ13CDIC is the photosynthetic discrimination against 13C combining the carboxylation of DIC and the effects of respiration and photorespiration, which is expressed as
where Vc′ is the rate of Rubisco carboxylation for DIC and given by
where ADIC_soil is the net photosynthetic rate for soil DIC. Empirically, ADIC is less than 10% of A in many species. Here, we assumed an initial value for ADIC_soil (ADIC_soil0) to be A/10. Although this assumption introduced some errors, it had little effect on the calculation of Δ13CDIC and the contribution of DIC to leaf total photosynthesis. Considering bicarbonate was the dominant species in soil DIC under high pH (7.09–7.75), δDIC in Equation (9) is derived from the δ13C of bicarbonate minus the discrimination related to the conversion between bicarbonate and CO2 (eb′, 9‰ at 25 °C).
However, studies showed that root-respired CO2 (C_R) can also dissolve in the xylem sap [50]. C_R is primarily reported to range from 0.01 to 10.98 mM and varies with species, height, season, etc. [51,52]. Therefore, we proposed a ratio of the concentration of soil CO2 or DIC (C_DIC) to C_R in the xylem, fs/x = C_DIC/(C_DIC + C_R). In the present study, we directly measured C_DIC and adopted the mean value of 5 mM for C_R. Thus, the δ13C of the mixed DIC in the xylem sap (δDIC_xylem) is calculated as
where δR is the carbon isotope composition of root-respired CO2 dissolved in the xylem sap. We assumed that the respiration of living tissues came from the recently fixed C, such as WSOM, which would carry the enriched isotopic signal to roots due to post-photosynthetic fractionation (−2‰, [53]) and then result in −1–−3‰ of respiratory fractionation in roots [54,55,56]. Here, we used the intermediate value −2‰ for respiratory fractionation; thus, δR is calculated as δR = δWSOM + 4‰ − am.
Considering that both atmospheric CO2 and soil DIC could be used for leaf photosynthesis, the mixture of photosynthates from two substrates determined the value of δWSOM, which is expressed as a two-end-member mixing model:
where fDIC_xylem is the proportion of xylem DIC contributing to δWSOM; δA′ is recalculated with Equation (9) but in which δDIC is replaced by δDIC_xylem. Finally, the contribution of soil DIC to the total leaf photosynthesis (fDIC_soil) is calculated as
2.7. Statistical Analysis
The comparison of mean values between species and altitudes was determined by one-way ANOVA with a t-test. Linear regression was used to investigate the relationship between fDIC_soil and δA-δWSOM. Pearson’s correlation was conducted to evaluate the relationships between physiological or environmental factors and fDIC_soil. All statistical analyses were performed using SPSS statistical software for Windows 21 (SPSS Inc., Chicago, IL, USA). The tests were considered significant at the p < 0.05 level. All data were expressed as the mean ± 1SE.
3. Results
3.1. Leaf Gas Exchange
All gas-exchange parameters exhibited significant differences within nine species (Table 2). However, no clear trend was observed between two life forms (tree and shrub). The maximum and minimum values of A were 18.24 ± 0.92 μmol m−2 s−1 in B. papyrifera and 9.81 ± 0.92 μmol m−2 s−1 in R. cymosa, respectively. The gs of nine species ranged from 0.14 to 0.28 mol m−2 s−1, suggesting mild-to-moderate drought stress. In contrast, there were small variations of E, ci/ca, and WUEi among nine species, with mean values of 3.91 mol H2O m−2 s−1, 0.67, and 3.27 μmol H2O mol−1, respectively.

Table 2.
Leaf gas exchange of nine species across six plots. A, net photosynthetic rate; gs, stomatal conductance; E, transpiration rate; ci/ca, ratio of intercellular to ambient partial pressure of CO2; WUEi, instantaneous water use efficiency. Values represent mean ± SE. N = 4 for each species in the specific plot. Capital letters indicate significant differences (p < 0.05) among species.
3.2. Characteristics of Soil DIC and CO2
The average concentrations of DIC (or C_DIC) in the rhizosphere of four tree species were similar (around 9.26 mM), whereas in five shrub species, they exhibited large variations, ranging from 8.10 mM in R. cymosa to 10.50 mM in Z. armatum (Figure 2A). In contrast, the mean concentrations of soil CO2 varied from 3919.91 ppm in P. longipes to 5279.83 ppm in B. papyrifera, higher than the mean value of 3454.42 ppm in all shrub species. According to Henry’s law, the partial pressure of CO2 over a solution was proportional to the concentration of the CO2 in the solution. The calculated quantity of CO2 dissolved in soil water ([CO2*]) was in the range of 0.55 to 0.92 mM at pH 7 and 25 °C across nine species, much lower than the mean value of [CO2*] in xylem sap (or C_R) investigated in many species. Thus, it was unlikely that soil CO2 could be fixed by plant roots due to a CO2 gradient from roots to the soil. In addition, the interspecies differences in the δ13C of soil DIC and CO2 were both less than 2‰ (Figure 2B). The mean values of soil DIC and CO2 among nine species were −10.19‰ and −20.01%, respectively.
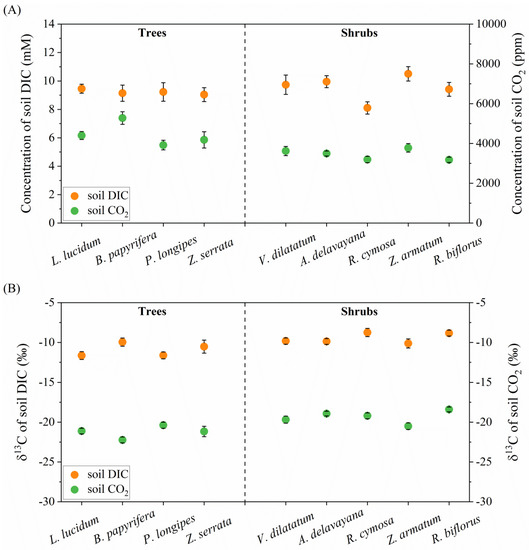
Figure 2.
Concentrations (A) and δ13C (B) of soil DIC and CO2 in the root zones of nine species across three altitudes. N = 4 for each species in the specific plot.
3.3. δ13C of Photosynthates
The δ13C of the newly formed photosynthates in leaves could be determined by measuring the isotope signals of WSOM, or predicted with Eqn. 1 that only accounted for the assimilation of atmospheric CO2 (Figure 3A). δWSOM varied all among species by about 3–4‰ and was generally lower than δA in all species. The discrepancy between δA and δWSOM (δA-δWSOM) was species-specific. Figure 3B displayed the distribution of δA-δWSOM in leaves of nine species under varying altitudes. The highest frequency (approximate 30%) of δA-δWSOM was located in the range of 1 to 2‰. Furthermore, half of the δA-δWSOM was distributed between 2‰ and 6‰. This phenomenon clearly illustrated the fact that there were other carbon sources, for example, soil DIC, which participated in leaf photosynthesis. The photosynthates originating from the mixture of soil DIC and xylem [CO2*] carried more 13C-depleted signal (δA′, see Supplementary Data), thus resulting in δWSOM systematically lower than δA.
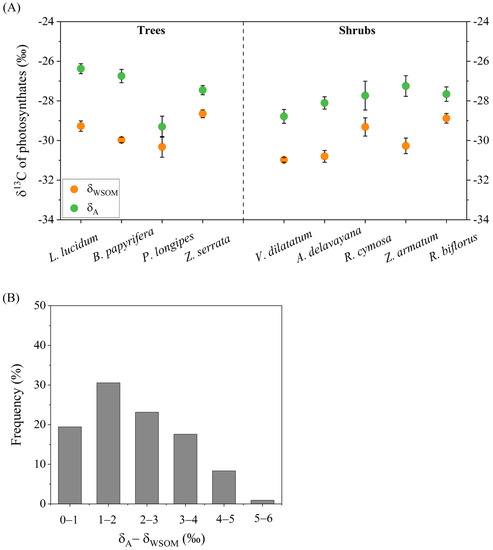
Figure 3.
δ13C of photosynthates determined by measuring isotope signals of leaf WSOM (orange closed circle) or predicted with Equation (1) that only accounted for assimilation of atmospheric CO2 (green closed circle) of nine species across three altitudes (A); frequency distribution of the discrepancy between δA and δWSOM (B).
3.4. Interspecies Difference in fDIC_soil
The potential contribution of soil DIC to leaf total photosynthesis (fDIC_soil) considerably varied with species across the six plots (Figure 4). In tree species, the average values of fDIC_soil in L. lucidum and B. papyrifera were 8.93% and 9.54%, respectively, significantly higher than those in P. longipes and Z. serrata (both less than 2.2%). By contrast, the mean values of fDIC_soi within the shrub species varied from 2.48% to 9.99%, with the highest value in Z. armatum and the lowest value in R. biflorus. Furthermore, fDIC_soil strongly correlated with δA-δWSOM (p < 0.001), with the coefficient of determination (R2) as 0.99. For the nine species, a higher value of δA-δWSOM generally indicated a higher value of fDIC_soil (Figure 5). This tight relation further demonstrated the plants’ use of soil DIC in the karst habitats. Additionally, fDIC_soi significantly correlated with A, ci/ca, and WUEi (Table 3).
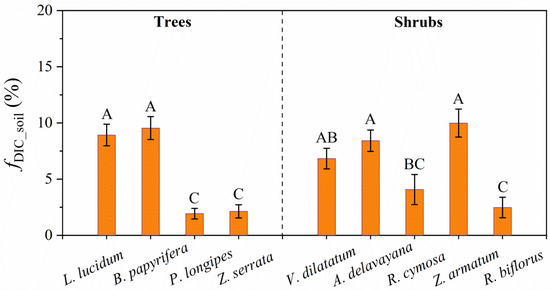
Figure 4.
Contribution of soil DIC to leaf total photosynthesis (fDIC_soil) in nine species across three altitudes. N = 4 for each species in the specific plot. Capital letters on the error bar indicate significant differences (p < 0.05) among species.
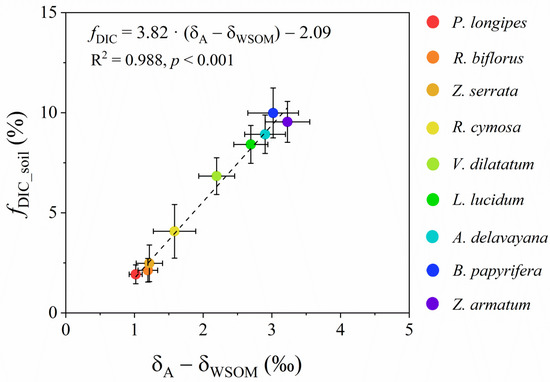
Figure 5.
Relationship between (δA-δWSOM) and fDIC_soil among nine species across three altitudes. N = 4 for each species in the specific plot.

Table 3.
Relationships between physiological or environmental factors and fDIC_soil analyzed by Pearson’s correlation. N = 108.
3.5. Variations in fDIC_soil at Different Altitudes
The impact of altitude on fDIC_soil was investigated in one tree (L. lucidum) and two shrub species (V. dilatatum and A. delavayana), which all appeared at three altitudes (Table 1; Figure 6). In L. lucidum and A. delavayana, fDIC_soil tended to increase from lower to higher altitude (p < 0.05), whereas in V. dilatatum, the pattern was opposite.
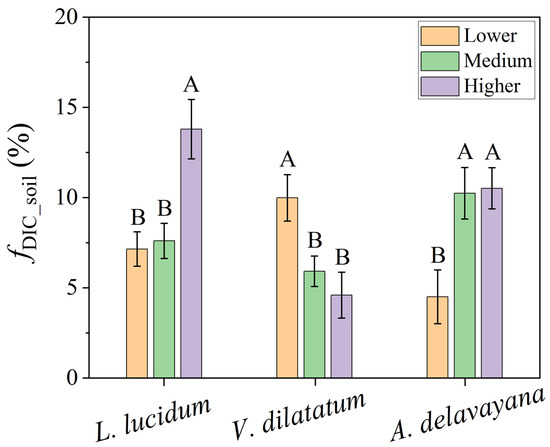
Figure 6.
fDIC_soil of one tree species (L. lucidum) and two shrub species (V. dilatatum and A. delavayana) at different altitudes. N = 4 for each species in the specific plot. Capital letters on the error bar indicate significant differences (p < 0.05) among altitudes.
4. Discussion
4.1. Isotope Evidence for the Utilization of Soil DIC by Karst Plants
Two issues needed to be addressed in field trials: (1) whether the natural abundance of 13C signals of soil DIC could be detected in leaves, and (2) what the major carbon source supplying the plant roots was. A few studies showed that even if the plants were irrigated with 13CO2 or H13CO3− solution, the C isotope signals were too weak to be detected [20,57]. This is probably due to a low concentration of the labeled species dissolved in the solution (usually less than 1 mM), resulting in less 13CO2 or H13CO3− taken up by roots as the diffusion against the concentration gradient from roots to the soil was minimal [15]. However, in the karst habitats, the high concentration of soil DIC made it possible to be fixed by plants. In addition, labeling experiments usually have a single type of carbon source [16,17,20], whereas, in field conditions, the major form of carbon source depends on soil pH, humidity, temperature, root respiration, microbial activity, etc. [58,59]. For this reason, we measured both the concentrations of soil DIC and CO2 (Figure 2). The result suggested that soil DIC rather than soil CO2 was the main source supplying the roots. However, direct evidence is needed to support this idea, especially in field conditions. Then we examined whether soil DIC could affect the δWSOM. The experimental data confirmed our speculation that δWSOM largely deviated from the predicted δ13C of photosynthates (δA) solely assimilated from atmospheric CO2 across nine species (Figure 3), consistent with our previous finding [26]. As the values of δA-δWSOM were all positive and half of them were larger than 2‰ (Figure 3B and Figure 5), the isotope measurement error (less than 0.1‰) and diurnal variations in δWSOM (less than 1‰) [60] are not sufficient to explain the discrepancy. When combined with the very negative 13C signal of soil DIC (δA′, see Supplementary Data), this large and positive deviation of δA from δWSOM suggests the participation of soil DIC in leaf photosynthesis. Therefore, both atmospheric CO2 and soil DIC contributed to the newly assimilated carbon (i.e., leaf WSOM). The contribution of soil DIC (fDIC_soil) to leaf total photosynthesis could be quantified with a two-end-member mixing model (Equation (13)). We further showed that δA-δWSOM was closely linked to fDIC_soil among all species (Figure 5). That is, the more the soil DIC was involved in photosynthesis, the more δA deviated from δWSOM, and the larger the proportion of soil DIC contributed to leaf WSOM.
However, uncertainties remain concerning the estimation of fDIC_soil. For example, we considered the tissues-respired CO2 dissolved in the xylem sap in the calculation of fDIC_soil. Although we assigned a mean value of 5 mM for the C_R, species might differ in C_R due to their different physiological statues and anatomical characteristics [15,52,61]. We also assumed that the source of respired CO2 came from the newly assimilated carbon and used the value of δWSOM to infer the δ13C of CO2 respired by living tissues (δR). Nevertheless, recent studies [62,63,64,65] showed that vegetation pools respired carbon of a wide range of ages in many species. For instance, the respired carbon in stems and roots was, on average, older than 1 year [65,66]. When plants were exposed to stress, respiration almost completely relied on the old carbon [64]. Therefore, in this study, there was a high probability that the investigated species used reserved carbon for respiration under the condition of frequent water limitation in karst habitats. To the best of our knowledge, the δ13C of reserved carbon has a close relationship with that of respired CO2 [56]. Our unpublished data showed that the maximum range of δ13C in the storage carbon of some karst-adaptable species was 4.13‰ during the growing season, which implied a similar or less variation in the range of δ13C for respired CO2 in stems and roots. Some studies also showed that seasonal or annual variability of δ13C in respired CO2 in stems and roots ranged within 2.0–4.5‰ [67,68,69]. In this study, taking L. lucidum for example, a change of 4.5‰ in δ13C of leaf WSOM only led to 0.5% of the change in fDIC_soil, indicating a limited influence of the source of carbon used for respiration on the quantification of plants’ utilization of soil DIC.
4.2. Interspecies Difference in Plants’ Use of Soil DIC
An assessment of fDIC_soil between two plant life forms, namely trees and shrubs, showed no clear trend (Figure 4), indicating that the variation of fDIC_soil was mainly resulted from the interspecies differences. In most species, the values of fDIC_soil were comparable with those reported in the laboratory experiments [24,25,26], suggesting that soil DIC was easily accessible to plants in the karst habitats. In this study, nine species were all native, most of which were deciduous trees or shrubs. Although species differed in height, biomass, ages, etc., our measurements and sampling were all conducted with newly expanded leaves and within the root zones of the same soil layer. As leaves and roots control the uptake of atmospheric CO2 and soil DIC, respectively, the species-specific variation in fDIC_soil might be related to leaf gas-exchange traits (Table 2) and soil conditions (Figure 2).
fDIC_soil positively correlates with A (p = 0.003) and WUEi (p < 0.001) but negatively with ci/ca (p < 0.001). At first sight, it was surprising that fDIC_soil increased with an increase in A, which was inconsistent with the results reported by our previous study [26]. For instance, when plants were confronted with moderate or severe water limitation, it would drastically reduce A and gs [7,29] and thus increased the proportion of root or soil-derived DIC to support photosynthesis [26,29]. The fact was that the change of A was not proportional to that of ADIC_soil or E [26], thus arithmetically promoting the value of fDIC_soil. In this study, the level of gs indicated mild drought stress in many species (Table 2). Among these species, higher A corresponded to higher gs and E, which benefited the long-distance transport of DIC in the xylem sap [70,71]. Similarly, higher WUEi also implied a higher contribution of soil DIC to leaf photosynthesis, although the correlation between E and fDIC_soil was not significant (Table 3). The negative correlation between fDIC_soil and ci/ca corresponded to the reverse change of fDIC_soil and the contribution of atmospheric CO2 to leaf total photosynthesis (1-fDIC_soil) because higher ci/ca usually suggested higher Δ13Ccom (Equation (2)) and lower A and E [32]. In addition, there was no significant correlation between fDIC_soil, C_DIC, and δDIC. This could be explained by less variation of these indicators in comparison with that of leaf gas-exchange parameters among species (Figure 2; Table 2).
4.3. Effect of Altitude on Plants’ Use of DIC
As the altitude increases, the number of tree species reduces and that of shrub species increases (Table 1), suggesting a change in the plant community. In this study, only three species presented in all plots and thus were available for evaluating the effect of altitude on fDIC_soil (Figure 6). Among these species, fDIC_soil of L. lucidum and A. delavayana increased from the lower to higher altitude, whereas in V. dilatatum, fDIC_soil decreased along the altitudes. The impact of altitude on fDIC_soil was a little bit confusing. However, altitude did not directly affect fDIC_soil, but through imposing influences on site-characteristic related microclimate and soil conditions [35,72]. For example, the altitude could constrain daily mean temperature and vapor pressure deficit [72], and produce considerable variations in soil moisture and nutrient availability [35]. However, in the present study, the air temperature and relative humidity recorded by LI-6400 did not linearly change with altitude (see Supplementary Data) but exhibited a high degree of spatial heterogeneity. The reasons might be that the altitude gradient was not large enough, and some occasional factors, e.g., wind, shading, and vegetation coverage, could redistribute these resources, e.g., light and vapor [72,73]. Our previous study reported decreasing patterns for soil water content, organic matter, and some nutrients with the altitudes in the same area and same season of 2015 [34]. However, the increasing trend of fDIC_soil along the altitude gradient in L. lucidum and A. delavayana was opposite to that in V. dilatatum (Figure 6), implying that the differences in soil conditions could not solely explain the variation of fDIC_soil. Therefore, we speculated that all these variabilities were combined to influence the leaf gas-exchange and subsequent estimation of fDIC_soil. Future studies may choose a large range of environmental gradients and exclude the influences of some occasional factors.
5. Conclusions
In the present study, we aimed to investigate whether soil DIC could be assimilated through leaf photosynthesis in the karst habitats, and whether this process, if taken place, varied between species and altitudes. Firstly, we showed that all plots in the study sites had a high content of soil DIC in the root zones of nine species. This implied a high possibility that soil DIC could be fixed by karst plants through photosynthesis. Secondly, we observed that there were large discrepancies between the measured and predicted δ13C of newly formed photosynthates (δA-δWSOM), and this systematic difference could not be explained by measurement errors or diurnal variations in δWSOM alone. Therefore, we accounted for the involvement of soil DIC in photosynthesis and thus deviating δWSOM from δA. Thirdly, we applied a two-end-member mixing model to estimate the contribution of soil DIC to leaf total photosynthesis (fDIC_soil). The values of fDIC_soil largely differed among species and some of which were influenced by altitudes. The present study improved our understanding of how plants adapted to alkaline soil conditions of karst habitats and acquired additional carbon for growth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102489/s1.
Author Contributions
Conceptualization, S.R. and Y.W.; experimentation, S.R.; writing, S.R.; data analysis, S.R. and Y.W.; review and editing, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Support Plan Projects of Science and Technology of Guizhou Province (No. (2021)YB453) and the National Key Research and Development Program of China (No. 2021YFD1100300).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this article can be found in the online version.
Acknowledgments
We deeply thank W. Lin, two anonymous reviewers for their valuable comments and suggestions that greatly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bonacci, O.; Pipan, T.; Culver, D.C. A framework for karst ecohydrology. Environ. Geol. 2009, 56, 891–900. [Google Scholar] [CrossRef]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
- Yuan, D.X. On the karst ecosystem. Acta Geol. Sin.-Engl. Ed. 2001, 75, 336–338. [Google Scholar]
- Wang, S.; Liu, Q.; Zhang, D. Karst rocky desertification in southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, D.; Tong, L.; Azim, M.; Yang, H.; Huang, F. An overview of karst ecosystem in southwest china: Current state and future management. J. Resour. Ecol. 2015, 6, 247–256. [Google Scholar]
- Yu, F.; Huang, X.; Liang, Q.; Yao, P.; Li, X.; Liao, Z.; Duan, C.; Zhang, A.; Shao, H. Ecological water demand of regional vegetation: The example of the 2010 severe drought in Southwest China. Plant Biosyst. 2015, 149, 100–110. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Comparative ecophysiological responses to drought of two shrub and four tree species from karst habitats of southwestern China. Trees 2011, 25, 537–549. [Google Scholar] [CrossRef]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Rowland, L.; da Costa, A.C.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef]
- Msilini, N.; Attia, H.; Bouraoui, N.; M’rah, S.; Ksouri, R.; Lachaâl, M.; Ouerghi, Z. Responses of Arabidopsis thaliana to bicarbonate-induced iron defciency. Acta Physiol. Plant. 2009, 31, 849–853. [Google Scholar] [CrossRef]
- Du, Y.; Pan, G.; Li, L.; Hu, Z.; Wang, X. Leaf N/P ratio and nutrient reuse between dominant species and stands: Predicting phosphorus deficiencies in karst ecosystems, southwestern China. Environ. Earth Sci. 2011, 64, 299–309. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; Wang, H.; Peng, J.; Meersmans, J.; Green, S.; Quine, T.A.; Wu, X.; Song, Z. Bedrock geochemistry influences vegetation growth by regulating the regolith water holding capacity. Nat. Commun. 2020, 11, 2392. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Y.; Xing, D.; Hang, H.; Xie, X.; Yang, X.; Zhang, K.; Rao, S. Biomass production of three biofuel energy plants’ use of a new carbon resource by carbonic anhydrase in simulated karst soils: Mechanism and capacity. Energies 2017, 10, 1370. [Google Scholar] [CrossRef]
- Wu, Y.; Hong, W.; Chen, Y. Leaf physiological and anatomical characteristics of two indicator species in the limestone region of southern China under drought stress. Pak. J. Bot. 2018, 50, 1335–1342. [Google Scholar]
- Bloemen, J.; Teskey, R.O.; McGuire, M.A.; Aubrey, D.P.; Steppe, K. Root xylem CO2 flux: An important but unaccounted-for component of root respiration. Trees 2016, 30, 343–352. [Google Scholar] [CrossRef]
- Shimono, H.; Kondo, M.; Evans, J.R. Internal transport of CO2 from the root-zone to plant shoot is pH dependent. Physiol. Plant. 2019, 165, 451–463. [Google Scholar] [CrossRef]
- Tarvainen, L.; Wallin, G.; Linder, S.; Nasholm, T.; Oren, R.; Löfvenius, M.O.; Rantfors, M.; Tor-Ngern, P.; Marshall, J.D. Limited vertical CO2 transport in stems of mature boreal Pinus sylvestris trees. Tree Physiol. 2020, 41, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.L.; De Roo, L.; Bodé, S.; Boeckx, P.; Steppe, K. Efflux and assimilation of xylem-transported CO2 in stems and leaves of tree species with different wood anatomy. Plant Cell Environ. 2021, 44, 3494–3508. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, D.P.; Teskey, R.O. Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol. 2009, 184, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.R.; Wurzburger, N.; Hendrick, R.L.; Teskey, R.O. Soil DIC uptake and fixation in Pinus taeda seedlings and its C contribution to plant tissues and ectomycorrhizal fungi. Tree Physiol. 2007, 27, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Wu, Y.; Wang, R. Bicarbonate stimulates non-structural carbohydrate pools of Camptotheca acuminata. Physiol. Plant. 2019, 165, 780–789. [Google Scholar] [CrossRef]
- Liang, F.; Yang, W.; Xu, L.; Ji, L.; He, Q.; Wu, L.; Ran, Y.; Yan, S. Closing extra CO2 into plants for simultaneous CO2 fixation, drought stress alleviation and nutrient absorption enhancement. J. CO2 Util. 2020, 42, 101319. [Google Scholar] [CrossRef]
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2020, 101, 1001–1015. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, D. Effect of bicarbonate treatment on photosynthetic assimilation of inorganic carbon in two plant species of Moraceae. Photosynthetica 2012, 50, 587–594. [Google Scholar] [CrossRef]
- Hang, H.; Wu, Y. Quantification of photosynthetic inorganic carbon utilisation via a bidirectional stable carbon isotope tracer. Acta Geochim. 2016, 35, 130–137. [Google Scholar] [CrossRef]
- Rao, S.; Wu, Y. Root-derived bicarbonate assimilation in response to variable water deficit in Camptotheca acuminata seedlings. Photosynth. Res. 2017, 134, 59–70. [Google Scholar] [CrossRef]
- Valipour, M.; Khoshgoftarmanesh, A.H.; Baninasab, B. Physiological responses of hawthorn (Crataegus persica Pojark.) and quince (Cydonia oblonga Mill.) rootstocks to bicarbonate-induced iron deficiency in nutrient solution. J. Plant Nutr. Soil Sci. 2018, 181, 905–913. [Google Scholar] [CrossRef]
- Han, X.; Jing, Y.; Xu, C.; Gao, L.; Li, M.; Liu, Y.; Qi, H. Root-zone CO2 concentration affects partitioning and assimilation of carbon in oriental melon seedlings. Int. J. Mol. Sci. 2022, 23, 10694. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y. The increase in the karstification–photosynthesis coupled carbon sink and its implication for carbon neutrality. Agronomy 2022, 12, 2147. [Google Scholar] [CrossRef]
- Göttlicher, S.; Knohl, A.; Wanek, W.; Buchmann, N.; Richter, A. Short-term changes in carbon isotope composition of soluble carbohydrates and starch: From canopy leaves to the root system. Rapid Commun. Mass Spectrom. 2006, 20, 653–660. [Google Scholar] [CrossRef]
- Yang, Y.; Siegwolf, R.T.; Körner, C. Species specific and environment induced variation of δ13C and δ15N in alpine plants. Front. Plant Sci. 2015, 6, 423. [Google Scholar] [CrossRef][Green Version]
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.; Marshall, J.D.; Farquhar, G.D. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef]
- Marshall, J.D.; Dawson, T.E.; Ehleringer, J.R. Integrated nitrogen, carbon, and water relations of a xylem-tapping mistletoe following nitrogen fertilization of the host. Oecologia 1994, 100, 430–438. [Google Scholar] [CrossRef]
- Rao, S.; Wu, Y. Comparison of physiological and morphological traits of Platycarya longipes in different slope locations in karst area. Earth Environ. 2017, 45, 10–17. [Google Scholar]
- Zhang, Z.H.; Hu, G.; Ni, J. Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, southwestern China. J. Mt. Sci. 2013, 10, 95–104. [Google Scholar] [CrossRef]
- Brandes, E.; Kodama, N.; Whittaker, K.; Weston, C.; Rennenberg, H.; Keitel, C.; Adams, M.A.; Gessler, A. Short-term variation in the isotopic composition of organic matter allocated from the leaves to the stem of Pinus sylvestris: Effects of photosynthetic and postphotosynthetic carbon isotope fractionation. Glob. Chang. Biol. 2006, 12, 1922–1939. [Google Scholar] [CrossRef]
- Gessler, A.; Tcherkez, G.; Peuke, A.D.; Ghashghaie, J.; Farquhar, G.D. Experimental evidence for diel variations of the carbon isotope composition in leaf, stem and phloem sap organic matter in Ricinus communis. Plant Cell Environ. 2008, 31, 941–953. [Google Scholar] [CrossRef]
- Ghashghaie, J.; Badeck, F.W.; Girardin, C.; Sketriené, D.; Lamothe-Sibold, M.; Werner, R.A. Changes in δ13C of dark respired CO2 and organic matter of different organs during early ontogeny in peanut plants. Isot. Environ. Health Stud. 2015, 51, 93–108. [Google Scholar] [CrossRef]
- Yousfi, S.; Serret, M.D.; Araus, J.L. Comparative response of δ13C, δ18O and δ15N in durum wheat exposed to salinity at the vegetative and reproductive stages. Plant Cell Environ. 2013, 36, 1214–1227. [Google Scholar] [CrossRef]
- Studer, M.S.; Siegwolf, R.T.; Leuenberger, M.; Abiven, S. Multi-isotope labelling of organic matter by diffusion of 2H/18OH2O vapour and 13C-CO2 into the leaves and its distribution within the plant. Biogeosciences 2015, 12, 1865–1879. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Offermann, C.A.; Gessler, A.; Winkler, J.B.; Ferrio, J.P.; Buchmann, N.; Barnard, R.L. Drought effects on allocation of recent carbon: From beech leaves to soil CO2 efflux. New Phytol. 2009, 184, 950–961. [Google Scholar] [CrossRef]
- Zuo, Y.; Ren, L.; Zhang, F.; Jiang, R. Bicarbonate concentration as affected by soil water content controls iron nutrition of peanut plants in a calcareous soil. Plant Physiol. Biochem. 2007, 45, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon-dioxide concentration in leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Ubierna, N.; Holloway-Phillips, M.M.; Farquhar, G.D. Using stable carbon isotopes to study C3 and C4 photosynthesis: Models and calculations. In Photosynthesis; Humana Press: New York, NY, USA, 2018; pp. 155–196. [Google Scholar]
- Evans, J.R.; von Caemmerer, S. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ. 2013, 36, 745–756. [Google Scholar] [CrossRef]
- Ubierna, N.; Cernusak, L.A.; Holloway-Phillips, M.M.; Busch, F.A.; Cousins, A.B.; Farquhar, G.D. Critical review: Incorporating the arrangement of mitochondria and chloroplasts into models of photosynthesis and carbon isotope discrimination. Photosynth. Res. 2019, 141, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G. How large is the carbon isotope fractionation of the photorespiratory enzyme glycine decarboxylase? Funct. Plant Biol. 2006, 33, 911–920. [Google Scholar] [CrossRef]
- Gillon, J.; Yakir, D. Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2. Science 2001, 291, 2584–2587. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef]
- Teskey, R.O.; McGuire, M.A. Measurement of stem respiration of sycamore (Platanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ. 2007, 30, 570–579. [Google Scholar] [CrossRef]
- Levy, P.E.; Meir, P.; Allen, S.J.; Jarvis, P.G. The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol. 1999, 19, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef]
- Bowling, D.R.; Pataki, D.E.; Randerson, J.T. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 2008, 178, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Keitel, C.; Kodama, N.; Weston, C.; Winters, A.J.; Keith, H.; Grice, K.; Leuning, R.; Farquhar, G.D. δ13C of organic matter transported from the leaves to the roots in Eucalyptus delegatensis: Short-term variations and relation to respired CO2. Funct. Plant Biol. 2007, 34, 692–706. [Google Scholar] [CrossRef]
- Kodama, N.; Barnard, R.L.; Salmon, Y.; Weston, C.; Ferrio, J.P.; Holst, J.; Werner, R.A.; Saurer, M.; Rennenberg, H.; Buchmann, N.; et al. Temporal dynamics of the carbon isotope composition in a Pinus sylvestris stand: From newly assimilated organic carbon to respired carbon dioxide. Oecologia 2008, 156, 737–750. [Google Scholar] [CrossRef]
- Bathellier, C.; Badeck, F.W.; Ghashghaie, J. Carbon isotope fractionation in plant respiration. In Plant Respiration: Metabolic Fluxes and Carbon Balance; Springer: Cham, Switzerland, 2017; pp. 43–68. [Google Scholar]
- Ubierna, N.; Kumar, A.S.; Cernusak, L.A.; Pangle, R.E.; Gag, P.J.; Marshall, J.D. Storage and transpiration have negligible effects on δ13C of stem CO2 efflux in large conifer trees. Tree Physiol. 2009, 29, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, A.; Malakouti, M.J.; Fallahi, E. Effects of bicarbonate content of irrigation water on nutritional disorders of some apple varieties. J. Plant Nutr. 2005, 28, 1663–1678. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barceló, J. Transport and use of bicarbonate in plants: Current knowledge and challenges ahead. Int. J. Mol. Sci. 2018, 19, 1352. [Google Scholar] [CrossRef] [PubMed]
- Dubbert, M.; Rascher, K.G.; Werner, C. Species-specific differences in temporal and spatial variation in δ13C of plant carbon pools and dark-respired CO2 under changing environmental conditions. Photosynth. Res. 2012, 113, 297–309. [Google Scholar] [CrossRef]
- Werner, C.; Gessler, A. Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: A review of dynamics and mechanisms. Biogeosciences 2011, 8, 2437–2459. [Google Scholar] [CrossRef]
- Sierra, C.A.; Ceballos-Núñez, V.; Hartmann, H.; Herrera-Ramírez, D.; Metzler, H. Ideas and perspectives: Allocation of carbon from net primary production in models is inconsistent with observations of the age of respired carbon. Biogeosciences 2022, 19, 3727–3738. [Google Scholar] [CrossRef]
- Gao, D.; Joseph, J.; Werner, R.A.; Brunner, I.; Zürcher, A.; Hug, C.; Wang, A.; Zhao, C.; Bai, E.; Meusburger, K.; et al. Drought alters the carbon footprint of trees in soils—Tracking the spatio-temporal fate of 13C-labelled assimilates in the soil of an old-growth pine forest. Glob. Chang. Biol. 2021, 27, 2491–2506. [Google Scholar] [CrossRef]
- Huang, J.; Hammerbacher, A.; Gershenzon, J.; van Dam, N.M.; Sala, A.; McDowell, N.G.; Chowdhury, S.; Gleixner, G.; Trumbore, S.; Hartmann, H. Storage of carbon reserves in spruce trees is prioritized over growth in the face of carbon limitation. Proc. Natl. Acad. Sci. USA 2021, 118, e2023297118. [Google Scholar] [CrossRef]
- Hilman, B.; Muhr, J.; Helm, J.; Kuhlmann, I.; Schulze, E.D.; Trumbore, S. The size and the age of the metabolically active carbon in tree roots. Plant Cell Environ. 2021, 44, 2522–2535. [Google Scholar] [CrossRef]
- Muhr, J.; Trumbore, S.; Higuchi, N.; Kunert, N. Living on borrowed time–Amazonian trees use decade-old storage carbon to survive for months after complete stem girdling. New Phytol. 2018, 220, 111–120. [Google Scholar] [CrossRef]
- Brændholt, A.; Ibrom, A.; Ambus, P.; Larsen, K.S.; Pilegaard, K. Combining a quantum cascade laser spectrometer with an automated closed-chamber system for δ13C measurements of forest soil, tree stem and tree root CO2 fluxes. Forests 2019, 10, 432. [Google Scholar] [CrossRef]
- Kuptz, D.; Fleischmann, F.; Matyssek, R.; Grams, T.E. Seasonal patterns of carbon allocation to respiratory pools in 60-yr-old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytol. 2011, 191, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Wang, A.; Yuan, F.; Guan, D.; Dai, G.; Wu, J. Environmental effects on carbon isotope discrimination from assimilation to respiration in a coniferous and broad-leaved mixed forest of Northeast China. Forests 2020, 11, 1156. [Google Scholar] [CrossRef]
- Bloemen, J.; McGuire, M.A.; Aubrey, D.P.; Teskey, R.O.; Steppe, K. Assimilation of xylem-transported CO2 is dependent on transpiration rate but is small relative to atmospheric fixation. J. Exp. Bot. 2013, 64, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Stutz, S.S.; Hanson, D.T. Contribution and consequences of xylem-transported CO2 assimilation for C3 plants. New Phytol. 2019, 223, 1230–1240. [Google Scholar] [CrossRef]
- Jucker, T.; Hardwick, S.R.; Both, S.; Elias, D.M.; Ewers, R.M.; Milodowski, D.T.; Swinfield, T.; Coomes, D.A. Canopy structure and topography jointly constrain the microclimate of human-modified tropical landscapes. Glob. Chang. Biol. 2018, 24, 5243–5258. [Google Scholar] [CrossRef]
- Burgess, A.J.; Retkute, R.; Preston, S.P.; Jensen, O.E.; Pound, M.P.; Pridmore, T.P.; Murchie, E.H. The 4-dimensional plant: Effects of wind-induced canopy movement on light fluctuations and photosynthesis. Front. Plant Sci. 2016, 7, 1392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).