Assessing the Effect of Irrigation Using Different Water Resources on Characteristics of Mild Cadmium-Contaminated Soil and Tomato Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Sample Collection
2.3.2. Soil Physicochemical Analysis
2.3.3. Plant Analysis
2.3.4. DNA Extraction
2.3.5. PCR Amplification
2.3.6. Library Construction and MiSeq Sequencing
2.4. Data Analysis
3. Results
3.1. Changes in Soil Physicochemical Properties under Drip Irrigation with Different Water Sources
3.2. Effect of Different Treatments on Yield and Quality of Tomatoes
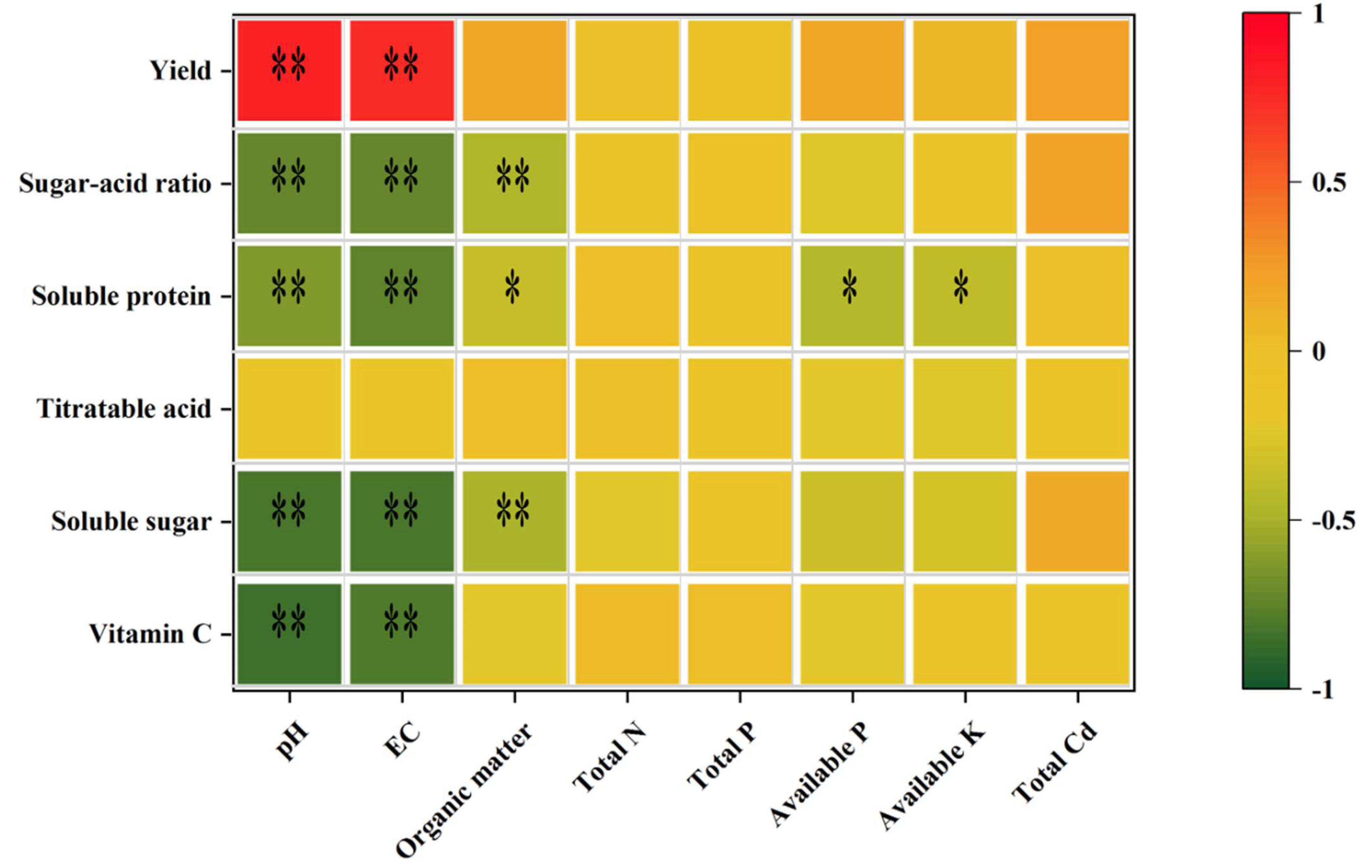
3.3. Interrelationship between Soil Physicochemical Properties and Tomato Quality Indicators after Irrigation
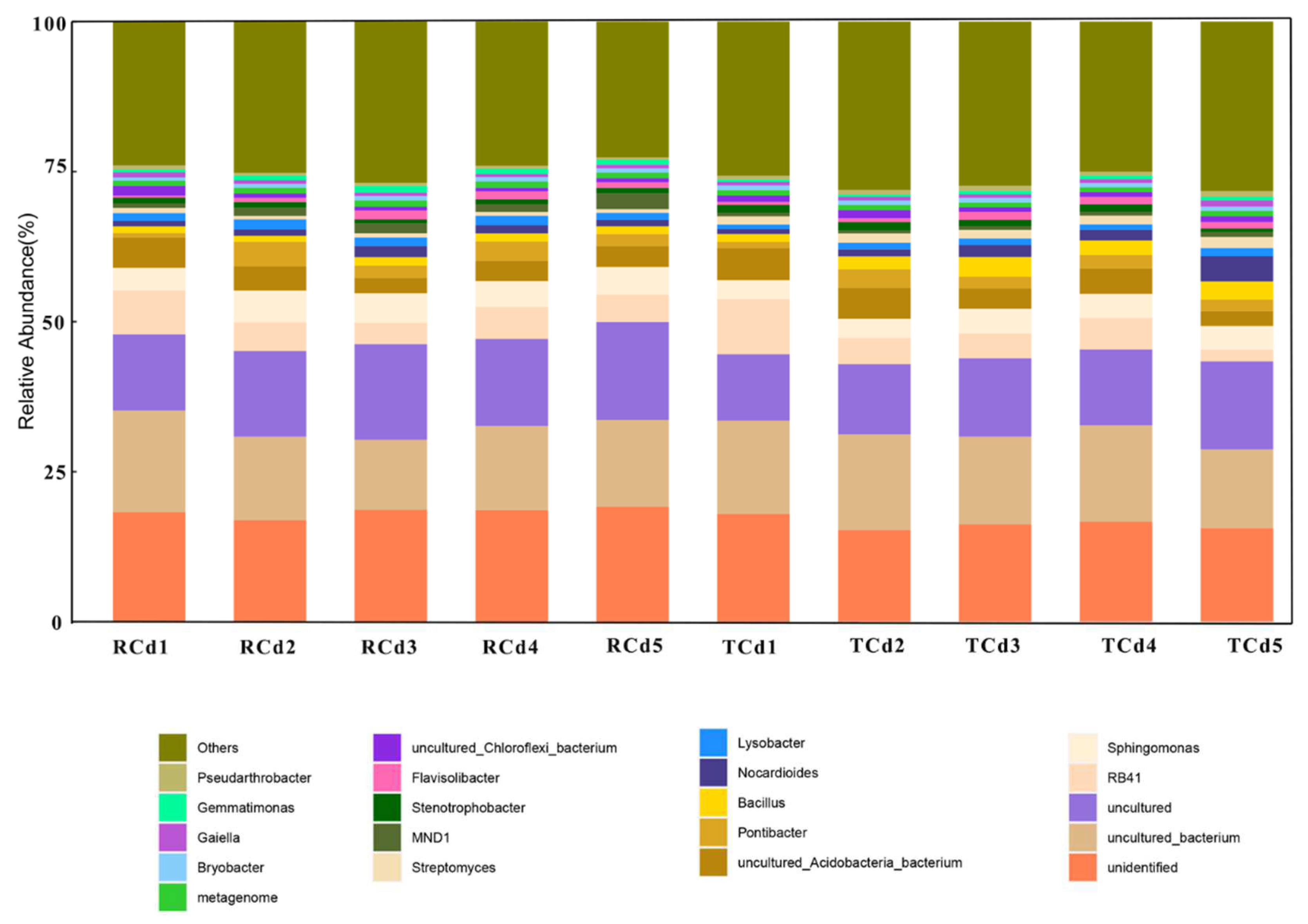
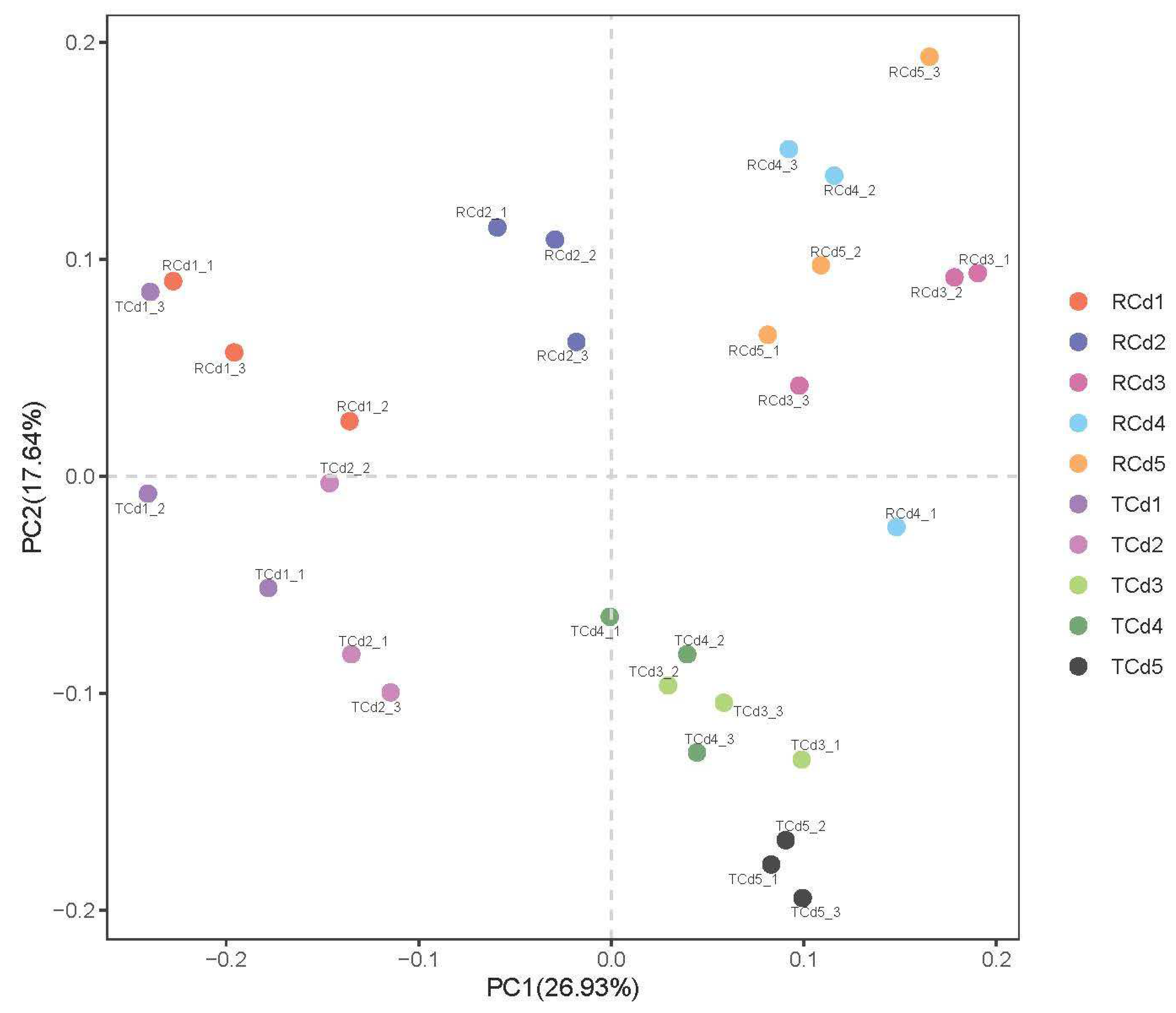
3.4. Analysis of the Diversity and Structure of the Soil Bacterial Community
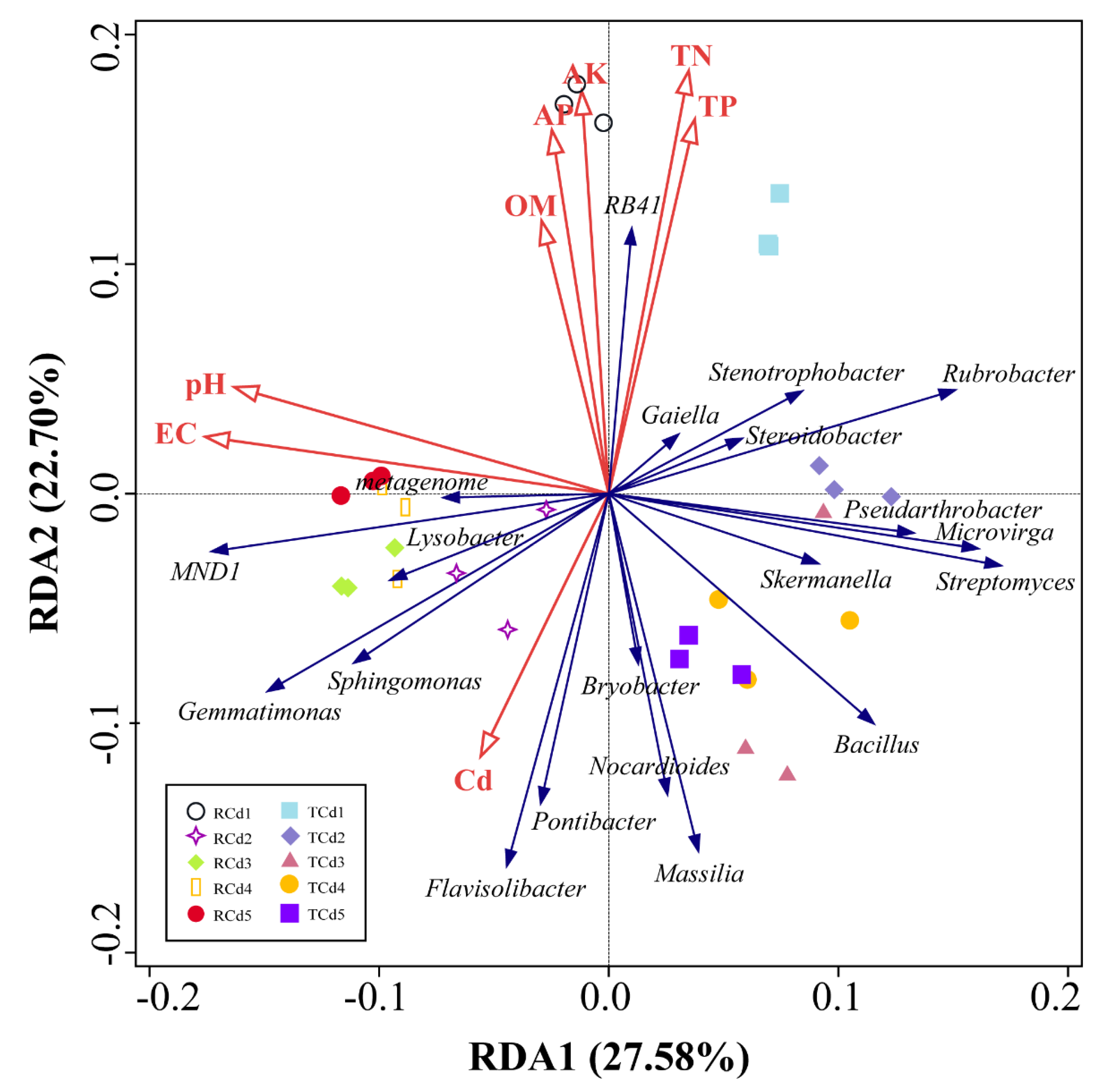
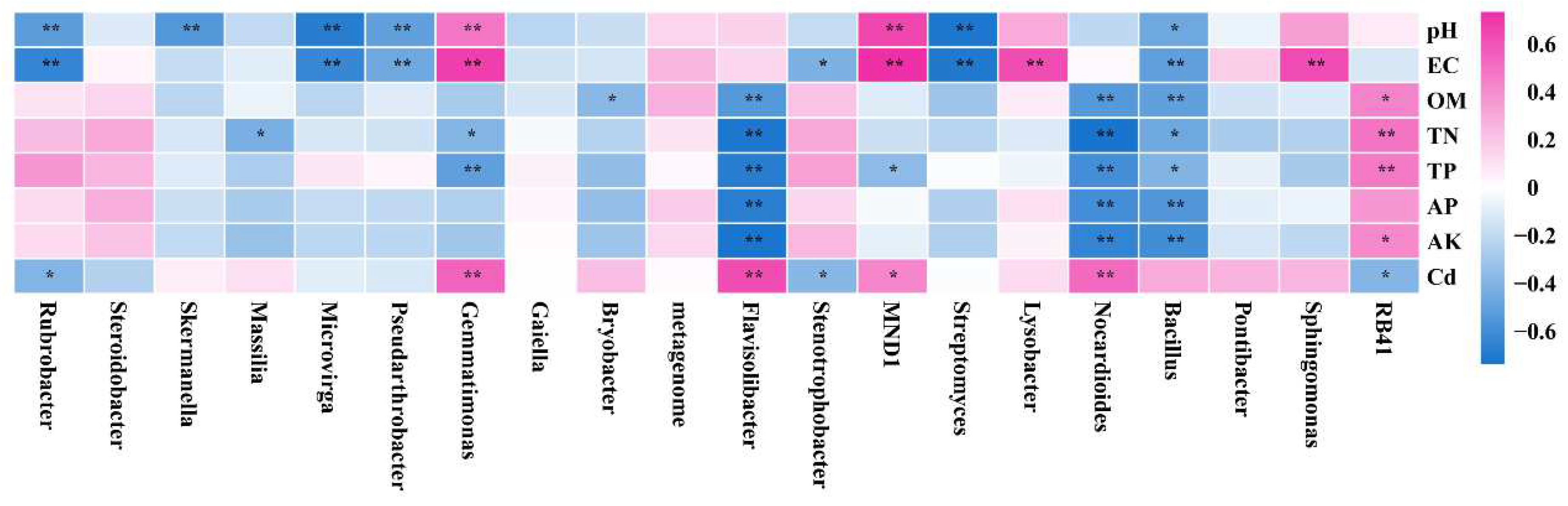
3.5. Correlation Analysis between Soil Bacterial Community Clustering Characteristics and Environmental Factors
4. Discussion
4.1. Changes in Physicochemical Properties of Tomato Soils Contaminated with Different Cadmium Concentrations by Different Irrigation Water Sources
4.2. Effect of Different Irrigation Water Sources on the Yield and Quality of Tomatoes Grown in Soils Contaminated with Different Cadmium Concentrations
4.3. Changes in Tomato Rhizosphere Soil Microbial Community by Different Irrigation Water Sources
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasreen, N.; Ikramullah, K.; Bakhtiar, G.; Gohar, A.; Farooq, J.; Nawaz, J.; Muhammad, S. Response of tomato (Lycopersicon esculentum Mill.) growth to different phosphorous levels and sowing dates. Acta Ecol. Sin. 2019, 39, 30–35. [Google Scholar] [CrossRef]
- Lu, Q. Tomato Quality Traits Diversity Analysis and Agronomic Characters Identification; Northwest A&F University: Xi’an, China, 2021. [Google Scholar]
- You, Q. Genetic Diversity Analysis and Comprehensive Evaluation of Phenotypic Traits in Dwarf Tomato Germplasm Resources; College of Horticulture Science and Technology, Hebei Normal University Of Science & Technology: Qinhuangdao, China, 2021. [Google Scholar]
- Almaroaia, Y.A.; Eissa, M.A. Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci. Hortic. 2020, 265, 109210. [Google Scholar] [CrossRef]
- Ding, F.; Liu, S.; Luo, D.; Wang, G. Different Sensitivity of 23 Common Crop Species to Cadmium Toxicity. Environ. Sci. 2011, 32, 277–283. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Fang, W.; Yang, Z. Variations of Cd absorption and accumulation of 36 Lycopersicon esculentum cultivars. Acta Ecol. Sin. 2006, 26, 4071–4081. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. China Ecological Environment Bulletin; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2020.
- Deng, L.; Zeng, G.; Fan, C.; Lu, L.; Chen, X.; Chen, M.; Wu, H.; He, X.; He, Y. Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Appl. Microbiol. Biotechnol. 2015, 99, 8259–8269. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, M.; Li, W.; Qiu, Y.; Wang, H.; Lv, X. Cotton straw biochar and Bacillus compound biofertilizer decreased Cd migration in alkaline soil: Insights from relationship between soil key metabolites and key bacteria. Ecotoxicol. Env. Saf. 2022, 232, 113293. [Google Scholar] [CrossRef]
- Demirezen Yilmaz, D.; Temizgül, A. Determination of Heavy-Metal Concentration with Chlorophyll Contents of Wheat (Triticum aestivum) Exposed to Municipal Sewage Sludge Doses. Commun. Soil Sci. Plant Anal. 2014, 45, 2754–2766. [Google Scholar] [CrossRef]
- Wu, X.; He, Y.; Huang, Z.; Zhang, D.; Zheng, H.; Ding, J. Effects of different treatment levels of sewage on bacterial community structure and enzyme activity. J. Agro-Environ. Sci. 2020, 39, 2026–2035. [Google Scholar] [CrossRef]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Abadía, J.; Romero-Trigueros, C.; Ruiz-Navarro, A.; Alarcon, J.J.; Garcia, C.; Nicolas, E. Comparing the impacts of drip irrigation by freshwater and reclaimed wastewater on the soil microbial community of two citrus species. Agric. Water Manag. 2018, 203, 53–62. [Google Scholar] [CrossRef]
- Mahjoub, O.; Mauffret, A.; Michel, C.; Chmingui, W. Use of groundwater and reclaimed water for agricultural irrigation: Farmers’ practices and attitudes and related environmental and health risks. Chemosphere 2022, 295, 133945. [Google Scholar] [CrossRef]
- García-Orenes, F.; Caravaca, F.; Morugán-Coronado, A.; Roldán, A. Prolonged irrigation with municipal wastewater promotes a persistent and active soil microbial community in a semiarid agroecosystem. Agric. Water Manag. 2015, 149, 115–122. [Google Scholar] [CrossRef]
- Dang, Q.; Tan, W.; Zhao, X.; Li, D.; Li, Y.; Yang, T.; Li, R.; Zu, G.; Xi, B. Linking the response of soil microbial community structure in soils to long-term wastewater irrigation and soil depth. Sci. Total Env. 2019, 688, 26–36. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Liang, P. Influence of drip irrigation by reclaimed water on the dynamic change of the nitrogen element in soil and tomato yield and quality. J. Clean. Prod. 2016, 139, 561–566. [Google Scholar] [CrossRef]
- Wu, W.; Liao, R.; Hu, Y.; Wang, H.; Liu, H.; Yin, S. Quantitative assessment of groundwater pollution risk in reclaimed water irrigation areas of northern China. Env. Pollut. 2020, 261, 114173. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. National Soil Pollution Survey Bulletin; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China; Ministry of Land and Resources of the People’s Republic of China: Beijing, China, 2014.
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bacterial bulk and rhizosphere communities studied by denaturing gradient gel electrophoresis of PCR-amplified fragments of 16S rRNA genes: Plantdependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 2001, 67, 4742–4751. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Y.; Li, G.; Zhang, W.; Lin, H.; Lin, Z.; Zhen, Z. Effects of rice straw biochar on tomato yield and quality in farmland affected by Cd contamination. J. Ofagro-Environ. Sci. 2022, 41, 492–503. [Google Scholar] [CrossRef]
- Bao, S. The Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Chen, G.; Li, S. Plant Physiology Experiment; Higher Education Press: Beijing, China, 2016. [Google Scholar]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liao, X.; Li, J.; Xu, L.; Liu, M.; Du, B.; Wang, Y. Influence of long-term sewage irrigation on the distribution of organochlorine pesticides in soil–groundwater systems. Chemosphere 2013, 92, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, S.; Pan, N.; Wang, Y.; Wu, L. Impact of reclaimed water irrigation on soil health in urban green areas. Chemosphere 2015, 119, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Devau, N.; Cadre, E.L.; Hinsinger, P.; Jaillard, B.; Gérard, F. Soil pH controls the environmental availability of phosphorus: Experimental and mechanistic modelling approaches. Appl. Geochem. 2009, 24, 2163–2174. [Google Scholar] [CrossRef]
- Rusan, M.J.M.; Hinnawi, S.; Rousan, L. Long term effect of wastewater irrigation of forage crops on soil and plant quality parameters. Desalination 2007, 215, 143–152. [Google Scholar] [CrossRef]
- Jiao, Z.; Huang, Z.; Li, Y.; Wang, W.; Yan, B.; Peng, L.; Li, H. The Effect of Reclaimed Water Irrigation on Soil Performance and the Microorganism. J. Agro-Environ. Sci. 2010, 29, 319–323. [Google Scholar]
- Li, Z.; Fan, X.; Qi, X.; Qiao, D.; Li, P.; Zhao, Z. Effect of reclaimed municipal wastewater on ryegrass growth and soil phosphorus conversion. Chin. J. Eco-Agric. 2012, 20, 1072–1076. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, H.; Wang, Y. Influences of Reclaimed Water Irrigation on Crop-soil System. S. N. Water Divers. Water Sci. Technol. 2011, 9, 120–124. [Google Scholar]
- Wang, Y.; Tang, D.; Zhang, X.; Yuan, X.; Xu, L. Effects of corn-straw biochar on cadmium adsorption, nutrient contents, and chemical forms in red soil. J. Agro-Environ. Sci. 2017, 36, 2445–2452. [Google Scholar] [CrossRef]
- Zalacain, D.; Martínez-Perez, S.; Bienes, R.; García-Díaz, A.; Sastre-Merlín, A. Turfgrass biomass production and nutrient balance of an urban park irrigated with reclaimed water. Chemosphere 2019, 237, 124481. [Google Scholar] [CrossRef]
- Zaragoza, C.A.; García, I.F.a.; García, I.M.; Poyato, E.C.; Díaz, J.A.R. Spatio-temporal analysis of nitrogen variations in an irrigation distribution network using reclaimed water for irrigating olive trees. Agric. Water Manag. 2022, 262, 107353. [Google Scholar] [CrossRef]
- Gatta, G.; Libutti, A.; Gagliardi, A.; Beneduce, L.; Brusetti, L.; Borruso, L.; Disciglio, G.; Tarantino, E. Treated agro-industrial wastewater irrigation of tomato crop: Effects on qualitative/quantitative characteristics of production and microbiological properties of the soil. Agric. Water Manag. 2015, 149, 33–43. [Google Scholar] [CrossRef]
- Yin, S.; Wu, W.; Liu, H.; Zhang, X. Spatial variability of groundwater nitrate-nitrogen and cause analysis of its pollution for irrigation area with reclaimed water. Trans. Chin. Soc. Agric. Eng. 2012, 28, 200–207. [Google Scholar] [CrossRef]
- Cuartero, J.; FernaÂndez-MunÄoz, R. Tomato and salinity. Sci. Hortic. 1999, 78, 83–125. [Google Scholar] [CrossRef]
- Maestre-Valero, J.F.; Gonzalez-Ortega, M.J.; Martinez-Alvarez, V.; Gallego-Elvira, B.; Conesa-Jodar, F.J.; Martin-Gorriz, B. Revaluing the nutrition potential of reclaimed water for irrigation in southeastern Spain. Agric. Water Manag. 2019, 218, 174–181. [Google Scholar] [CrossRef]
- Xue, K. Molecular Identification and Diseases Resistance Analysis of Resistant Germplam Resources in Tomato; Shanghai Jiao Tong University: Shanghai, China, 2020. [Google Scholar]
- Li, Y.; Wen, J.; Li, J. Effects of Drip Irrigation Schemes and Water Quality of Reclaimed Water on Tomato Yield and Fruit Quality. J. Irrig. Drain. 2014, 33, 204–208. [Google Scholar]
- Xue, Y.; Yang, P.; Ren, S.; Liu, H.; Wu, W.; Su, Y.; Fang, Y. Effects of irrigation with treated wastewater on nutrient distribution in cucumber and tomato plants and their fruit quality. Chin. J. Appl. Ecol. 2011, 22, 395–401. [Google Scholar] [CrossRef]
- Vivaldi, G.A.; Camposeo, S.; Romero-Trigueros, C.; Pedrero, F.; Caponio, G.; Lopriore, G.; Alvarez, S. Physiological responses of almond trees under regulated deficit irrigation using saline and desalinated reclaimed water. Agric. Water Manag. 2021, 258, 107172. [Google Scholar] [CrossRef]
- Qaryouti, M.; Bani-Hani, N.; Abu-Sharar, T.; Shnikat, I.; Hiari, M.; Radiadeh, M. Effect of using raw waste water from food industry on soil fertility, cucumber and tomato growth, yield and fruit quality. Sci. Hortic. 2015, 193, 99–104. [Google Scholar] [CrossRef]
- Chen, H. Effects of Reclaimed Water Irrigation on Growth, Yield and Quality of Wolfberry and Tomato; Ningxia University: Yinchuan, China, 2021. [Google Scholar]
- Chen, Y.; Mi, T.; Liu, Y.; Li, S.; Zhen, Y. Microbial Community Composition and Function in Sediments from the Pearl River Mouth Basin. J. Ocean Univ. China 2020, 19, 941–953. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Wang, P.; Chen, J.; Wang, X.; Yuan, Q.; Ma, J. How sediment bacterial community shifts along the urban river located in mining city. Environ. Sci. Pollut. Res. Int. 2021, 28, 42300–42312. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Heuer, H.; Smalla, K. Dynamics of bacterial communities in two unpolluted soils after spiking with phenanthrene: Soil type specific and common responders. Front. Microbiol. 2012, 3, 290. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, Č.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. Int. Soc. Microb. Ecol. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Hemmat-Jou, M.H.; Safari-Sinegani, A.A.; Mirzaie-Asl, A.; Tahmourespour, A. Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicology 2018, 27, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yu, L. Effects of Cd or/and Pb on soil enzyme activities and microbial community structure. Ecol. Eng. 2011, 37, 1889–1894. [Google Scholar] [CrossRef]
- Cui, B.; Gao, F.; Hu, C.; Li, Z.; Fan, X.; Cui, E. Effect of Different Reclaimed Water Irrigation Methods on Bacterial Community Diversity and Pathogen Abundance in the Soil-Pepper Ecosystem. Environ. Sci. 2019, 40, 5151–5163. [Google Scholar]
- Cui, B.; Cui, E.; Liu, C.; Hu, C.; Fan, X.; Li, Z.; Gao, F. Effects of Soil Amendments on the Bacterial Diversity and Abundances of Pathogens and Antibiotic Resistance Genes in Rhizosphere Soil Under Drip Irrigation with Reclaimed Water. Environ. Sci. 2022, 43, 4765–4778. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- DeBruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global Biogeography and Quantitative Seasonal Dynamics of Gemmatimonadetes in Soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef]
- Li, S.; Fan, X.; Erping, C.; Feng, G.; Wu, H.; Li, S.; Cui, B.; Hu, C. Effects of Dripping Rate with Reclaimed Water on Typical Microbial Community Structure in the Root Zone Soil of Tomato. J. Irrig. Drain. 2021, 40, 26–35. [Google Scholar] [CrossRef]

| Water Source for Irrigation | Irrigation Level Symbol | pH | EC/ μs·cm−1 | TN/mg·L−1 | TP/mg·L−1 | COD/mg·L−1 | Cd/mg·L−1 | Treatment |
|---|---|---|---|---|---|---|---|---|
| Reclaimed water | R | 7.33 | 1298 | 8.9 | 0.75 | <15 | <0.0001 | RCd1 |
| RCd2 | ||||||||
| RCd3 | ||||||||
| RCd4 | ||||||||
| RCd5 | ||||||||
| Tap water | T | 8.08 | 249.67 | 0.6 | 0.2 | <10 | <0.001 | TCd1 |
| TCd2 | ||||||||
| TCd3 | ||||||||
| TCd4 | ||||||||
| TCd5 |
| Treatment | pH | EC Value μs·cm−1 | Organic Matter Content/g·kg−1 | Total N Content/mg·g−1 | Total P Content/mg·g−1 | Available P Content/mg·kg−1 | Available K Content/mg·kg−1 | Total Cd Content/mg·kg−1 |
|---|---|---|---|---|---|---|---|---|
| Cd1 | 8.13 | 360.50 | 31.10 | 1.00 | 0.90 | 68.62 | 324.90 | 0.30 |
| Cd2 | 8.20 | 338.40 | 24.60 | 0.79 | 0.73 | 63.95 | 276.60 | 0.69 |
| Cd3 | 8.28 | 394.30 | 18.97 | 0.70 | 0.59 | 53.03 | 245.40 | 1.20 |
| Cd4 | 8.20 | 505.80 | 25.74 | 0.67 | 0.58 | 62.54 | 251.60 | 2.07 |
| Cd5 | 8.22 | 459.80 | 20.26 | 0.64 | 0.62 | 50.15 | 248.50 | 2.74 |
| Treatment | pH | EC Value/μs·cm−1 | Organic Matter Content/g·kg−1 | Total N Content/mg·g−1 | Total P Content/mg·g−1 | Available P Content/mg·kg−1 | Available K Content/mg·kg−1 | Total Cd Content/mg·kg−1 |
|---|---|---|---|---|---|---|---|---|
| RCd1 | 8.6 ± 0.01 ab | 490.33 ± 11.02 bc | 34.49 ± 0.09 a | 0.86 ± 0.01 a | 0.95 ± 0.02 a | 52.95 ± 0.47 a | 259.37 ± 1.5 a | 0.28 ± 0.02 e |
| RCd2 | 8.44 ± 0.07 c | 574.00 ± 33.15 a | 32.10 ± 2.36 ab | 0.64 ± 0.01 b | 0.72 ± 0.04 bc | 43.79 ± 2.23 b | 224.42 ± 2.19 b | 1.00 ± 0.08 d |
| RCd3 | 8.60 ± 0.01 ab | 510.00 ± 7.00 b | 31.77 ± 1.36 abc | 0.56 ± 0.02 bc | 0.56 ± 0.02 def | 37.87 ± 1.46 cd | 215.27 ± 3.96 c | 1.23 ± 0.06 c |
| RCd4 | 8.57 ± 0.02 b | 485.00 ± 2.65 c | 28.47 ± 0.56 cde | 0.58 ± 0.03 bc | 0.59 ± 0.02 d | 36.79 ± 0.47 cd | 214.56 ± 0.46 c | 2.31 ± 0.13 b |
| RCd5 | 8.64 ± 0.02 a | 450.33 ± 8.62 d | 27.77 ± 0.54 de | 0.57 ± 0.01 bc | 0.53 ± 0.03 ef | 35.88 ± 0.41 cd | 204.00 ± 3.61 d | 2.99 ± 0.01 a |
| TCd1 | 8.33 ± 0.01 e | 216.33 ± 0.58 f | 32.15 ± 0.50 ab | 0.80 ± 0.01 a | 0.76 ± 0.02 b | 42.43 ± 1.48 b | 254.37 ± 4.83 a | 0.28 ± 0.02 e |
| TCd2 | 8.40 ± 0.04 cd | 191.9 ± 1.91 g | 30.13 ± 1.58 bcd | 0.63 ± 0.01 b | 0.7 ± 0.06 c | 39.57 ± 4.28 bc | 220.37 ± 1.39 bc | 0.91 ± 0.09 d |
| TCd3 | 8.44 ± 0.01 c | 231.67 ± 10.12 ef | 28.33 ± 3.61 cde | 0.60 ± 0.13 bc | 0.51 ± 0.07 f | 27.96 ± 1.44 e | 173.23 ± 1.07 e | 1.30 ± 0.11 c |
| TCd4 | 8.35 ± 0.04 de | 240.33 ± 3.06 e | 25.37 ± 3.18 e | 0.55 ± 0.01 bc | 0.58 ± 0.01 de | 35.33 ± 5.59 cd | 202.47 ± 8.3 d | 2.26 ± 0.13 b |
| TCd5 | 8.34 ± 0.04 de | 273.33 ± 10.21 d | 24.89 ± 1.99 e | 0.52 ± 0.05 c | 0.52 ± 0.02 ef | 33.83 ± 1.08 d | 200.11 ± 3.86 d | 2.89 ± 0.17 a |
| Treatment | Vitamin C Content/mg·kg−1 | Soluble Sugar Content/% | Titratable Acid Content/% | Soluble Protein Content/mg·kg−1 | Sugar-Acid Ratio/% | Yield/g |
|---|---|---|---|---|---|---|
| RCd1 | 311.72 ± 17.16 c | 2.94 ± 0.1 d | 0.75 ± 0.02 bc | 16.58 ± 2.39 cd | 3.92 ± 0.13 de | 195.33 ± 69.67 ab |
| RCd2 | 320.01 ± 4.90 c | 2.85 ± 0.39 d | 0.91 ± 0.09 ab | 15.75 ± 0.86 de | 3.14 ± 0.36 e | 186.53 ± 28.66 ab |
| RCd3 | 275.83 ± 17.28 d | 2.52 ± 0.26 d | 0.89 ± 0.1 ab | 13.61 ± 0.65 e | 2.83 ± 0.31 e | 205.48 ± 2.28 ab |
| RCd4 | 254.81 ± 10.35 d | 2.37 ± 0.28 d | 0.61 ± 0.06 c | 16.45 ± 2.86 cd | 3.88 ± 0.53 de | 214.45 ± 45.26 ab |
| RCd5 | 263.83 ± 13.91 d | 2.51 ± 0.35 d | 0.84 ± 0.15 ab | 14.78 ± 0.48 e | 3.07 ± 0.91 e | 243.57 ± 6.45 a |
| TCd1 | 385.53 ± 21.43 a | 4.06 ± 0.17 c | 0.86 ± 0.11 ab | 20.26 ± 1.22 b | 4.82 ± 0.85 cd | 116.02 ± 13.98 a |
| TCd2 | 354.20 ± 8.22 b | 4.67 ± 0.19 b | 0.85 ± 0.1 ab | 18.61 ± 0.93 bc | 5.55 ± 0.65 bc | 95.92 ± 29.63 ab |
| TCd3 | 400.29 ± 22.21 a | 4.4 ± 0.24 bc | 0.96 ± 0.09 a | 25.11 ± 1.56 a | 4.64 ± 0.65 cd | 135.16 ± 29.05 a |
| TCd4 | 393.92 ± 29.39 a | 5.55 ± 0.26 a | 0.75 ± 0.04 bc | 20.19 ± 0.62 b | 7.44 ± 0.68 a | 125.86 ± 45.01 ab |
| TCd5 | 383.93 ± 2.12 a | 5.61 ± 0.58 a | 0.87 ± 0.02 ab | 21.29 ± 1.56 b | 6.43 ± 0.64 ab | 128.24 ± 6.83 b |
| Treatment | Chao1 | Goods Coverage | Observed Species | PD Whole Tree | Shannon | Simpson |
|---|---|---|---|---|---|---|
| RCd1 | 3676.65 ab | 0.96 a | 2612.2 bc | 225.37 bc | 9.57 ab | 1 a |
| RCd2 | 3949.29 a | 0.96 a | 2795.17 a | 240.65 a | 9.72 a | 1 a |
| RCd3 | 3823.74 ab | 0.96 a | 2696.93 abc | 235.94 ab | 9.67 ab | 1 a |
| RCd4 | 3837.97 ab | 0.96 a | 2714.6 abc | 241.92 a | 9.64 ab | 1 a |
| RCd5 | 3897.8 a | 0.96 a | 2750.73 ab | 241.59 a | 9.72 a | 1 a |
| TCd1 | 3598.63 b | 0.96 a | 2569.87 c | 218.58 c | 9.41 b | 0.99 b |
| TCd2 | 3759.83 ab | 0.96 a | 2657.27 abc | 223.65 bc | 9.67 ab | 1 a |
| TCd3 | 3794.19 ab | 0.96 a | 2675.57 abc | 223.61 bc | 9.67 ab | 1 a |
| TCd4 | 3744.05 a | 0.96 a | 2718.23 abc | 230.1 abc | 9.56 ab | 1 a |
| TCd5 | 3868.75 ab | 0.96 a | 2757.77 ab | 230.52 abc | 9.72 a | 1 a |
| pH | EC | OM | TN | TP | AP | AK | Cd | ||
|---|---|---|---|---|---|---|---|---|---|
| RB41 | r | 0.432 | 0.486 | 0.467 | 0.412 | −0.538 | |||
| p | 0.017 | 0.006 | 0.009 | 0.024 | 0.002 | ||||
| Sphingomonas | r | 0.632 | |||||||
| p | 0.0001 | ||||||||
| Bacillus | r | −0.461 | −0.505 | −0.040 | −0.462 | −0.403 | −0.555 | −0.604 | |
| p | 0.010 | 0.004 | 0.004 | 0.010 | 0.027 | 0.001 | 0.0004 | ||
| Nocardioides | r | −0.543 | 0.740 | −0.598 | −0.600 | −0.650 | 0.522 | ||
| p | 0.002 | 0.00000293 | 0.0005 | 0.0004 | 0.0000988 | 0.003 | |||
| Streptomyces | r | −0.723 | −0.706 | ||||||
| p | 0.00000653 | 0.0000132 | |||||||
| MND1 | r | 0.648 | 0.732 | −0.362 | 0.427 | ||||
| p | 0.0001 | 0.00000428 | 0.049 | 0.019 | |||||
| Stenotrophobacter | r | −0.414 | −0.382 | ||||||
| p | 0.023 | 0.037 | |||||||
| Flavisolibacter | r | −0.541 | −0.720 | −0.688 | −0.682 | −0.732 | 0.626 | ||
| p | 0.002 | 0.00000726 | 0.000027 | 0.0000337 | 0.00000424 | 0.0002 | |||
| Gemmatimonas | r | 0.478 | 0.679 | −0.400 | −0.505 | 0.543 | |||
| p | 0.007 | 0.0000371 | 0.028 | 0.004 | 0.002 | ||||
| Pseudarthrobacter | r | −0.500 | −0.464 | ||||||
| p | 0.004 | 0.010 | |||||||
| Microvirga | r | −0.687 | −0.634 | ||||||
| p | 0.0000279 | 0.0002 | |||||||
| Massilia | r | −0.428 | |||||||
| p | 0.018 | ||||||||
| Skermanella | r | −0.548 | |||||||
| p | 0.002 | ||||||||
| Rubrobacter | r | −0.526 | −0.647 | −0.399 | |||||
| p | 0.003 | 0.0001 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Li, P.; Qi, X.; Guo, W.; Rahman, S.U. Assessing the Effect of Irrigation Using Different Water Resources on Characteristics of Mild Cadmium-Contaminated Soil and Tomato Quality. Agronomy 2022, 12, 2721. https://doi.org/10.3390/agronomy12112721
Cui J, Li P, Qi X, Guo W, Rahman SU. Assessing the Effect of Irrigation Using Different Water Resources on Characteristics of Mild Cadmium-Contaminated Soil and Tomato Quality. Agronomy. 2022; 12(11):2721. https://doi.org/10.3390/agronomy12112721
Chicago/Turabian StyleCui, Jiaxin, Ping Li, Xuebin Qi, Wei Guo, and Shafeeq Ur Rahman. 2022. "Assessing the Effect of Irrigation Using Different Water Resources on Characteristics of Mild Cadmium-Contaminated Soil and Tomato Quality" Agronomy 12, no. 11: 2721. https://doi.org/10.3390/agronomy12112721
APA StyleCui, J., Li, P., Qi, X., Guo, W., & Rahman, S. U. (2022). Assessing the Effect of Irrigation Using Different Water Resources on Characteristics of Mild Cadmium-Contaminated Soil and Tomato Quality. Agronomy, 12(11), 2721. https://doi.org/10.3390/agronomy12112721









