Molecular Mechanisms Regulating the Oil Biosynthesis in Olive (Olea europaea L.) Fruits Revealed by Transcriptomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Oil Content Analysis of Olive Fruits
2.3. RNA Extraction and Nanopore Sequencing
2.4. Transcriptomics Raw Data Processing and Analysis
2.5. Gene Expression Quantification and Differential Expression Analysis
2.6. Functional Annotation and Enrichment Analysis of Differentially Expressed Genes
2.7. Prediction of Transcription Factors and Construction of Co-Expression Network
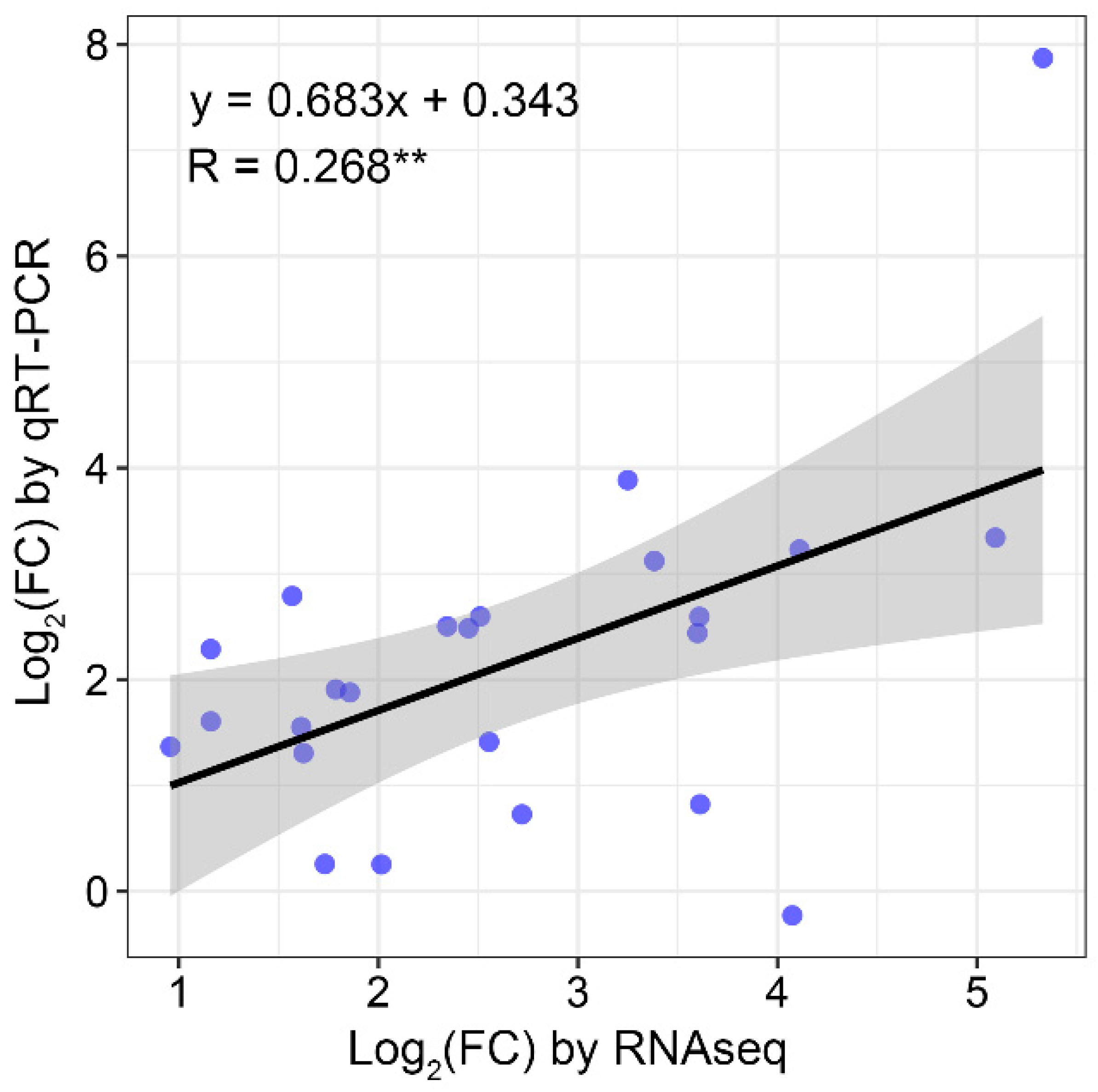
2.8. Verification of the Gene Expression Patterns Based on Nanopore Sequencing by Quantitative Real-Time PCR
3. Results
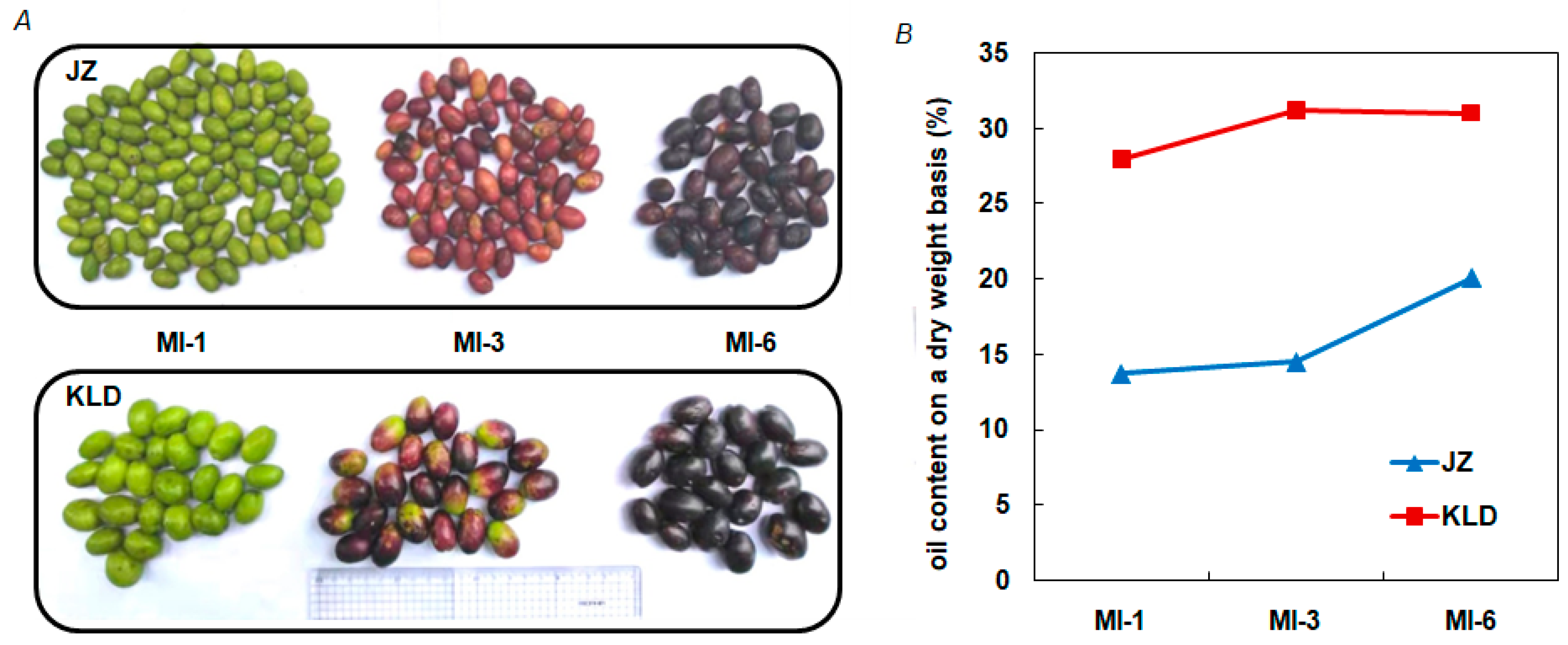
3.1. Variations in Phenotype and Oil Content of Olive Cultivars Kalinjot (JZ) and Coratina (KLD)
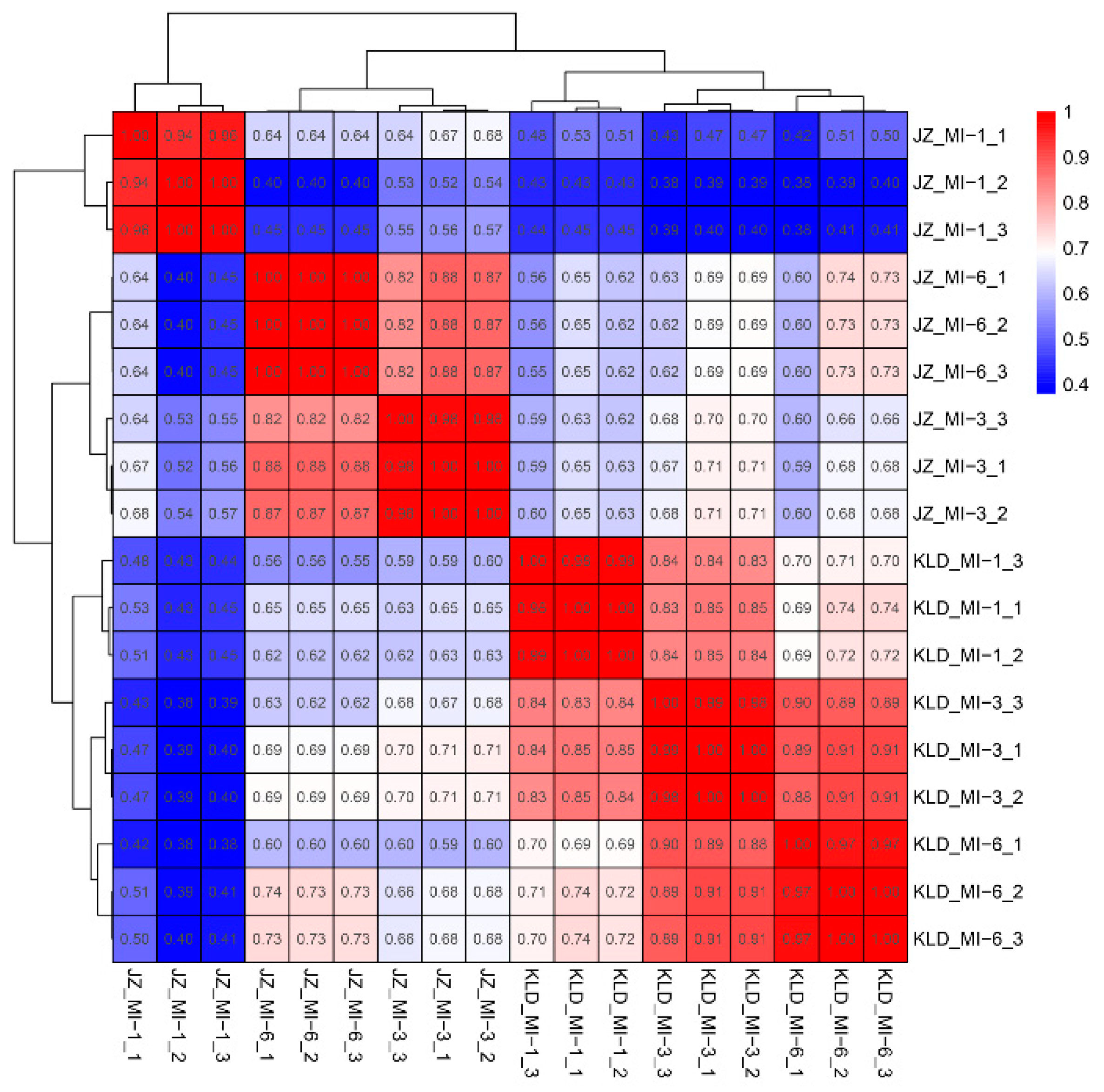
3.2. Transcriptomics Analysis of Olive Cultivars Kalinjot (JZ) and Coratina (KLD)
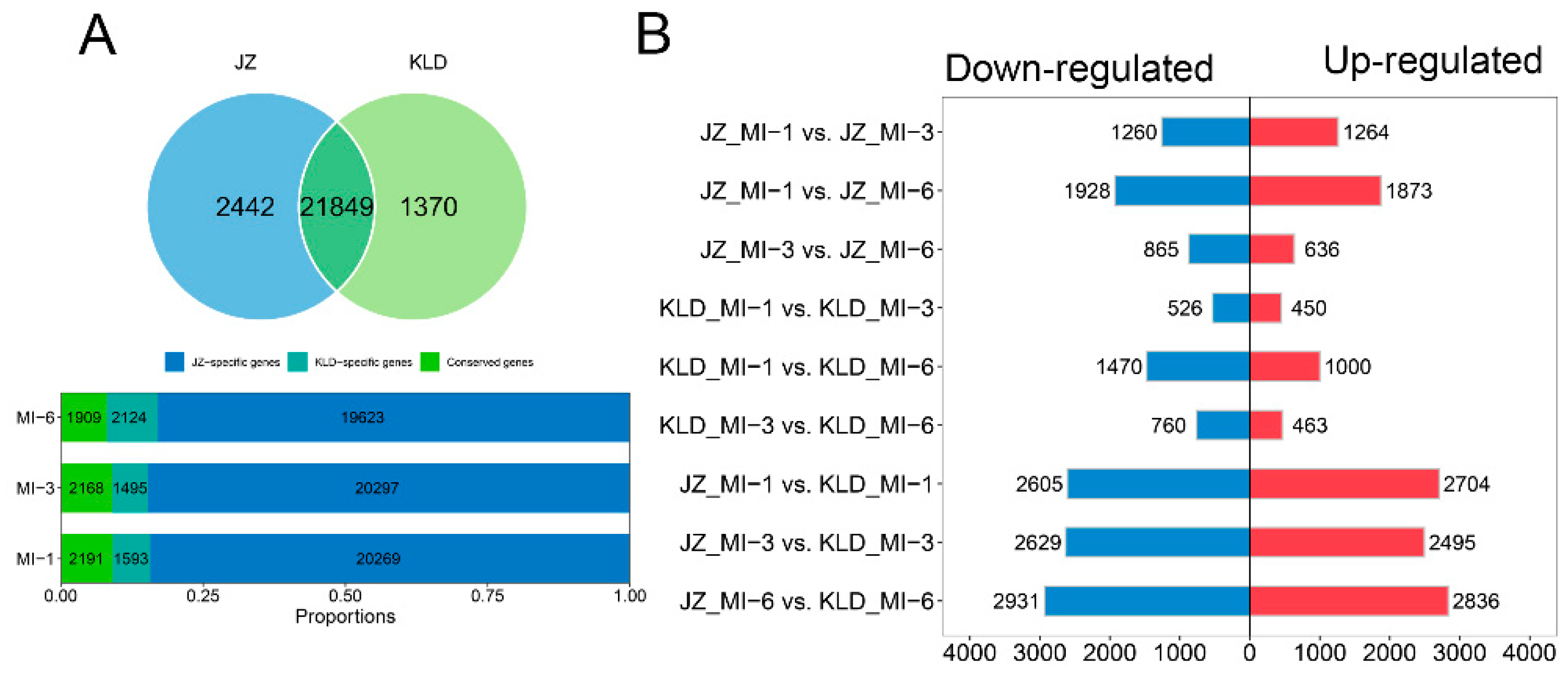
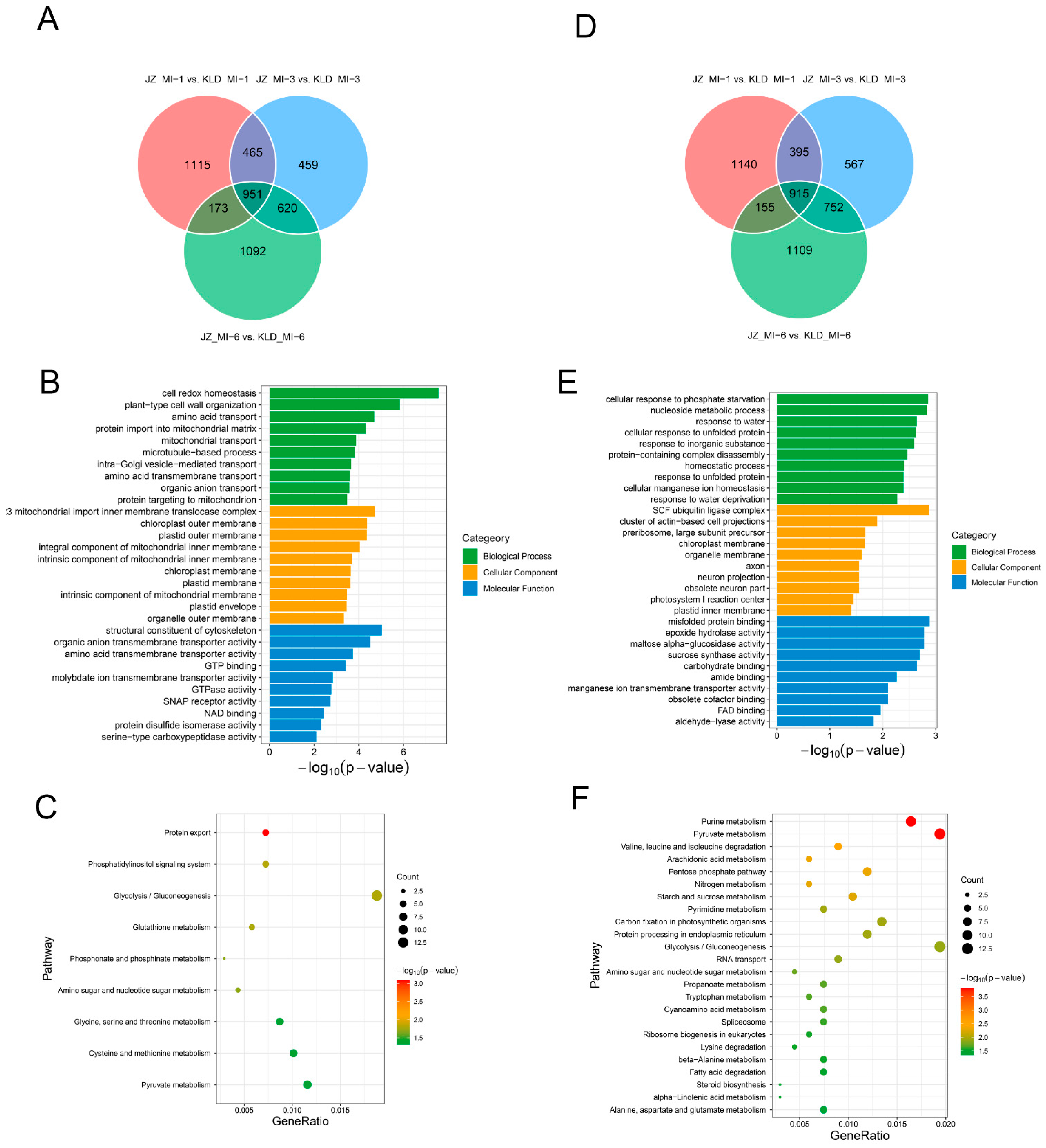
3.3. Identification of Differentially Expressed Eenes in Olive Cultivars Kalinjot (JZ) and Coratina (KLD)
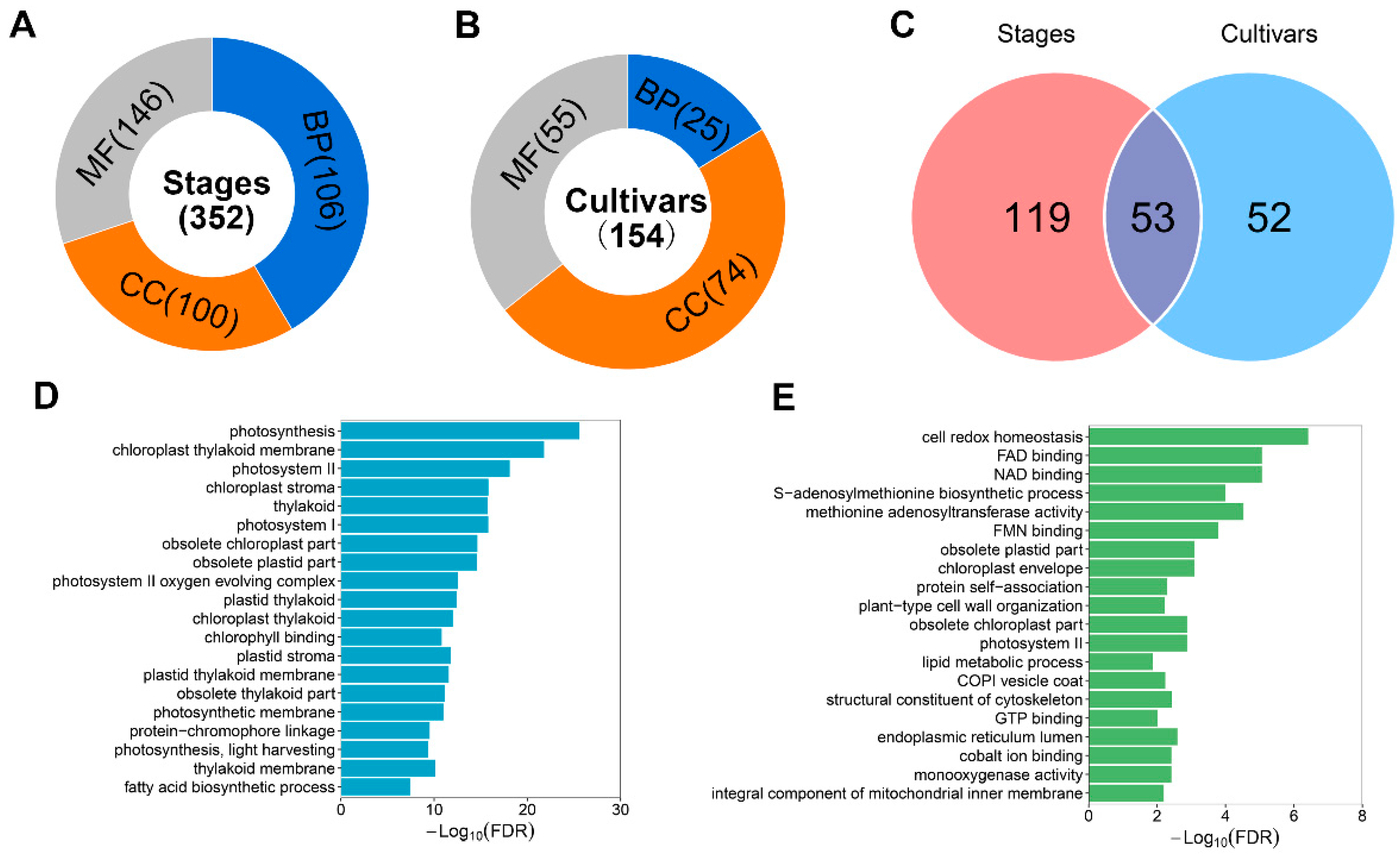
3.4. Functional Annotation of DEGs Based on Gene Ontology Database
3.5. Functional Annotation and Enrichment Analysis of Conserved DEGs in Olive Cultivars Kalinjot (JZ) and Coratina (KLD)
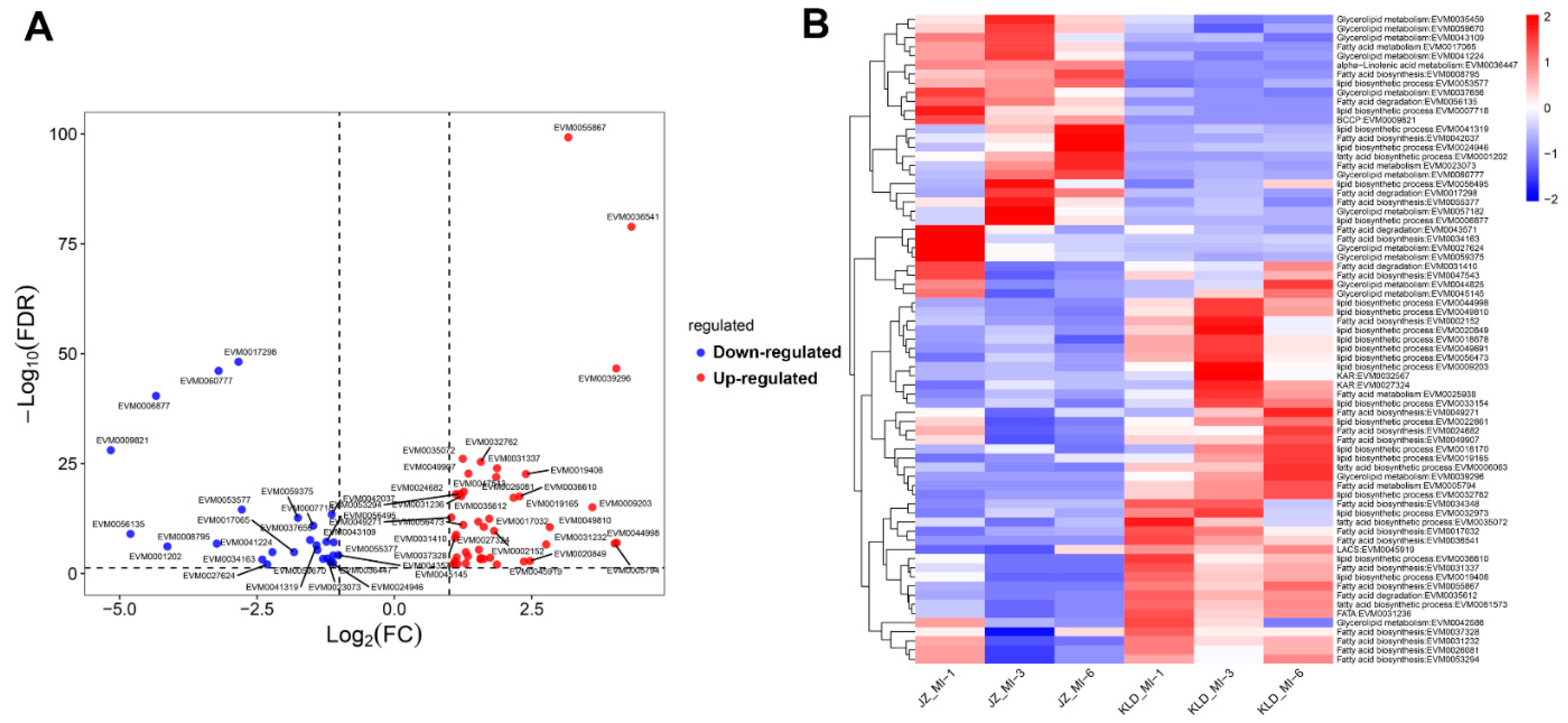
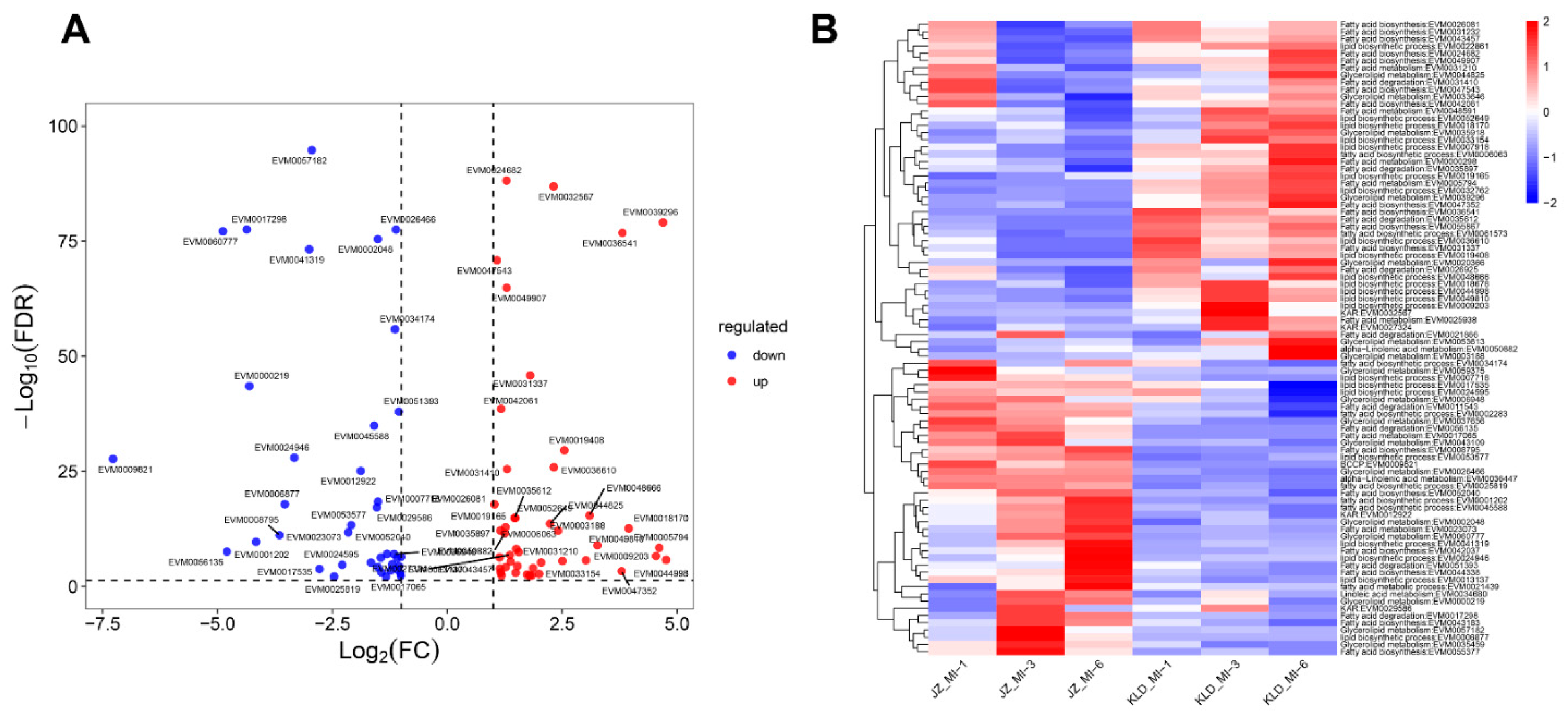
3.6. DEGs Involved in Oil Biosynthesis Pathway of Olive Cultivars Kalinjot (JZ) and Coratina (KLD) at Three Maturity Stages
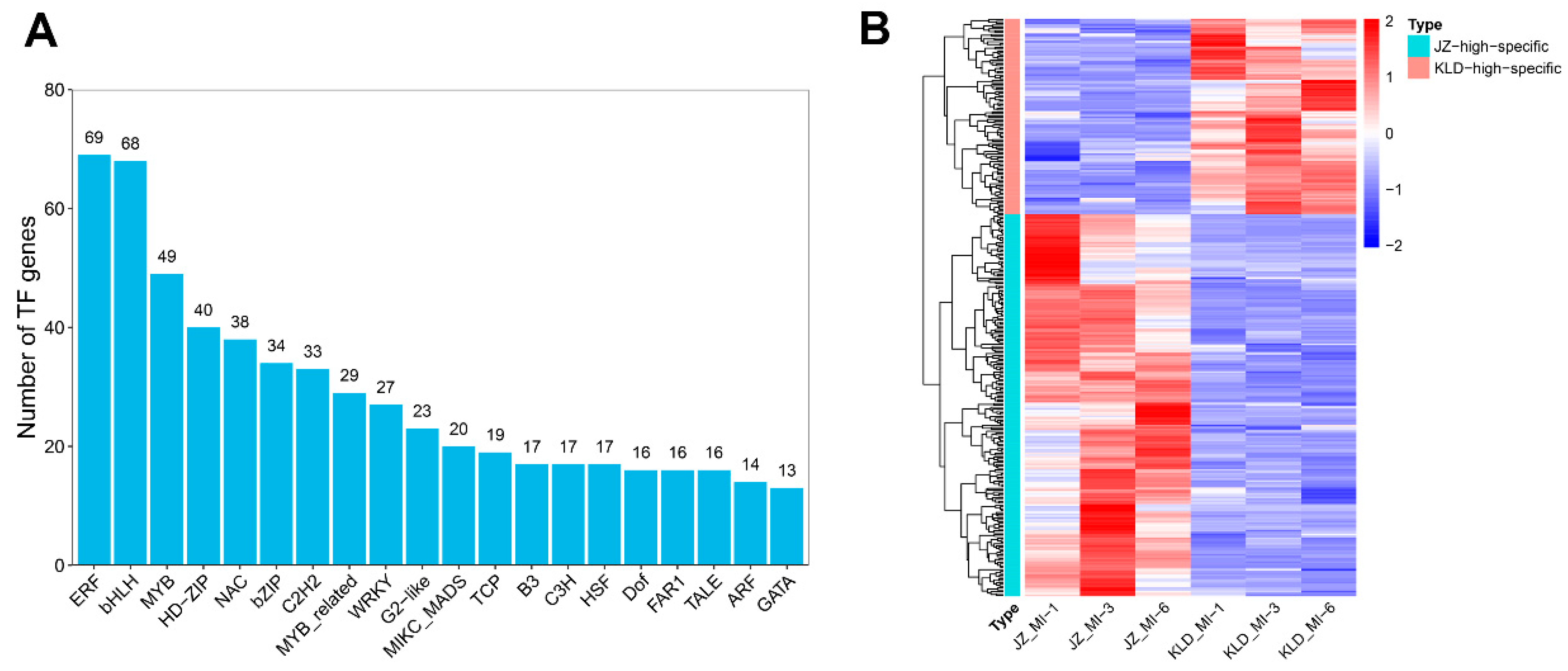
3.7. Transcription Factors in Olive Fruits
3.8. Co-Expression Network of Oil Biosynthesis Based on Transcription Factors and Their Binding Sites
4. Discussion
4.1. Full-Length Nanopore Sequencing of Olive Cultivars Kalinjot (JZ) and Coratina (KLD)
4.2. Identification of Genes Involved in Oil Biosynthesis of Olive
4.3. Co-Expression Network of Oil Biosynthesis Based on Transcription Factors and Their Target Genes in Olive
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bervillé, A.; Breton, C. Genetic and environmental features for oil composition in olive varieties. OCL 2014, 21, D504. [Google Scholar] [CrossRef]
- Diez, C.M.; Trujillo, I.; Martinez-Urdiroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. Olive domestication and diversification in the Mediterranean Basin. New Phytol. 2015, 206, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Sordini, B.; Esposto, S.; Taticchi, A.; Urbani, S.; Sebastiani, L. Metabolomics of Olive Fruit: A Focus on the Secondary Metabolites. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 123–139. [Google Scholar]
- Contreras, C.; Mariotti, R.; Mousavi, S.; Baldoni, L.; Guerrero, C.; Roka, L.; Cultrera, N.; Pierantozzi, P.; Maestri, D.; Gentili, L.; et al. Characterization and validation of olive FAD and SAD gene families: Expression analysis in different tissues and during fruit development. Mol. Biol. Rep. 2020, 47, 4345–4355. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.L.; Sicardo, M.D.; Alfonso, M.; Martínez-Rivas, J.M. Transcriptional Regulation of Stearoyl-Acyl Carrier Protein Desaturase Genes in Response to Abiotic Stresses Leads to Changes in the Unsaturated Fatty Acids Composition of Olive Mesocarp. Front. Plant Sci. 2019, 10, 251. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G. Improvement of bioactive phenol content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Rico, A.; Salvador, M.D.; Fregapane, G. Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur. Food Res. Technol. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- Parvini, F.; Zeinanloo, A.A.; Ebrahimie, E.; Tahmasebi-Enferadi, S.; Hosseini-Mazinani, M. Differential expression of fatty acid desaturases in Mari and Shengeh olive cultivars during fruit development and ripening. Eur. J. Lipid Sci. Technol. 2015, 117, 523–531. [Google Scholar] [CrossRef]
- Hernández, M.L.; Mancha, M.; Martínez-Rivas, J.M. Molecular cloning and characterization of genes encoding two microsomal oleate desaturases (FAD2) from olive. Phytochemistry 2005, 66, 1417–1426. [Google Scholar] [CrossRef]
- Hernández, M.L.; Sicardo, M.D.; Martínez-Rivas, J.M. Differential Contribution of Endoplasmic Reticulum and Chloroplast ω-3 Fatty Acid Desaturase Genes to the Linolenic Acid Content of Olive (Olea europaea) Fruit. Plant Cell Physiol. 2016, 57, 138–151. [Google Scholar] [CrossRef]
- Unver, T.; Wu, Z.; Sterck, L.; Turktas, M.; Lohaus, R.; Li, Z.; Yang, M.; He, L.; Deng, T.; Escalante, F.J.; et al. Genome of wild olive and the evolution of oil biosynthesis. Proc. Natl. Acad. Sci. USA 2017, 114, E9413–E9422. [Google Scholar] [CrossRef]
- Nissim, Y.; Shlosberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. A High Temperature Environment Regulates the Olive Oil Biosynthesis Network. Plants 2020, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.L.; Padilla, M.N.; Sicardo, M.D.; Mancha, M.; Martínez-Rivas, J.M. Effect of different environmental stresses on the expression of oleate desaturase genes and fatty acid composition in olive fruit. Phytochemistry 2011, 72, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Akeson, M.; Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 2016, 34, 518–524. [Google Scholar] [CrossRef]
- VanBuren, R.; Bryant, D.; Edger, P.P.; Tang, H.; Burgess, D.; Challabathula, D.; Spittle, K.; Hall, R.; Gu, J.; Lyons, E.; et al. Single-molecule sequencing of the desiccation-tolerant grass Oropetium thomaeum. Nature 2015, 527, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Renner, T.; Ibarra-Laclette, E.; Farr, K.M.; Chang, T.H.; Cervantes-Pérez, S.A.; Zheng, C.; Sankoff, D.; Tang, H.; Purbojati, R.W.; et al. Long-read sequencing uncovers the adaptive topography of a carnivorous plant genome. Proc. Natl. Acad. Sci. USA 2017, 114, E4435–E4441. [Google Scholar] [CrossRef]
- Chen, X.; Bracht, J.R.; Goldman, A.D.; Dolzhenko, E.; Clay, D.M.; Swart, E.C.; Perlman, D.H.; Doak, T.G.; Stuart, A.; Amemiya, C.T.; et al. The architecture of a scrambled genome reveals massive levels of genomic rearrangement during development. Cell 2014, 158, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Karki, A.; Horvath, D.P.; Sutton, F. Induction of DREB2A pathway with repression of E2F, jasmonic acid biosynthetic and photosynthesis pathways in cold acclimation-specific freeze-resistant wheat crown. Funct. Integr. Genom. 2013, 13, 57–65. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, X.; Wang, W.; Pan, Y.; Huang, L.; Liu, X.; Zong, Y.; Zhu, L.; Yang, D.; Fu, B. Comparative transcriptome profiling of chilling stress responsiveness in two contrasting rice genotypes. PLoS ONE 2012, 7, e43274. [Google Scholar] [CrossRef]
- Xu, Z.; Peters, R.J.; Weirather, J.; Luo, H.; Liao, B.; Zhang, X.; Zhu, Y.; Ji, A.; Zhang, B.; Hu, S.; et al. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. Plant J. 2015, 82, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Chai, H.; Yin, H.; Yang, M.; Hu, G.; Guo, M.; Yi, R.; Zhang, P. Full-length transcriptome sequencing reveals the low-temperature-tolerance mechanism of Medicago falcata roots. BMC Plant Biol. 2019, 19, 575. [Google Scholar] [CrossRef] [PubMed]
- Briegas, B.; Corbacho, J.; Parra-Lobato, M.C.; Paredes, M.A.; Labrador, J.; Gallardo, M.; Gomez-Jimenez, M.C. Transcriptome and Hormone Analyses Revealed Insights into Hormonal and Vesicle Trafficking Regulation among Olea europaea Fruit Tissues in Late Development. Int. J. Mol. Sci. 2020, 21, 4819. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Zhang, J.; Liu, X.; Li, X.; Wang, C. Combined Metabolome and Transcriptome Profiling Reveal Optimal Harvest Strategy Model Based on Different Production Purposes in Olive. Foods 2021, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, L.; Zhang, J.; Xue, L.; Luo, Y.; Rao, G. Integrated Analysis of Fatty Acid Metabolism and Transcriptome Involved in Olive Fruit Development to Improve Oil Composition. Forests 2021, 12, 1773. [Google Scholar] [CrossRef]
- Hassan, H.; El-Rahman, A.; Attia, M. Color Properties of Olive Fruits During Its Maturity Stages Using Image Analysis. AIP Conf. Proc. 2011, 1380, 101–106. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Rao, G.; Zhang, J.; Liu, X.; Lin, C.; Xin, H.; Xue, L.; Wang, C. De novo assembly of a new Olea europaea genome accession using nanopore sequencing. Hortic. Res. 2021, 8, 64. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W. Benjamini–Hochberg Method. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; p. 78. [Google Scholar]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Yang, D.C.; Meng, Y.Q.; Jin, J.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef] [PubMed]
- Lyerly, S.B. The average spearman rank correlation coefficient. Psychometrika 1952, 17, 421–428. [Google Scholar] [CrossRef]
- Hernández, M.L.; Padilla, M.N.; Mancha, M.; Martínez-Rivas, J.M. Expression analysis identifies FAD2-2 as the olive oleate desaturase gene mainly responsible for the linoleic acid content in virgin olive oil. J. Agric. Food Chem. 2009, 57, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rao, X.; Dixon, R.A. Co-expression networks for plant biology: Why and how. Acta Biochim. Biophys. Sin. 2019, 51, 981–988. [Google Scholar] [CrossRef]
- Zainal-Abidin, R.A.; Harun, S.; Vengatharajuloo, V.; Tamizi, A.A.; Samsulrizal, N.H. Gene Co-Expression Network Tools and Databases for Crop Improvement. Plants 2022, 11, 1625. [Google Scholar] [CrossRef]
- Rugini, E.; Cristofori, V.; Silvestri, C. Genetic improvement of olive (Olea europaea L.) by conventional and in vitro biotechnology methods. Biotechnol. Adv. 2016, 34, 687–696. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, H.; Yao, X.; Wang, K.; Chang, J. Full-length transcriptome analysis of pecan (Carya illinoinensis) kernels. G3 2021, 11, jkab182. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, H.; Luo, H.; Fan, Y.; Zhou, Z.; Chen, R.; Luo, T.; Li, X.; Liu, X.; Li, Y.; et al. Characterization of full-length transcriptome in Saccharum officinarum and molecular insights into tiller development. BMC Plant Biol. 2021, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, F.; Chen, Y.; Bai, G.; Liu, Y.; Jin, J.; Wang, Q. Full-length transcriptome analysis provides new insights into the early bolting occurrence in medicinal Angelica sinensis. Sci. Rep. 2021, 11, 13000. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, M.; Xiao, Y.; Wu, X.; Wang, J. The activation of gene expression and alternative splicing in the formation and evolution of allopolyploid Brassica napus. Hortic. Res. 2022, 9, uhab075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, M.; Wang, J.; Lei, M.; Li, C.; Zhao, D.; Huang, J.; Li, W.; Li, S.; Li, J.; et al. PacBio full-length cDNA sequencing integrated with RNA-seq reads drastically improves the discovery of splicing transcripts in rice. Plant J. 2019, 97, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D. Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Kafkaletou, M.; Tsantili, E. Oil content and composition in relation to leaf photosynthesis, leaf sugars and fruit sugars in maturing Koroneiki olives—The mannitol effect on oil. J. Appl. Bot. Food Qual. 2016, 89, 1–10. [Google Scholar] [CrossRef]
- Proietti, P. Phtosynthesis and respiration in olive fruit. Acta Hortic. 1990, 286, 211–214. [Google Scholar] [CrossRef]
- Sánchez, J. Olive Oil Biogenesis. Contribution of Fruit photosynthesis. In Plant Lipid Metabolism; Kader, J.-C., Mazliak, P., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 564–566. [Google Scholar]
- Bengana, M.; Bakhouche, A.; Lozano-Sánchez, J.; Youcef, A.; Youyou, A.; Segura Carretero, A.; Fernández-Gutiérrez, A. Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef]
- Hecker, M.; Lambeck, S.; Toepfer, S.; van Someren, E.; Guthke, R. Gene regulatory network inference: Data integration in dynamic models—A review. Biosystems 2009, 96, 86–103. [Google Scholar] [CrossRef]
- Haque, S.; Ahmad, J.S.; Clark, N.M.; Williams, C.M.; Sozzani, R. Computational prediction of gene regulatory networks in plant growth and development. Curr. Opin. Plant Biol. 2019, 47, 96–105. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, M.L.; Castillo-Jiménez, A.; Martínez-García, J.C.; Álvarez-Buylla, E.R. Multi-level gene regulatory network models to understand complex mechanisms underlying plant development. Curr. Opin. Plant Biol. 2020, 57, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Jiang, T.; Liu, Y.X.; Bai, Y.C.; Reed, J.; Qu, B.; Goossens, A.; Nützmann, H.W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Goh, H.; Azodi, C.B.; Krishnamoorthi, S.; Liu, M.J.; Urano, D. Evolutionarily conserved hierarchical gene regulatory networks for plant salt stress response. Nat. Plants 2021, 7, 787–799. [Google Scholar] [CrossRef]
- Ueda, Y.; Ohtsuki, N.; Kadota, K.; Tezuka, A.; Nagano, A.J.; Kadowaki, T.; Kim, Y.; Miyao, M.; Yanagisawa, S. Gene regulatory network and its constituent transcription factors that control nitrogen-deficiency responses in rice. New Phytol. 2020, 227, 1434–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, C.; Song, L.; Shang, Z.; Yang, S.; Zhang, D.; Sun, W.; Shen, Q.; Zhao, D. RNA Sequencing and Coexpression Analysis Reveal Key Genes Involved in α-Linolenic Acid Biosynthesis in Perilla frutescens Seed. Int. J. Mol. Sci. 2017, 18, 2433. [Google Scholar] [CrossRef]
- Lee, H.G.; Park, B.-Y.; Kim, H.U.; Seo, P.J. MYB96 stimulates C18 fatty acid elongation in Arabidopsis seeds. Plant Biotechnol. Rep. 2015, 9, 161–166. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.G.; Yoon, S.; Kim, H.U.; Seo, P.J. The Arabidopsis MYB96 Transcription Factor Is a Positive Regulator of ABSCISIC ACID-INSENSITIVE4 in the Control of Seed Germination. Plant Physiol. 2015, 168, 677–689. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Chen, Z.; Wang, B.; Feng, S.; Tong, Z.; Chen, T.; Zhou, L.; Peng, Z.; Ding, C. Molecular Mechanisms Regulating the Oil Biosynthesis in Olive (Olea europaea L.) Fruits Revealed by Transcriptomic Analysis. Agronomy 2022, 12, 2718. https://doi.org/10.3390/agronomy12112718
Qu J, Chen Z, Wang B, Feng S, Tong Z, Chen T, Zhou L, Peng Z, Ding C. Molecular Mechanisms Regulating the Oil Biosynthesis in Olive (Olea europaea L.) Fruits Revealed by Transcriptomic Analysis. Agronomy. 2022; 12(11):2718. https://doi.org/10.3390/agronomy12112718
Chicago/Turabian StyleQu, Jipeng, Zhenyong Chen, Bixia Wang, Shiling Feng, Zhaoguo Tong, Tao Chen, Lijun Zhou, Zhengsong Peng, and Chunbang Ding. 2022. "Molecular Mechanisms Regulating the Oil Biosynthesis in Olive (Olea europaea L.) Fruits Revealed by Transcriptomic Analysis" Agronomy 12, no. 11: 2718. https://doi.org/10.3390/agronomy12112718
APA StyleQu, J., Chen, Z., Wang, B., Feng, S., Tong, Z., Chen, T., Zhou, L., Peng, Z., & Ding, C. (2022). Molecular Mechanisms Regulating the Oil Biosynthesis in Olive (Olea europaea L.) Fruits Revealed by Transcriptomic Analysis. Agronomy, 12(11), 2718. https://doi.org/10.3390/agronomy12112718







