Improving Fodder Yields and Nutritive Value of Some Forage Grasses as Animal Feeds through Intercropping with Egyptian Clover (Trifolium alexandrinum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Agronomic Cultivation and Evaluation
2.2. Laboratory Analysis
2.3. In Vitro Assay
2.4. Rumen Fermentation and Degradability
2.5. Statistical Analysis
3. Results
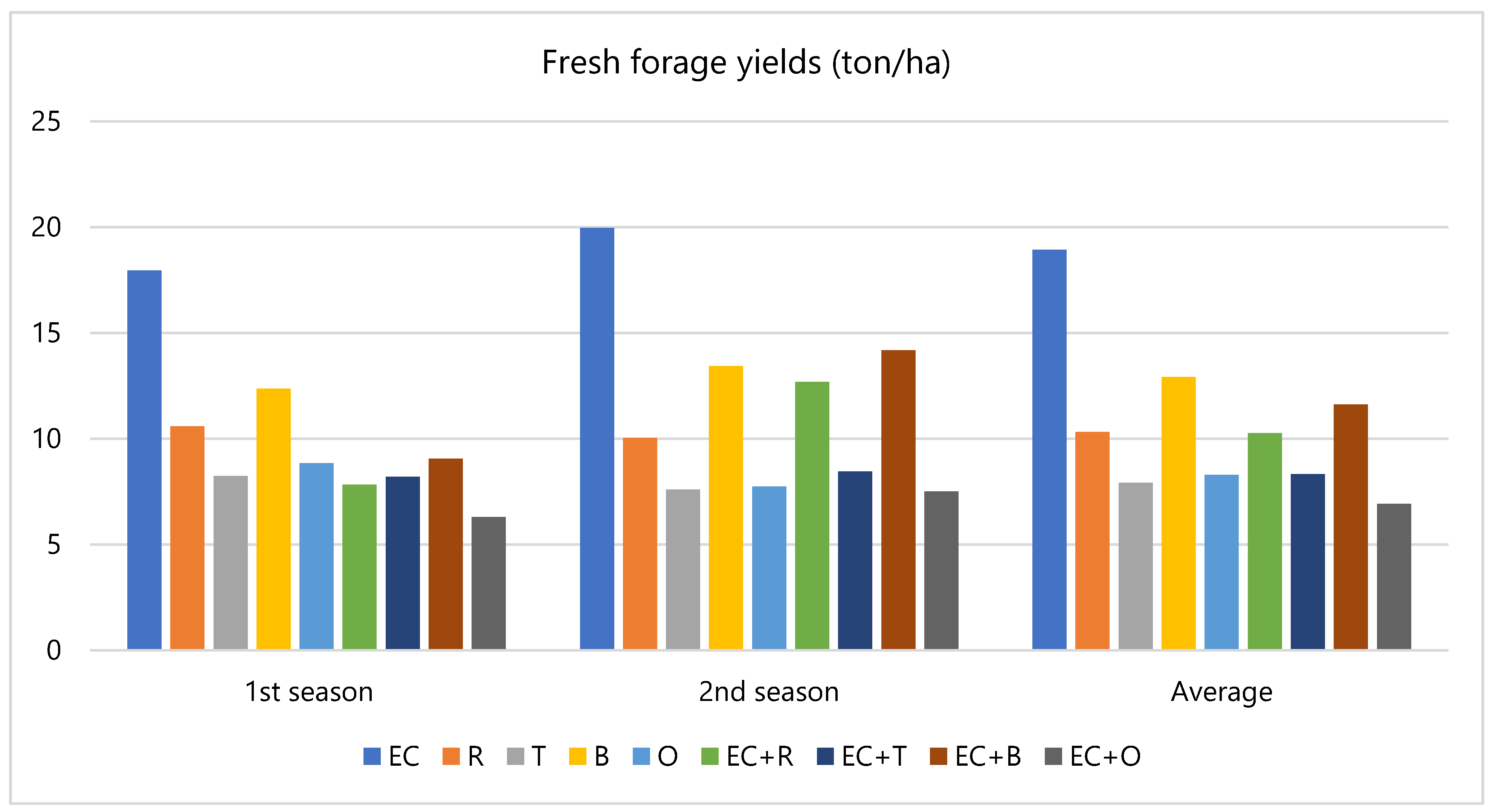
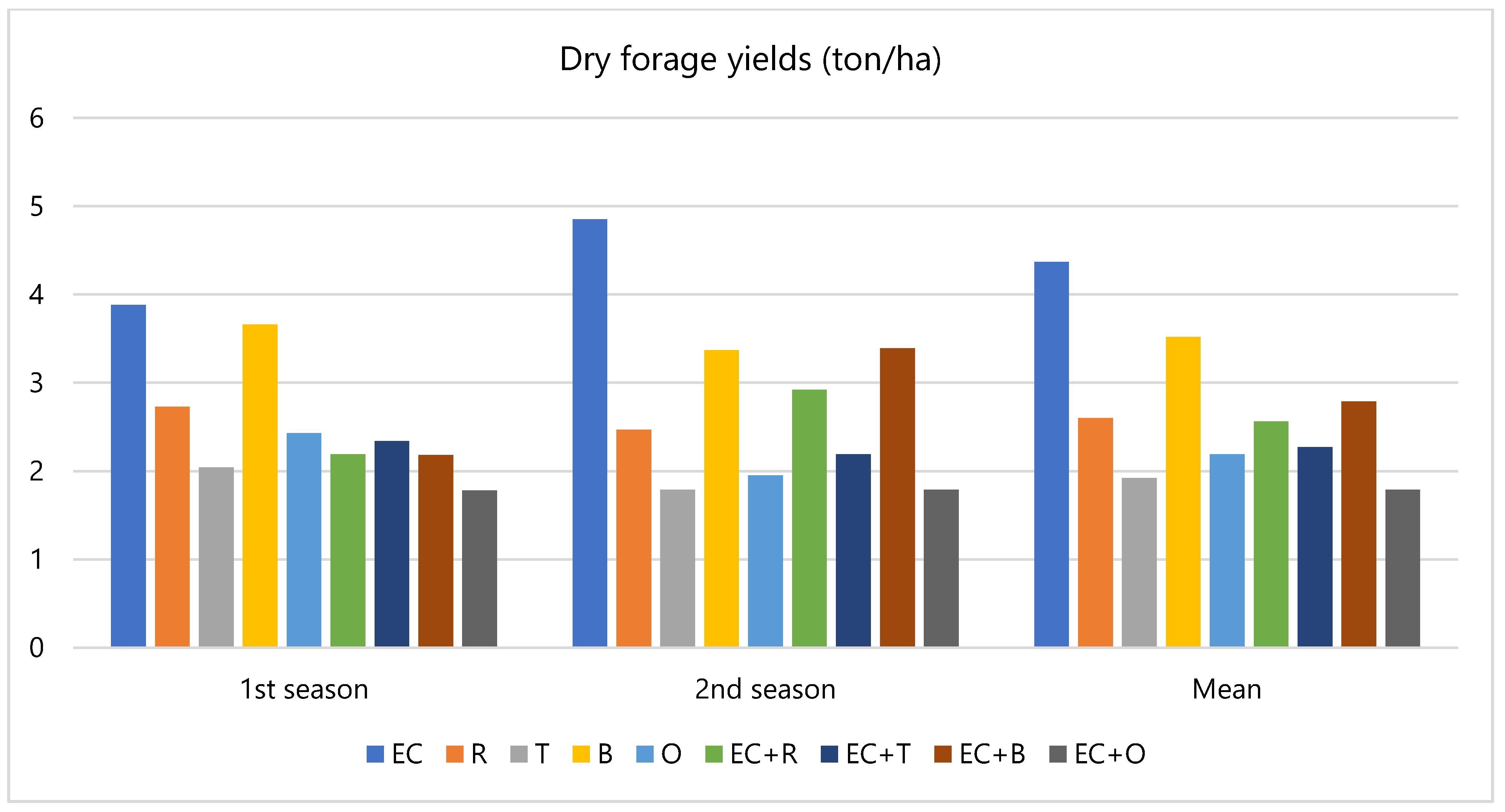
3.1. Field Evaluation
3.2. Chemical Composition and Fermentation
4. Discussion
4.1. Field Evaluation
4.2. Chemical Composition and Fermentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SADS Egypt. Sustainable Agricultural Development Strategy Towards 2030; Agricultural Research and Development Council, Ministry of Agriculture and Land Reclamation: Cairo, Egypt, 2009. [Google Scholar]
- CAPMAS. Egypt Statistical Yearbook; CAPMAS: Cairo, Egypt, 2019. [Google Scholar]
- Muhammad, D.; Misri, B.; EL-Nahrawy, M.; Khan, S.; Serkan, A. Egyptian Clover (Trifolium Alexandrinum)-King of Forage Crops; FAO Regional Office for the Near East and North Africa: Cairo, Egypt, 2014. [Google Scholar]
- Kholif, A.E.; Gouda, G.A.; Olafadehan, O.A.; Abdo, M.M. Effects of replacement of Moringa oleifera for berseem clover in the diets of Nubian goats on feed utilisation, and milk yield, composition and fatty acid profile. Animal 2018, 12, 964–972. [Google Scholar] [CrossRef]
- Tufail, M.S.; Krebs, G.L.; Southwell, A.; Piltz, J.W.; Norton, M.R.; Wynn, P.C. Enhancing performance of berseem clover genotypes with better harvesting management through farmers’ participatory research at smallholder farms in Punjab. Sci. Rep. 2020, 10, 3545. [Google Scholar] [CrossRef]
- Haq, S.A.; Korieng, K.J.; Shiekh, T.; Bahar, F.; Dar, K.; Raja, W.; Wani, R.A.; Khuroo, N. Yield and quality of winter cereal-legume fodder mixtures and their pure stand under temperate conditions of Kashmir Valley, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3626–3631. [Google Scholar] [CrossRef]
- Heba, S.A.S.; Hala, H.B.; Salama, H.S.A.; Badry, H.H. Influence of variable mixing rates and nitrogen fertilization levels on the fodder quality of Egyptian clover (Trifolium alexandrinum L.) and annual ryegrass (Lolium multiflorum Lam.). Afr. J. Agric. Res. 2015, 10, 4858–4864. [Google Scholar] [CrossRef]
- El-Karamany, M.F.; Bakry, B.A.; Elewa, T.A.E.-F. Integrated action of mixture rates and nitrogen levels on quantity and quality of forage mixture from Egyptian clover and barley in sandy soil. Agric. Sci. 2014, 5, 1539–1546. [Google Scholar] [CrossRef][Green Version]
- Salem, M.O.M.; Barsoum, M.S.; Attia, M.A. Studies on mixture forage between clover and barley crops under rainfed conditions. J. Plant Prod. 2015, 6, 1491–1505. [Google Scholar] [CrossRef]
- Gill, K.S.; Omokanye, A.T. Potential of Spring Barley, Oat and Triticale Intercrops with Field Peas for Forage Production, Nutrition Quality and Beef Cattle Diet. J. Agric. Sci. 2018, 10, 1–17. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Kaul, H.-P. Nitrogen uptake, use and utilization efficiency by oat–pea intercrops. Field Crop. Res. 2015, 179, 113–119. [Google Scholar] [CrossRef]
- Ansar, M.; Ahmed, Z.I.; Malik, M.A.; Nadeem, M.; Majeed, A.; Rischkowsky, B.A. Forage yield and quality potential of winter cereal-vetch mixtures under rainfed conditions. Emir. J. Food Agric. 2010, 22, 25–36. [Google Scholar] [CrossRef]
- Sultan, F.M.; Shafie, W.W. Intercropping barley with berseem clover under different seeding rates. Egypt. J. Agric. Res. 2015, 93, 1195–1207. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Keco, R.X.; Dima, A.K.; Paschalidis, K.; Gatsis, T.D. Forage yield and competition indices of faba bean intercropped with oat. Grass Forage Sci. 2014, 69, 376–383. [Google Scholar] [CrossRef]
- Moyo, M.; Bhiya, S.T.; Katamzi, M.; Nsahlai, I.V. Evaluation and Prediction of the Nutritive Value of Underutilised Forages as Potential Feeds for Ruminants. In Forage Groups; IntechOpen: London, UK, 2019; ISBN 978-1-78985-682-8. [Google Scholar]
- Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Barbabosa, A.; Márquez, O.; Odongo, N.E. The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J. Agric. Sci. 2017, 155, 494–507. [Google Scholar] [CrossRef]
- Pizzol, J.D.; Ribeiro-Filho, H.; Quereuil, A.; Le Morvan, A.; Niderkorn, V. Complementarities between grasses and forage legumes from temperate and subtropical areas on in vitro rumen fermentation characteristics. Anim. Feed Sci. Technol. 2017, 228, 178–185. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Niderkorn, V.; Baumont, R.; Le Morvan, A.; Macheboeuf, D. Occurrence of associative effects between grasses and legumes in binary mixtures on in vitro rumen fermentation characteristics. J. Anim. Sci. 2011, 89, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Elghandour, M.M.Y.; Chagoyán, J.C.V.; Salem, A.Z.M.; Kholif, A.E.; Castañeda, J.S.M.; Camacho, L.M.; Cerrillo-Soto, M.A. Effects of Saccharomyces cerevisiae at direct addition or pre-incubation on in vitro gas production kinetics and degradability of four fibrous feeds. Ital. J. Anim. Sci. 2014, 13, 3075. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Márquez-Molina, O.; Vázquez-Armijo, J.F.; Puniya, A.K.; Salem, A.Z.M. Influence of individual or mixed cellulase and xylanase mixture on in vitro rumen gas production kinetics of total mixed rations with different maize silage and concentrate ratios. Turk. J. Vet. Anim. Sci. 2015, 39, 435–442. [Google Scholar] [CrossRef]
- Sadreddine, B. Yield and quality of dual-purpose barley and triticale in a semi-arid environment in Tunisia. Afr. J. Agric. Res. 2016, 11, 2730–2735. [Google Scholar] [CrossRef][Green Version]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of AOAC International, 18th ed.; AOAC International: Washington, DC, USA, 2005; ISBN 0935584544. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Cabral Filho, S.L.; Gobbo, S.P.; Louvandini, H.; Vitti, D.M.S.S.; Abdalla, A.L. Influence of inoculum source in a gas production method. Anim. Feed. Sci. Technol. 2005, 123–124, 95–105. [Google Scholar] [CrossRef]
- Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Dehority, B.A. Laboratory Manual for Classification and Morphology of Rumen Ciliate Protozoa, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993; ISBN 9781351073912. [Google Scholar]
- Palmquist, D.; Conrad, H. Origin of plasma fatty acids in lactating cows fed high grain or high fat diets. J. Dairy Sci. 1971, 54, 1025–1033. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1980. [Google Scholar]
- Bakheit, B.; Ali, M.; Helmy, A. The influence of temperature, genotype and genotype x temperature interaction on seed yield of berseem clover (Trifolium alexandrinum L.). Asian J. Crop Sci. 2012, 4, 63–71. [Google Scholar] [CrossRef]
- Bacchi, M.; Monti, M.; Calvi, A.; Lo Presti, E.; Pellicanò, A.; Preiti, G. Forage potential of cereal/legume intercrops: Agronomic performances, yield, quality forage and LER in two harvesting times in a Mediterranean environment. Agronomy 2021, 11, 121. [Google Scholar] [CrossRef]
- Mona, B.H. Intercropped canopies of berseem clover and barley ameliorate the forage quantity and quality beside n-transfer to the neighboured cereal. Int. J. Environ. 2019, 8, 1–15. [Google Scholar]
- Yucel, C.; Inal, I.; Yucel, D.; Hatipoglu, R. Effects of mixture ratio and cutting time on forage yield and silage quality of intercropped berseem clover and Italian ryegrass. Legum. Res. Int. J. 2018, 41, 846–853. [Google Scholar] [CrossRef]
- Shoaib, M.; Ayub, M.; Shehzad, M.; Akhtar, N.; Tahir, M.; Arif, M. Dry Matter Yield and Forage Quality of Oat, Barley and Canola Mixture. Pak. J. Agric. Sci. 2014, 51, 433–439. [Google Scholar]
- Darapuneni, M.K.; Morgan, G.D.; Shaffer, O.J. Effect of Planting Date on Distribution of Seasonal Forage Yields in Dual-Purpose Wheat, Oats, and Ryegrass Crops. Crop. Forage Turfgrass Manag. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Eskandari, H.; Ghanbari, A.; Javanmard, A. Intercropping of Cereals and Legumes for Forage Production. Not. Sci. Biol. 2009, 1, 7–13. [Google Scholar] [CrossRef]
- Johnston, J.; Wheeler, B. Forage Production from Spring Cereals and Cereal-Pea Mixtures; Ministry of Agriculture, Food and Rural Affairs: Guelph, ON, Canada, 1998. [Google Scholar]
- Butler, T.J.; Muir, J.P. Perspective on forage legume systems for the tallgrass and mixed-grass prairies of the Southern Great Plains of Texas and Oklahoma. Crop Sci. 2012, 52, 1971–1979. [Google Scholar] [CrossRef]
- El Kramany, M.F.; Elewa, T.A.; Bakry, A.B. Effect of mixture rates on forage mixture of Egyptian clover (Trifolium Alexandrinum L.) with triticale (× Triticosecale Wittmack) under newly reclaimed sandy soil. Aust. J. Basic Appl. Sci. 2012, 6, 40–44. [Google Scholar]
- Rizk, T.Y.; El-Agroudy, M.H.; Al-Aswad, A.M. Effect of irrigation regimes on forage quality and nutritive values of berseem in binary mixtures with barley and annual ryegrass. In Proceedings of the 8th African Crop Science Society Conference, El-Minia, Egypt, 27–31 October 2007; pp. 195–199. [Google Scholar]
- Hathout, M.K.; Swidan, F.Z.; El-Sayes, M.F.; Eid, H.A. Quality evaluation of feed resources at the newly reclaimed area in Egypt. Egypt. J. Appl. Sci. 1997, 13, 80–96. [Google Scholar]
- Lithourgidis, A.; Vasilakoglou, I.; Dhima, K.; Dordas, C.; Yiakoulaki, M. Forage yield and quality of common vetch mixtures with oat and triticale in two seeding ratios. Field Crop. Res. 2006, 99, 106–113. [Google Scholar] [CrossRef]
- Denčić, S.; Mladenov, N.; Kobiljski, B. Effects of genotype and environment on breadmaking quality in wheat. Int. J. Plant Prod. 2011, 5, 71–82. [Google Scholar] [CrossRef]
- Ceyhan, E.; Kahraman, A.; Önder, M. The Impacts of Environment on Plant Products. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 48–51. [Google Scholar] [CrossRef][Green Version]
- Ball, D.; Collins, M.; Lacefield, G.; Martin, N.; Mertens, D.; Olson, K.; Putnam, D.; Undersander, D.; Wolf, M. Understanding Forage Quality; American Farm Bureau Federation Publication: Park Ridge, IL, USA, 2001. [Google Scholar]
- Hamid, P.; Akbar, T.; Hossein, J.; Ali, M.G. Nutrient digestibility and gas production of some tropical feeds used in ruminant diets estimated by the in vivo and in vitro gas production techniques. Am. J. Anim. Vet. Sci. 2007, 2, 108–113. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Hernández, J.; Mariezcurrena, M.; Lopez, S.; Camacho, L.; Márquez, O.; Salem, A.Z.M. Influence of the addition of exogenous xylanase with or without pre-incubation on the in vitro ruminal fermentation of three fibrous feeds. Czech J. Anim. Sci. 2016, 61, 262–272. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Bastida, A.Z.; Martínez, D.L.P.; Salem, A.Z.M. In vitro gas production of five rations of different maize silage and concentrate ratios influenced by increasing levels of chemically characterized extract of Salix babylonica. Turk. J. Vet. Anim. Sci. 2015, 39, 186–194. [Google Scholar] [CrossRef]
- Li, Q.; Xue, B.; Zhao, Y.; Wu, T.; Liu, H.; Yi, X.; Sun, C.; Wang, Z.; Zou, H.; Yan, T. In situ degradation kinetics of 6 roughages and the intestinal digestibility of the rumen undegradable protein. J. Anim. Sci. 2018, 96, 4835–4844. [Google Scholar] [CrossRef]
- Ivan, M.; Neill, L.; Forster, R.; Alimon, R.; Rode, L.; Entz, T. Effects of Isotricha, Dasytricha, Entodinium, and total fauna on ruminal fermentation and duodenal flow in wethers fed different diets. J. Dairy Sci. 2000, 83, 776–787. [Google Scholar] [CrossRef]
- Newbold, C.J.; de La Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The role of ciliate protozoa in the rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Gouda, G.A.; Patra, A.K. The sustainable mitigation of in vitro ruminal biogas emissions by ensiling date palm leaves and rice straw with lactic acid bacteria and Pleurotus ostreatus for cleaner livestock production. J. Appl. Microbiol. 2022, 132, 2925–2939. [Google Scholar] [CrossRef]
- Harikrishna, C.; Mahender, M.; Reddy, Y.; Prakash, M.; Sudhakar, K.; Pavani, M. Evaluation of in vitro gas production and nutrient digestibility of complete diets supplemented with different levels of thermotolerant yeast in Nellore rams. Vet. World 2012, 5, 477–485. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Gouda, G.A.; Anele, U.Y.; Galyean, M.L. Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim. Feed Sci. Technol. 2016, 217, 45–55. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-06997-7. [Google Scholar]
- Ahmed, M.H.; Elghandour, M.M.Y.; Salem, A.Z.M.; Zeweil, H.; Kholif, A.E.; Klieve, A.; Abdelrassol, A. Influence of Trichoderma reesei or Saccharomyces cerevisiae on performance, ruminal fermentation, carcass characteristics and blood biochemistry of lambs fed Atriplex nummularia and Acacia saligna mixture. Livest. Sci. 2015, 180, 90–97. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Dietary strategies to enrich milk with healthy fatty acids—A review. Ann. Anim. Sci. 2022, 22, 523–536. [Google Scholar] [CrossRef]
- Rigout, S.; Hurtaud, C.; Lemosquet, S.; Bach, A.; Rulquin, H. Lactational effect of propionic acid and duodenal glucose in cows. J. Dairy Sci. 2003, 86, 243–253. [Google Scholar] [CrossRef]

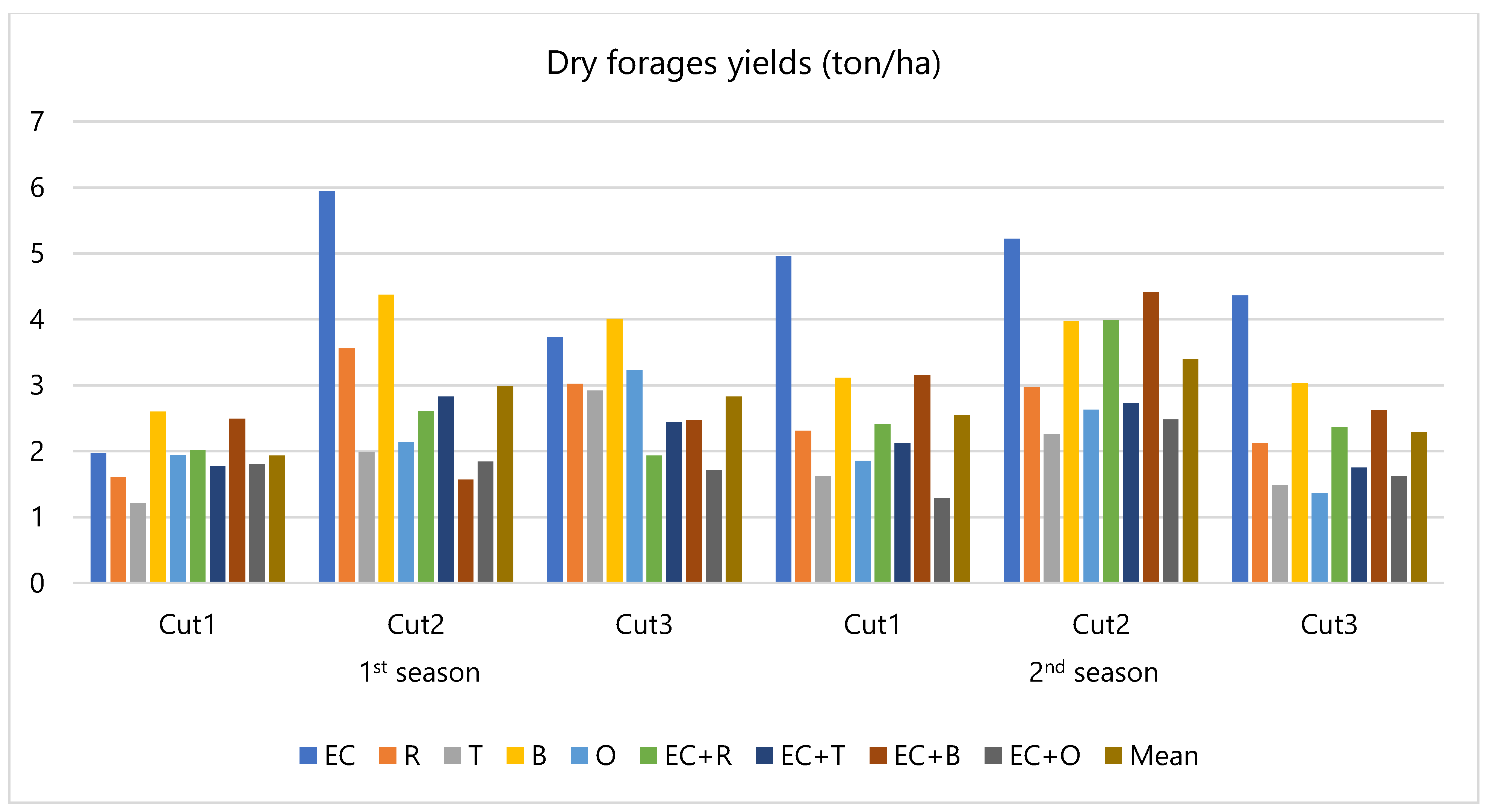
| Serial | Treatment | Designation | Mixing Ratio | |
|---|---|---|---|---|
| Egyptian Clover % | Grass % | |||
| 1 | Egyptian clover monoculture (Control) | EC | 100% | - |
| 2 | Ryegrass monoculture | R | - | 100% |
| 3 | Triticale monoculture | T | - | 100% |
| 4 | Barley monoculture | B | - | 100% |
| 5 | Oat monoculture | O | - | 100% |
| 6 | Egyptian clover + ryegrass intercropping | EC+R | 75% | 25% |
| 7 | Egyptian clover + triticale intercropping | EC+T | 75% | 25% |
| 8 | Egyptian clover + barley intercropping | EC+B | 75% | 25% |
| 9 | Egyptian clover + bats intercropping | EC+O | 75% | 25% |
| Source of Variance | Degree of Freedom | Mean Squares | |
|---|---|---|---|
| Fresh Forage Yield | Dry Forage Yield | ||
| Years | 1 | 49.56 ** | 0.74 n.s |
| Replications × years interaction | 2 | 2.33 | 1.33 |
| Cutting | 2 | 47.28 ** | 8.56 ** |
| Years × cutting interaction | 2 | 0.43 n.s | 3.34 ** |
| Error | 4 | 1.63 | 0.78 |
| Forages | 8 | 160.78 ** | 8.03 ** |
| Years × forages interaction | 8 | 15.69 ** | 1.19 ** |
| Cutting × forages interaction | 16 | 4.54 ** | 0.47 n.s |
| Years × cutting × forages interaction | 16 | 2.11 n.s | 0.88 * |
| Error | 48 | 1.71 | 0.31 |
| OM | CP | EE | NSC | NDF | ADF | Hemicellulose | Cellulose | Lignin | |
|---|---|---|---|---|---|---|---|---|---|
| EC | 862.5 c | 155.3 a | 17.1 cd | 136.9 cd | 553.2 d | 490.1 e | 63.1 bc | 335.0 d | 155.1 b |
| R | 872.7 bc | 114.1 cd | 13.4 f | 214.0 a | 531.3 e | 472.0 f | 59.3 c | 290.5 e | 181.5 a |
| T | 897.8 ab | 50.7 f | 23.0 b | 139.0 cd | 685.0 a | 621.3 b | 63.7 bc | 520.1 a | 101.2 d |
| B | 901.8 ab | 99.4 de | 16.0 de | 196.2 ab | 590.2 bc | 541.5 c | 48.7 c | 410.5 b | 131.0 bc |
| O | 910.9 a | 83.0 e | 25.8 a | 110.6 e | 691.6 a | 646.7 a | 44.8 c | 533.9 a | 112.9 cd |
| EC+R | 875.7 bc | 134.5 ab | 12.7 f | 118.0 d | 610.4 b | 530.1 cd | 80.3 ab | 397.8 bc | 132.3 bc |
| EC+T | 889.8 abc | 145.0 ab | 18.88 | 154.7 cd | 571.2 cd | 520.5 d | 50.7 c | 389.7 bc | 130.8 bc |
| EC+B | 876.2 bc | 125.1 bc | 14.5 ef | 166.4 bc | 570.1 cd | 522.0 d | 48.1 c | 381.8 c | 140.2 b |
| EC+O | 893.6 ab | 143.8 ab | 23.9 b | 113.8 d | 612.0 b | 525.0 d | 87.1 a | 384.5 c | 140.5 b |
| SEM | 9.35 | 6.67 | 0.64 | 12.56 | 7.31 | 4.92 | 5.79 | 6.86 | 7.41 |
| p value | 0.030 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 |
| Net GP | TDDM | TDOM | pH | NH3-N | Protozoa | PF | |
|---|---|---|---|---|---|---|---|
| EC | 168.0 b | 759.4 a | 733.9 a | 6.31 | 20.8 a | 3.00 d | 3.70 bc |
| R | 180.2 a | 710.6 b | 683.5 b | 6.12 | 15.4 bc | 4.80 bc | 3.25 cd |
| T | 165.4 b | 547.5 f | 532.5 fg | 6.14 | 16.6 bc | 6.50 ab | 2.95 d |
| B | 172.5 b | 637.2 d | 620.1 d | 6.10 | 15.3 bc | 3.55 cd | 3.27 cd |
| O | 137.2 cd | 533.1 f | 508.2 g | 6.09 | 16.9 bc | 5.85 ab | 3.68 bc |
| EC+R | 123.5 e | 576.1 e | 544.0 f | 6.20 | 14.7 c | 6.50 ab | 3.93 ab |
| EC+T | 132.6 d | 599.4 e | 574.1 e | 6.19 | 16.0 bc | 6.90 a | 4.16 ab |
| EC+B | 141.4 c | 686.1 bc | 657.2 bc | 6.16 | 16.9 bc | 5.05 abc | 4.36 a |
| EC+O | 138.5 cd | 659.6 cd | 630.4 cd | 6.12 | 18.8 ab | 6.40 ab | 4.30 a |
| SEM | 2.52 | 9.77 | 10.30 | 0.051 | 1.17 | 0.577 | 0.163 |
| p value | <0.001 | <0.001 | <0.001 | 0.095 | 0.007 | 0.009 | <0.001 |
| Total | Acetate | Propionate | Isobutyrate | Butyrate | Isovalerate | Valerate | |
|---|---|---|---|---|---|---|---|
| EC | 44.1 c | 28.4 d | 7.66 d | 0.93 | 3.74 cd | 2.56 | 0.80 d |
| R | 49.9 b | 31.8 b | 8.95 ab | 1.00 | 4.51 b | 2.76 | 0.89 c |
| T | 43.4 c | 27.4 d | 7.52 d | 0.92 | 4.35 b | 2.60 | 0.65 ef |
| B | 52.4 a | 33.0 a | 9.52 a | 0.94 | 5.17 a | 2.71 | 1.03 a |
| O | 43.9 c | 27.9 d | 7.81 d | 0.86 | 4.21 bc | 2.37 | 0.83 d |
| EC+R | 44.6 c | 28.3 d | 7.48 d | 0.95 | 4.53 b | 2.66 | 0.71 e |
| EC+T | 51.3 a | 32.8 a | 8.62 b | 1.02 | 5.06 a | 2.84 | 0.93 b |
| EC+B | 45.5 c | 28.8 d | 8.17 c | 0.90 | 4.27 c | 2.53 | 0.88 c |
| EC+O | 47.2 bc | 30.5 c | 8.24 c | 0.92 | 4.06 d | 2.58 | 0.92 b |
| SEM | 2.34 | 1.22 | 0.314 | 0.196 | 0.230 | 0.659 | 0.090 |
| p value | 0.004 | 0.030 | 0.014 | 0.088 | 0.044 | 0.921 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rady, A.M.S.; Attia, M.F.A.; Kholif, A.E.; Sallam, S.M.A.; Vargas-Bello-Pérez, E. Improving Fodder Yields and Nutritive Value of Some Forage Grasses as Animal Feeds through Intercropping with Egyptian Clover (Trifolium alexandrinum L.). Agronomy 2022, 12, 2589. https://doi.org/10.3390/agronomy12102589
Rady AMS, Attia MFA, Kholif AE, Sallam SMA, Vargas-Bello-Pérez E. Improving Fodder Yields and Nutritive Value of Some Forage Grasses as Animal Feeds through Intercropping with Egyptian Clover (Trifolium alexandrinum L.). Agronomy. 2022; 12(10):2589. https://doi.org/10.3390/agronomy12102589
Chicago/Turabian StyleRady, Asmaa M. S., Marwa F. A. Attia, Ahmed E. Kholif, Sobhy M. A. Sallam, and Einar Vargas-Bello-Pérez. 2022. "Improving Fodder Yields and Nutritive Value of Some Forage Grasses as Animal Feeds through Intercropping with Egyptian Clover (Trifolium alexandrinum L.)" Agronomy 12, no. 10: 2589. https://doi.org/10.3390/agronomy12102589
APA StyleRady, A. M. S., Attia, M. F. A., Kholif, A. E., Sallam, S. M. A., & Vargas-Bello-Pérez, E. (2022). Improving Fodder Yields and Nutritive Value of Some Forage Grasses as Animal Feeds through Intercropping with Egyptian Clover (Trifolium alexandrinum L.). Agronomy, 12(10), 2589. https://doi.org/10.3390/agronomy12102589







