The Effects of Agricultural Plastic Waste on the Vermicompost Process and Health Status of Eisenia fetida
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Set-Up
2.2.1. Feedstock Characteristics
2.2.2. Earthworms
2.2.3. Plastic Material
2.2.4. Biofilm Sample
2.3. Analytical Methods
2.3.1. Vermicompost Physicochemical Parameters
2.3.2. Vermicompost and Biofilm Enzymatic Activity
2.3.3. Eisenia fetida Survival and Body Weight
2.3.4. Earthworm Biomarkers
2.3.5. Statistical Analysis
3. Results
3.1. Effect of AWP on Vermicompost Physicochemical Parameters
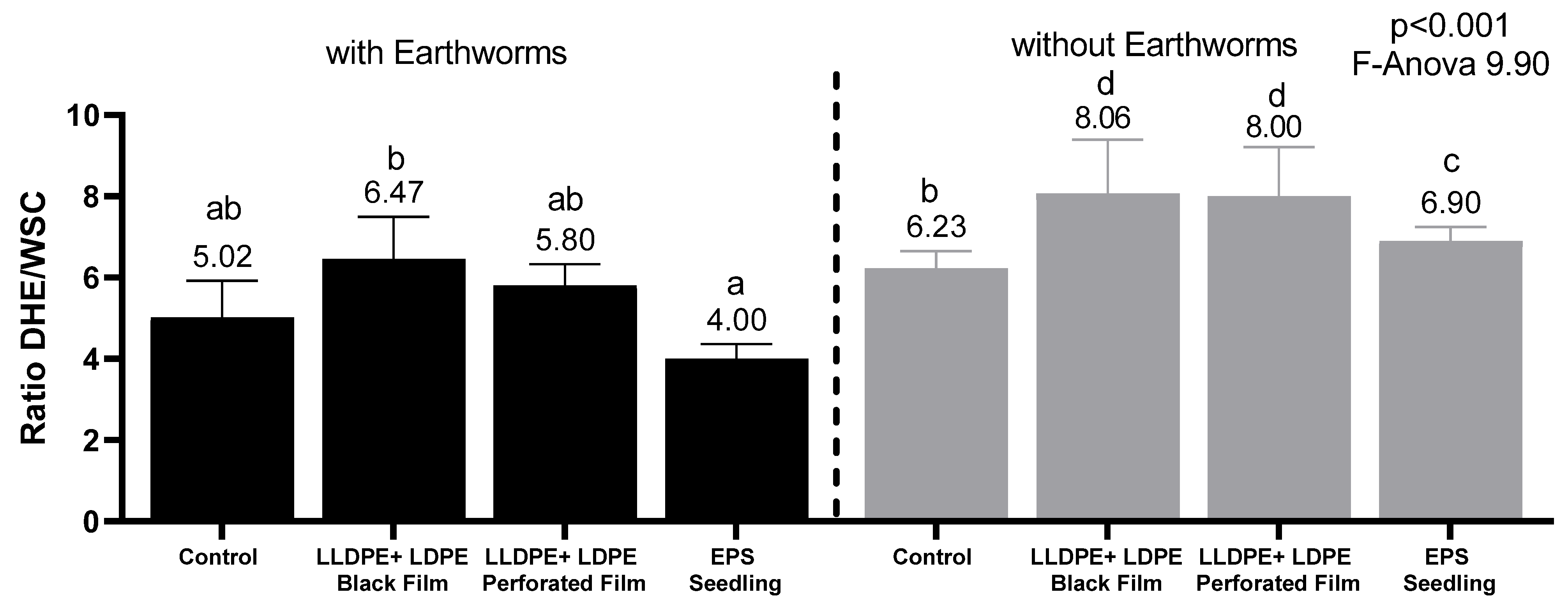
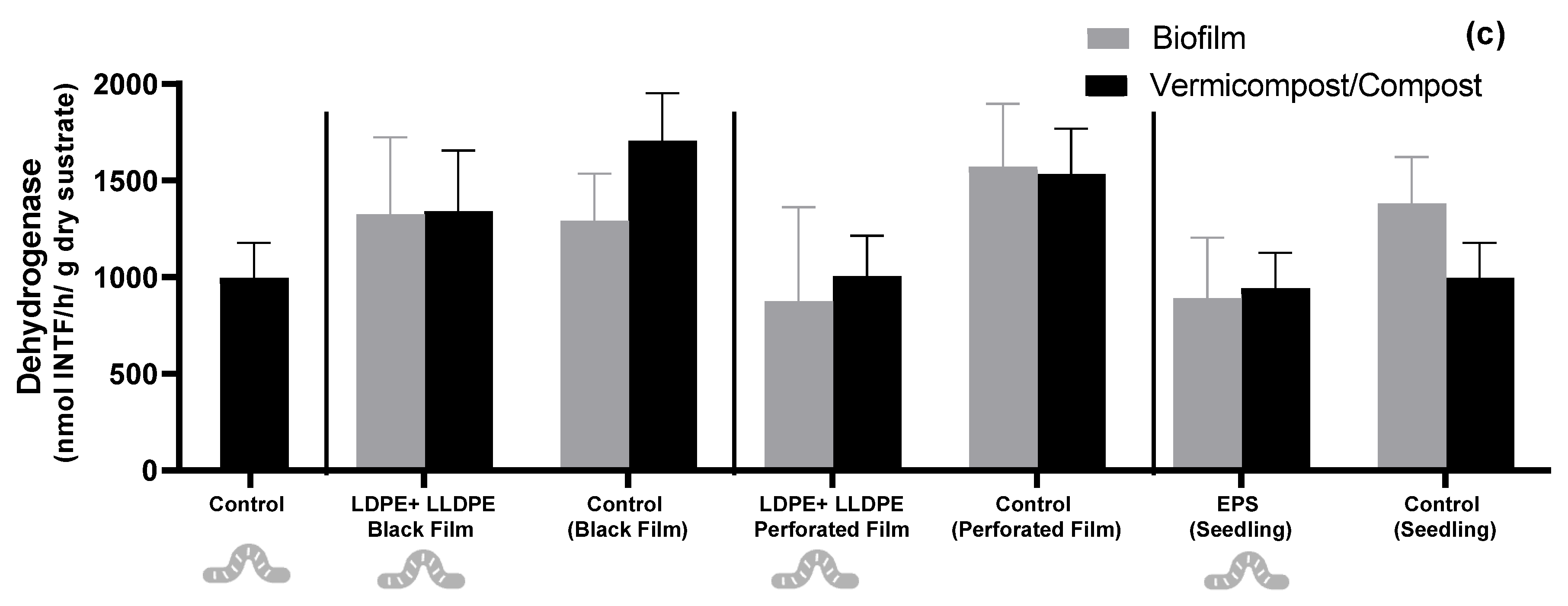
3.2. Vermicompost and Biofilm Exoenzymatic Activity
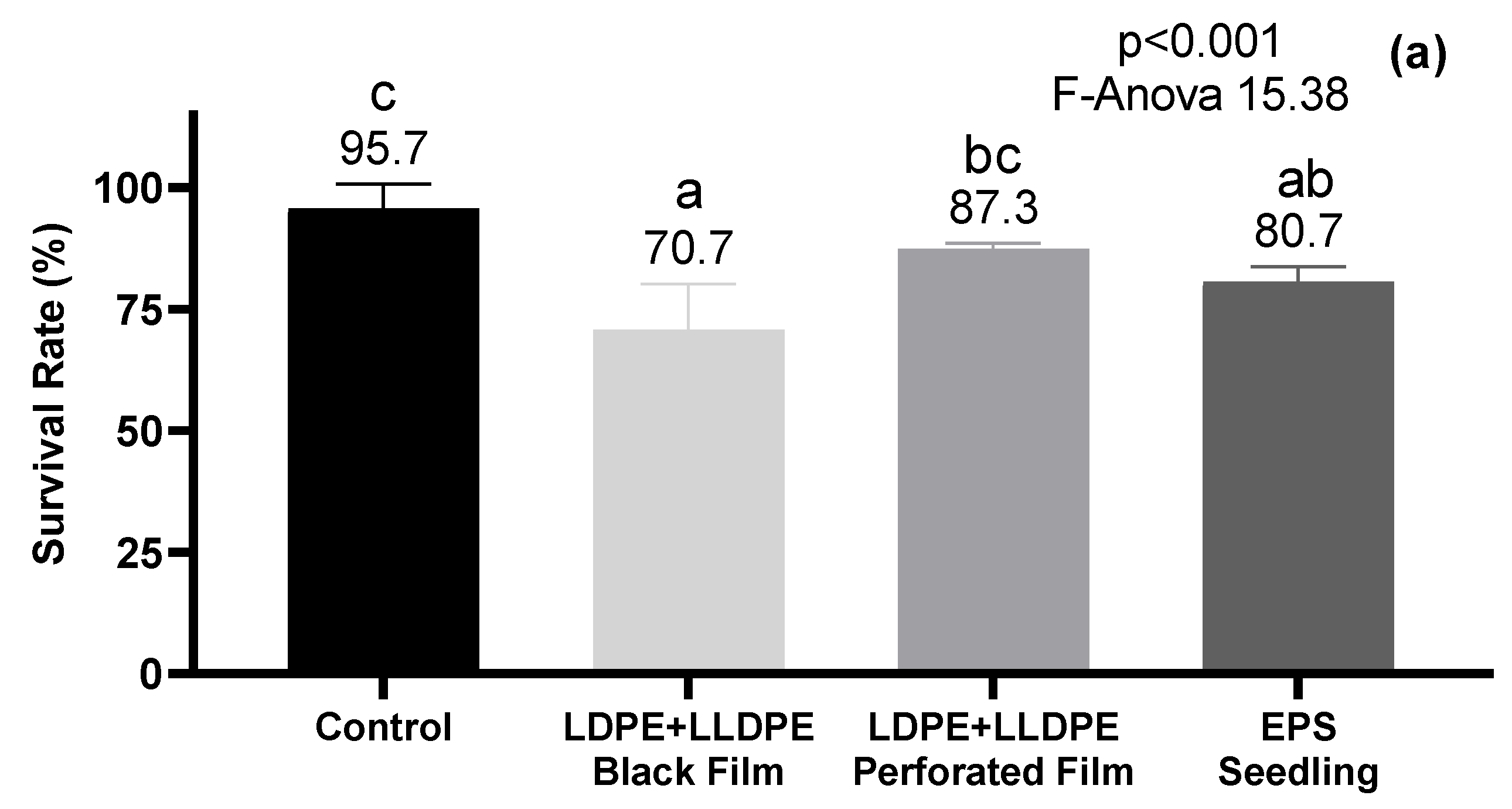
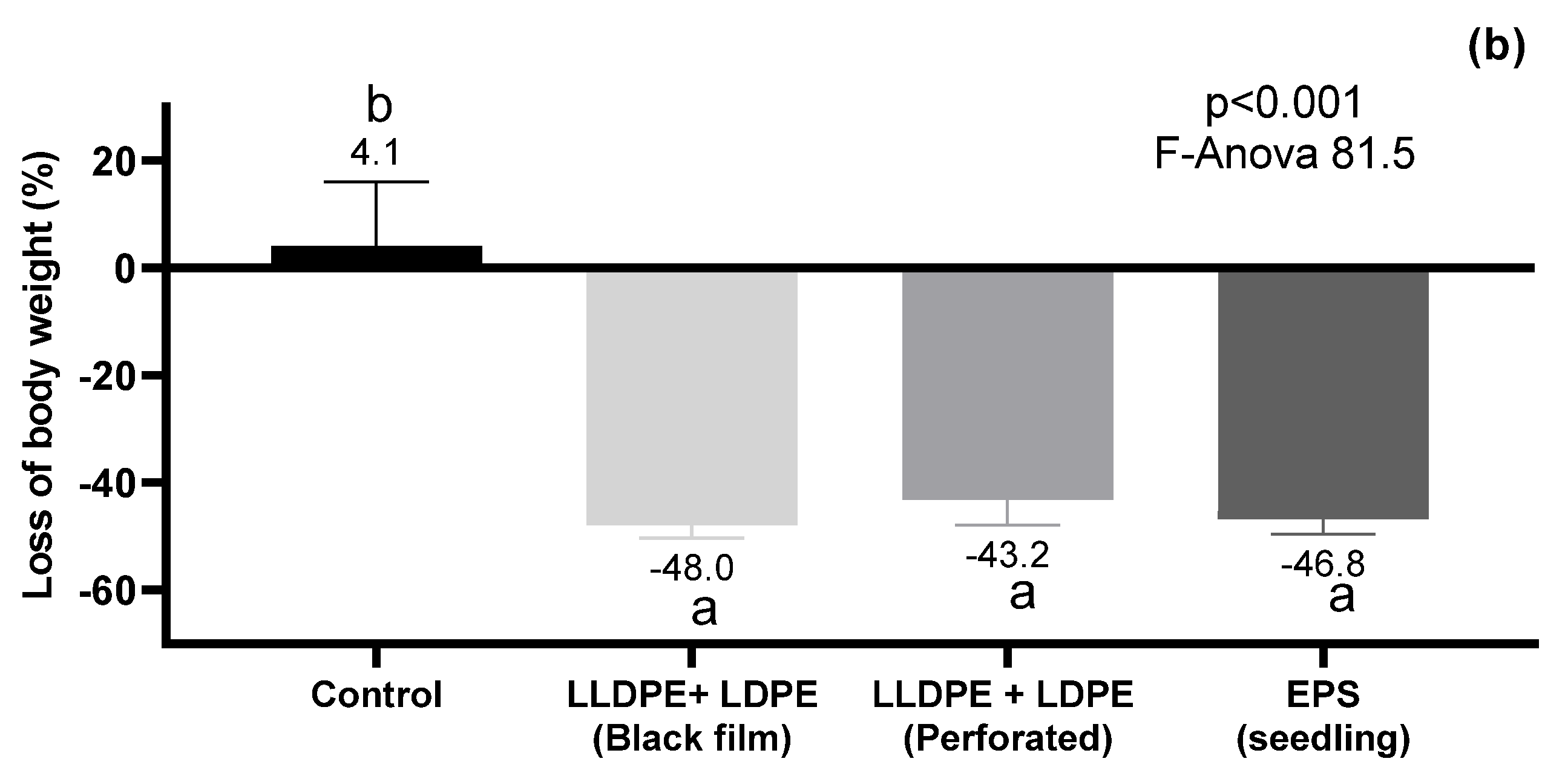
3.3. Eisenia fetida Survival and Body Weight
3.4. Earthworm Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lee, H.; Lee, H. Abundance and characteristics of microplastics in soils with different agricultural practices: Importance of sources with internal origin and environmental fate. J. Hazard. Mater. 2021, 403, 123997. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Fambri, L.; Lonardi, C.; Slouf, M.; Kolarik, J. Linear low density polyethylene/cycloolefin copolymer blends. Express Polym. Lett. 2011, 5, 23–37. [Google Scholar] [CrossRef]
- Ren, S.; Kong, S.; Ni, H. Contribution of mulch film to microplastics in agricultural soil and surface water in China. Environ. Pollut. 2021, 291, 118227. [Google Scholar] [CrossRef]
- Zhang, J.; Siyang, R.; Xu, W.; Liang, C.; Li, J.; Zhang, H.; Li, Y.; Liu, X.L.; Jones, D.; Chadwick, D.; et al. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater. 2022, 435, 129065. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Linb, H.; Chenb, H.; Jin, X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.; Löder, M.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, W. Biodegradation of Plastics in Tenebrio Genus (Mealworms). In Microplastics in Terrestrial Environments; Springer: Berlin/Heidelberg, Germany, 2020; pp. 385–422. [Google Scholar]
- Mintenig, S.M.; Int-Veen, I.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane arraybased micro-Fourier-transform infrared imaging. Water 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assesment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Villar, I.; Alves, D.; Salustiano, M. Product quality and microbial dynamics during vermicomposting and maturation of compost from pig manure. Waste Manag. 2017, 69, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Gajst, T. Analysis of Plastic Residues in Commercial Compost. Bachelors Thesis, University Nova Gorica, Nova Gorica, Slovenia, 2016. [Google Scholar]
- Lazcano, C.; Gómez-Brandón, M.; Domínguez, J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 2008, 72, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; Mendoza-Hernández, D.; García-de-la-Fuente, R.; Abad, M.; Belda, R.M. Composting versus vermicomposting: A comparative study of organic matter evolution through straight and combined processes. Bioresour. Technol. 2012, 118, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Villar, I.; Alves, D.; Pérez-Díaz, D.; Mato, S. Changes in microbial dynamics during vermicomposting of fresh and composted sewage sludge. Waste Manag. 2016, 48, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Villar, I.; Alves, D.; Garrido, J.; Mato, S. Evolution of microbial dynamics during the maturation phase of the composting of dyferent type of waste. Waste Manag. 2016, 54, 83–92. [Google Scholar] [CrossRef]
- Rochman, C.; Hoh, E.; Hentschel, B.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Balbi, T.; Salis, A.; Damonte, G.; Cortese, K.; Caratto, V.; Monopoli, M.; Dawson, K.; et al. Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: Role of soluble hemolymph proteins. Environ. Res. 2016, 150, 73–81. [Google Scholar] [CrossRef]
- Iñiguez, M.; Conesa, J.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 8620. [Google Scholar] [CrossRef]
- Sharifinia, M.; Bahmanbeigloo, Z.; Keshavarzifard, M.; Khanjani, M.; Lyons, B. Microplastic pollution as a grand challenge in marine research: A closer look at their adverse impacts on the immune and reproductive systems. Ecotoxicol. Environ. Saf. 2020, 204, 111109. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; da Costa, J.; Rocha-Santos, T.; Duarte, A.; Pereira, R. Oxidative stress, energy metabolism and molecular responses of earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics. Environ. Sci. Pollut. Res. 2018, 25, 33599–33610. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Adams, C.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127–531. [Google Scholar] [CrossRef]
- de Souza Machado, A.; Lau, C.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard. Mater. 2020, 392, 122–273. [Google Scholar] [CrossRef]
- Pathan, S.; Arfaioli, P.; Bardelli, T.; Ceccherini, M.; Nannipieri, P.; Pietramellara, G. Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard. Sustainability 2020, 12, 7255. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Benali, S.; Cyriaque, V.; Moins, S.; Raquez, J.; Gobert, S.; Wattiez, R. Microbial biofilm composition and polymer degradation of compostable and non-compostable plastics immersed in the marine environment. J. Hazard. Mater. 2021, 419, 126526. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals, Section 2. Available online: https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-2-effects-on-biotic-systems_20745761 (accessed on 30 June 2022).
- Ramos-Martinez, J.I.; Bartolomé, T.R.; Pernas, R.V. Purification and properties of glutathione reductase from hepatopancreas of Mytilus edulis L. Comp. Biochem. Physiol. 1983, 75, 689–692. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Yadav, K.; Tare, V.; Ahammed, M. Vermicomposting of source-separated human faeces by Eisenia fetida: Effect of stocking density on feed consumption rate, growth characteristics and vermicompost production. Waste Manag. 2011, 31, 1162–1168. [Google Scholar] [CrossRef]
- Macci, C.; Masciandaro, G.; Ceccanti, B. Vermicomposting of olive oil mill wastewaters. Waste Manag. Res. J. A Sustain. Circ. Econ. 2009, 28, 738–747. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.C. Chapter 11-Pesticide Biomarkers in Terrestrial Invertebrates. In Pesticides in the Modern World—Pests Control and Pesticides Exposure and Toxicity Assessment; IntechOpen Limited: London, UK, 2011. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.C.; Mazzia, C.; Capowiez, Y.; Raul, M. Carboxylesterase activity in earthworm gut contents: Potential (eco) toxicological implications. Comp. Biochem. Physiol. 2009, 150, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Godínez, E.; Lagunes-Zarate, J.; Corona-Hernández, J.; Barajas-Aceves, M. Growth and reproductive potential of Eisenia foetida (Sav) on various zoo animal dungs after two methods of pre-composting followed by vermicomposting. Waste Manag. 2017, 64, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, H.; Hatti, S. Earthworms diversity of Koppal district with the updated information on genus Thatonia of Hyderabad-Karnataka region, Karnataca, India. J. Asia-Pac. Biodivers. 2018, 11, 482–493. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, C.; Prater, I.; Angst, Š.; Frouz, J.; Jílková, V.; Peterse, F.; Nierop, K.G.J. Earthworms act as biochemical reactors to convert labile plant compounds into stabilized soil microbial necromass. Commun. Biol. 2019, 2, 441. [Google Scholar] [CrossRef]
- Nogales, R.; Romero, E.; Benítez, E.; Polo, A.Y. Reciclaje de residuos orgánicos. In Ciencias y Medio Ambiente; Valladares, F., Ed.; CCMA-CSIC: Madrid, Spain, 2002; pp. 115–124. [Google Scholar]
- Khalil, H.; Sanaa, S. Application of Sewage Sludge in Composting Technology for Eradication of Pathogenic Bacteria. Aust. J. Basic Appl. Sci. 2022, 3, 4591–4600. [Google Scholar]
- Fernández-Gómez, M.; Romero, E.; Nogales, R. Feasibility of vermicomposting for vegetable greenhouse waste recycling. Bioresour. Technol. 2010, 101, 9654–9660. [Google Scholar] [CrossRef]
- Huang, K.; Xia, H.; Cui, G.; Li, F. Effects of earthworms on nitrification and ammonia oxidizers in vermicomposting systems for recycling of fruit and vegetable wastes. Sci. Total Environ. 2017, 578, 337–345. [Google Scholar] [CrossRef]
- Nogales, R.; Saavedra Fecci, M.; Benitez, E. Recycling of wet olive cake “alperujo” through treatment with fungi and subsequent vermicomposting. Fresenius Environ. Bull. 2008, 17, 1822–1827. [Google Scholar]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of composts in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef]
- Domínguez, J.; Aira, M.; Gómez-Brandón, M. Vermicomposting: Earthworms enhances the work of microbes. In Microbes at Work: From Wastes to Resources; Insam, H., Franke-Whittle, I., Goberna, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 93–114. [Google Scholar]
- García-Sánchez, M.; Taušnerová, H.; Hanč, A.; Tlustoš, P. Stabilization of different starting materials through vermicomposting in a continuous-feeding system: Changes in chemical and biological parameters. Waste Manag. 2017, 62, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Nogales, R.; Fernandez-Gómez, J.; Delgado –Moreno, L.; Castillo-Diaz, J.M.; Romero, E. Eco-fiendly vermitechnological winery waste management: A pilot-scale study. Springer Nat. 2020, 2, 653. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, Z.; Qian, C.; Yu, H.Q. Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Eviron. Pollut. 2018, 233, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Riah, W.; Laval, K.; Laroche-Ajzenberg, E.; Mougin, C.; Latour, X.; Trinsoutrot-Gattin, I. Effects of pesticides on soil enzymes: A review. Environ. Chem. Lett. 2014, 12, 257–273. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Sandoval, M.; Pierart, A. Short-term response of soil enzyme activities in a chlorpyrifos-treated mesocosm: Use of enzyme-based indexes. Ecol. Indic. 2017, 73, 525–535. [Google Scholar] [CrossRef]
- Serrano-García, N.; Vaca, R.; Lugo, J.; Aguila, P. Effects of residual sludge and vermicompost organic residues on inorganic indicators and catalase. Rev. Int. Contam. Ambient. 2022, 33, 173–179. [Google Scholar]
- Ouni, Y.; Lakhdar, A.; Scelza, R.; Scotti, R.; Abdelly, C.; Barhoumi, Z.; Rao, M. Effects of two composts and two grasses on microbial biomass and biological activity in a salt-affected soil. Ecol. Eng. 2013, 60, 363–369. [Google Scholar] [CrossRef]
- Malinska, K.; Zabochnicka-Swiatek, M.; Cáceres, R.; Marfà, O. The effect of precomposted sewage sludge mixture amended with biochar on the growth and reproduction of Eisenia fetida during laboratory vermicomposting. Ecol. Eng. 2016, 90, 35–41. [Google Scholar] [CrossRef]
- Sarker, A.; Deepo, D.M.; Nandi, R.; Rana, J.; Islam, S.; Rahman, S.; Hossain, M.N.; Islam, M.S.; Baroi, A.; Kim, J.-E. A review of microplastics pollution in the soil and terrestrial ecosystems: A global and Bangladesh perspective. Sci. Total Environ. 2020, 733, 139–296. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X.; Li, H. Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci. Total Environ. 2020, 708, 134–841. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense responses in earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics in soils. Ecotoxicol. Environ. Saf. 2020, 187, 109–788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113–896. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Seijo, A.; Santos, B.; Ferreira da Silva, E.; Cachada, A.; Pereira, R. Low-density polyethylene microplastics as a source and carriers of agrochemicals to soil and earthworms. Environ. Chem. 2019, 16, 8. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Impact of microplastic beads and fibers on waterflea (Ceriodaphnia dubia) survival, growth and reproduction: Implications of single and mixture exposures. Environ. Sci. Technol. 2017, 51, 13397–13406. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raleysusman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis Elegans. Sci. Total Environ. 2018, 619, 1–8. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Capowiez, Y.; Ro, K. Potential Use of Earthworms to Enhance Decaying of Biodegradable Plastics. ACS Sustain. Chem. Eng. 2020, 8, 4292–4316. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Luís, G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei: Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Lackman, C.; Velki, M.; Simic, A.; Müller, A.; Braun, U.; Ecimovic, S.; Hollert, H. Two types of mircoplastics (polystyrene-HBCD and car tire abrasion) affect oxidative stress-related biomarkers in earthworm Eisenia Andrei in time-dependent manner. Environ. Int. 2022, 163, 107190. [Google Scholar] [CrossRef]
- Li, B.; Song, W.; Cheng, Y.; Zhang, K.; Tian, H.; Du, Z.; Wang, J.; Wang, J.; Zhang, W.; Zhu, L. Ecotoxicological effects of different size ranges of industrial-grade polyethylene and propylene microplastics on earthworms Eisenia fetida. Sci. Total Environ. 2021, 783, 147007. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Coffin, S.; Sun, C.; Schlenk, D.; Gan, J. Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida. Soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Gagné, F.; André, C.; Della Torre, C.; Auclair, J.; Hanana, H.; Parenti, C.C.; Bonasoro, F.; Binelli, A. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 2018, 631–632, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrenemicroplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, F.; Cui, Y.; Li, M. Ecotoxicological effects of earthworm following longterm Dechlorane Plus exposure. Chemosphere 2016, 144, 2476–2481. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.-L.; Shi, Y.; Chen, C.; Yang, Z.; Li, Y.; Feng, Y. Antioxidant and metabolic responses induced by cadmium and pyrene in the earthworm Eisenia fetida in two different systems: Contact and soil tests. Chem. Ecol. 2009, 25, 205–215. [Google Scholar] [CrossRef]

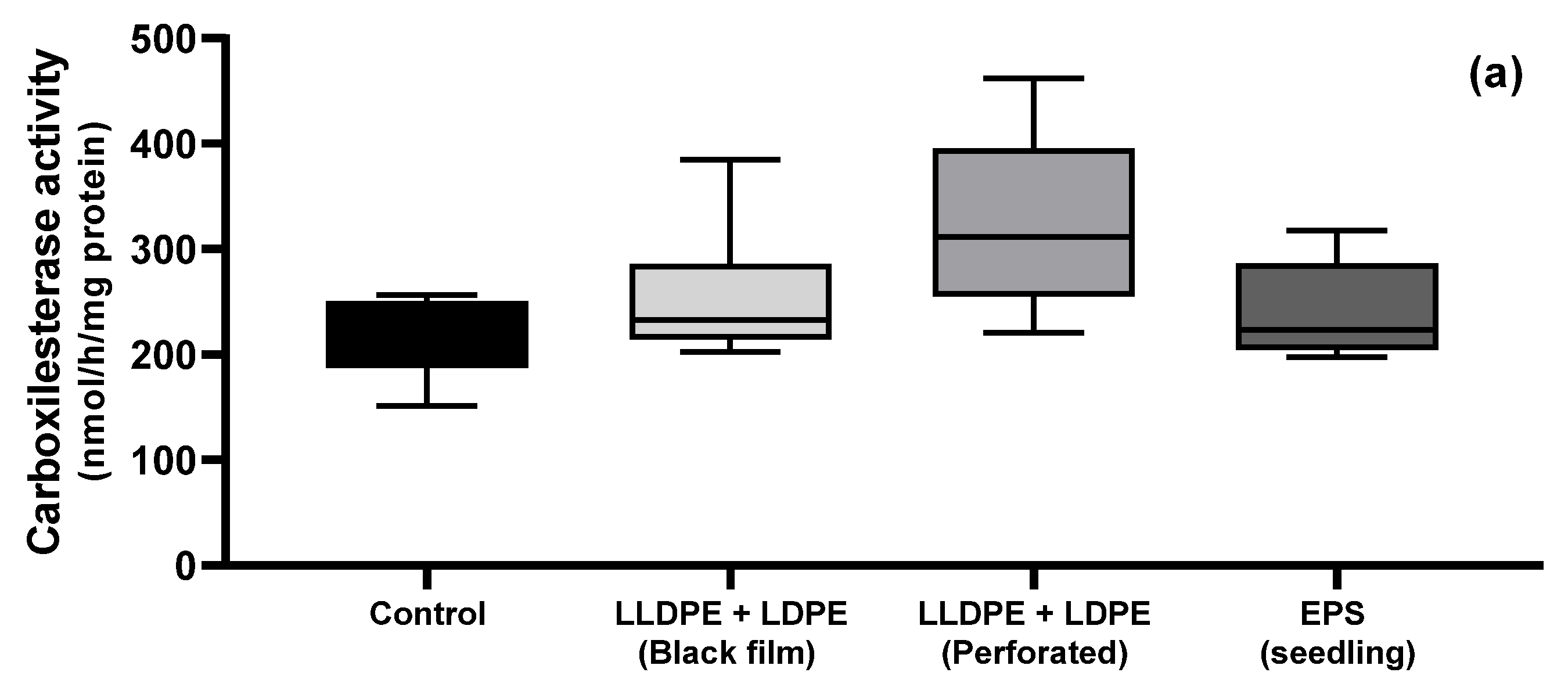
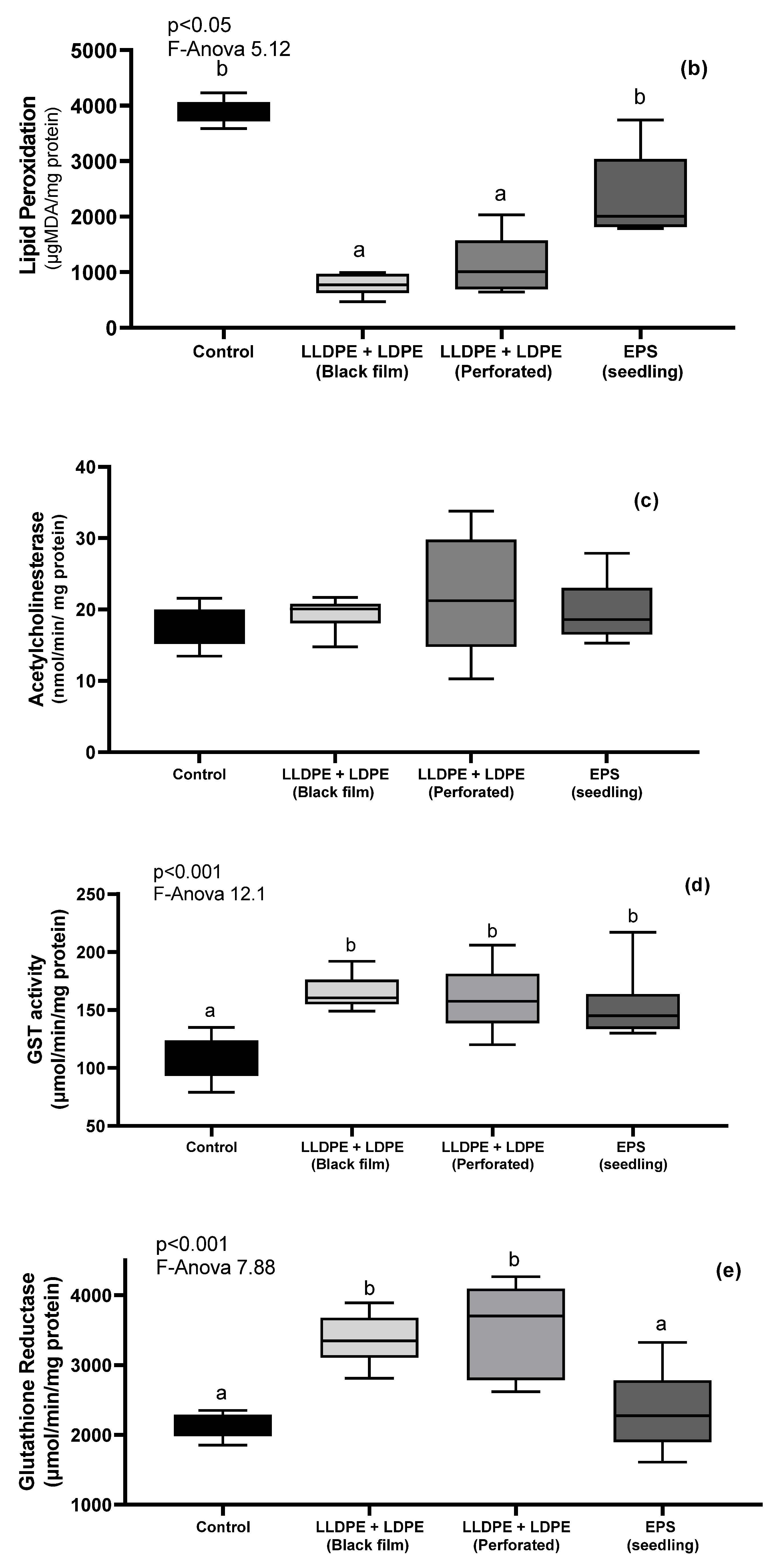
| Physicochemical Parameters | Macronutrients | Mature Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | EC (dS m−1) | BD (g L−1) | TOM (%) | TOC (g kg−1) | TN (%) | P (%) | K (%) | GI (%) | CHA (%) | CFA (%) | CEC (meq 100 g−1 MO) |
| 7.8 | 4.5 | 486 | 63.0 | 225 | 2.13 | 0.41 | 1.01 | 108 | 1.93 | 2.27 | 128 |
| E. fetida Presence | Type of APW | pH | EC (dS m−1) | TOM (%) | TOC (%) | TN (%) | P (%) | K (%) | WSC (g kg−1) | CFA (%) | CHA (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No plastic t = 45 d | 7.42 a | 5.42 | 52.9 | 23.7 b | 2.24 a | 0.81 | 1.28 | 8.83 a | 3.66 c | 3.58 |
| LDPE + LLDPE black film | 7.24 a | 4.81 | 53.6 | 26.2 c | 2.31 ab | 0.82 | 1.27 | 9.00 a | 2.63 a | 4.11 | |
| LDPE + LLDPE perforated film | 7.46 a | 4.42 | 55.7 | 24.4 b | 2.29 ab | 0.82 | 1.16 | 8.36 a | 2.47 a | 3.27 | |
| EPS seedling | 7.21 a | 4.56 | 57.1 | 24.6 b | 2.24 a | 0.81 | 1.19 | 8.33 a | 2.45 a | 3.17 | |
| No | No plastic t = 45 days | 7.88 b | 4.17 | 55.3 | 27.3 c | 2.53 c | 0.72 | 1.36 | 9.89 b | 2.57 a | 3.16 |
| LDPE + LLDPE black film | 8.00 bc | 3.26 | 52.4 | 24.3 b | 2.38 b | 0.83 | 1.08 | 10.3 b | 3.31 b | 4.96 | |
| LDPE + LLDPE perforated film | 8.20 c | 3.20 | 54.4 | 24.0 b | 2.34 b | 0.81 | 1.12 | 9.23 b | 3.01 b | 3.76 | |
| EPS seedling | 8.20 c | 2.90 | 55.2 | 22.6 a | 2.25 a | 0.81 | 1.11 | 10.2 b | 2.71 ab | 3.87 | |
| Main effects | |||||||||||
| E. fetida presence | Yes | 7.35 a | 4.92 b | 54.4 | 24.8 b | 2.26 a | 0.81 | 1.22 | 8.41 a | 3.01 b | 3.52 a |
| No | 8.07 b | 3.38 a | 54.3 | 24.4 a | 2.37 b | 0.79 | 1.19 | 9.82 b | 2.86 a | 3.89 b | |
| Type of APW | No plastic t = 45 d | 7.57 a | 5.0 c | 53.7 ab | 24.9 b | 2.33 b | 0.78 | 1.28 | 8.83 | 3.32 c | 3.42 a |
| LDPE + LLDPE black film | 7.61 a | 4.04 b | 53.0 a | 25.3 b | 2.34 b | 0.82 | 1.18 | 9.65 | 2.97 b | 4.53 b | |
| LDPE + LLDPE perforated film | 7.83 c | 3.81 a | 55.0 ab | 24.2 a | 2.31 b | 0.81 | 1.14 | 8.80 | 2.74 a | 3.52 a | |
| EPS seedling | 7.70 b | 3.73 a | 56.1 b | 23.6 a | 2.24 a | 0.81 | 1.16 | 9.26 | 2.58 a | 3.58 a | |
| Statistical significance | |||||||||||
| E. fetida presence | *** | *** | ns | *** | ** | ns | ns | *** | *** | * | |
| Type of APW | *** | *** | * | *** | *** | ns | ns | ns | *** | ** | |
| E. fetida x APW | *** | ns | ns | ** | *** | ns | ns | *** | *** | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez, J.A.; Pedraza Torres, A.M.; Blesa Marco, Z.E.; Andreu-Rodríguez, F.J.; Marhuenda-Egea, F.C.; Martínez-Sabater, E.; López, M.J.; Suarez-Estrella, F.; Moral, R. The Effects of Agricultural Plastic Waste on the Vermicompost Process and Health Status of Eisenia fetida. Agronomy 2022, 12, 2547. https://doi.org/10.3390/agronomy12102547
Sáez JA, Pedraza Torres AM, Blesa Marco ZE, Andreu-Rodríguez FJ, Marhuenda-Egea FC, Martínez-Sabater E, López MJ, Suarez-Estrella F, Moral R. The Effects of Agricultural Plastic Waste on the Vermicompost Process and Health Status of Eisenia fetida. Agronomy. 2022; 12(10):2547. https://doi.org/10.3390/agronomy12102547
Chicago/Turabian StyleSáez, José A., Angie M. Pedraza Torres, Zbigniew Emil Blesa Marco, Francisco Javier Andreu-Rodríguez, Frutos C. Marhuenda-Egea, Encarnación Martínez-Sabater, María J. López, Francisca Suarez-Estrella, and Raúl Moral. 2022. "The Effects of Agricultural Plastic Waste on the Vermicompost Process and Health Status of Eisenia fetida" Agronomy 12, no. 10: 2547. https://doi.org/10.3390/agronomy12102547
APA StyleSáez, J. A., Pedraza Torres, A. M., Blesa Marco, Z. E., Andreu-Rodríguez, F. J., Marhuenda-Egea, F. C., Martínez-Sabater, E., López, M. J., Suarez-Estrella, F., & Moral, R. (2022). The Effects of Agricultural Plastic Waste on the Vermicompost Process and Health Status of Eisenia fetida. Agronomy, 12(10), 2547. https://doi.org/10.3390/agronomy12102547







