Abstract
Biostimulants constitute an emerging group of crop management products used to enhance productivity under abiotic stress conditions. The ability of some biostimulant products, such as seaweed extracts (SE), to enhance crop tolerance to salinity stress has been documented. SE contain a series of bioactive compounds and signaling molecules, as well as mineral and organic nutrients, that greatly benefit plants. A greenhouse experiment was conducted in order to evaluate SE-mediated tolerance mechanisms in tomato plants under salinity stress. The experiment was divided into two developmental phases (vegetative and reproductive) and included four treatments: control (plants with neither treatment), SE (plants treated with seaweed extract), NaCl (plants irrigated with 300 mM NaCl), and SE + NaCl (plants treated with seaweed extract and irrigated with 300 mM NaCl). Tomato plants treated with the SE from Padina gymnospora showed an increase in root and shoot length (18 cm and 13 cm), root and shoot area (33 cm2 and 98 cm2), and shoot and root fresh weight (1.0 and 3.8 g) under the control and salinity stress conditions. The decrease in productivity (number of fruits) associated with salinity stress was reduced from 28.7% to only 3.4% in SE-treated plants. The positive effects of SE application also included early flowering and enhanced fruit weight and quality. Our findings suggest that optimized photosynthetic performance and antioxidant defense systems (proline, total phenols, and flavonoids) appear to be major factors modulating SE responses to salinity tolerance in tomato plants with promising agricultural applications.
1. Introduction
Abiotic stress constitutes a threat to plant growth and productivity in modern agricultural practices. Salinity stress is of particular concern, as it continues to be responsible for substantial crop losses worldwide [1]. In fact, salinity stress is one of the most serious limiting factors for crop production worldwide. Globally, over 900 million hectares or 6% of land are affected by saline [2]. It is very difficult to prevent salinity stress and even more difficult to reclaim soils once they have become salinized. As a matter of global food security, solutions must be found to prevent and treat soil salinization and to support plant growth and productivity under conditions of salinity stress.
In addition, ~20% of irrigated agricultural land is classified as saline, and ~1% of agricultural land (2 million ha) is deteriorated by salinity annually, which diminishes or halts crop production [2]. In Latin America and the Caribbean, approximately 18.4 million ha are affected by irrigation-induced soil salinization [3]. In Mexico, approximately 6 million ha of all irrigated agricultural lands (i.e., ~20%) are affected by soil salinity stress [3]. This is incredibly problematic given the importance of many crops that are produced within the country. According to the Observatory of Economic Complexity (OEC), one of the top exporters of tomatoes worldwide in 2020 was Mexico (USD 2.62 B), which produced ~4 million metric tons in that year that required ~93,000 ha of agricultural land [3,4].
Tomato Solanum lycopersicum L. is a high-value fruit that is extensively cultivated worldwide. Tomatoes are commonly cultivated in open fields or under greenhouse conditions. At present, tomato cultivars have the genetic potential to tolerate mild-to-medium salinity stress [5]. However, tomato plants are not exempt from the negative effects associated with salinity stress during the stages of germination and vegetative growth, which inevitably affects the yield, quality, and fruit production [6,7,8,9]. In addition, tomato plants appear to be particularly sensitive to salinity stress during the early growth stages [7,10].
The occurrence of abiotic stresses, e.g., high salt concentrations in soils, produces osmotic stress, which, in turn, hinders the water and nutrient uptake by plants. The rates of transpiration and photosynthesis also decrease under conditions of osmotic stress, and the opening and closing of stomata are generally quite quick. In addition, ion toxicity (a high influx of Na+ ions resulting in the displacement of K+ ions from many enzymes and cofactors), membrane instability, and mineral limitation can be observed under these conditions, along with the inhibition of multiple enzymes and metabolic pathways [11].
The overall results of salinity stress can be seen in impaired plant growth and physiological functions and decreased crop yields, although these depend on the severity of the stress, the time scale of the response, and whether or not the onset of stress was abrupt or gradual [12]. Salinity stress also induces the production of reactive oxygen species (ROS), which causes additional oxidative stress. Although plants can activate some protective mechanisms (e.g., deploying antioxidant compounds) to defend themselves from stressful conditions, severe and long-term exposure to stress can cause physiological disturbances in metabolic processes [12,13].
Plants employ multiple mechanisms to adjust and adapt to stressful conditions, including those involved in recovery and signaling, stabilizing proteins and protein complexes in the chloroplast and cytosol, and protecting the photosynthetic apparatus [14]. The photosynthetic apparatus is an important site for the production of ROS which include superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals [15]. These ROS disrupt normal cellular metabolism by altering membranes and damaging lipids, proteins, and nucleic acids [15].
Plants have developed complex defense mechanisms to mitigate the deleterious effects of oxidative damage that involve a series of enzymatic antioxidants, such as catalase (CAT), glutathione (GSH), superoxide dismutase (SOD), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX), and nonenzymatic antioxidants, which include ascorbate, carotenoids, flavonoids, and other phenolics [16]. In addition, it is widely understood that the biosynthesis of many primary and secondary metabolites is an important component of plant defense responses to stressful conditions [17].
Moreover, natural plant antioxidant enzymes and nonenzymatic antioxidants induced by abiotic stresses such as climate change (temperature, water deficit as drought, salinity stress, and nutrient deficiency); heavy metals; fungicide; and pesticides can mitigate harmful effect in different ways [18,19,20].
Recent crop studies have revealed that salinity tolerance during the early growth stages is more important than during the subsequent plant growth stages [7,10]. Growth maintenance, which can be defined as an increase in mass that mainly occurs during the early growth stages, has been widely used as an important indicator of salinity tolerance [21]. Many studies have also suggested different approaches to identifying genotypes tolerant to abiotic stress based on stress indices or the selection criteria of biomass production or the yield, the latter being of greater interest to the agricultural industry [10,22].
To prevent low yields associated with salinity stress, plant biostimulants (PBs) are being increasingly integrated into production systems to favorably modify physiological processes and enhance plant productivity [23]. A plant biostimulant is “a formulated product of biological origin that improves plant productivity as a consequence of the novel or emergent properties of the constituents and not as a sole consequence of the presence of known essential plant nutrients, plant growth regulators, or plant protective compounds” [23]. According to this claim-oriented definition, PBs are products that must be able to improve one or more of the following plant or plant rhizosphere characteristics: (i) nutrient use efficiency, (ii) tolerance to abiotic stressors, (iii) quality traits, or (iv) the availability of confined nutrients in the soil or rhizosphere [24].
Plant biostimulants are commonly used in horticulture to increase the yield quantity and quality and improve stress tolerance. Plant biostimulants can be effective in promoting the accumulation of osmolytes, which helps the plant counteract the negative effects of saline stress, thus limiting production losses [25]. Plant biostimulants derived from seaweed extracts (SE) contain plant hormones such as cytokinins, auxins, and other hormone-like substances [26,27].
Seaweed extracts (SEs) also contain many mineral and bioactive compounds, including complex polysaccharides and phycocolloids (e.g., fucoidan, alginate, and carrageenan), that have been proven to enhance salinity tolerance in plants [28,29,30]. There are numerous reports of the beneficial effects of the application of SEs related to increases in plant growth and the yield parameters [31,32]. In tomato plants, SEs have been shown to increase the tolerance to salinity stress [33]. Tomato seeds soaked in natural SEs that were allowed to germinate showed improved plant productivity and growth under conditions of salinity stress [34,35]. In addition, the application of SEs was found to enhance the qualitative characteristics of tomato fruits while reducing the accumulation of toxic ions under conditions of salinity stress [36].
Seaweed extracts were also found to significantly increase the amount of total chlorophyll in tomato plants while enhancing the photosynthetic activity, transpiration rate, stomatal conductance, and antioxidant activity, which were found to be strongly and positively correlated with an increase in fresh weight and fruit yield [37,38]. As such, the application of SEs may enhance the plant growth, yield, and quality by inducing the accumulation of secondary metabolites (e.g., proline, simple sugars, alcohols, abscisic acid, and antioxidant compounds) and/or activating metabolic pathways, thus mitigating the negative effects of salinization [39,40,41]. It should be noted that the number of SE applications will depend on the species and salinity stress conditions.
The seaweed extract from Padina gymnospora constitutes PBs that promote tomato plant growth and nutrient uptake and absorption [42,43]. However, the mechanisms by which tomato plants respond to SE treatments and improve their physiological performance under conditions of stress have not been fully elucidated. To this end, the current study aimed to investigate the morphological, physiological, and biochemical mechanisms involved in the response of tomato plants treated with an SE from P. gymnospora to prolongated irrigation with saline (NaCl) water to further explain the stress–performance relationship.
2. Materials and Methods
2.1. Algae Material and SE Preparation
The seaweed extract was prepared as reported by Hernández-Herrera et al. [43]. Briefly, 2 g of dry powder from the brown seaweed Padina gymnospora (Holmes 1896) was added to 1 L of distilled water, constantly stirred for 15 min, and autoclaved at 121 °C for 1 h at 1.21 kg cm−2. The hot extract was filtered through Whatman No. 40 filter paper from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and stored at −4 °C until further use. The chemical composition of the alga powder and seaweed extract from P. gymnospora are shown in Supplementary Tables S1 and S2.
2.2. Plant Material and Growing Conditions
Solanum lycopersicum L. cv. Rio Fuego seeds (Kristen Seed, San Diego, CA, USA) were sown in peat moss (Sunshine Mix 3™) and grown for 15 d. The seedlings were then transferred to individual plastic 3-L pots with a soil mixture (2:2:2: v/v/v) composed of vermiculite (Termolita S.A., Santa Catarina, NL, Mexico), peat moss (Sunshine Mix 3™), and organic soil. The plants were cultivated under natural light conditions from February to June 2021 at the greenhouse facility of the University Center of Biological and Agricultural Sciences (CUCBA) of the University of Guadalajara (Guadalajara, Mexico). The average daily temperature (day: 30 ± 2 °C; night: 16 ± 2 °C) and relative humidity (day/night ~85%) in the greenhouse were carefully monitored.
2.3. Experimental Design
The experiment was divided into two developmental phases: vegetative and reproductive (Figure 1). Phase one began with 15-day-old plants and concluded with 46-day-old plants. At the end of the first phase of this study, plants in the vegetative stage were assessed for morphological traits (i.e., shoot length, root length, leaf area, and root area); physiological characteristics (i.e., photosynthetic performance and chlorophyll content); biochemical compounds (i.e., proline, reducing sugars, phenols, flavonoids, and carotenoids); DPPH (2,2-diphenyl-1-picrylhydrazy) and 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) antioxidant activity; the superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) antioxidant enzymes; and Na+, K+, and Cl− ion concentrations. The second phase began 47 days after germination (DAG) and ended at 85 DAG. In the second phase, the number of flowers and fruits were recorded, and the yield, stress tolerance, and sensitivity indices were determined. Inflorescence topping practices were conducted to homogenize and increase the fruit size. In total, five flower clusters per plant were assessed in each harvest.
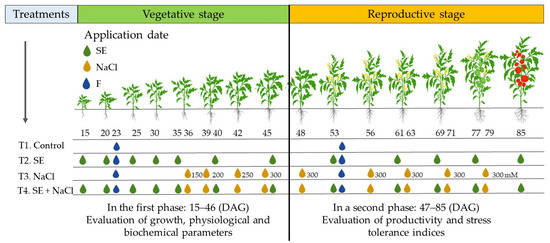
Figure 1.
Schematic representation of phases one and two of the experiment. Each treatment is graphically represented by its own timeline. The vertical line in the middle of the timelines separates phase one from phase two. The ages of the plants are given in days after germination (DAG) that appear above the timelines directly below the plant icons. The application days of the seaweed extract (SE) from Padina gymnospora, sodium chloride (NaCl), and soluble fertilizer (F) are represented by the colored drops along each timeline and are given in terms of plant age (DAG). The NaCl concentration is shown next to each orange drop icon and is the same for each application day for treatments 3 and 4.
2.4. SE Application and Salinity (NaCl) Stress Experiments
At the start of phase one, tomato plants (n = 60; 15 DAG) were randomly divided into four groups of 15 experimental unit plots. The experiment included four treatments (Figure 1): (1) control (plants with neither SE or NaCl), (2) SE (plants treated with SE), (3) NaCl (plants irrigated with 300 mM NaCl), and (4) SE + NaCl (plants treated with SE and irrigated with 300 mM NaCl). A total of 100 mL of soluble fertilizer 20N–20P–20K (Peters Professional; Scotts-Sierra Horticultural Products Co., Marysville, OH, USA) was added to all treatments in the form of a soil drench at 23 DAG. Plants from treatments 2 and 4 were treated with SE (100 mL of P. gymnospora 0.2% w/v) immediately after being transplanted (15 DAG). They received seven applications of SE that were supplied directly to the substrate every 5 days (up to 45 DAG). Twenty days after being transplanted (35 DAG), 100 mL of NaCl solution was administered to the plants from treatments 3 and 4 every 3 days with progressively increasing concentrations of 150, 200, 250, and 300 mM.
In a second phase of this study (46–85 DAG), SE was used help the tomato plants acclimate to salinity stress as the plants matured. The tomato plants kept in 3-L pots during phase one were transplanted at 46 DAG into 8-L pots for phase two. These plants were kept in the same four treatments and substrate and greenhouse conditions as those in phase one. A total of 100 mL of soluble fertilizer 20N–20P–20K was added to all treatments in the form of a soil drench at 53 DAG. Plants from treatments 3 and 4 continued to be irrigated with 100 mL of the salt solution (300 mM NaCl; 12.82 dS/cm2). The SE was administered directly to the substrate of the plants in treatments 2 and 4 and applied every eight days. All plants were watered every third day at field capacity until the end of the experiment (Figure 1).
2.5. Physiological Measurements
2.5.1. Photosynthetic Performance
Fifteen plants in the vegetative stage from each treatment were assessed for photosynthetic performance via nonintrusive pulse amplitude-modulated chlorophyll fluorometry using a Junior PAM fluorometer (Heinz Walz GmbH, Effeltrich, Germany). The leaves were dark-acclimated with leaf clips for 20 min before the start of a rapid light curve (RLC) routine. Actinic illumination in the RLC trial was increased in 12-step increments from 5 to 1500 μmol photons m−2 s−1, with each light level lasting 30 s. The maximum electron transport rate (ETRMAX) was calculated from the RLC according to the methodology of Murchie and Lawson [44] and was used as a proxy for plant photosynthetic performance. In the same fluorescence trial, the maximum photochemical quantum yield efficiency of PSII in a dark-adapted state FV/FM, nonphotochemical quenching (NPQ), and the light saturation index (EK) were also obtained to evaluate the photoinhibition state, photoprotection capacity, and light use efficiency of the plants due to a SE addition.
2.5.2. Chlorophyll Measurements
Using the same plants as those in Section 2.5.1, the chlorophyll content in the leaves was measured with a portable SPAD 502 Plus gauge (Minolta; Spectrum Technologies Inc., Aurora, IL, USA). The optical density difference measurement method was employed with the wavelengths of 650 nm and 940 nm. The leaves were sampled at the center of the lamina, avoiding the midvein under shaded conditions to prevent the SPAD from being directly exposed to solar radiation. Once the photosynthetic performance and chlorophyll were measured, the plants were harvested to assess their growth and biochemical composition.
2.6. Plant Growth Measurements
The plants described in Section 2.5.1 (46 DAG) were carefully removed from their plastic pots and immediately submerged in bowls filled with 20 °C water for 20 min. The roots and foliar system were carefully washed to eliminate soil particles. The plants (i.e., 15 per treatment) were weighted and photographed, and the growth characteristics (shoot length, root length, leaf area, and root area) were measured using ImageJ v.1.52a software (http://imagej.nih.gov/ij/download.html, accessed on 4 May 2021). In addition, the mean fresh weight was obtained with an electronic balance. After the plant growth variables were photographed, leaf samples were collected from each plant, frozen in liquid nitrogen, and stored at −70 °C until use.
2.7. Biochemical Measurements
The frozen leaf samples were ground in a mortar and then lyophilized. The lyophilized plant tissue (n = 12 plants) was used to analyze biochemical compounds i.e., metabolites, antioxidant capacity, and antioxidant enzyme activity. All biochemical measurements were performed for six replicates (each replicate consisted of a mix of two plants).
2.7.1. Metabolite Analyses
The proline assay was conducted as described by Palmeros-Suárez et al. [45]. Briefly, leaves were harvested, flash-frozen in liquid nitrogen, pulverized, and lyophilized. A total of 25 mg of powdered tissue from each sample was mixed with 100 µL of the proline extraction buffer (80% ethanol, 100 mM HEPES N-(2-Hydroxyethyl) piperazine-N0-(2-ethane sulfonic acid) buffer at pH 7.4, and 5 mM MgC l2). The mixture was incubated at 4 °C for 10 min with shaking and then centrifuged at 10,000× g at 4 °C for 10 min. The supernatant was transferred to a new tube and concentrated by vacuum centrifugation using a Maxi Dry Lyo (Heto-Holten, Allerød, Denmark). The precipitate was dissolved in 100 µL of HEPES buffer (100 mM, pH 7.4) with MgCl2 (5 mM). A 100-µL aliquot of the former solution was added to 200 µL of a solution containing glacial acetic acid 60% (v/v), ethanol 20% (v/v), and ninhydrin 1% (w/v). The mixture was boiled at 95 °C for 20 min, cooled to room temperature, and the optical density was recorded at 520 nm in the MultiskanTM GO microplate reader (Thermo Fisher Scientific, Tokyo, Japan).
The reducing sugar, phenol, and flavonoid contents were determined from acidified ethanol extractions conducted with fresh frozen leaves (six plants per treatment) that were pulverized and lyophilized (ILSHIN BIOBASE Table Top Freeze Dryer). A total of 25 mg of lyophilized leaves was extracted in 70% ethanol (acidified with 0.1% HCl) for 24 h at 4 °C in the dark. The mixture was centrifuged at 4 °C for 20 min at 10,000× g, and the supernatant was collected for a metabolite analysis.
The reducing sugars were determined according to the methodology of Negrulescu et al. [46] with some modifications. A 100-µL aliquot of aqueous extract and 1000 µL of DNS reagent (3,5-dinitrosalicylic acid (1%) and potassium sodium tartrate (30%) in sodium hydroxide (0.4M)) were combined, and the solution was mixed and incubated for 10 min at 99 °C in a water bath. Subsequently, the mixture was cooled to 4 °C and incubated for 4 min, and 200 µL was placed in a microplate. The absorbance was read at 540 nm with the MultiskanTM GO microplate reader (Thermo Fisher Scientific, Tokyo, Japan). A glucose calibration curve ranging from 1 to 10 mg/mL was used.
The total phenol content was determined using the Folin–Ciocalteu method at 730 nm according to the methodology of Singleton and Rossi [47] after adjusting the reaction volume to the microplate format. A 100-µL aliquot of the sample extract and 500-μL aliquot of fresh Folin–Ciocalteu reagent 1N in water (1:1 v/v) were mixed with 100 μL of ethanolic extract or different concentrations (10–200 g/mL) of gallic acid as a standard. After incubation for 4 min at room temperature, 400 μL of 15% Na2CO3 was added. The solution was then mixed thoroughly and incubated for 10 min at 45 °C. A total of 200 µL of the reaction mixture was placed into a 96-well plate. The absorbance was measured at 730 nm in a microplate reader (MultiskanTM GO Microplate Spectrophotometer, Thermo Fisher Scientific, Waltham, MA, USA) after mixing and an 80-min incubation in the dark. A standard curve using gallic acid was recorded (10–200 µg/mL). The phenol content was expressed as gallic acid equivalents (GAE, mg) per gram of DW (mg GAE/g DW).
The total flavonoid content was determined using the colorimetric method of Borrás-Linares [48] with modifications. A 100-μL aliquot of ethanolic extract was mixed with 300 μL of ethanol (99.8%; Sigma-Aldrich), 20 μL of 10% AlCl3, 20 μL of 1M potassium acetate (Sigma-Aldrich), and 560 μL of distilled water. The mixture was left to sit at room temperature for 30 min. The absorbance was then measured at 415 nm. Quercetin was used as the standard, with concentrations ranging from 10 to 200 g/mL. The total flavonoid content was expressed as quercetin equivalents (Scharlau, Barcelona, Spain) per gram of dry weight (mg of QE/g DW). The carotenoid content was assessed according to the method described by Osuna-Ruiz et al. [49]. Pigments were extracted from 300 mg of fresh, frozen leaves in 90% acetone for 24 h at 4 °C and then centrifuged at 3220× g for 10 min. The supernatants were recovered and stored at −20 °C until use. Pigment quantification was performed with a 1200 infinity HPLC System (Agilent Technologies, Santa Clara, CA, USA).
2.7.2. Antioxidant Capacity
Lyophilized plant tissue (0.25 mg) was mixed for 60 s with 70% ethanol (1:75 v/v), the and extraction was assisted by shaking for 24 h at 4 °C in the dark. The samples were centrifuged for 20 min at 21,952× g at 4 °C (Sigma 3–18 K, Sigma Laboratory Centrifuge, Berlin, Germany), and the supernatant was recovered. This procedure was performed twice. The extracts were stored at −20 °C until the total antioxidant activity analysis. The methodology of Brand-Williams et al. [50] was used to identify the total antioxidant activity using a DPPH (2,2-diphenyl-1-picrylhydrazy) scavenging assay with modifications. A 100-μL aliquot of the extract of each sample was taken, and 900 μL of DPPH solution was added. The mixtures were incubated for 30 min, and their absorbance was determined at 515 nm. The results were expressed as micromolar equivalents of Trolox per gram of dry weight (mg ET/g DW). The calibration curve was calculated at different Trolox concentrations that included ten points between 1 and 500 μM mL−1. Extracts were stored at −20 °C until analysis of the total antioxidant activity by the 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method, which was carried out according to the methodology of Re et al. [51].
2.7.3. Antioxidant Enzyme Activity
After eight days of salinity stress, 25 mg of ground lyophilized leaf tissue were homogenized with an extraction buffer containing 1 mM ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich), 1% (w/v) polyvinylpyrrolidone (PVPP), 1 mM phenylmethylsulphonyl fluoride (PMSF), and 0.05% Triton X-100 in 50-mM potassium phosphate buffer (pH = 7.0). The samples were then centrifuged at 14,000× g for 10 min at 4 °C. The resultant supernatant was separated and used for the enzymatic analysis. All data were collected via three replicates (n = 15 plants). The protein content in the enzyme extracts was determined according to the Bradford assay [52], using bovine serum albumin (BSA) as the standard. The enzyme activity of superoxide dismutase (SOD, E.C. 1.15.1.1) and catalase (CAT, E.C. 1.11.1.6) were determined as described by Palmeros-Suárez et al. [45]. Ascorbate peroxidase (APX EC 1.11.1.11) activity was assayed according to the method of Nagano and Asada [53].
2.7.4. Na+, K+, and Cl− Concentrations
The Na+ and K+ concentrations were measured according to the methodology of Zhang et al. [54]. Plant leaf tissues were dried at 60 °C for 72 h. Dry samples (0.5 g) were incinerated in a muffle furnace at 500 °C for 6 h. The ashes were dissolved in 5 mL of concentrated nitric acid with 500 mL distilled water. The ion concentrations were determined using Atomic Absorption Spectrometer 240FS AA (PerkinElmer, Santa Clara, CA, USA), and the Mohr method [55] was used to determine the chloride concentration. All determinations were carried out in triplicate (each replicate consisted of a mix of five plants).
2.8. Productivity Evaluation and Stress Tolerance Indices
The number of flowers and the total number and weights of the fruits were obtained from a single harvest at the end of phase two (86 DAG). The categories of fruit size (cm) and weight (g) were estimated according to the Saladette product category guidelines for imports of the Premier Horticulture Group of Mexico. To identify plants tolerant to salinity stress in the long term, we opted to use stress indices or selection criteria based on biomass production or yield. These stress indices are thought to provide a reliable evaluation of the performance of the whole crop under conditions of stress [10,22,56]. To assess the salt tolerance of the genotype of Solanum lycopersicum cv. Rio Fuego, we used the following stress indices: stress susceptibility index (SSI), stress tolerance index (STI), mean productivity index (MPI), and yield stability index (YSI). These indices were based on fruit yields and employed the equations shown in Table 1.

Table 1.
Stress tolerance indices based on the fruit yield in this study.
2.9. Statistical Analysis
All data are given as the means ± SD of three independent replicates. In all the experiments, a one-way analysis of variance (ANOVA) was used to compare treatments, and a least significant different (LSD) multiple range comparison test was used to evaluate differences between means (p ≤ 0.05). All data analyses were performed using Statgraphics Centurion XIX for Windows.
3. Results
3.1. Effect of the SE on the Physiological Responses of Tomato Plants
The photosynthetic characteristics of the tomato plants indicated that the SE induced protection of the photosynthetic apparatus and stimulated photosynthesis under salinity stress. The plants grown under control conditions that were treated with the extract (SE+) or not (SE−) showed similar photochemical quantum efficiency (FV/FM) values. Both FVFM values were slightly lower than the known threshold value for unstressed plants (i.e., 0.83) due to the greenhouse conditions (Table 2). Plants subjected to irrigation with saline water that were not treated with the SE (NaCl SE−) showed FV/FM values that were ~6% lower than those of the control plants. Interestingly, the SE limited the drop in the FV/FM of plants under salinity stress (NaCl SE+), and the resulting FV/FM value was only ~3% lower than the FV/FM value of the control plants. Given that FV/FM is the quantum efficiency of the whole photosynthetic apparatus, even a slight reduction in its value is likely to significantly affect the productivity and photosynthetic performance of a plant. Therefore, SE confers protection to PSII under salinity stress conditions. The electron transport rate in plants under salinity stress that were not treated with the SE (NaCl SE−) was reduced by ~25% compared to that of the control plants when no SE was added (Table 2). However, the effect observed in plants under salinity stress that were treated with the SE was an almost complete attenuation of the drop in ETR, which resulted in only a 4% reduction in ETR when compared to that of the control plants. Therefore, the SE stimulates a photosynthetic performance under salinity stress conditions. The concentration of the photosynthetic pigment chlorophyll explains the results of the photosynthetic parameters. The chlorophyll a (Chl a) concentration in the control plants was similar whether or not the SE was applied. However, under conditions of salinity stress, a significant reduction in Chl a was found whether or not SE was applied. Interestingly, the SE attenuated the reduction of Chl a under salinity stress. An SE-induced stimulation of chlorophyll synthesis seems to explain the superior photosynthetic performance of tomato plants under salinity stress conditions (Table 2). Moreover, the chlorophyll concentration also explains the light saturation irradiance (EK) values of each treatment, as the light needed to reach the maximum photosynthesis is highly related to the chlorophyll concentration.

Table 2.
Photosynthetic parameters of the tomato plants grown under salinity stress.
The parameter related to the photoprotective capacity of the tomato plants via the thermal dissipation of energy in the photosynthetic antenna (nonphotochemical quenching; NPQ) was similar in the SE− and SE+ treated control plants. However, under conditions of salinity stress, NPQ was reduced by the same amount in SE− and SE+ treated plants (~33%), which implies that no effect of the SE over NPQ photoprotection was present (Table 2). These results show that the SE supports the photosynthetic performance of tomato plants and appears to be an important factor that modulates photosynthetic responses to salinity.
3.2. Effects of the SE on the Morphological Attributes of Tomato Plants
The SE from P. gymnospora significantly (p ≤ 0.001) promoted the growth of the tomato plants under different irrigation conditions, namely under normal conditions without stress (Control) and under conditions of salinity stress (Table 3 and Figure 2). The SE applications given to tomato plants that were grown under normal conditions significantly increased the morphological parameters of the treated plants (i.e., shoot length, root length, leaf area, and fresh weight) when compared to those of the tomato plants not treated with the SE. As expected, irrigation with 300 mM NaCl without any SE extract caused a significant decrease in all shoot growth parameters with regards to the length (14.3 cm root and 10.3 cm shoot), area (18 cm2 root and 63 cm2 shoot), and fresh weight (0.55 g root and 2.2 g shoot). However, no significant changes were found in the roots with respect to those of the control plants, which was unexpected. Although slightly less pronounced, a decrease in the shoot parameters was also observed between control and SE-treated plants under salinity stress with regards to length (18 cm root and 12 cm shoot), area (33.1 cm2 root and 98.6 cm2 shoot), and fresh weight (1.09 g root and 3.8 g shoot) (Table 3). Interestingly, the root parameters were even observed to significantly increase in SE-treated plants (up to 18 cm in length and 33.1 cm2 in area) under salinity stress when compared to those of the SE-treated control plants (16.9 and 28.3 cm2, respectively).

Table 3.
Growth parameters of the tomato plants.
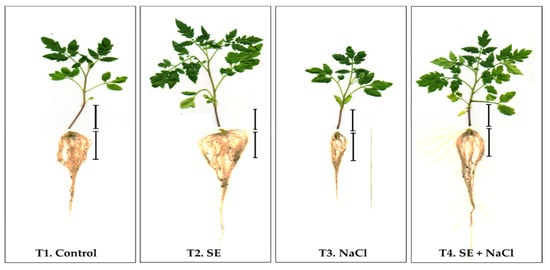
Figure 2.
Tomato plant growth 46 days after germination (DAG). Treatments included T1 (Control plants grown under normal conditions), T2 (plants treated with the application of the P. gymnospora extract; 0.2% seaweed extract (SE)), T3 (plants grown under salinity stress conditions; 300 mM NaCl), and T4 (plants grown under salinity stress conditions (300 mM NaCl) and treated with the SE). Bar: 5 cm.
These results clearly show that the application of SE significantly enhanced all growth parameters in SE-treated plants under the control and salinity stress conditions. In the presence of salinity stress, treatment with the SE was particularly useful in improving the root length and root area of the tomato plants (up to 26% and 84%, respectively) when compared to those of non-SE-treated plants, which highlights the applicability of the SE in this study as a tool to boost the salinity tolerance of tomato plants (Table 3).
3.3. Effects of the SE on the Biochemical Characteristics of Tomato Plants
3.3.1. Metabolites
Plants employ different strategies to cope with salinity stress, one of which is to increase osmolytes, such as proline and sugars, which also serve as essential stress markers. Therefore, we analyzed the levels of these osmolytes in the leaves of the tomato plants of the different treatments (Table 4) at the end of phase one (vegetative stage; 46 DAG). The proline levels were found to significantly increase in tomato plants when subjected to salinity stress when compared to plants grown under the control conditions. Interestingly, the increase in metabolites was 3.6-fold higher in plants treated with the SE (4.5-fold) compared to that of the untreated plants, which may account for the observed enhanced performance in both growth and yield (Table 4). The content of total reducing sugars (TRS) was affected by treatment with the SE and by saline irrigation. Under saline-free irrigation conditions, the accumulation of TRS in tomato plants significantly increased (two-fold) in SE-treated plants when compared to those of plants not treated with the extract. Interestingly, irrigation with NaCl only induced higher TRS production in plants that were not treated with the SE. Meanwhile, the TRS content in SE+ tomato plants under salinity stress conditions decreased as a result of the application of the extract (Table 4). Another important means by which plants cope with salinity stress is to strengthen the antioxidant activity.

Table 4.
Metabolites present in the tomato plants at the vegetative stage.
Phenols and carotenoids are among the most relevant antioxidant metabolites known to respond to salinity stress. When we evaluated the levels of these metabolites, we found that the metabolite content increased in plants grown under salinity stress conditions compared to that of the control plants. Interestingly, the irrigation of tomato plants with saline water resulted in a higher total phenol content in plants grown without the SE when compared to those treated with the extract (Table 4).
Moreover, the phenol levels were found to slightly but significantly increase in SE-treated plants compared to those of plants not treated with the extract under control irrigation conditions. The fact that phenols increased due to the application of the SE and did not increase as much in plants not treated with the extract under salinity stress conditions may indicate that a protective effect of the SE was already present in the plants, which may have ameliorated further responses (Table 4). Meanwhile, the content of flavonoids in the tomato plants grown under salinity stress conditions only increased significantly as a result of the SE application. In general, the total phenolics and flavonoid content tended to increase in plants under salinity stress conditions as a way to mitigate stress by scavenging free radicals (Table 4).
On the other hand, the carotenoid content was found to be higher in plants grown under salinity stress conditions compared to that of the control plants. However, the content of carotenoids decreased in SE-treated plants. Carotenoids are important antioxidant metabolites with special functions at the level of photosynthetic machinery (Table 4). The carotenoid content was found to be higher in both SE treatments under salinity stress when compared to those of the SE treatments.
3.3.2. Antioxidant Activity
Tomato plants grown under salinity stress had significantly higher total antioxidant activity when compared to the control plants (Table 5), regardless of whether or not they were treated with the SE. Moreover, the antioxidant activity (DPPH) had already increased in the control plants grown under control conditions that were treated with the SE. Under salinity conditions, plants treated with (SE+) or without (SE−) the extract exhibited similar values, reflecting the high antioxidant activity of DDPH. Interestingly, a significant increase in ABTS of 34.7 mg ET/g DW was noted in SE+ plants grown under salinity conditions compared to what was observed in SE plants. The application of the SE significantly increased the total antioxidant activity when compared to the activity of the control plants (Table 5).

Table 5.
Antioxidant activity in the tomato plants at the vegetative stage.
3.3.3. Antioxidant Enzyme Activity
The activity of SOD, CAT, and APX in tomato plants grown under the control conditions was significantly affected by the application of the SE. The SE-treated tomato plants showed a significant increase in SOD and CAT activity (67.9 and 4.0 Umg−1 protein, respectively) compared to those of the control plants that were not treated with the extract (50.4 and 3.5 Umg−1 protein, respectively). However, SE-treated plants under salinity stress showed similar trends with regards to SOD and APX activity, which reduced in response to the application of the SE. There were no significant differences in CAT activity among the treatments under salinity stress (Figure 3a,b).
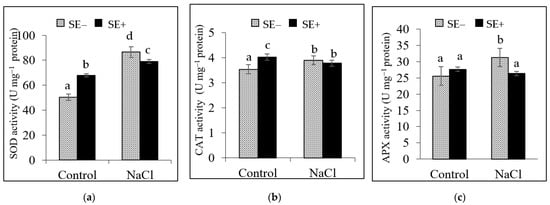
Figure 3.
Activity of the antioxidant enzymes (a) superoxide dismutase (SOD), (b) catalase (CAT), and (c) ascorbate peroxidase (APX) in tomato plants grown under the control conditions (Control) and salinity stress conditions (300 mM NaCl) with the application of the extract from Padina gymnospora (0.2%, SE+) or without the extract (SE−). Values are the means ± SD of six replicates (n = 12 plants). Different letters indicate significant differences among treatments according to the least significant differences (LSD) mean comparison test (p ≤ 0.001).
3.3.4. Measurement of Na+, K+, and Cl− Concentrations
Salinity stress caused a significant (p ≤ 0.005) accumulation of ions. The accumulation of both toxic Na+ and Cl- ions in the leaves of tomato plants was observed (2.9- and 11.16-fold higher than that of the control plants, respectively; Figure 4a,c). The K+ concentration significantly increased up to 1.2-fold with respect to that of the control plants (Figure 4b).
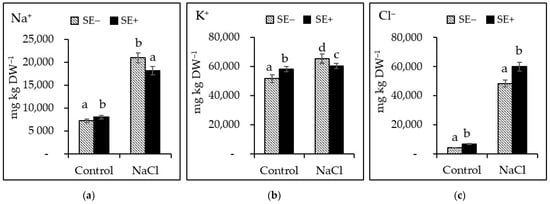
Figure 4.
The ion concentrations of (a) sodium (Na+), (b) potassium (K+), and (c) chlorides (Cl−) in the leaves of tomato plants grown under the control (Control) and salinity stress (300 mM NaCl) conditions with the application of the P. gymnospora extract (0.2%; SE+) or without the extract (SE−). Values are the means ± SD of three replicates (n = 15 plants). Different letters indicate significant differences among treatments according to the least significant differences (LSD) mean comparison test (p ≤ 0.001).
The application of SE at 0.2% in plants grown under salinity stress conditions led to a significant (p ≤ 0.005) increase in the Na+ and Cl- concentrations (2.2- and 8.9-fold, respectively). Moreover, the K+ concentration increased by 1.1-fold compared to that of the control, although this increase was not significant (Figure 4b).
3.4. Effects of the SE on the Reproductive Attributes of the Tomato Plants
At the end of phase two (85 DAG), the application of the SE (0.2%) was found to significantly (p < 0.005) improve different reproductive attributes of the SE-treated plants when compared to those grown under the control and salinity stress conditions. The control plants that were treated with the SE showed a significant increase in productivity in terms of the number of flowers and fruits and the fresh weight of the fruits when compared to those grown under control and salinity stress conditions.
As expected, in plants that were not treated with the SE, salinity caused a reduction in the number of flowers and fruits and the fresh weight of the fruits (Figure 5a–c). Salinity caused a significant (p < 0.005) decline in different reproductive parameters, as evidenced by a decrease in productivity due to a reduction of up to 28.7% in fruit number in the plants not treated with the SE. However, in SE-treated plants, the number of fruits per plant was not affected by salinity, and only a 3.4% loss in productivity was recorded (Figure 5b). Although the fresh weight of fruits was observed to decrease in the presence of salinity, the reduction in productivity was significantly less in SE-treated plants (Figure 5c).
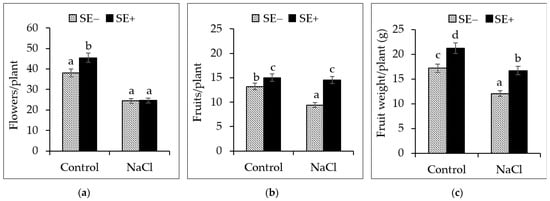
Figure 5.
Productivity at harvest. (a) Number of flowers per plant, (b) number of fruits per plant, and (c) fresh weight of fruits per plant grown under normal conditions (Control) and salinity stress conditions (300 mM NaCl) with the application of the seaweed extract from P. gymnospora (0.2%; SE+) and without the extract (SE−). Different letters indicate significant differences among treatments according to the least significant differences (LSD) mean comparison test (p ≤ 0.001).
The SE-treated plants showed a 21.4% loss in fruit weight, whereas untreated tomato plants showed a 30% loss in fruit weight. The SE from P. gymnospora seemed to improve the salinity tolerance of the tomato plants, which was reflected in a significantly better total productivity per plant when compared with that of the plants not treated with the SE. Salinity was also found to affect the fruit size.
The proportion of small tomato fruits of plants growing under salinity stress conditions was 91–95% compared to tomato plants grown in normal conditions (81–83%) (Figure 6). The proportions of medium tomato fruits in SE-treated control plants were 15% compared to plants grown in salinity stress (4%). The large tomato fruits (up to 3%) was reported only in SE-treated control plants (Figure 6 a,b).
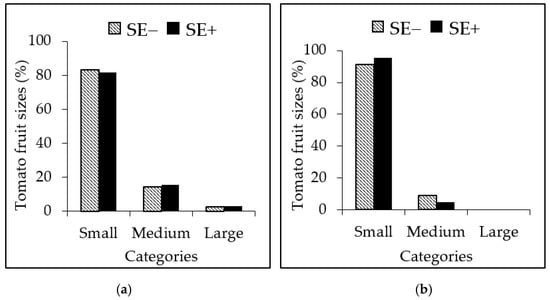
Figure 6.
Proportions of tomato fruits according to size at harvest reported as percentages. Plants grown under (a) normal conditions (Control) and (b) under salinity stress conditions (300 mM NaCl) with the application of the P. gymnospora extract (0.2%; SE+) and without the extract (SE−). Fruit size and mass categories for small (2.5–3.4 cm and 20–59 g), medium (3.5–4.4 cm and 60–83 g), and large (4.5–5.4 cm and 84–100 g) tomato fruits were used to classify fruits at harvest.
3.5. Effects of the SE on the Tolerance and Productivity of Tomato Plants as Determined by Stress Indices
To better account for the observed traits, we calculated the productivity and stress-related indices (Table 6). Overall, we observed increased tolerance and productivity in tomato plants treated with the SE under salinity stress conditions compared to those of plants that were not treated with the SE, as shown by the higher values of the STI, MPI, and YSI. Furthermore, plants grown under salinity stress conditions without the SE were found to be more susceptible to stress, as indicated by the lower levels of SSI and the reduction in the yield.

Table 6.
Tolerance and productivity of tomato plants as determined by stress indices.
4. Discussion
Seaweed extracts are gaining attraction as low-cost alternatives to traditional fertilizers, because they are both eco-friendly and nontoxic [43,62,63]. The SEs from brown algae have been commercialized in recent years as PBs that improve plant productivity. This improvement has mainly been attributed to the provision of essential nutrients [40,41,64,65] and the attenuation of the negative effects of salinity stress. In addition, SEs have been shown to enhance root morphology. In fact, Hernández-Herrera et al. [43] proposed that treatments with SE stimulate root proliferation, germination, and growth by making plants more able to adequately extract nutrients from the deeper soil layers. Furthermore, SEs have been shown to induce the build-up of nonstructural carbohydrates that improve energy storage while boosting the metabolism, in addition to improving leaf growth by enhancing leaf water retention, membrane permeability, and the transport of osmolytes/ions, which improves tolerance to abiotic stress [66].
In this study, a greenhouse experiment was conducted to evaluate the potential of the SE from Padina gymnospora to support the growth and productivity of tomatoes (Solanum lycopersicum L.) under salinity stress during both the vegetative and reproductive developmental stages of the plants. Padina spp. are the most common brown marine algae in Mexico due to their widespread distribution along the tropical Pacific and, as such, have notable economic potential [63]. A prior chemical composition analysis of this SE from P. gymnospora indicated that the extract is rich in minerals while being a source of antioxidants (e.g., phenols and proline; Supplementary Table S2). These compounds counteract environmental stress and play important roles in preventing toxic free radicals from accumulating while improving crop growth and productivity by increasing the nutrient uptake and availability [4].
The results of this study indicate that the SE from P. gymnospora was able to significantly improve both the vegetative and reproductive growth attributes of the tomato plants grown under control conditions (Control) and salinity stress conditions (300 mM NaCl), while a clear decline in yield was detected due to the drastic effects of salinity stress. Salinity stress reduces shoot growth in tomato plants due to a direct inhibition of cell division and expansion [61]. Treatment with the SE was found to mitigate this NaCl-induced inhibition of plant growth. The promotion of tomato plant growth under both control and salinity stress conditions due to the application of the SE from P. gymnospora could be attributed to the presence of growth-promoting substances, total carbohydrates, proline, and phenolic compounds. Our findings are also consistent with those of previous studies performed with okra [40], canola [67], and tomato [26] plants, which have reported positive impacts of SE applications that have resulted in optimum yields [29,37,67].
The SE from P. gymnospora is an efficient PB under normal growth conditions that noticeably improves plant outcomes. The tomato plants grown under stress conditions and treated with the SE showed enhanced vegetative growth characteristics and improved functioning of the photosynthetic apparatus, which corresponded to an increase in the levels of photosynthetic pigments (chlorophyll a). The application of SE enhances the photosynthetic potential of the plant by improving the chlorophyll concentration in leaves [40,68,69,70,71,72]. It has also been established that the application of low concentrations of SE from Sargsum polycystum, S. latifolium, and S. wightii on Cajanus cajan, Rosmarinus officinalis Cyamopsis tetrangonolaba, and Abelmoschus esculentus resulted in an increase in the level of chlorophyll due to the high absorption of magnesium ions, which are major components of chlorophyll biosynthesis. In addition, the liquid extract from S. wightii contained high levels of iron, magnesium, and nitrogen, which helped to increase the chlorophyll synthesis in A. esculentus under stress [40]. Additionally, Elansary et al. [73] studied the effects of SE from A. nodosum on Paspalum vaginatum under salinity stress conditions and observed a high amount of total nonstructural carbohydrates (related to enhanced photochemical efficiency and proline).
Similarly, tomato plants supplemented with SE from P. gymnospora might increase their cellular metabolic rates and retard senescence by slowing or preventing chlorophyll destruction and/or increasing chlorophyll biosynthesis. The high content of N and Fe (structural components of chlorophyll) in SE-treated plants may enhance chlorophyll accumulation, which would lead to higher rates of photosynthesis [4]. Therefore, higher ETRMAX values were reflected in higher plant growth under these conditions. Moreover, higher ETRMAX values may indicate an increase in the translocation rates of different molecules that are produced via photosynthesis to fruits, which would thus increase fruit yields. Substrate amendments with P. gymnospora protect chlorophylls from the harmful effects of salinity. The beneficial effects of SE treatment on the chlorophyll content under normal and salinity stress conditions indicated that higher photosynthetic activity was present in these treatments with regard to that of the control. Similar results have been reported in canola, okra, and soybean plants [4,40,68,74].
Treatment with SE resulted in an enhanced acquisition of Cl ions in the leaves of the tomato plants grown under salinity stress, which likely resulted in a decrease in the water potential [36]. In this study, the Na+ concentration strongly increased under salinity stress and reduced after the application of SE. According to Dell´Aversana et al. [75], an increase in cellular Na+ was not able to interfere with K+ translocation from the roots to shoots and with its transport through the plasma membrane. Therefore, no significant decrease in cytosolic K was found in Bougainvillea treated with biostimulants under salinity stress [76]. The Na+ concentration in plant tissues can also increase while the cytosolic K+ concentration is maintained at a constant level or even increased by the release of vacuolar K [77]. The compartmentalizing of Na+ in the vacuole, which is osmotically balanced by K+ in the cytosol, could be another essential mechanism by which tomato plants tolerate salinity stress [78]. As demonstrated by the substantial contribution of K+ to the total osmolality, an increase in the K concentration in the cytosol could be enough to balance the mesophyll cells without the need for other metabolites.
Secondary metabolites and antioxidants that alleviate stress act as the first line of defense for plants when confronted by abiotic stress [17]. The widespread use of SEs has been used to reduce the damage cause by salinity stress, which is mainly due to oxidative damage. Elansary et al. [73] conducted a study employing seaweed extracts from Ascophyllum nodosum on Spiraea nipponica “Snowmound” and Pittosporum eugenioides “Variegatum” and observed that the phenolic and flavonoid contents, antioxidant capacity, and proline accumulation increased. They attributed these results to growth-stimulating activities and the composition of the SE itself. The SE contains macroelements, microelements, vitamins, auxins, and phytohormones, which have antioxidant properties. The current study found that the SE from P. gymnospora showed high antioxidant activities of flavonoid compounds. The bioactive compounds of the SE from P. gymnospora, such as phenols, carbohydrates, protein, and proline, positively impact the overall plant growth by optimizing metabolite production (stress alleviating amino acids), membrane permeability, and the transport of osmolytes/ions, which enhance the abiotic stress tolerance by modifying the water capacity and turgor pressure in plants [40]. Proline (an osmolyte) plays a vital role in osmoregulation and has been shown to have antioxidant activity. As an ROS scavenger, proline contributes to cellular homeostasis, protects the photosynthetic apparatus, functions as a protein precursor, and acts as an energy source for the stress recovery process [79,80,81]. At the same time, proline metabolism plays a key role in ROS production and the signaling pathways [82]. It is the most abundant amino acid in plants under salinity stress, so it has been suggested that proline is a marker molecule that responds to salinity, as its concentration increases considerably under salinity stress [83].
As in previous studies, an increase in the proline content was detected in the tomato plants when they were exposed to salinity stress. The concentration of osmolytes and antioxidant activity varies between plant species, and studies have reported this phenomenon in tomatoes [84], potatoes [85], and melons [86]. These authors also observed proline accumulation in salt-tolerant varieties and suggested that it is due to the elevated activity of proline synthesis enzymes and a reduction in proline dehydrogenase activity (PDH) and not protein degradation [87]. Additionally, these authors stated that proline is a stress indicator and therefore does not increase the salinity tolerance [84]. Bacha et al. [16] suggested that the application of proline successfully improved stress tolerance in plants. In this study, we observed an increase in proline concentration when tomato plants were exposed to salinity stress. Nevertheless, this increase was higher when tomato plants were treated with the SE, which could be due to the proline content in the SE. We determined that these plants were less stressed compared to their untreated counterparts due to the application of the SE.
A different situation was observed with the concentration of reducing sugars under salinity stress conditions in which a decrease in the accumulation of these sugars was observed when the SE was applied. The increase in the reducing sugar content in plants under salinity stress conditions suggests that soluble sugars may help maintain the osmotic balance and stabilize cell membranes. However, the fact that TRS did not increase in SE-treated plants suggests that membrane damage is reduced due to the application of the SE (i.e., the increase in proline metabolites; Table 4).
Seaweed extracts have been found to positively affect the antioxidant enzyme activity and increase the antioxidant defensive mechanisms, such as CAT, SOD, and APX activity. This has been observed in wheat and chickpea plants with various SE from Ulva rigida, Sargassum muticum, and Jania rubens under conditions of salinity stress [88,89]. In those studies, the results were attributed to enhanced levels of photosynthetic pigments (carotenoids and Chl a and b), phenolic content, and metabolites (e.g., soluble sugars and proline), which provide the energy required to activate defense strategies. Additionally, the authors attributed this positive effect to a coordinated increase in the activities of SOD, CAT, and APX, which are known ROS scavengers. In addition, Zou et al. [29] demonstrated that an SE from Lessonia nigrescens enriched with polysaccharides could improve plant growth under conditions of salinity stress by increasing the chlorophyll and proline content and supporting osmoregulation and ROS scavenging via an increase in SOD and CAT activity.
In the present study, the SE-treated tomato plants showed a significant increase in SOD and CAT activity compared to those of the control plants that were not treated with the SE. However, SE-treated plants under salinity stress showed higher SOD and APX activity, which reduced in response to the application of the SE. CAT activity did not improve with the application of the SE. This response of CAT has been previously reported with Rosmarinus officinalis [73], although the responses of CAT activity to salinity stress have been variable and range from large increases in activity [90] to a lack of change [91] to severe deactivation [92].
We found that the SE has the potential to enhance the productivity of plants under normal conditions. In addition, the application of the SE allowed plants to better adapt to irrigation with saline water, which ultimately promoted their growth and yield under salinity stress. The SE from P. gymnospora at 0.2% tended to alleviate the inhibitory effects of up to 300 mM NaCl on the growth and fruit yield of tomato plants. Moreover, the productivity of the tomato crop treated with the SE under salinity stress was significantly greater (4.7%) than the productivity of tomato plants that were not treated with the SE but subjected to salinity stress.
The chemical composition analysis of the SE revealed that it is rich in antioxidants that counteract environmental stress and play important roles in preventing the formation of toxic-free radicals, which improves the growth and productivity of crops by increasing the nutrient availability and uptake [93] and enhancing the tolerance to salinity stress [29,34,36,38,74]. In addition, the observed increase in tomato plant productivity may be due to the inorganic compounds of the SE, which include nitrogen, phosphorous, potassium, calcium, sulfates, iron, zinc, magnesium, copper, and boron [4]. The SE also contains compounds such as cytokinins, auxin-like growth substances, carbohydrates, polyphenols, proline, and amino acids [94], all of which support cellular metabolism and lead to enhanced growth and crop yields while enhancing the tolerance to abiotic stress. The mediation of the activities of the enzymes and genes involved in efficient nutrient assimilation has also been proposed as a mode of action of the SE that contributes to an overall increase in growth [23,95,96].
Previous studies have demonstrated that the application of SE formulations induces early flowering in tomato plants [97,98]. In the present study, a beneficial effect of the SE from P. gymnospora on flowering was recorded. The SE-treated plants tended to flower earlier (at least 7 d) than the control plants. Recently, Dookie et al. [98] provided additional molecular evidence of the effectiveness of SEs from Sargassum sp. and A. nodosum in enhancing the expression of the major flowering genes in tomatoes. The increased expression of these genes implies that the application of SE not only improves the growth and yield but also enhances the regulatory aspects of architectural development in plants. The positive effects observed during the reproductive stage, such as enhanced flowering, can also be attributed to favorable changes in the hormonal levels, the mobilization of nutrients, improved photosynthetic efficiency, and delayed senescence, all of which have been hypothesized to be induced by the application of SEs [64,95,99,100,101].
To assess the tolerance of the genotype of Solanum lycopersicum cv. Rio Fuego to salinity stress, we employed various stress indices (i.e., SSI, STI, MPI, and YSI) that were based on the fruit yield. We measured the plant performance under salinity stress and normal conditions and took into account the results of previous studies to calculate the aforementioned indices [57,60,62,102,103,104,105]. With these indices, decreases in performance under salinity stress were able to be compared with those under normal conditions, which were then used to determine the severity of the stress. These indices are usually based on the resistance or sensitivity of a genotype to stress [58]. Rosielle and Hamblin [59] defined the difference in plant performance under normal conditions (Yp) and stress conditions (Ys) as stress tolerance (TOL) and defined the average performance under both conditions as the mean productivity (MP). Although all these indices are mathematical derivations of the same yield data, the selection of a combination of different indices may provide more useful criteria to improve the tolerance to salinity stress in tomato plants. Interestingly, SE-treated plants displayed higher levels of tolerance compared to untreated plants, as indicated by the STI, MPI, and YSI. However, an accurate screening index is not yet available that can be used to evaluate tolerance to abiotic stress and high yields due to SE application in the presence and absence of stress.
The present study highlights the beneficial effects of the SE from P. gymospora by the significant increase in plant productivity and fruit quality reported herein. These yield results agree with those of Rouphael and Colla [68]. Under normal and stressful environmental conditions, SE application may result in higher crop yields and productivity. Additionally, the application of Ascophyllum nodosum-based algal extracts enhanced the qualitative aspects of the tomato fruits and reduced the accumulation of toxic ions under salinity stress [36]. In addition, a significant increase in the total chlorophyll and antioxidant compounds in wheat plants irrigated with brackish water has been previously reported, which highlights a strong positive correlation with the increase in fresh weight, grain weight, and yield components [69].
Our results showed that the application of the SE from P. gymnospora positively affected the photosynthetic performance, improved the antioxidant defense system, and reduced the oxidative damage in tomato plants. The beneficial effects of SEs may be due to the synergistic activity of the extract components. These results provide the means to ensure high crop yields and plant development under adverse conditions. Moreover, these findings indicate that the SE from P. gymnospora can be used as an environmentally friendly and multifunctional PB in agricultural practices to support sustainable production methods.
5. Conclusions
The seaweed extract from P. gymnospora reduced the negative effects induced by irrigation with saline water. Tomato plants treated with the SE from P. gymnospora showed (1) an increase in the length, area, and fresh weights of shoot and roots; (2) an increase in ETRMAX, the photochemical quantum efficiency of PSII (FV/FM), and a photosynthetic pigment (chlorophyll); (3) a reduction of the accumulation of Na+ and K+, along with the improved retention of Cl-; and (4) an increase in the proline osmolyte and flavonoid contents. During the reproductive stage, the positive effects of SE application were identified, such as early flowering and an increase in yield (number, weight, and quality of fruits). Given the increased use of SEs in agriculture, it is essential to understand which active ingredients and their functional combinations in SE and PB formulations elicit beneficial responses in terms of plant growth, yield, and stress tolerance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102495/s1. Table S1: The composition analysis of Padina gymnospora powder. Values represent means ± standard deviations of triplicates. DW: dry weight. Table S2: Physicochemical composition of seaweed extract of Padina gymnospora, DW: dry weight.
Author Contributions
Conceptualization, R.M.H.-H. and C.V.S.-H.; methodology, P.A.P.-S. and H.O.-A.; software, R.M.H.-H.; validation, F.S.-R., I.D.M.-C. and H.O.-A.; formal analysis, A.B.-E.; investigation, R.M.H.-H.; resources, C.V.S.-H. and R.M.H.-H.; writing—original draft preparation, R.M.H.-H.; writing—review and editing, I.D.M.-C.; visualization, H.O.-A. and A.B.-E.; supervision, F.S.-R.; and project administration, R.M.H.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the journal reviewers for their valuable comments, suggestions, and guidance. The author gratefully acknowledges Andrea Liévana MacTavish for her English language review. We thank Miguel Ángel Hurtado Oliva for their support on the HPLC analysis of carotenoids. AB-E acknowledges CONACYT for its support through the program Researchers for Mexico (No. 2196).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, J.; Takhur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer Nature Singapore Private Ltd.: Singapore, 2018; pp. 27–46. [Google Scholar]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production [Internet]; Hasanuzzaman, M., Filho, M.C.M.T., Fujita, M., Nogueira, T.A.R., Eds.; IntechOpen: London, UK, 2020; pp. 1–21. [Google Scholar]
- FAO & ITPS Status of the World’s Soil Resources (SWSR)—Main Report. Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils, Rome, Italy. 2015. Available online: http://www.fao.org/3/a-i5199e.pdf (accessed on 16 May 2022).
- Simoes, A.J.G.; Hidalgo, C.A. The Economic Complexity Observatory: An Analytical Tool for Understanding the Dynamics of Economic Development. 2022. Available online: https://oec.world/en/profile/hs/tomatoes?redirect=true (accessed on 24 July 2022).
- Singh, J.; Sastry, E.V.D.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol. Mol. Biol. Plants 2011, 18, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Devkar, V.; Thirumalaikumar, V.P.; Xue, G.P.; Vallarino, J.G.; Turěcková, V.; Strnad, M.; Fernie, A.R.; Hoefgen, R.; Mueller-Roeber, B.; Balazadeh, S. Multifaceted regulatory function of tomato SlTAF1 in the response to salinity stress. New Phytol. 2020, 225, 1681–1698. [Google Scholar] [CrossRef]
- Pailles, Y.; Awlia, M.; Julkowska, M.M.; Passone, L.; Zemmouri, K.; Negrão, S.; Schmöckel, S.M.; Tester, M. Diverse traits contribute to salinity tolerance of wild tomato seedlings from the Galapagos Islands. Plant Physiol. 2020, 182, 534–546. [Google Scholar] [CrossRef]
- Ibrahimova, U.F.; Mammadov, A.C.; Feyziyev, Y.M. The effect of NaCl on some physiological and biochemical parameters in Triticum aestivum L. genotypes. Plant Physiol. Rep. 2019, 24, 370–375. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salinity stress under the scalpel—Dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef]
- Massange-Sánchez, J.A.; Sánchez-Hernández, C.V.; Hernández-Herrera, R.M.; Palmeros-Suárez, P.A. The Biochemical Mechanisms of Salt Tolerance in Plants. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: London, UK, 2021. [Google Scholar]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafque, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortifed composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef]
- Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi, A. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Microtom leaves. South. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Borusiewicz, A.; Łozowicka, B. Fluazinam and its mixtures induce diversified changes of crucial biochemical and antioxidant profile in leafy vegetable. Sci. Hortic. 2022, 298. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Derbali, W.; Goussi, R.; Koyro, H.W.; Abdelly, C.; Manaa, A. Physiological and biochemical markers for screening salt tolerant quinoa genotypes at early seedling stage. J. Plant Interact. 2020, 15, 27–38. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Andreotti, C.; Rouphael, Y.; Colla, G.; Basile, B. Rate and timing of application of biostimulant substances to enhance fruit tree tolerance toward environmental stresses and fruit quality. Agronomy 2022, 12, 603. [Google Scholar] [CrossRef]
- De Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and Their Role in Improving Plant Growth under Abiotic Stresses. In Biostimulants in Plant Scienc; Mirmajlessi, S.M., Radhakrishnan, R., Eds.; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Drira, M.; Mohamed, J.B.; Hlima, H.B.; Hentati, F.; Michaud, P.; Abdelkafi, S.; Fendri, I. Improvement of Arabidopsis thaliana salt tolerance using a polysaccharidic extract from the brown algae Padina pavonica. Algal Res. 2021, 56, 102324. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salinity stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef]
- Bi, F.; Iqbal, S.; Arman, M.; Ali, A.; Hassan, M.-U. Carrageenan as an elicitor of induced secondary metabolites and its effects on various growth characters of chickpea and maize plants. J. Saudi. Chem. Soc. 2011, 15, 269–273. [Google Scholar] [CrossRef]
- Layek, J.; Das, A.; Idapuganti, R.G.; Sarkar, D.; Ghosh, A.; Zodape, S.T.; Lal, R.; Yavad, G.S.; Panwar, A.S.; Ngachan, S.; et al. Seaweed extract as organic bio-stimulant improves productivity and quality of rice in eastern Himalayas. J. Appl. Phycol. 2018, 30, 547–558. [Google Scholar] [CrossRef]
- Sharma, L.; Banerjee, M.; Malik, G.C.; Gopalakrishnan, V.A.K.; Zodape, S.T.; Ghosh, A. Sustainable agro-technology for enhancement of rice production in the red and lateritic soils using seaweed based biostimulants. J. Clean. Prod. 2017, 149, 968–975. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Hussein, M.H.; Eltanahy, E.; Al Bakry, A.F.; Elsafty, N.; Elshamy, M.M. Seaweed extracts as prospective plant growth bio-stimulant and salinity stress alleviator for Vigna sensis and Zea mays. J. Appl. Phycol. 2021, 33, 1273–1291. [Google Scholar] [CrossRef]
- Kasim, W.A.E.A.; Saad-Allah, K.M.; Hamouda, M. Seed priming with extracts of two seaweeds alleviates the physiological and molecular impacts of salinity stress on radish (Raphanus sativus). Int. J. Agric. Biol. 2016, 18, 653–660. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Gul, H.; Rauf, M.; Arif, M.; Hamayun, M.; Ud-Din, A.; Sajid, Z.A.; Khilji, S.; Rehman, A.; Tabassum, A.; et al. Sargassum wightii aqueous extract improved salinity stress tolerance in Abelmoschus esculentus by mediating metabolic and ion rebalance. Front. Mar. Sci. 2022, 9, 853272. [Google Scholar] [CrossRef]
- Rai, N.; Rai, S.P.; Sarma, B.K. Prospects for abiotic stress tolerance in crops utilizing phyto- and bio-stimulants. Front. Sustain. Food. Syst. 2021, 5, 754853. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Palmeros-Suárez, P.A.; Massange-Sánchez, J.A.; Martínez-Gallardo, N.A.; Montero-Vargas, J.M.; Gómez-Leyva, J.F.; Délano-Frier, J.P. The overexpression of an Amaranthus hypochondriacus NF-YC gene modifies growth and confers water deficit stress resistance in Arabidopsis. Plant Sci. 2015, 240, 25–40. [Google Scholar] [CrossRef]
- Negrulescu, A.; Patrulea, V.; Mincea, M.M.; Cosmin, I.; Beatrice, A.V.; Vasile, O. Adapting the reducing sugars method with dinitrosalicylic acid to microtiter plates and microwave heating. J. Braz. Chem. Soc. 2012, 23, 2176–2182. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutierrez, A.; Gomez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crops. Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Osuna-Ruiz, I.; Nieves-Soto, M.; Manzano-Sarabia, M.M.; Hernández-Garibay, E.; Lizardi-Mendoza, J.; Burgos-Hernández, A.; Hurtado-Oliva, M.A. Gross chemical composition, fatty acids, sterols, and pigments in tropical seaweed species off Sinaloa, Mexico. Cienc. Mar. 2019, 45, 101–120. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar]
- Zhang, J.; Zeng, L.; Chen, S.; Sun, H.; Ma, S. Transcription profile analysis of Lycopersicum esculentum leaves, unravels volatile emissions and gene expression under salinity stress. Plant Physiol. Biochem. 2018, 126, 11–21. [Google Scholar] [CrossRef]
- Mohr, C.F. Neue massanalytische Bestimmung des chlors in Verbindungen. Ann.Chem. Pharm. 1856, 97, 335–338. [Google Scholar]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early growth stage characterization and the biochemical responses for salinity stress in tomato. Plant 2021, 10, 712. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars: I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetable and Other Food Crops in Temperature and Water Stress, Tainan, Taiwan, 13–18 August 1992; pp. 257–270. [Google Scholar]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non- stress environment. Crop. Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybean. Part 1: Evaluation of three screening techniques for heat and drought tolerance. Crop. Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Golestani Araghi, S.; Assad, M.T. Evaluation of four screening techniques for drought resistance and their relationship to yield reduction ratio in wheat. Euphytica 1998, 103, 293–299. [Google Scholar] [CrossRef]
- Sohn, S.I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in seaweeds: An overview on biosynthesis to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Briceño-Domínguez, D.R.; Filippo-Herrera, D.A.; Hernández-Carmona, G. Seaweed as potential plant growth stimulants for agriculture in Mexico. Hidrobiologica 2018, 28, 129–140. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. Dynamic Physiological Phenotyping of Drought-Stressed Pepper Plants Treated with “Productivity-Enhancing” and “Survivability-Enhancing” Biostimulants. Front. Plant. Sci. 2019, 10, 905. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mansour, H.A.; El-Khawas, S.A.; Hassanein, R.A. The potentiality of marine macro-algae as bio-fertilizers to improve the productivity and salinity stress tolerance of canola (Brassica napus L.) plants. Agronomy 2019, 9, 146. [Google Scholar]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; Hussein, M.M.; El-Baroty, G.S. Algal extracts improve antioxidant defense abilities and salt tolerance of wheat plant irrigated with sea water. Afr. J. Biomed. Res. 2008, 2, 151–164. [Google Scholar]
- Erulan, V.; Thirumaran, G.; Soundarapandian, P.; Ananthan, G. Studies on the effect of Sargassum polycystum (C. Agardh, 1824) extract on the growth and biochemical composition of Cajanus Cajan (L.) Mill sp. Am. -Eurasian J. Agric. Environ Sci. 2009, 6, 392–399. [Google Scholar]
- Ramya, S.S.; Nagaraj, S.; Vijayanand, N. Biofertilizing Efficiency of brown and green algae on growth, biochemical and yield parameters of Cyamopsis Tetragonolaba (L.) Taub. Recent Res. Sci. Technol. 2010, 2, 45–52. [Google Scholar]
- Gharib, F.A.E.L.; Zeid, I.M.; Salem, O.M.A.H.; Ahmed, E.Z. Effects of Sargassum latifolium extract on growth, oil content and enzymatic activities of rosemary plants under salinity stress. Life Sci. 2014, 11, 933–945. [Google Scholar]
- Elansary, H.O.; Skalicka-Woźniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Biochem. 2016, 105, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Ramarajan, S.; Joseph, L.H.; Ganthi, A.S. Effect of seaweed liquid fertilizer on the germination and pigment concentration of soybean. J. Crop Sci. Technol. 2012, 1, 1–5. [Google Scholar]
- Dell’Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum based extracts counteract salinity stress in tomato by remodeling leaf nitrogen metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. Trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Becker, D.F. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015, 6, 552. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salinity stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Jaarsma, R.; de Vries, R.S.; de Boer, A.H. Effect of salinity stress on growth, Na+ accumulation and proline metabolism in potato (Solanum tuberosum) cultivars. PLoS ONE 2013, 8, e60183. [Google Scholar]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-González, A.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the proline metabolism and glycine betaine on tolerance to salinity stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Chernane, H.; Latique, S.; Mansori, M.; El Kaoua, M. Salinity stress tolerance and antioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. J. Agric. Vet. 2015, 8, 36–44. [Google Scholar]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Gossett, D.R.; Millhollon, E.P.; Lueas, C.; Banks, S.W.; Marney, M.M. The effects of NaCI on antioxidant enzyme activities in callus tissue of salt-tolerant and salt-sensitive cotton genotypes (Gossypium hirsutum L.). Plant Cell. Rep. 1994, 13, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Fadzilla, N.A.M.; Finch, R.P.; Burdon, R.H. Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J. Exp. Bot. 1997, 48, 325–331. [Google Scholar] [CrossRef]
- Shalata, A.; Neumann, P.M. Exogenous ascorbic acid (vitamin C) increases resistance to salinity stress and reduces lipid peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chowdhury, N. Salt stress in plants and amelioration strategies: A critical review. In Abiotic Stress in Plants; Fahad, S., Saudy, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2020; pp. 1–23. [Google Scholar]
- Shanmuganathan, B.; Pandima-Devi, K. Evaluation of the nutritional profile and antioxidant and anti-cholinesterase activities of Padina gymnospora (Phaeophyceae). Eur. J. Phycol. 2016, 51, 482–490. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant. Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Venkatesan, K.; Selvakumari, P. Seasonal influence of seaweed gel on growth and yield of tomato (Solanum lycopersicum Mill.) Hybrid COTH 2. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 55–66. [Google Scholar] [CrossRef]
- Dookie, M.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci. Hortic. 2021, 276, 109715. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory Activities of Ascophyllum nodosum Extract in Tomato and Sweet Pepper Crops in a Tropical Environment. PLoS ONE 2019, 14, e0216710. [Google Scholar]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Jayaraman, J.; Ali, N. Use of Seaweed Extracts for Disease Management of Vegetable Crops. In Sustainable Crop Disease Management Using Natural Products; CABI: Wallingford, UK, 2015; pp. 160–173. [Google Scholar]
- Mitra, J. Genetics and genetic improvement of drought resistance in crop plants. Curr. Sci. 2001, 80, 758–762. [Google Scholar]
- Porch, T.G. Application of stress indices for heat tolerances screening of common bean. J. Agron. Crop. Sci. 2006, 192, 390–394. [Google Scholar] [CrossRef]
- Singh, S.; Sengar, R.S.; Kulshreshtha, N.; Datta, D.; Tomar, R.S.; Rao, V.P.; Garg, D.; Ojha, A. Assessment of multiple tolerance indices for salinity stress in bread wheat (Triticum aestivum L.). J. Agric. Sci. 2015, 7, 49–57. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Gautam, R.K.; Sharma, P.C.; Sharma, D.K. Effect of different salinity stresses on agro-morphological traits and utilization of salinity stress indices for reproductive stage salt tolerance in rice. Field Crops Res. 2016, 190, 26–33. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).