Ultrasonic Treatment Enhances Germination and Affects Antioxidant Gene Expression in Soybean (Glycine max L. Merr)
Abstract
1. Introduction
2. Materials and Methods
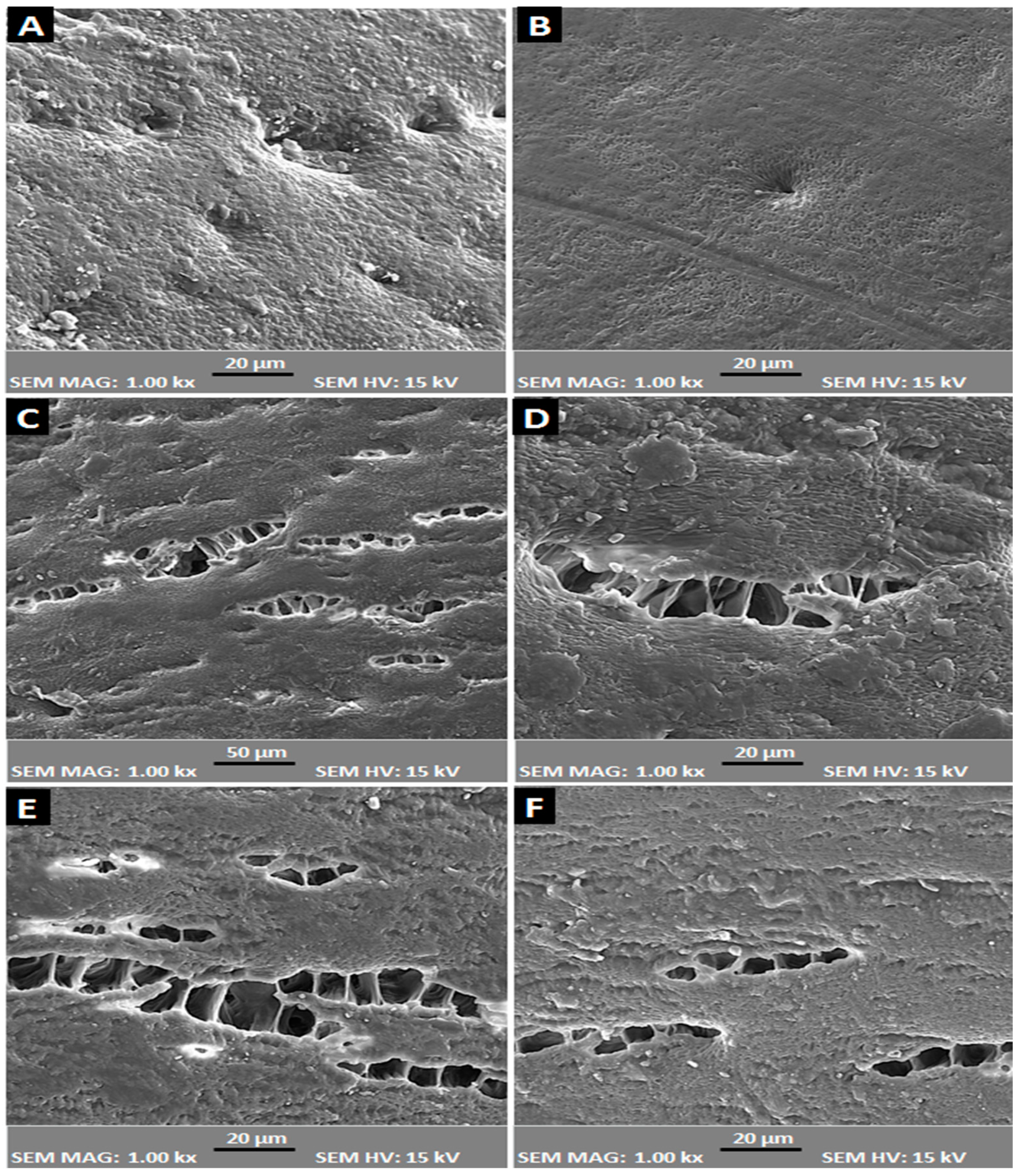
2.1. Scanning Electron Microscopy (SEM)
2.2. Germination Percentage (GP%)
2.3. Root Length
2.4. Shoot Length
2.5. Seedling Dry Matter
2.6. Seedling Vigor Index (SVI)
2.7. Real-Time Polymerase Chain Reaction (RT-PCR)
2.8. Statistical Analysis
3. Results
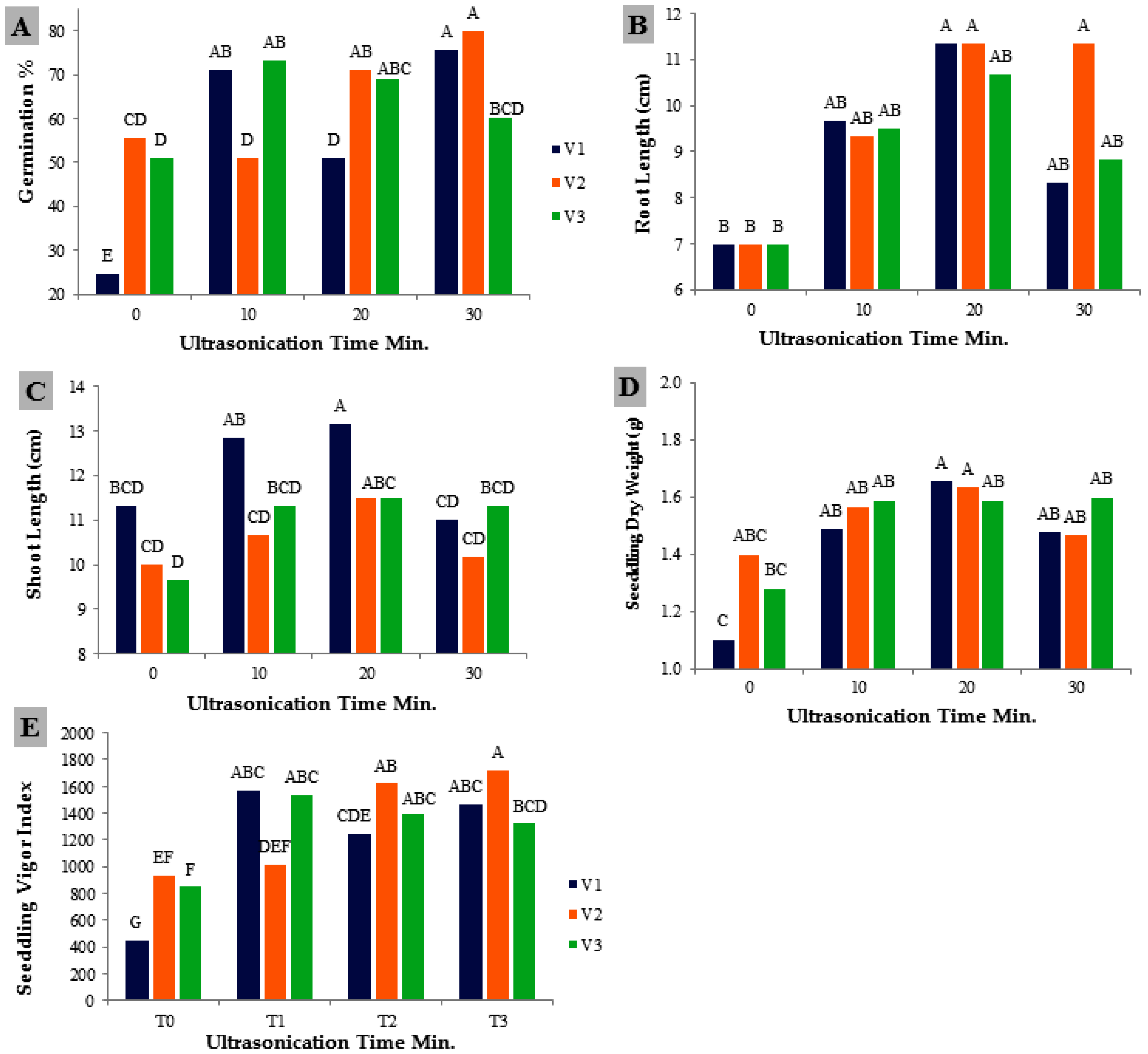
3.1. Seedling Growth Parameters
3.2. Antioxidant Gene Expression
3.3. Scanning Electronic Microscope (SEM)
4. Discussion
4.1. Seedling Growth Parameters
4.2. Antioxidant Gene Expression
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weerasekara, I.; Sinniah, U.R.; Namasivayam, P.; Nazli, M.H.; Abdurahman, S.A.; Ghazali, M.N. Priming with Humic Acid to Reverse Aging Damage in Soybean [Glycine max (L.) Merrill.] Seeds. Agriculture 2021, 11, 966. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Zhao, S.; Yang, C.; Meng, Y.; Shuai, H.; Luo, X.; Dai, Y.; Yin, H.; Du, J.; et al. DA-6 promotes germination and seedling establishment from aged soybean seeds by mediating fatty acid metabolism and glycometabolism. J. Exp. Bot. 2019, 70, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Q.; Karagić, Đ.; Liu, X.; Cui, J.; Gui, J.; Gu, M.; Gao, W. Effects of ultrasonication on increased germination and improved seedling growth of aged grass seeds of tall fescue and Russian wildrye. Sci. Rep. 2016, 6, 22403. [Google Scholar] [CrossRef] [PubMed]
- Mahjabin, S.B.; Abidi, A.B. Physiological and biochemical changes during seed deterioration: A review. Int. J. Recent Sci. Res. 2015, 6, 3416–3422. [Google Scholar]
- Kapoor, N.; Arya, A.; Siddiqui, M.A.; Kumar, H.; Amir, A. Physiological and biochemical changes during seed deterioration in aged seeds of rice (Oryza sativa L.). Am. J. P. Phys. 2011, 6, 28–35. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Alfalahi, A.O. Melatonin regulates antioxidant gene expression in soybean Glycine max L. Merr. Plant Cell Biotech. Mol. Biol. 2020, 21, 47–54. [Google Scholar]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Phys. 2015, 183, 1–12. [Google Scholar] [CrossRef]
- Lopez-Ribera, I.; Vicient, C.M. Use of ultrasonication to increase germination rates of Arabidopsis seeds. Plant Methods 2017, 13, 31. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Drochioiu, G.; Ciobanu, C.I.; Bancila, S.; Ion, L.; Petre, B.A.; Andries, C.; Murariu, M. Ultrasound-based protein determination in maize seeds. Ultrasonicssonochemistry 2016, 29, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Yaldagard, M.; Mortazavi, S.A.; Tabatabaie, F. Application of ultrasonic waves as a priming technique for accelerating and enhancing the germination of barley seed: Optimization of method by the Taguchi approach. J. Inst. Brew. 2008, 114, 14–21. [Google Scholar] [CrossRef]
- Kim, H.J.; Feng, H.; Kushad, M.M.; Fan, X. Effects of ultrasound, irradiation, and acidic electrolyzed water on germination of alfalfa and broccoli seeds and Escherichia coli O157: H7. J. Food Sci. 2006, 71, M168–M173. [Google Scholar] [CrossRef]
- Chin, Y.K.; Baque, M.A.; Elghamedi, S.; Lee, E.J.; Paek, K.Y. Effects of Activated Charcoal, Plant Growth Regulators and Ultrasonic Pre-treatments on ’invitro’ Germination and Protocorm Formation of ‘Calanthe’ Hybrids. Aust. J. Crop Sci. 2011, 5, 582. [Google Scholar]
- Goussous, S.J.; Samarah, N.H.; Alqudah, A.M.; Othman, M.O. Enhancing Seed Germination of Four Crop Species Using an Ultrasonic Technique. Exp. Agric. 2010, 46, 231–242. [Google Scholar] [CrossRef]
- Scouten, A.J.; Beuchat, L.R. Combined effects of chemical, heat and ultrasound treatments to kill Salmonella and Escherichia coli O157: H7 on alfalfa seeds. J. Appl. Microb. 2002, 92, 668–674. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.; Yersaiyiti, H.; Liu, Y.; Cui, J.; Wu, C.; Zhang, Y.; He, X. Modeling Analysis on Germination and Seedling Growth Using Ultrasound Seed Pretreatment in Switchgrass. PLoS ONE 2012, 7, e47204. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Liu, Q.; Gao, Y.N.; Zhou, W.N.; Cui, X.W.; Wang, Q.Z. Effects of ultrasound on the germination and seedling growth of three aged forage seeds. J. Appl. Ecol. 2018, 29, 1857–1866. [Google Scholar] [CrossRef]
- Powell, A.A.; Matthews, S. Towards the validation of the controlled deterioration vigour test for small seeded vegetables. Seed Test. Int. 2005, 129, 21–24. Available online: https://www.seedtest.org/upload/cms/user/STI12921-24.pdf (accessed on 15 March 2022).
- Weerasekara, I.; Sinniah, U.R.; Namasivayam, P.; Nazli, M.H.; Abdurahman, S.A.; Ghazali, M.N. The Influence of Seed Production Environment on Seed Development and Quality of Soybean [Glycine max (L.) Merrill.]. Agronomy 2021, 11, 1430. [Google Scholar] [CrossRef]
- Calonego, J.C.; Rosolem, C.A. Soybean root growth and yield in rotation with cover crops under chiseling and no-till. Euro. J. Agron. 2010, 33, 242–249. [Google Scholar] [CrossRef]
- Afrakhteh, S.; Frahmandfar, E.; Hamidi, A.; Ramandi, H.D. Evaluation of growth characteristics and seedling vigor in two varieties of soybean dried under different temperature and fluidized bed dryer. Int. J. Agric. Crop Sci. 2013, 5, 2537. Available online: http://ijagcs.com/../2537-2544.pdf (accessed on 15 March 2022).
- Zilli, C.G.; Santa-Cruz, D.M.; Yannarelli, G.G.; Noriega, G.O.; Tomaro, M.L.; Balestrasse, K.B. Hemeoxygenase contributes to alleviate salinity damage in Glycine max L. leaves. Int. J. Cell Biol. 2009, 9, 848516. [Google Scholar] [CrossRef]
- Machikowa, T.; Kulrattanarak, T.; Wonprasaid, S. Effects of ultrasonic treatment on germination of synthetic sunflower seeds. In Proceedings of World Academy of Science, Engineering and Technology; World Academy of Science, Engineering and Technology (WASET): Paris, France, 2013; Volume 73, p. 53. [Google Scholar] [CrossRef]
- Shekari, F.; Mustafavi, S.H.; Abbasi, A. Sonication of seeds increase germination performance of sesame under low temperature stress. Acta Agric. Slov. 2015, 105, 203–212. [Google Scholar] [CrossRef]
- Nazari, M.; Eteghadipour, M. Impacts of ultrasonic waves on seeds: A mini-review. Agric Res Tech. Open Access J. 2017, 6, 5. [Google Scholar] [CrossRef]
- Yıldıztugay, E.; Sekmen, A.H.; Turkan, I.; Kucukoduk, M. Elucidation of physiological and biochemical mechanisms of an endemic halophyte Centaurea tuzgoluensis under salt stress. Plant Physiol. Biochem. 2011, 49, 816–824. [Google Scholar] [CrossRef]
- Lee, K.J.; Baek, D.-Y.; Lee, G.-A.; Cho, G.-T.; So, Y.-S.; Lee, J.-R.; Ma, K.-H.; Chung, J.-W.; Hyung, D.Y. Phytochemicals and Antioxidant Activity of Korean black Soybean [Glycine max (L.) Merrill.] landraces. Antioxidants 2020, 9, 213. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Yoon, H.; Shin, M.-J.; Lee, Y.; Hur, O.S.; Lee, B.C.; Ha, B.-K.; Wang, X.; Desta, K.T. Metabolite Contents and Antioxidant Activities of Soybean [Glycine max (L.) Merrill.] Seeds of Different Seed Coat Colors. Antioxidants 2021, 10, 1210. [Google Scholar] [CrossRef]


| Treatments | Number of Copies | Gene Expression vs. Control (%) | |||
|---|---|---|---|---|---|
| SOD | CAT | SOD | CAT | ||
| V1 | T1 | 11.73 | 18.36 | −19.2 | 43.8 |
| T2 | 93.94 | 80.50 | 547.1 | 530.6 | |
| T3 | 50.73 | 23.36 | 249.4 | 83.0 | |
| V2 | T1 | 60.91 | 64.70 | 407.4 | 119.5 |
| T2 | 92.02 | 71.89 | 666.6 | 143.9 | |
| T3 | 14.27 | 28.36 | 18.9 | −3.8 | |
| V3 | T1 | 49.70 | 36.38 | 243.3 | 71.9 |
| T2 | 16.89 | 23.67 | 16.6 | 56.8 | |
| T3 | 18.36 | 26.77 | 26.8 | 61.8 | |
| T0 | V1 | 14.52 | 12.76 | - | - |
| V2 | 12.00 | 29.48 | - | - | |
| V3 | 14.48 | 10.23 | - | - | |
| Mean | SOD | T0: 13.67 | T1: 40.78 | T2: 67.62 | T3: 27.79 |
| CAT | T0: 17.49 | T1: 39.81 | T2: 58.69 | T3: 26.16 | |
| SOD | V1: 52.13 | V2: 55.73 | V3: 28.32 | ||
| CAT | V1: 40.74 | V2: 54.98 | V3: 28.94 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfalahi, A.O.; Alobaidy, B.S.; Almarie, A.A.; Dhanoon, O.M.; Qasem, J.R.; Almehemdi, A.F.; Najda, A. Ultrasonic Treatment Enhances Germination and Affects Antioxidant Gene Expression in Soybean (Glycine max L. Merr). Agronomy 2022, 12, 2446. https://doi.org/10.3390/agronomy12102446
Alfalahi AO, Alobaidy BS, Almarie AA, Dhanoon OM, Qasem JR, Almehemdi AF, Najda A. Ultrasonic Treatment Enhances Germination and Affects Antioxidant Gene Expression in Soybean (Glycine max L. Merr). Agronomy. 2022; 12(10):2446. https://doi.org/10.3390/agronomy12102446
Chicago/Turabian StyleAlfalahi, Ayoob Obaid, Bushra Shaker Alobaidy, Ahmed Abdulwahid Almarie, Omar Mahmood Dhanoon, Jamal Ragheb Qasem, Ali Fadaam Almehemdi, and Agnieszka Najda. 2022. "Ultrasonic Treatment Enhances Germination and Affects Antioxidant Gene Expression in Soybean (Glycine max L. Merr)" Agronomy 12, no. 10: 2446. https://doi.org/10.3390/agronomy12102446
APA StyleAlfalahi, A. O., Alobaidy, B. S., Almarie, A. A., Dhanoon, O. M., Qasem, J. R., Almehemdi, A. F., & Najda, A. (2022). Ultrasonic Treatment Enhances Germination and Affects Antioxidant Gene Expression in Soybean (Glycine max L. Merr). Agronomy, 12(10), 2446. https://doi.org/10.3390/agronomy12102446









