Impacts of Alexandrian Clover Living Mulch on the Yield, Phenolic Content, and Antioxidant Capacity of Leek and Shallot
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Shallot
2.3. Leek
2.4. Weather Conditions
2.5. Yield and Morphological Parameter Evaluation
2.6. Sample Preparation and Analyses
2.7. Extraction
2.8. Total Phenolics Measurement
2.9. HPLC Analysis
2.10. Determination of Total Antioxidant Capacity
2.11. Chemicals and Standards
2.12. Statistical Analysis
3. Results
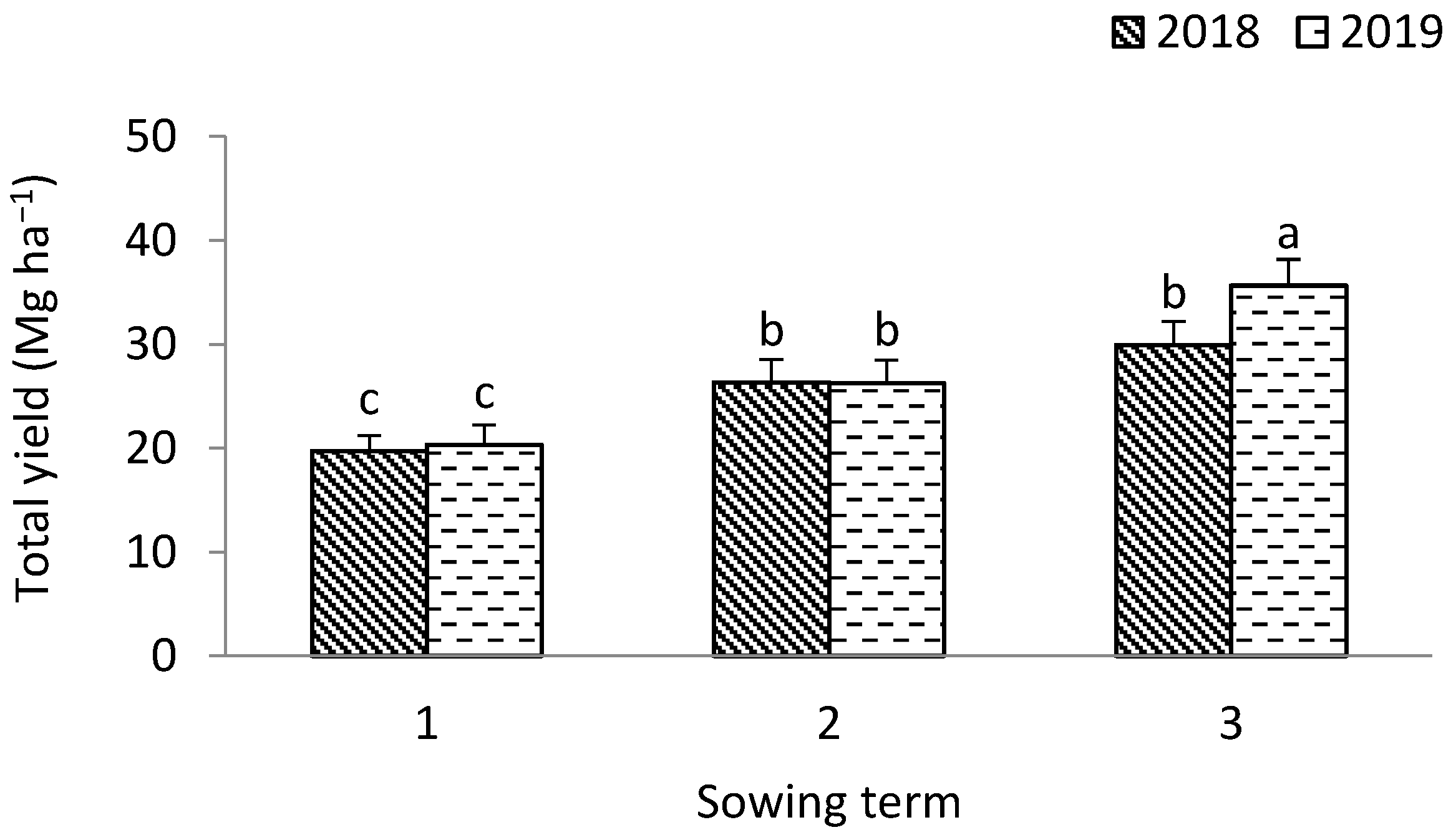
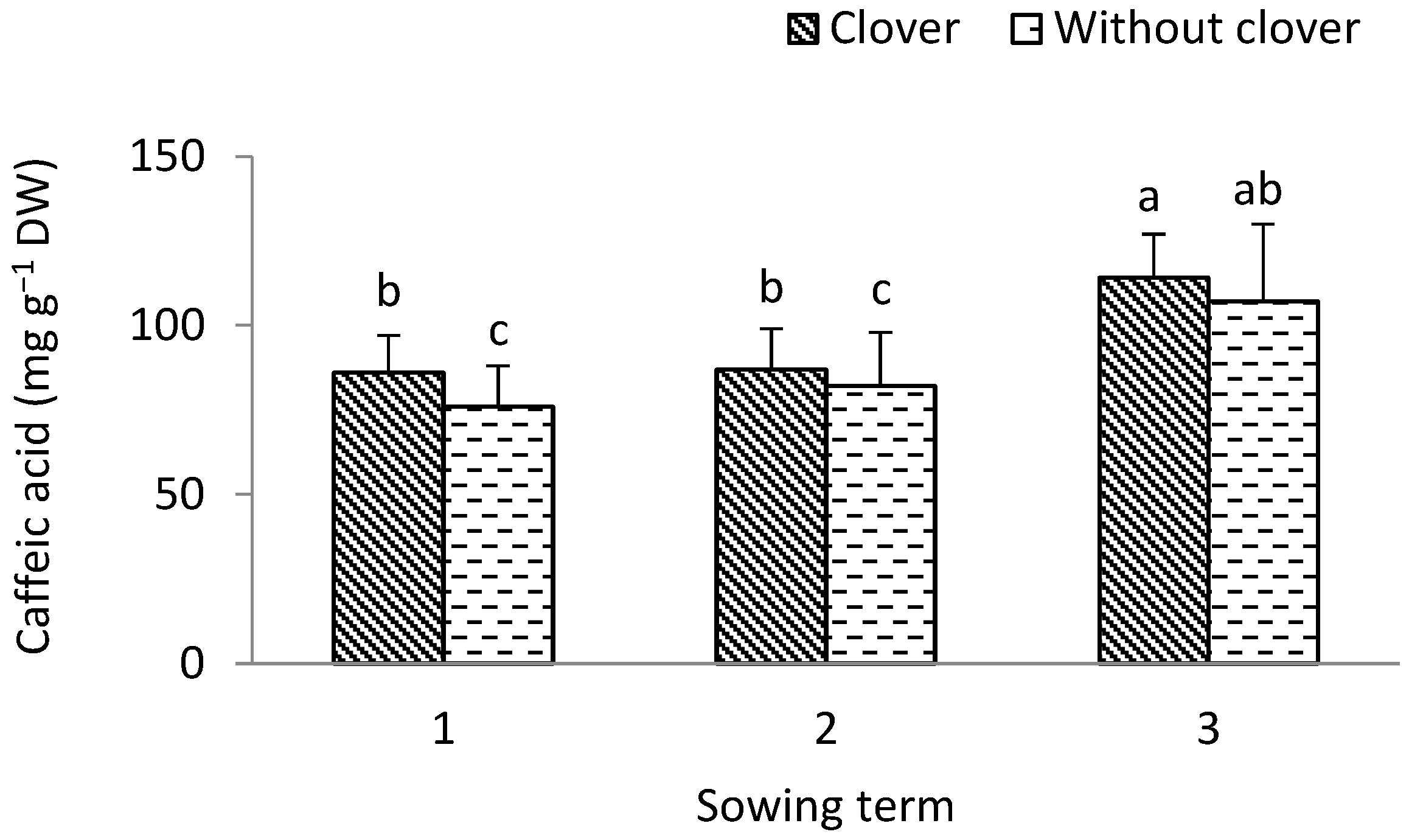
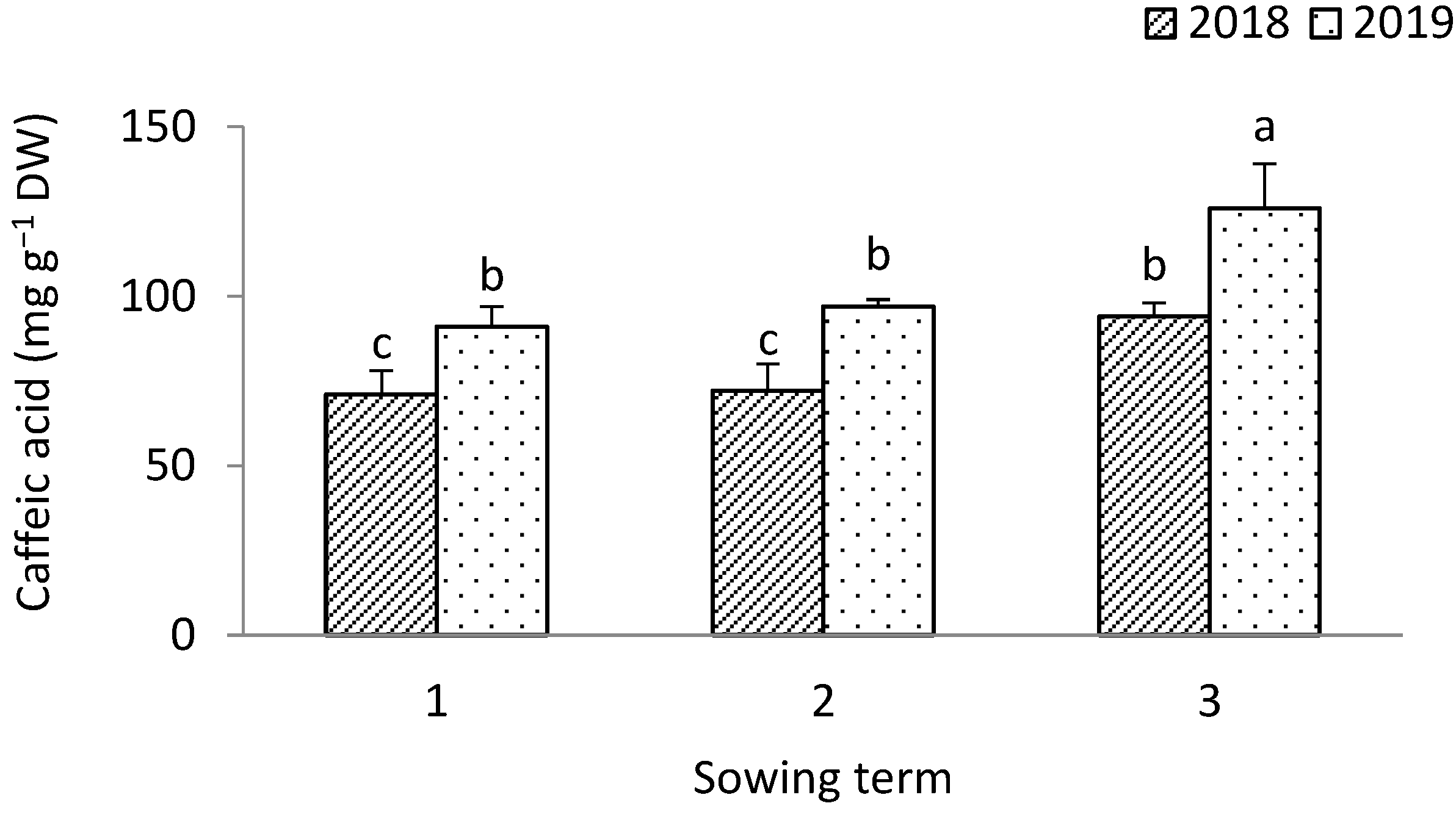
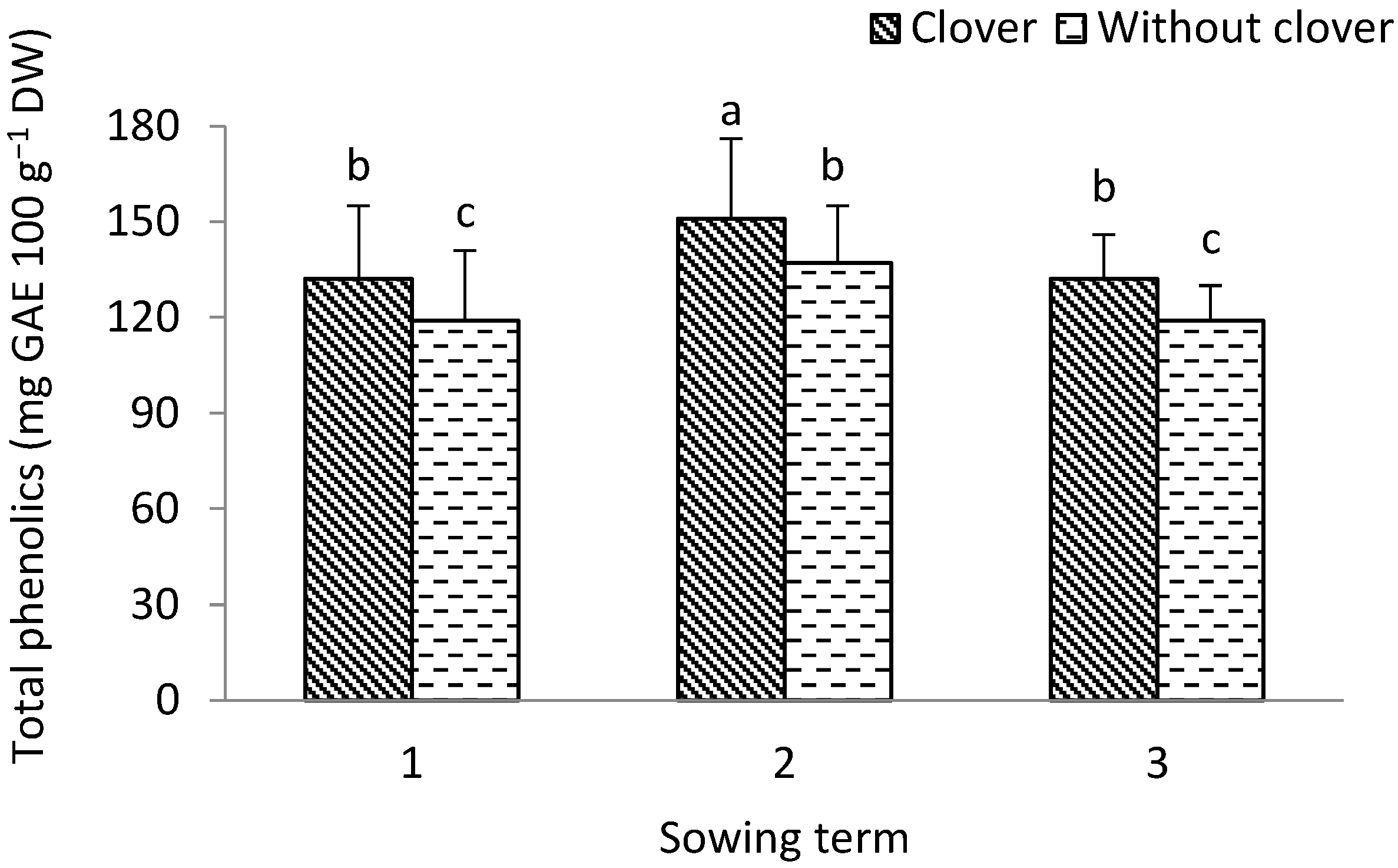
3.1. Leek
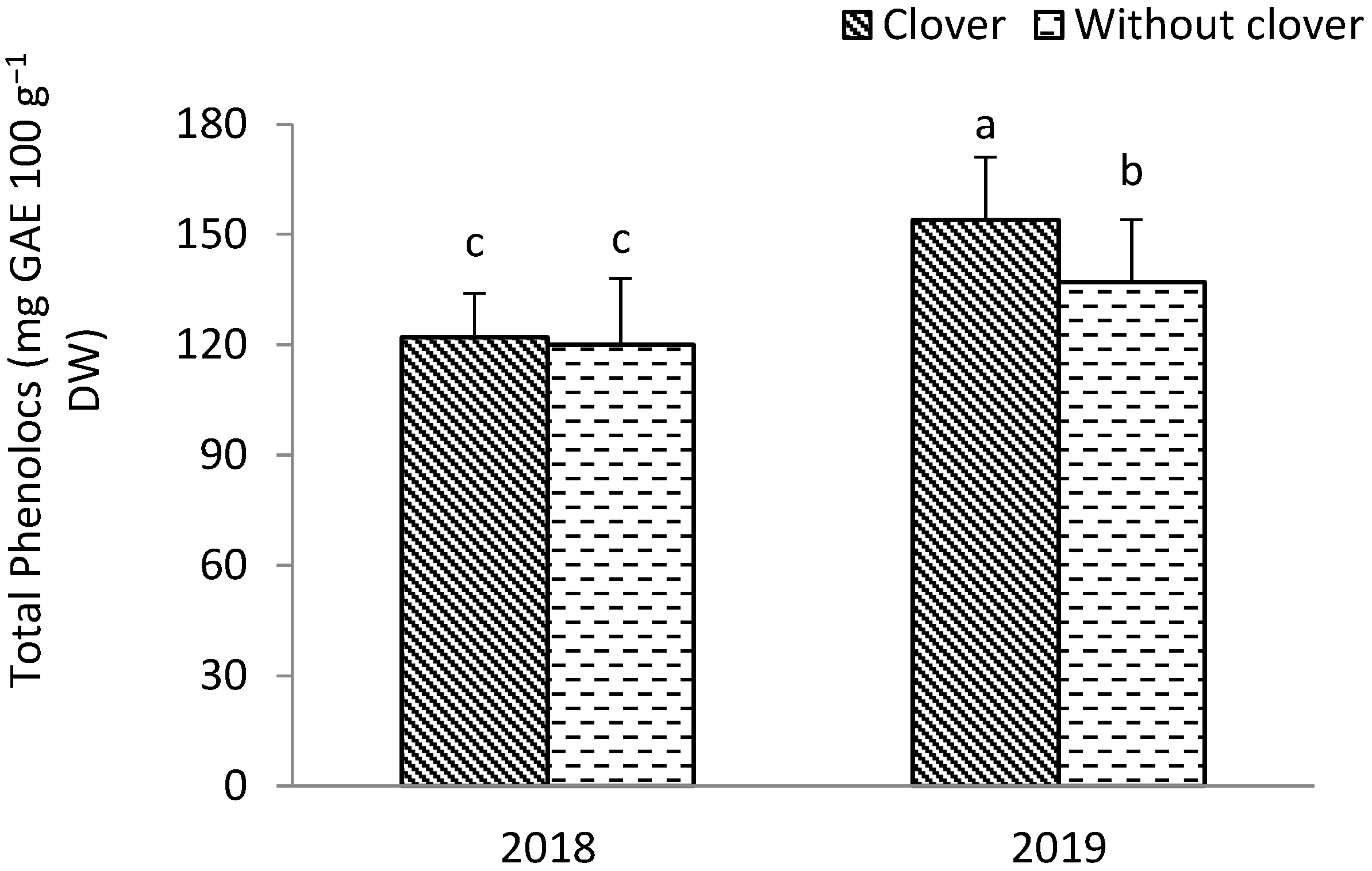
3.2. Shallot
4. Discussion
4.1. The Yield of Leek Above-Ground Mass and the Yield of Shallot Bulbs
4.2. The Biological Value of Leek Pseudostems and Shallot Bulbs
4.3. Antioxidant Value
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. World Food and Agriculture—Statistical Yearbook. Rome. 2021. Available online: https://www.fao.org/documents/card/en/c/cb4477en/ (accessed on 22 April 2022).
- Tendaj, M.; Mysiak, B.; Gruszecki, R. Plon cebul i zawartość wybranych składników pokarmowych u kilku odmian cebuli zwyczajnej i szalotki. Ann. Hortic. 2014, 24, 1–8. [Google Scholar]
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essential oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [PubMed]
- Monika, N.; Sakthi Abirami, M. Allium porrum: A review. World J. Pharm. Life Sci. 2018, 4, 28–40. [Google Scholar]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from allium extracts on HepG2; PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Irena Budić-Leto, I.; Bilušić, T.; Čikeš-Čulić, V.; Puizina, J. Chemical composition and biological activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.A.; Young, B.G. Utility of winter rye living mulch for weed management in zucchini squash production. Weed Technol. 2008, 22, 724–728. [Google Scholar] [CrossRef]
- Leary, J.; DeFrank, J. Living mulches for organic farming systems. HortTechnology 2000, 10, 692–698. [Google Scholar] [CrossRef]
- Siller, A.R.; Albrecht, K.A.; Jokela, W.E. Soil erosion and nutrient runoff in corn silage production with kura clover living mulch and winter rye. Agron. J. 2016, 108, 989–999. [Google Scholar] [CrossRef]
- Robačer, M.; Canali, S.; Kristensen, H.L.; Bavec, F.; Mlakar, S.G.; Jakop, M.; Bavec, M. Cover crops in organic field vegetable production. Sci. Hortic. 2016, 208, 104–110. [Google Scholar] [CrossRef]
- Swenson, J.A.; Walters, S.A.; Chong, S.K. Influence of tillage and mulching systems on soil water and tomato fruit yield and quality. J. Veg. Crop Prod. 2004, 10, 81–95. [Google Scholar] [CrossRef]
- Andrews, J.; Sanders, Z.; Cabrera, M.; Hill, N.; Radcliffe, D. Estimating nitrate leaching covers cropped and perennial living mulch corn production systems annually. J. Soil Water Conserv. 2020, 75, 91–102. [Google Scholar] [CrossRef]
- Boyd, N.S.; Gordon, R.; Asiedu, S.K.; Martin, R.C. The effect of living mulches on potato tuber yield (Solanum tuberosum L.). Biol. Agric. Hortic. 2000, 18, 203–220. [Google Scholar] [CrossRef]
- Qi, Z.; Helmers, M.J.; Christianson, R.D.; Pederson, C.H. Nitrate-nitrogen losses through subsurface drainage under various agricultural land covers. J. Environ. Qual. 2011, 40, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.; Cordeau, S.; Chauvel, B.; Bohan, D.; Guillemin, J.-P.; Steinberg, C. Biodiversity-based options for arable weed management. A review. Agron. Sustain. Dev. 2018, 38, 48. [Google Scholar] [CrossRef]
- Médiène, S.; Valantin-Morison, M.; Sarthou, J.-P.; De Tourdonnet, S.; Gosme, M.; Bertrand, M.; Roger-Estrade, J.; Aubertot, J.-N.; Rusch, A.; Motisi, N.; et al. Agroecosystem management and biotic interactions: A review. Agron. Sustain. Dev. 2011, 31, 491–514. [Google Scholar] [CrossRef]
- Pouryousef, M.; Yousefi, A.R.; Oveisi, M.; Asadi, F. Intercropping of fenugreek as living mulch at different densities for weed suppression in coriander. Crop Prot. 2015, 69, 60–64. [Google Scholar] [CrossRef]
- Pfeiffer, A.; Silva, E.; Colquhoun, J. Living mulch cover crops for weed control in small-scale application. Renew. Agric. Food Syst. 2015, 31, 309–317. [Google Scholar] [CrossRef]
- Akemo, M.; Bennett, M.; Regnier, E. Tomato growth in spring-sown cover crops. HortScience 2000, 35, 843–848. [Google Scholar] [CrossRef]
- Bhaskar, V.; Bellinder, R.R.; Reiners, S.; Westbrook, A.S.; Di Tommaso, A. Significance of herbicide order in sequential applications to target weeds in sun hemp living mulch. Weed Technol. 2021, 35, 565–573. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Kołota, E. Application of living mulches to pepper cultivation. Zesz. Nauk. AR Bydg. 2001, 234, 7–11, (In Polish with English summary). [Google Scholar]
- Carruthers, K.; Prithiviraj, B.; Fe, Q.; Cloutier, D.; Martin, R.C.; Smith, D.L. Intercropping corn with soybean; lupin and forages: Yield component responses. Eur. J. Agron. 2020, 12, 103–115. [Google Scholar] [CrossRef]
- Bhaskar, V.; Bellinder, R.R.; Reiners, S.; DiTommaso, A. Reduced herbicide rates for control of living mulch and weeds in fresh market tomato. Weed Technol. 2020, 34, 55–63. [Google Scholar] [CrossRef]
- Båth, B.; Kristensen, H.L.; Thorup-Kristensen, K. Root pruning reduces root competition and increases crop growth in a living mulch cropping system. J. Plant Interact. 2008, 3, 211–221. [Google Scholar] [CrossRef]
- Gibson, K.D.; Mcmillan, J.; Hallett, S.G.; Jordan, T.; Weller, S.C. Effect of a living mulch on weed seed banks in tomato. Weed Technol. 2011, 25, 245–251. [Google Scholar] [CrossRef]
- Kołodziejczyk, M. The effect of living mulches and conventional methods of weed control on weed infestation and potato yield. Sci. Hortic. 2015, 191, 127–133. [Google Scholar] [CrossRef]
- Ciaccia, C.; Kristensen, H.L.; Campanelli, G.; Xie, Y.; Testani, E.; Leteo, F.; Canali, S. Living mulch for weed management in organic vegetable cropping systems under the Mediterranean and North European conditions. Renew. Agric. Food Syst. 2017, 32, 248–262. [Google Scholar] [CrossRef]
- Borowy, A. Growth of onion (Allium cepa L.) under no-tillage cultivation with rye (Secale cereale L.) as a cover crop. In Proceedings of the 27th International Horticultural Congress & Exhibition, Lueven, Belgium, 13–19 August 2006. [Google Scholar]
- Faradonbeh, M.M.; Mashhadi, A.A.; Bakhshandeh, A.; Jalalabadi, A.L. Evaluation of the effects of different material on quantity and quality yield of garlic populations (Allium sativum L.). Int. Agric. Crop Sci. 2013, 5, 2660–2665. [Google Scholar]
- Adamczewska-Sowińska, K.; Kołota, E.; Winiarska, S. Living mulches in field cultivation of vegetables. Veg. Crops Res. Bull. 2009, 70, 19–29. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K. Wpływ żywych ściółek na plonowanie i wartość biologiczną papryki. Zesz. Probl. Post. Nauk Rol. 2008, 527, 59–65. [Google Scholar]
- Adamczewska-Sowińska, K.; Kołota, E. Wartość nawozowa żywych ściółek stosowanych w uprawie pomidora i papryki. Zesz. Probl. Post. Nauk Rol. 2009, 539, 23–29. [Google Scholar]
- Adamczewska-Sowińska, K.; Kołota, E. Yielding and nutritive value of field cultivated eggplant using living and synthetic mulches. Acta Sci. Pol. Hortorum Cultus 2010, 9, 191–199. [Google Scholar]
- Campanelli, G.; Canali, S. Crop production and environmental effects in conventional and organic vegetable farming systems: The case of a long-term experiment in Mediterranean conditions (Central Italy). J. Sustain. Agric. 2012, 36, 599–619. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; update 2015. International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Reports No.106; FAO: Rome, Italy, 2015; p. 192. [Google Scholar]
- PN-90/A-75101/03; Przetwory Owocowe i Warzywne. Oznaczanie zawartości suchej masy []. PKN: Warszawa, Poland, 1990. (In Polish)
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, 151–183. [Google Scholar] [CrossRef] [PubMed]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Dewanto, V.X.; Wu, K.; Adom, K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 97, 654–660. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes; onions; lettuce; and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Antioxidant potential of Artemisia argentea L. L’Hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Seredin, T.M.; Antoshkina, M.S.; Kosheleva, O.V.; Teliban, G.C.; Caruso, G. Yield, quality, antioxidants and elemental composition of new leek cultivars under organic or conventional system. Horticulture 2018, 4, 39. [Google Scholar] [CrossRef]
- Xie, Y.; Tittarelli, F.; Von Fragstein, P.; Bavec, M.; Canali, S.; Kristensen, H. Can living mulches in intercropping systems reduce the potential nitrate leaching? Studies of organic cauliflower (Brassica oleracea L. var. botrytis) and leek (Allium porrum L.) production across European conditions. Renew. Agric. Food Syst. 2017, 32, 224–239. [Google Scholar] [CrossRef]
- Trinchera, A.; Tewstani, E.; Roccuzzo, G.; Campanelli, G.; Ciaccia, C. Agroecological service crops drive plant mycorrhization in organic horticultural systems. Microorganisms 2021, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Canali, S.; Campanelli, G.; Ciaccia, C.; Diacono, M.; Leteo, F.; Fiore, A.; Montemurro, F. Living mulch strategy for organic cauliflower (Brassica oleracea L.) production in central and southern Italy. Ital. J. Agron. 2015, 10, 90–96. [Google Scholar] [CrossRef]
- Vanek, S.; Wien, H.C.; Rangarajan, A. Time of interseeding of lana vetch and winter rye cover strips determines competitive impact on pumpkins grown using organic practices. HortScience 2005, 40, 1716–1722. [Google Scholar] [CrossRef]
- Brainard, D.C.; Bellinder, R.R.; Miller, A.J. Cultivation and interseeding for weed control in transplanted cabbage. Weed Technol. 2004, 188, 704–710. [Google Scholar] [CrossRef]
- Bernaert, N.; De Paepe, D.; Bouten, C.; De Clercq, H.; Stewart, D.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant capacity; total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chem. 2012, 134, 669–677. [Google Scholar] [CrossRef]
- García-Herrera, P.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Pardo-de-Santayana, M.; Molina, M.; Tardio, J. Nutrients, phytochemicals and antioxidant activity in wild populations of Allium ampeloprasum L., a valuable underutilized vegetable. Food Res. Int. 2014, 62, 272–279. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef]
- Lisiewska, Z.; Kmiecik, W.; Korus, A. Content of vitamin C; carotenoids; chlorophylls and polyphenols in green parts of dill (Anethum graveolens L.) depending on plant height. J. Food Compos. Anal. 2006, 19, 134–140. [Google Scholar] [CrossRef]
- Kołota, E.; Adamczewska-Sowińska, K. The effects of living mulches on yield; overwintering and biological value of leek. Acta Hortic. 2004, 638, 209–214. [Google Scholar] [CrossRef]
- Kaspar, S.; Matros, A.; Mockm, H.P. Proteome and flavonoid analysis reveals distinct responses of epidermal tissue and whole leaves upon UV-B radiation of barley (Hordeum vulgare L.) seedlings. J. Proteome Res. 2010, 9, 2402–2411. [Google Scholar] [CrossRef]
- Li, J.; Ou-Lee, T.M.; Raba, R.; Amundson, R.G.; Last, R.L. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Putri, F.; Arifin Aziz, S.; Andarwulan, N.; Melati, M.; Suwarto, S. Leaf pigment, phenolic content; and production of green shallot of five different shallot varieties. Planta Trop. J. Agrosains 2021, 9, 48–57. [Google Scholar] [CrossRef]
- Manukyan, A. Effects of PAR and UV-B radiation on herbal yield, bioactive compounds and their antioxidant capacity of some medicinal plants under controlled environmental conditions. Photochem. Photobiol. 2013, 89, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of meteorological conditions on antioxidant flavonoids in Portuguese cultivars of white and red onions. Food Chem. 2011, 124, 303–308. [Google Scholar] [CrossRef]
- Greenland, R.G. Optimum height at which to kill barley used as a living mulch in onions. HortScience 2000, 35, 853–855. [Google Scholar] [CrossRef]
- Bibi, N.; Shah, M.H.; Khan, N.; Al-Hashimi, A.; Elshikh, M.S.; Iqbal, A.; Ahmad, S.; Abbasi, A.M. Variations in total phenolic, total flavonoid contents, and free radicals’ scavenging potential of onion varieties planted under diverse environmental conditions. Plants 2022, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content; soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
| Years | Month | ||||||
|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | Average/Sum | |
| Temperature (°C) | |||||||
| 2018 | 7.5 | 16.7 | 18.8 | 20.6 | 20.8 | 15.5 | 16.7 |
| 2019 | 9.5 | 13.4 | 21.5 | 19.4 | 20.3 | 14.5 | 16.4 |
| 1951–2010 | 7.4 | 13.0 | 16.2 | 17.8 | 17.1 | 12.6 | 14.0 |
| Rainfall (mm) | |||||||
| 2018 | 40 | 56 | 65 | 124 | 72 | 68 | 425 |
| 2019 | 49 | 93 | 37 | 38 | 102 | 52 | 371 |
| 1951–2010 | 39 | 58 | 66 | 84 | 69 | 54 | 368 |
| Parameter | LM a | ST b | GS c | (LM) × (ST) | (LM) × (GS) | (ST) × (GS) | (LM) × (ST) × (GS) |
|---|---|---|---|---|---|---|---|
| Yield | 39.428 * | 9.328 * | 29.633 * | 1.326 NS | 1.502 NS | 7.972 * | 2.152 NS |
| Mean pseudo-stem weight | 3.613 * | 3.496 * | 5.185 * | 0.202 NS | 0.932 NS | 0.283 NS | 2.308 NS |
| Length of pseudo-stems | 0.00 NS | 4.99 * | 1.56 NS | 6.22 * | 0.00 NS | 0.01 NS | 0.69 NS |
| Diameter of pseudo-stems | 11.64 * | 25.95 * | 4.55 * | 7.14 * | 2.91 NS | 4.41 * | 3.59 * |
| Dry weight | 124.023 * | 12.235 * | 11.273 * | 7.156 * | 0.028 NS | 3.018 NS | 2.514 NS |
| Total phenolics | 58.257 * | 4.104 * | 16.541 * | 0.048 NS | 0.048 NS | 0.808 NS | 0.048 NS |
| Ferulic acid | 152.197 * | 0.452 NS | 65.724 * | 0.124 NS | 0.124 NS | 1.615 NS | 0.124 NS |
| Chlorogenic acid | 17.091 * | 30.158 * | 238.793 * | 0.047 NS | 0.426 NS | 0.047 NS | 0.426 NS |
| Caffeic acid | 2.02 NS | 111.44 * | 212.58 * | 9.45 * | 9.78 * | 3.51 * | 3.91 * |
| FRAP | 60.446 * | 0.638 NS | 3.021 NS | 2.418 NS | 0.269 NS | 0.486 NS | 0.269 NS |
| DPPH | 35.121 * | 1.830 NS | 0.173 NS | 0.103 NS | 0.026 NS | 3.978 * | 0.103 NS |
| Main Factor | Marketable Yield | Mean Pseudo-Stem Weight | Length of Pseudo-Stems | Diameter of Pseudo-Stems |
|---|---|---|---|---|
| Living mulch | ||||
| Clover | 38.32 ± 1.4 a | 249 ± 12 a | 9.07 ± 0.54 a | 3.21 ± 0.09 b |
| Without mulch | 24.38 ± 0.7 b | 190 ± 70 b | 9.07 ± 0.23 a | 3.30 ± 0.17 a |
| Sowing term | ||||
| 3 weeks before leek planting | 26.26 ± 0.9 b | 170 ± 11 b | 9.16 ± 0.47 ab | 3.17 ± 0.09 b |
| At time of leek planting | 30.01 ± 1.2 b | 219 ± 60 ab | 9.23 ± 0.15 a | 3.39 ± 0.11 a |
| 3 weeks after leek planting | 37.78 ± 1.5 a | 270 ± 11 a | 8.81 ± 0.43 b | 3.21 ± 0.13 b |
| Growing season | ||||
| 2018 | 25.31 ± 0.09 b | 185 ± 10 b | 9.14 ± 0.36 a | 3.28 ± 0.17 a |
| 2019 | 37.40 ± 0.14 a | 255 ± 10 a | 9.00 ± 0.45 a | 3.23 ± 0.11 a |
| Main Factor | DW * | TP | FER | CHL | CAF | FRAP | DPPH |
|---|---|---|---|---|---|---|---|
| Living mulch | |||||||
| Clover | 20.96 ± 3.13 a | 655 ± 1.87 a | 62.74 ± 0.08 a | 8.65 ± 0.23 a | 93.42 ± 1.51 a | 8.01 ± 0.11 a | 1.01 ± 0.11 a |
| Without mulch | 13.52 ± 3.21 b | 616 ± 1.85 b | 43.30 ± 0.07 b | 7.59 ± 0.24 b | 90.92 ± 2.43 a | 5.51 ± 0.08 b | 0.80 ± 0.08 b |
| Sowing term | |||||||
| 3 weeks before leek planting | 15.33 ± 5.23 b | 626 ± 2.50 b | 52.13 ± 0.12 a | 6.98 ± 0.21 c | 81.31 ± 1.23 b | 7.00 ± 0.20 a | 0.87 ± 0.20 a |
| At time of leek planting | 19.36 ± 2.87 a | 644 ± 2.45 a | 53.96 ± 0.12 a | 9.40 ± 0.23 a | 84.56 ± 1.41 b | 6.70 ± 0.14 a | 0.89 ± 0.14 a |
| 3 weeks after leek planting | 17.03 ± 5.64 a | 636 ± 3.01 ab | 52.96 ± 0.14 a | 7.98 ± 0.21 b | 110.65 ± 1.89 a | 6.57 ± 0.12 a | 0.95 ± 0.12 a |
| Growing season | |||||||
| 2018 | 16.12 ± 5.18 b | 625 ± 2.63 b | 46.63 ± 0.10 b | 10.10 ± 0.12 a | 79.35 ± 1.29 b | 6.48 ± 0.16 a | 0.90 ± 0.16 a |
| 2019 | 18.36 ± 4.46 a | 645 ± 2.40 a | 59.41 ± 0.11 a | 6.15 ± 0.13 b | 105.00 ± 1.76 a | 7.04 ± 0.15 a | 0.91 ± 0.15 a |
| Parameter | LM a | ST b | GS c | (LM) × (ST) | (LM) × (GS) | (ST) × (GS) | (LM) × (ST) × (GS) |
|---|---|---|---|---|---|---|---|
| Yield | 17.12 * | 12.58 * | 0.79 NS | 1.21 NS | 3.23 NS | 2.42 NS | 0.2 NS |
| Weight of 100 bulbs | 8.33 * | 7.58 * | 1.44 NS | 1.24 NS | 3.53 NS | 0.20 NS | 0.43 NS |
| Dry weight | 31.60 * | 14.63 * | 44.83 * | 0.48 NS | 0.40 NS | 1.06 NS | 0.05 NS |
| Total phenolics | 5.96 ** | 9.36 ** | 46.43 ** | 1.98 NS | 5.08 ** | 8.59 ** | 0.39 NS |
| p-coumaric acid | 4.43 ** | 1.72 NS | 72.01 ** | 0.11 NS | 0.03 NS | 1.94 NS | 0.61 NS |
| Quercetin | 7.86 ** | 3.58 ** | 6.87 ** | 1.11 NS | 3.98 NS | 0.65 NS | 2.57 NS |
| Chlorogenic acid | 1.30 NS | 0.37 NS | 92.26 ** | 0.22 NS | 0.00 NS | 0.31 NS | 0.01 NS |
| FRAP | 0.27 NS | 16.82 ** | 68.98 ** | 1.75 NS | 7.47 ** | 0.35 NS | 2.13 NS |
| DPPH | 0.33 NS | 0.48 NS | 121.11 ** | 0.89 NS | 23.25 ** | 12.78 ** | 6.75 ** |
| Main Factor | Yield | Weight of 100 Bulbs | Dry Weight |
|---|---|---|---|
| Living mulch | |||
| Clover | 26.24 ± 0.64 a | 8.04 ± 0.23 a | 13.41 ± 1.08 a |
| Without mulch | 20.20 ± 0.64 b | 6.44 ± 0.20 b | 12.42 ± 0.88 b |
| Sowing term | |||
| 3 weeks before shallot planting | 18.05 ± 0.69 b | 5.71 ± 0.21 b | 12.31 ± 0.89 b |
| At time of shallot planting | 25.55 ± 0.61 a | 7.98 ± 0.21 a | 13.47 ± 1.06 a |
| Three weeks after shallot planting | 26.05 ± 0.52 a | 8.03 ± 0.19 a | 12.99 ± 1.10 a |
| Growing season | |||
| 2018 | 23.02 ± 0.76 a | 6.91 ± 0.25 a | 12.33 ± 0.93 b |
| 2019 | 23.41 ± 0.65 a | 7.57 ± 0.21 a | 13.51 ± 0.92 a |
| Main Factor | TP * | COU | CHL | QUE | FRAP | DPPH |
|---|---|---|---|---|---|---|
| Living mulch | ||||||
| Clover | 137 ± 22.90 a | 5323 ± 53.48 a | 2.35 ± 0.67 a | 116 ± 6.12 a | 2.70 ± 0.76 a | 1.17 ± 0.06 a |
| Without mulch | 129 ± 18.84 a | 5122 ± 51.66 a | 2.22 ± 0.63 a | 96 ± 4.56 b | 2.65 ± 0.50 a | 1.13 ± 0.04 a |
| Sowing term | ||||||
| 3 weeks before shallot planting | 125 ± 23.10 b | 5102 ± 56.43 a | 2.24 ± 0.71 a | 94 ± 3.56 b | 2.26 ± 0.51 b | 1.14 ± 0.04 a |
| In time of shallot planting | 130 ± 22.80 b | 5311 ± 58.17 a | 2.35 ± 0.65 a | 107 ± 7.01 ab | 2.84 ± 0.61 a | 1.12 ± 0.06 a |
| 3 weeks after shallot planting | 144 ± 12.67 a | 5254 ± 44.52 a | 2.26 ± 0.61 a | 117 ± 6.23 a | 2.93 ± 0.61 a | 1.20 ± 0.04 a |
| Growing season | ||||||
| 2018 | 121 ± 14.81 b | 4818 ± 42.44 b | 1.74 ± 0.49 b | 97 ± 5.04 b | 2.25 ± 0.49 b | 0.78 ± 0.03 b |
| 2019 | 145 ± 19.56 a | 5627 ± 22.85 a | 2.82 ± 0.11 a | 115 ± 6.34 a | 3.10 ± 0.48 a | 1.53 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sałata, A.; Nurzyńska-Wierdak, R.; Kalisz, A.; Moreno-Ramón, H. Impacts of Alexandrian Clover Living Mulch on the Yield, Phenolic Content, and Antioxidant Capacity of Leek and Shallot. Agronomy 2022, 12, 2602. https://doi.org/10.3390/agronomy12112602
Sałata A, Nurzyńska-Wierdak R, Kalisz A, Moreno-Ramón H. Impacts of Alexandrian Clover Living Mulch on the Yield, Phenolic Content, and Antioxidant Capacity of Leek and Shallot. Agronomy. 2022; 12(11):2602. https://doi.org/10.3390/agronomy12112602
Chicago/Turabian StyleSałata, Andrzej, Renata Nurzyńska-Wierdak, Andrzej Kalisz, and Héctor Moreno-Ramón. 2022. "Impacts of Alexandrian Clover Living Mulch on the Yield, Phenolic Content, and Antioxidant Capacity of Leek and Shallot" Agronomy 12, no. 11: 2602. https://doi.org/10.3390/agronomy12112602
APA StyleSałata, A., Nurzyńska-Wierdak, R., Kalisz, A., & Moreno-Ramón, H. (2022). Impacts of Alexandrian Clover Living Mulch on the Yield, Phenolic Content, and Antioxidant Capacity of Leek and Shallot. Agronomy, 12(11), 2602. https://doi.org/10.3390/agronomy12112602







