Maize Breeding: From Domestication to Genomic Tools
Abstract
1. Introduction
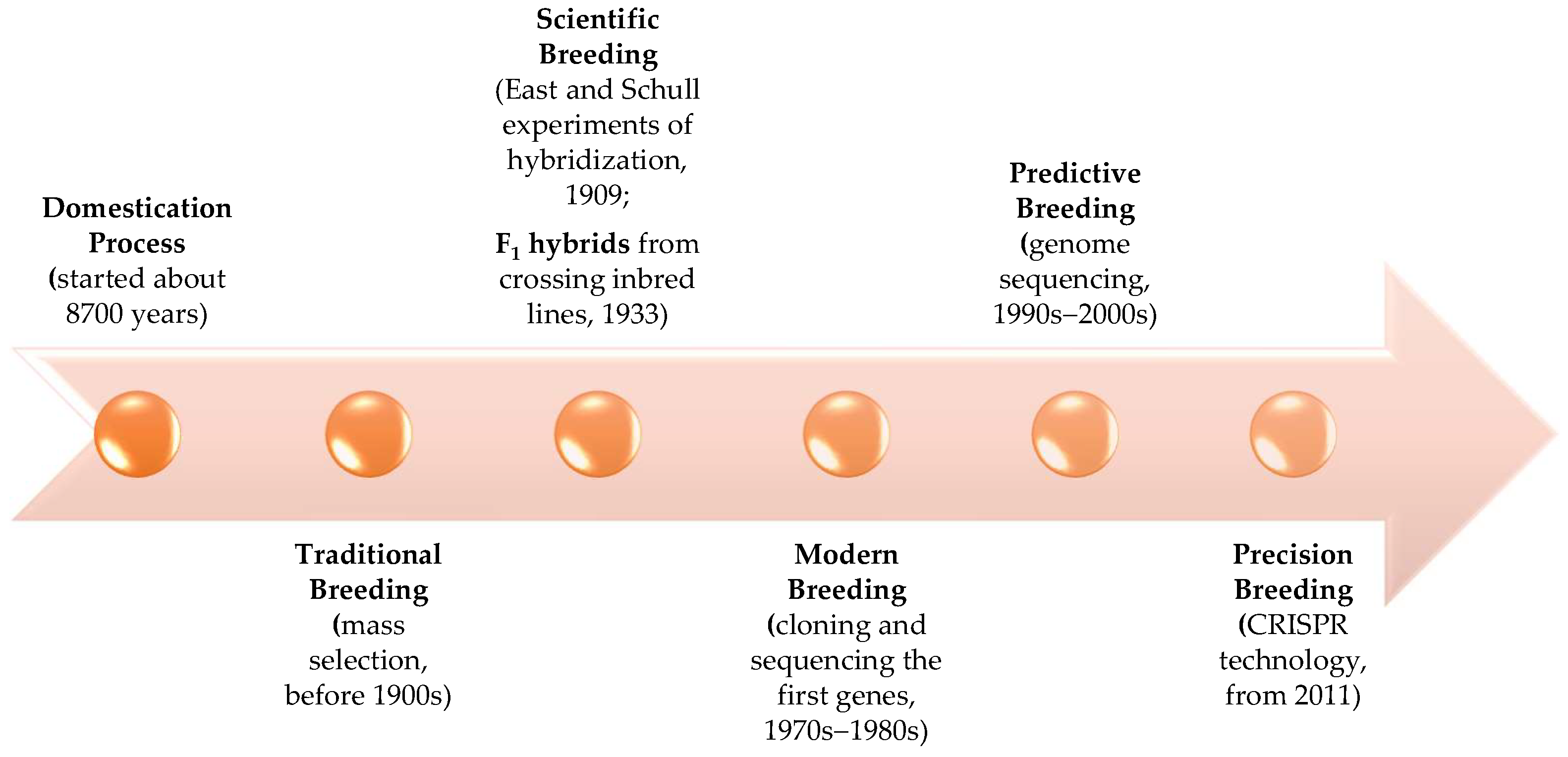
2. Evolution of Maize: From Domestication to Precise Breeding
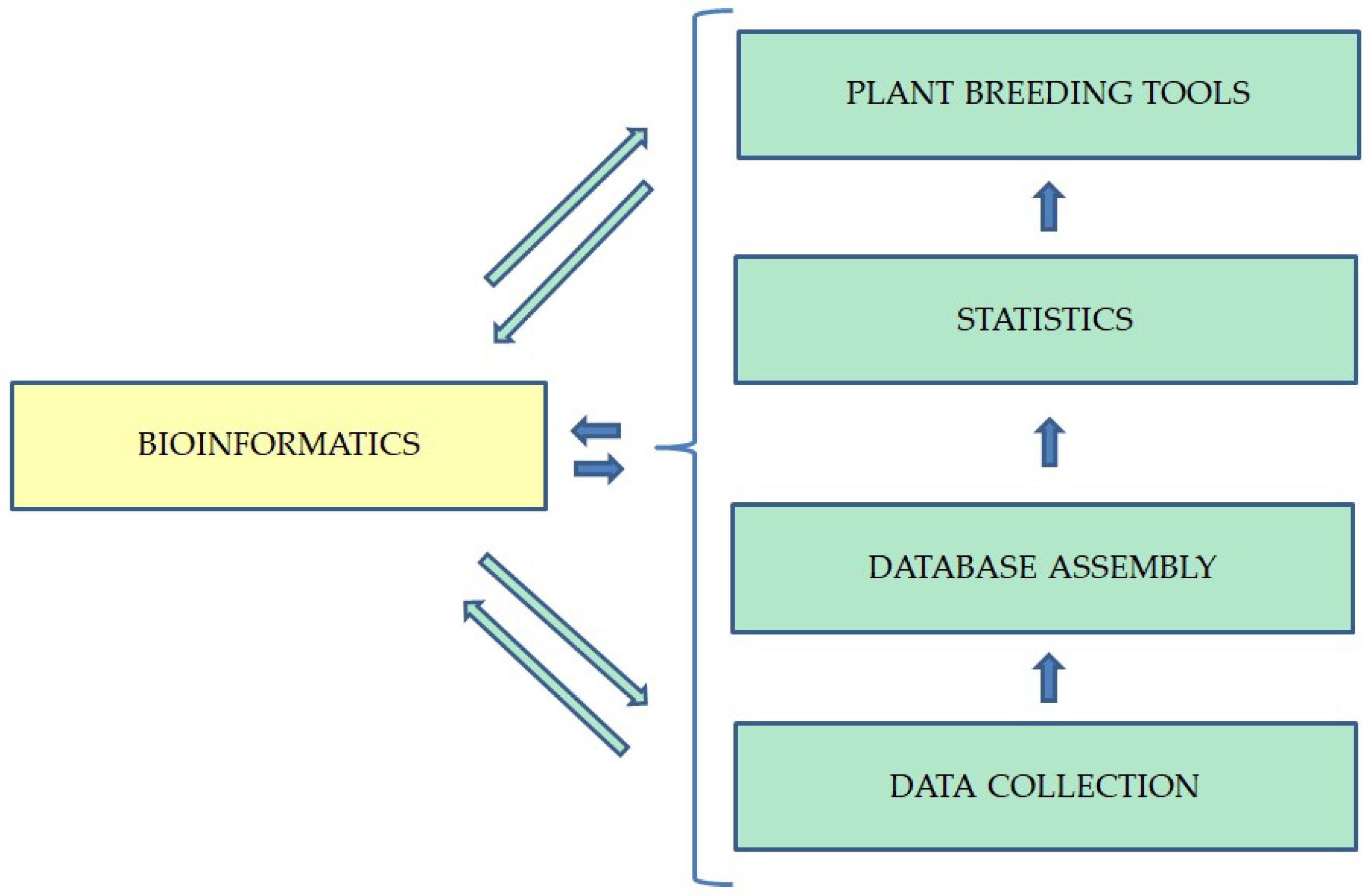
3. Bioinformatics: The Fundamental Tool Underlying Modern Methods of Breeding
4. Marker-Assisted Selection
5. Genomic Selection
6. Breeding by Design
7. Genome Sequencing
8. New Plant-Breeding Techniques
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roser, M. Future Population Growth. Our World in Data, 9 May 2013. [Google Scholar]
- Hu, H.; Scheben, A.; Edwards, D. Advances in Integrating Genomics and Bioinformatics in the Plant Breeding Pipeline. Agriculture 2018, 8, 75. [Google Scholar] [CrossRef]
- Merca, N.C.; Rusu, T.; Merca, I.; Ona, A.D. Agroecology: A Real Opportunity to Fight against the Climate Challenges. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2021, 21, 6. [Google Scholar]
- Shafi, A.; Zahoor, I.; Haq, E.; Fazili, K.M. Impact of Bioinformatics on Plant Science Research and Crop Improvement. In Essentials of Bioinformatics, Volume III: In Silico Life Sciences: Agriculture; Hakeem, K.R., Shaik, N.A., Banaganapalli, B., Elango, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 29–46. ISBN 978-3-030-19318-8. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 29 March 2022).
- Ghețe, A.B.; Duda, M.M.; Vârban, D.I.; Vârban, R.; Moldovan, C.; Muntean, S. Maize (Zea Mays), a Prospective Medicinal Plant in Romania. Hop Med. Plants 2018, 26, 44–51. [Google Scholar]
- Vârban, D.I.; Gheţe, A.; Chirițoiu, I.A.; Odagiu, A. Study of the Behavior of Some Maize Hybrids, in the Pedo- Climatic Conditions from Stănilești Commune Vaslui County. ProEnvironment 2020, 13, 43. [Google Scholar]
- Merca, N.C.; Rusu, T.; Merca, I.; Ona, A.D. Maize under the Climate Change Impact. Agricultura 2021, 118, 1–2. [Google Scholar] [CrossRef]
- Cristea, M. Importanţa economică, răspândirea geografică, producţia şi comerţul cu porumb. In Monografia Porumbului; Editura Academiei Române: București, Romania, 2004; Volume 1, pp. 17–27. ISBN 973-27-1055-1. [Google Scholar]
- Ufaz, S.; Galili, G. Improving the Content of Essential Amino Acids in Crop Plants: Goals and Opportunities. Plant Physiol. 2008, 147, 954–961. [Google Scholar] [CrossRef]
- Lone, A.A.; Dar, Z.A.; Gull, A.; Gazal, A.; Naseer, S.; Khan, M.H.; Ahangar, A.; Iqbal, A.M. Breeding Maize for Food and Nutritional Security; IntechOpen: London, UK, 2021; ISBN 978-1-83969-164-5. [Google Scholar]
- Poehlman, J.M.; Sleper, D.A. Breeding Field Crops; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cushman, J.C.; Bohnert, H.J. Genomic Approaches to Plant Stress Tolerance. Curr. Opin. Plant Biol. 2000, 3, 117–124. [Google Scholar] [CrossRef]
- Hafner, S. Trends in Maize, Rice, and Wheat Yields for 188 Nations over the Past 40 Years: A Prevalence of Linear Growth. Agric. Ecosyst. Environ. 2003, 1–3, 275–283. [Google Scholar] [CrossRef]
- Crouch, D.J.M.; Bodmer, W.F. Polygenic Inheritance, GWAS, Polygenic Risk Scores, and the Search for Functional Variants. Proc. Natl. Acad. Sci. USA 2020, 117, 18924–18933. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Kossmann, J. Improving Crops for a Changing World. Front. Plant Sci. 2021, 12, 728328. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Ashraf, M.; Younis, M.; Hu, X.; Kumar, A.; Akram, N.A.; Al-Qurainy, F. Role of Transgenic Plants in Agriculture and Biopharming. Biotechnol. Adv. 2012, 30, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Maghuly, F.; Myśków, B.; Till, B.J. Functional Genomics for Plant Breeding. Int. J. Mol. Sci. 2021, 22, 11854. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of Genetic Diversity Assessment in Crop Plants and Its Recent Advances: An Overview of Its Analytical Perspectives. Genet. Res. Int. 2015, 2015, e431487. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Meng, D.; Zhong, Y.; Chen, C.; Dong, X.; Xu, X.; Chen, B.; Li, W.; Li, L.; et al. A 4-Bp Insertion at ZmPLA1 Encoding a Putative Phospholipase a Generates Haploid Induction in Maize. Mol. Plant. 2017, 10, 520–522. [Google Scholar] [CrossRef]
- Piperno, D.R.; Ranere, A.J.; Holst, I.; Iriarte, J.; Dickau, R. Starch Grain and Phytolith Evidence for Early Ninth Millennium B.P. Maize from the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 5019–5024. [Google Scholar] [CrossRef]
- Ranere, A.J.; Piperno, D.R.; Holst, I.; Dickau, R.; Iriarte, J. The Cultural and Chronological Context of Early Holocene Maize and Squash Domestication in the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 5014–5018. [Google Scholar] [CrossRef]
- Tenaillon, M.I.; Charcosset, A. A European Perspective on Maize History. Comptes Rendus. Biol. 2011, 334, 221–228. [Google Scholar] [CrossRef]
- Schlegel, R.H.J. History of Plant Breeding; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lee, E.A.; Tracy, W.F. Modern Maize Breeding. In Handbook of Maize: Genetics and Genomics; Bennetzen, J.L., Hake, S., Eds.; Springer: New York, NY, USA, 2009; pp. 141–160. ISBN 978-0-387-77863-1. [Google Scholar]
- Byerlee, D. The Globalization of Hybrid Maize, 1921–1970. J. Glob. Hist. 2020, 15, 101–122. [Google Scholar] [CrossRef]
- Varshney, R.K.; Tuberosa, R. Genomics-Assisted Crop Improvement: An Overview. In Genomics-Assisted Crop Improvement: Vol. 1: Genomics Approaches and Platforms; Varshney, R.K., Tuberosa, R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 1–12. ISBN 978-1-4020-6295-7. [Google Scholar]
- Gengenbach, B.G.; Green, C.E. Selection of T-Cytoplasm Maize Callus Cultures Resistant to Helminthosporium Maydis Race T Pathotoxin1. Crop Sci. 1975, 15, 645–649. [Google Scholar] [CrossRef]
- Troyer, A.F. Development of Hybrid Corn and the Seed Corn Industry. In Handbook of Maize: Genetics and Genomics; Bennetzen, J.L., Hake, S., Eds.; Springer: New York, NY, USA, 2009; pp. 87–114. ISBN 978-0-387-77863-1. [Google Scholar]
- Lundmark, C. Genetically Modified Maize. BioScience 2007, 57, 996. [Google Scholar] [CrossRef][Green Version]
- Washburn, J.D.; Burch, M.B.; Franco, J.A.V. Predictive Breeding for Maize: Making Use of Molecular Phenotypes, Machine Learning, and Physiological Crop Models. Crop Sci. 2020, 60, 622–638. [Google Scholar] [CrossRef]
- Fritsche-Neto, R.; Galli, G.; Borges, K.L.R.; Costa-Neto, G.; Alves, F.C.; Sabadin, F.; Lyra, D.H.; Morais, P.P.P.; Braatz de Andrade, L.R.; Granato, I.; et al. Optimizing Genomic-Enabled Prediction in Small-Scale Maize Hybrid Breeding Programs: A Roadmap Review. Front. Plant Sci. 2021, 12, 658267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Kim, J.-Y. New Era of Precision Plant Breeding Using Genome Editing. Plant Biotechnol. Rep. 2019, 13, 419–421. [Google Scholar] [CrossRef]
- Waltz, E. CRISPR-Edited Crops Free to Enter Market, Skip Regulation. Nat. Biotechnol. 2016, 34, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Stewart, C.N. Functional Markers for Precision Plant Breeding. Int. J. Mol. Sci. 2020, 21, 4792. [Google Scholar] [CrossRef]
- Veillet, F.; Durand, M.; Kroj, T.; Cesari, S.; Gallois, J.-L. Precision Breeding Made Real with CRISPR: Illustration through Genetic Resistance to Pathogens. Plant Commun. 2020, 1, 100102. [Google Scholar] [CrossRef]
- Kushwaha, U.K.S.; Deo, I.; Jaiswal, J.P.; Prasad, B. Role of Bioinformatics in Crop Improvement. Glob. J. Sci. Front Res. D Agric. Vet. 2017, 17, 13–24. [Google Scholar]
- Hesper, B.; Hogeweg, P. Bio-Informatics: A Working Concept. A Translation of “Bio-Informatica: Een Werkconcept” by B. Hesper and P. Hogeweg. arXiv 2021, arXiv:2111.11832. [Google Scholar]
- Hogeweg, P. The Roots of Bioinformatics in Theoretical Biology. PLoS Comput. Biol. 2011, 7, e1002021. [Google Scholar] [CrossRef]
- Attwood, T.K.; Pettifer, S.R.; Thorne, D. Bioinformatics Challenges at the Interface of Biology and Computer Science: Mind the Gap; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-119-24344-1. [Google Scholar]
- Choudhuri, S. Bioinformatics for Beginners: Genes, Genomes, Molecular Evolution, Databases and Analytical Tools; Elsevier Science: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-410471-6. [Google Scholar]
- Jimenez-Gutierrez, L.R.; Barrios-Hernández, C.J.; Pedraza-Ferreira, G.R.; Vera-Cala, L.; Martinez-Perez, F. Importance of Databases of Nucleic Acids for Bioinformatic Analysis Focused to Genomics. J. Phys. Conf. Ser. 2016, 743, 012009. [Google Scholar] [CrossRef]
- Lawrence, C.J.; Dong, Q.; Polacco, M.L.; Seigfried, T.E.; Brendel, V. MaizeGDB, the Community Database for Maize Genetics and Genomics. Nucleic Acids Res. 2004, 32, D393–D397. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.J.; Seigfried, T.E.; Brendel, V. The Maize Genetics and Genomics Database. The Community Resource for Access to Diverse Maize Data. Plant Physiol. 2005, 138, 55–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harper, L.; Gardiner, J.; Andorf, C.; Lawrence, C.J. MaizeGDB: The Maize Genetics and Genomics Database. Methods Mol. Biol. 2016, 1374, 187–202. [Google Scholar] [CrossRef]
- Andorf, C.M.; Cannon, E.K.; Portwood, J.L.; Gardiner, J.M.; Harper, L.C.; Schaeffer, M.L.; Braun, B.L.; Campbell, D.A.; Vinnakota, A.G.; Sribalusu, V.V.; et al. MaizeGDB Update: New Tools, Data and Interface for the Maize Model Organism Database. Nucleic Acids Res. 2016, 44, D1195–D1201. [Google Scholar] [CrossRef]
- Dong, Q.; Roy, L.; Freeling, M.; Walbot, V.; Brendel, V. ZmDB, an Integrated Database for Maize Genome Research. Nucleic Acids Res. 2003, 31, 244–247. [Google Scholar] [CrossRef]
- Gai, X.; Lal, S.; Xing, L.; Brendel, V.; Walbot, V. Gene Discovery Using the Maize Genome Database ZmDB. Nucleic Acids Res. 2000, 28, 94–96. [Google Scholar] [CrossRef][Green Version]
- Cho, K.T.; Portwood, J.L.; Gardiner, J.M.; Harper, L.C.; Lawrence-Dill, C.J.; Friedberg, I.; Andorf, C.M. MaizeDIG: Maize Database of Images and Genomes. Front. Plant Sci. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Garnett, T.; Lawrence-Dill, C.J.; Pridmore, T.; Watt, M.; Pommier, C.; Pieruschka, R.; Ghamkhar, K. Phenotyping: From Plant, to Data, to Impact and Highlights of the International Plant Phenotyping Symposium—IPPS 2018; Frontiers Media SA: Lausanne, Switzerland, 2021; ISBN 978-2-88966-397-2. [Google Scholar]
- Zhou, W.; Wang, L.; Zheng, W.; Yao, W. MaizeSNPDB: A Comprehensive Database for Efficient Retrieve and Analysis of SNPs among 1210 Maize Lines. Comput. Struct. Biotechnol. J. 2019, 17, 1377–1383. [Google Scholar] [CrossRef]
- Gui, S.; Yang, L.; Li, J.; Luo, J.; Xu, X.; Yuan, J.; Chen, L.; Li, W.; Yang, X.; Wu, S.; et al. ZEAMAP, a Comprehensive Database Adapted to the Maize Multi-Omics Era. iScience 2020, 23, 101241. [Google Scholar] [CrossRef]
- Peng, Z.; Li, H.; Sun, G.; Dai, P.; Geng, X.; Wang, X.; Zhang, X.; Wang, Z.; Jia, Y.; Pan, Z.; et al. CottonGVD: A Comprehensive Genomic Variation Database for Cultivated Cottons. Front. Plant Sci. 2021, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Jiang, S.; Cheng, Q.; Wang, X.; Yan, J.; Zhang, R.; Qiao, F.; Ma, C.; Luo, J.; Li, W.; et al. The Genetic Mechanism of Heterosis Utilization in Maize Improvement. Genome Biol. 2021, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Canaran, P.; Jurkuta, R.; Fulton, T.; Glaubitz, J.; Buckler, E.; Doebley, J.; Gaut, B.; Goodman, M.; Holland, J.; et al. Panzea: A Database and Resource for Molecular and Functional Diversity in the Maize Genome. Nucleic Acids Res. 2006, 34, D752–D757. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Buckler, E.; Muse, S. Development of a Maize Molecular Evolutionary Genomic Database. Comp. Funct. Genom. 2003, 4, 246–249. [Google Scholar] [CrossRef]
- Canaran, P.; Buckler, E.S.; Glaubitz, J.C.; Stein, L.; Sun, Q.; Zhao, W.; Ware, D. Panzea: An Update on New Content and Features. Nucleic Acids Res. 2008, 36, D1041–D1043. [Google Scholar] [CrossRef]
- Gupta, P.; Naithani, S.; Tello-Ruiz, M.K.; Chougule, K.; D’Eustachio, P.; Fabregat, A.; Jiao, Y.; Keays, M.; Lee, Y.K.; Kumari, S.; et al. Gramene Database: Navigating Plant Comparative Genomics Resources. Curr. Plant Biol. 2016, 7–8, 10–15. [Google Scholar] [CrossRef]
- Jaiswal, P. Gramene Database: A Hub for Comparative Plant Genomics. Methods Mol. Biol. 2011, 678, 247–275. [Google Scholar] [CrossRef]
- Monaco, M.K.; Stein, J.; Naithani, S.; Wei, S.; Dharmawardhana, P.; Kumari, S.; Amarasinghe, V.; Youens-Clark, K.; Thomason, J.; Preece, J.; et al. Gramene 2013: Comparative Plant Genomics Resources. Nucleic Acids Res. 2014, 42, D1193–D1199. [Google Scholar] [CrossRef]
- Tello-Ruiz, M.K.; Naithani, S.; Gupta, P.; Olson, A.; Wei, S.; Preece, J.; Jiao, Y.; Wang, B.; Chougule, K.; Garg, P.; et al. Gramene 2021: Harnessing the Power of Comparative Genomics and Pathways for Plant Research. Nucleic Acids Res. 2021, 49, D1452–D1463. [Google Scholar] [CrossRef]
- Chen, F.; Dong, W.; Zhang, J.; Guo, X.; Chen, J.; Wang, Z.; Lin, Z.; Tang, H.; Zhang, L. The Sequenced Angiosperm Genomes and Genome Databases. Front. Plant Sci. 2018, 9, 418. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. The Universal Protein Resource (UniProt). Nucleic Acids Res. 2005, 33, D154–D159. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2005, 33, D34–D38. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Busby, B.; Mizrachi, I.K. Managing Sequence Data. In Bioinformatics; Keith, J.M., Ed.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2017; Volume 1525, pp. 79–106. ISBN 978-1-4939-6620-2. [Google Scholar]
- Leray, M.; Knowlton, N.; Ho, S.-L.; Nguyen, B.N.; Machida, R.J. GenBank Is a Reliable Resource for 21st Century Biodiversity Research. Proc. Natl. Acad. Sci. USA 2019, 116, 22651–22656. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.; van den Broek, A.; Camon, E.; Hingamp, P.; Sterk, P.; Stoesser, G.; Tuli, M.A. The EMBL Nucleotide Sequence Database. Nucleic Acids Res. 2000, 28, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Bolser, D.; Staines, D.M.; Pritchard, E.; Kersey, P. Ensembl Plants: Integrating Tools for Visualizing, Mining, and Analyzing Plant Genomics Data. In Plant Bioinformatics: Methods and Protocols; Edwards, D., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; pp. 115–140. ISBN 978-1-4939-3167-5. [Google Scholar]
- Kumawat, G.; Kumawat, C.K.; Chandra, K.; Pandey, S.; Chand, S.; Mishra, U.N.; Lenka, D.; Sharma, R. Insights into Marker Assisted Selection and Its Applications in Plant Breeding; IntechOpen: London, UK, 2020; ISBN 978-1-83968-310-7. [Google Scholar]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed Breeding Is a Powerful Tool to Accelerate Crop Research and Breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Watson, A.; Hickey, L.T.; Christopher, J.; Rutkoski, J.; Poland, J.; Hayes, B.J. Multivariate Genomic Selection and Potential of Rapid Indirect Selection with Speed Breeding in Spring Wheat. Crop Sci. 2019, 59, 1945–1959. [Google Scholar] [CrossRef]
- Stevens, R. Prospects for Using Marker-Assisted Breeding to Improve Maize Production in Africa. J. Sci. Food Agric. 2008, 88, 745–755. [Google Scholar] [CrossRef]
- Ramesh, P.; Mallikarjuna, G.; Sameena, S.; Kumar, A.; Gurulakshmi, K.; Reddy, B.V.; Reddy, P.C.O.; Sekhar, A.C. Advancements in Molecular Marker Technologies and Their Applications in Diversity Studies. J. Biosci. 2020, 45, 123. [Google Scholar] [CrossRef]
- Ribaut, J.; Hoisington, D. Marker-Assisted Selection: New Tools and Strategies. Trends Plant Sci. 1998, 3, 236–239. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Rahman, M.U.; Foolad, M.R. Marker-Assisted Selection in Plant Breeding for Salinity Tolerance. Methods Mol. Biol. 2012, 913, 305–333. [Google Scholar] [CrossRef] [PubMed]
- Francia, E.; Tacconi, G.; Crosatti, C.; Barabaschi, D.; Bulgarelli, D.; Dall’Aglio, E.; Valè, G. Marker Assisted Selection in Crop Plants. Plant Cell Tissue Organ Cult. 2005, 82, 317–342. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-Assisted Backcrossing: A Useful Method for Rice Improvement. Biotechnol. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Latif, M.A. Recurrent Parent Genome Recovery Analysis in a Marker-Assisted Backcrossing Program of Rice (Oryza Sativa L.). Comptes Rendus. Biol. 2015, 338, 83–94. [Google Scholar] [CrossRef]
- Helentjaris, T.; Slocum, M.; Wright, S.; Schaefer, A.; Nienhuis, J. Construction of Genetic Linkage Maps in Maize and Tomato Using Restriction Fragment Length Polymorphisms. Theoret. Appl. Genet. 1986, 72, 761–769. [Google Scholar] [CrossRef]
- Ristić, D.; Babić, V.; Anđelković, V.; Vančetović, J.; Mladenović-Drinić, S.; Ignjatović-Micić, D. Genetic Diversity in Maize Dent Landraces Assessed by Morphological and Molecular Markers. Genetika 2013, 45, 811–824. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, X.-Q.; Pan, G.-T. QTL Mapping of Fusarium Moniliforme Ear Rot Resistance in Maize. 1. Map Construction with Microsatellite and AFLP Markers. J. Appl. Genet. 2006, 47, 9–15. [Google Scholar] [CrossRef]
- Xia, X.C.; Reif, J.C.; Melchinger, A.E.; Frisch, M.; Hoisington, D.A.; Beck, D.; Pixley, K.; Warburton, M.L. Genetic Diversity among CIMMYT Maize Inbred Lines Investigated with SSR Markers: II. Subtropical, Tropical Midaltitude, and Highland Maize Inbred Lines and Their Relationships with Elite U.S. and European Maize. Crop Sci. 2005, 45, 2573–2582. [Google Scholar] [CrossRef]
- Owens, B.F.; Mathew, D.; Diepenbrock, C.H.; Tiede, T.; Wu, D.; Mateos-Hernandez, M.; Gore, M.A.; Rocheford, T. Genome-Wide Association Study and Pathway-Level Analysis of Kernel Color in Maize. Genetics 2019, 9, 1945–1955. [Google Scholar] [CrossRef]
- Kumar, A.; Longmei, N.; Kumar, P.; Kaushik, P. Molecular Marker Analysis of Genetic Diversity in Maize: A Review. OBM Genet. 2022, 6, 1. [Google Scholar] [CrossRef]
- Bentolila, S.; Guitton, C.; Bouvet, N.; Sailland, A.; Nykaza, S.; Freyssinet, G. Identification of an RFLP Marker Tightly Linked to TheHt1 Gene in Maize. Theoret. Appl. Genet. 1991, 82, 393–398. [Google Scholar] [CrossRef]
- Chandran, S.; Pukalenthy, B.; Adhimoolam, K.; Manickam, D.; Sampathrajan, V.; Chocklingam, V.; Eswaran, K.; Arunachalam, K.; Joikumarmeetei, L.; Rajasekaran, R.; et al. Marker-Assisted Selection to Pyramid the Opaque-2 (O2) and β-Carotene (CrtRB1) Genes in Maize. Front. Genet. 2019, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, S.; Kaur, A.; Vikal, Y.; Cheema, A.K.; Bains, B.K.; Kaur, N.; Gill, G.K.; Malhotra, P.K.; Kumar, A.; et al. Marker-Assisted Pyramiding of Lycopene-ε-Cyclase, β-Carotene Hydroxylase1 and Opaque2 Genes for Development of Biofortified Maize Hybrids. Sci. Rep. 2021, 11, 12642. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hua, J.; Wang, F.; Cheng, Z.; Meng, Q.; Chen, Y.; Han, X.; Tie, S.; Liu, C.; Li, X.; et al. Marker-Assisted Selection of QMrdd8 to Improve Maize Resistance to Rough Dwarf Disease. Breed Sci. 2020, 70, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.M.; Bayoumi, S.R.; Barakat, M.N. Identification of Molecular Markers Linked to Fusarium Ear Rot Genes in Maize Plants Zea Mays L. Biotechnol. Biotechnol. Equip. 2016, 30, 692–699. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Du, W.-P.; Xu, L.-Y.; Jiang, Y.; Zhang, J.; Xiang, X.-L.; Yu, G.-R. Identification, Mapping, and Molecular Marker Development for Rgsr8.1: A New Quantitative Trait Locus Conferring Resistance to Gibberella Stalk Rot in Maize (Zea Mays L.). Front. Plant Sci. 2017, 8, 1355. [Google Scholar] [CrossRef]
- Anderson, D.L. Functional Markers in Zea Mays L.; Iowa State University: Ames, IO, USA, 2021; p. 31. [Google Scholar]
- Duan, C.; Song, F.; Sun, S.; Guo, C.; Zhu, Z.; Wang, X. Characterization and Molecular Mapping of Two Novel Genes Resistant to Pythium Stalk Rot in Maize. Phytopathology 2019, 109, 804–809. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, X. Mapping Quantitative Trait Loci and Predicting Candidate Genes for Leaf Angle in Maize. PLoS ONE 2021, 16, e0245129. [Google Scholar] [CrossRef]
- Zuo, Z.; Lu, Y.; Zhu, M.; Chen, R.; Zhang, E.; Hao, D.; Huang, Q.; Wang, H.; Su, Y.; Wang, Z.; et al. Nucleotide Diversity of the Maize ZmCNR13 Gene and Association with Ear Traits. Front. Genet. 2021, 12, 773597. [Google Scholar] [CrossRef]
- Makarevitch, I.; Thompson, A.; Muehlbauer, G.J.; Springer, N.M. Brd1 Gene in Maize Encodes a Brassinosteroid C-6 Oxidase. PLoS ONE 2012, 7, e30798. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.J.; Goddard, M.E. Invited Review: Genomic Selection in Dairy Cattle: Progress and Challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Genomic Selection for Crop Improvement—Heffner—2009—Crop Science—Wiley Online Library. Available online: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2135/cropsci2008.08.0512 (accessed on 30 March 2022).
- Poland, J.A.; Rife, T.W. Genotyping-by-Sequencing for Plant Breeding and Genetics. Plant Genome 2012, 5, 92–102. [Google Scholar] [CrossRef]
- Heslot, N.; Yang, H.-P.; Sorrells, M.E.; Jannink, J.-L. Genomic Selection in Plant Breeding: A Comparison of Models. Crop Sci. 2012, 52, 146–160. [Google Scholar] [CrossRef]
- R2D2 Consortium; Fugeray-Scarbel, A.; Bastien, C.; Dupont-Nivet, M.; Lemarié, S. Why and How to Switch to Genomic Selection: Lessons from Plant and Animal Breeding Experience. Front. Genet. 2021, 12, 629737. [Google Scholar] [CrossRef]
- Atanda, S.A.; Olsen, M.; Burgueño, J.; Crossa, J.; Dzidzienyo, D.; Beyene, Y.; Gowda, M.; Dreher, K.; Zhang, X.; Prasanna, B.M.; et al. Maximizing Efficiency of Genomic Selection in CIMMYT’s Tropical Maize Breeding Program. Theor. Appl. Genet. 2021, 134, 279–294. [Google Scholar] [CrossRef]
- Robertsen, C.D.; Hjortshøj, R.L.; Janss, L.L. Genomic Selection in Cereal Breeding. Agronomy 2019, 9, 95. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, P.; Gianola, D.; González-Camacho, J.M.; Crossa, J.; Manès, Y.; Dreisigacker, S. Comparison Between Linear and Non-Parametric Regression Models for Genome-Enabled Prediction in Wheat. Genetics 2012, 2, 1595–1605. [Google Scholar] [CrossRef]
- Windhausen, V.S.; Atlin, G.N.; Hickey, J.M.; Crossa, J.; Jannink, J.-L.; Sorrells, M.E.; Raman, B.; Cairns, J.E.; Tarekegne, A.; Semagn, K.; et al. Effectiveness of Genomic Prediction of Maize Hybrid Performance in Different Breeding Populations and Environments. Genetics 2012, 2, 1427–1436. [Google Scholar] [CrossRef]
- Technow, F.; Bürger, A.; Melchinger, A.E. Genomic Prediction of Northern Corn Leaf Blight Resistance in Maize with Combined or Separated Training Sets for Heterotic Groups. Genetics 2013, 3, 197–203. [Google Scholar] [CrossRef]
- Massman, J.M.; Jung, H.-J.G.; Bernardo, R. Genomewide Selection versus Marker-Assisted Recurrent Selection to Improve Grain Yield and Stover-Quality Traits for Cellulosic Ethanol in Maize. Crop Sci. 2013, 53, 58–66. [Google Scholar] [CrossRef]
- Combs, E.; Bernardo, R. Genomewide Selection to Introgress Semidwarf Maize Germplasm into U.S. Corn Belt Inbreds. Crop Sci. 2013, 53, 1427–1436. [Google Scholar] [CrossRef]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic Selection in Plant Breeding: Methods, Models, and Perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, H.; Beyene, Y.; Semagn, K.; Liu, Y.; Cao, S.; Cui, Z.; Ruan, Y.; Burgueño, J.; San Vicente, F.; et al. Effect of Trait Heritability, Training Population Size and Marker Density on Genomic Prediction Accuracy Estimation in 22 Bi-Parental Tropical Maize Populations. Front. Plant Sci. 2017, 8, 1916. [Google Scholar] [CrossRef]
- Shepherd, R.K.; Meuwissen, T.H.; Woolliams, J.A. Genomic Selection and Complex Trait Prediction Using a Fast EM Algorithm Applied to Genome-Wide Markers. BMC Bioinform. 2010, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Plant Breeding with Genomic Selection: Gain per Unit Time and Cost—Heffner—2010—Crop Science—Wiley Online Library. Available online: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2135/cropsci2009.11.0662 (accessed on 30 March 2022).
- Jannink, J.-L.; Lorenz, A.J.; Iwata, H. Genomic Selection in Plant Breeding: From Theory to Practice. Brief. Funct. Genom. 2010, 9, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Beyene, Y.; Gowda, M.; Pérez-Rodríguez, P.; Olsen, M.; Robbins, K.R.; Burgueño, J.; Prasanna, B.M.; Crossa, J. Application of Genomic Selection at the Early Stage of Breeding Pipeline in Tropical Maize. Front. Plant Sci. 2021, 12, 685488. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Filho, J.B.M.; Carena, M.J. Breeding Plans. In Quantitative Genetics in Maize Breeding; Carena, M.J., Hallauer, A.R., Miranda Filho, J.B., Eds.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2010; pp. 577–653. ISBN 978-1-4419-0766-0. [Google Scholar]
- Fritsche-Neto, R.; Akdemir, D.; Jannink, J.-L. Accuracy of Genomic Selection to Predict Maize Single-Crosses Obtained through Different Mating Designs. Theor. Appl. Genet. 2018, 131, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.R.; Lipka, A.E. Diversifying Maize Genomic Selection Models. Mol. Breed. 2021, 41, 33. [Google Scholar] [CrossRef]
- Xu, J. Breeding by Design for Future Rice: Genes and Genome Technologies. Crop J. 2021, 6, 491–496. [Google Scholar] [CrossRef]
- Peleman, J.D.; van der Voort, J.R. Breeding by Design. Trends Plant Sci. 2003, 8, 330–334. [Google Scholar] [CrossRef]
- Zhang, G. Target Chromosome-Segment Substitution: A Way to Breeding by Design in Rice. Crop J. 2021, 9, 658–668. [Google Scholar] [CrossRef]
- Pérez-de-Castro, A.M.; Vilanova, S.; Cañizares, J.; Pascual, L.; Blanca, J.M.; Díez, M.J.; Prohens, J.; Picó, B. Application of Genomic Tools in Plant Breeding. Curr. Genom. 2012, 13, 179–195. [Google Scholar] [CrossRef]
- Hake, S.; Ross-Ibarra, J. Genetic, Evolutionary and Plant Breeding Insights from the Domestication of Maize. eLife 2015, 4, e05861. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.P.; Emery, M. Maize: Overview. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-08-100596-5. [Google Scholar]
- Haberer, G.; Young, S.; Bharti, A.K.; Gundlach, H.; Raymond, C.; Fuks, G.; Butler, E.; Wing, R.A.; Rounsley, S.; Birren, B.; et al. Structure and Architecture of the Maize Genome. Plant Physiol. 2005, 139, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Messing, J.; Bharti, A.K.; Karlowski, W.M.; Gundlach, H.; Kim, H.R.; Yu, Y.; Wei, F.; Fuks, G.; Soderlund, C.A.; Mayer, K.F.X.; et al. Sequence Composition and Genome Organization of Maize. Proc. Natl. Acad. Sci. USA 2004, 101, 14349–14354. [Google Scholar] [CrossRef] [PubMed]
- Chandler, V.L.; Brendel, V. The Maize Genome Sequencing Project. Plant Physiol. 2002, 130, 1594–1597. [Google Scholar] [CrossRef]
- Xu, Y.; Skinner, D.J.; Wu, H.; Palacios-Rojas, N.; Araus, J.L.; Yan, J.; Gao, S.; Warburton, M.L.; Crouch, J.H. Advances in Maize Genomics and Their Value for Enhancing Genetic Gains from Breeding. Int. J. Plant Genom. 2009, 2009, e957602. [Google Scholar] [CrossRef]
- Palmer, L.E.; Rabinowicz, P.D.; O’Shaughnessy, A.L.; Balija, V.S.; Nascimento, L.U.; Dike, S.; de la Bastide, M.; Martienssen, R.A.; McCombie, W.R. Maize Genome Sequencing by Methylation Filtration. Science 2003, 302, 2115–2117. [Google Scholar] [CrossRef]
- Mascher, M.; Gerlach, N.; Gahrtz, M.; Bucher, M.; Scholz, U.; Dresselhaus, T. Sequence and Ionomic Analysis of Divergent Strains of Maize Inbred Line B73 with an Altered Growth Phenotype. PLoS ONE 2014, 9, e96782. [Google Scholar] [CrossRef]
- Stojaković, M.; Ivanović, M.; Bekavac, G.; Stojaković, Ž. Grain Yield of B73 x Mo17-Type Maize Hybrids from Different Periods of Breeding. Cereal Res. Commun. 2010, 38, 440–448. [Google Scholar] [CrossRef]
- Stojaković, M.; Bekavac, G.; Vasić, N. B73 and Related Inbred Lines in Maize Breeding. Genetika 2005, 37, 245–252. [Google Scholar] [CrossRef]
- Hufford, M.B.; Seetharam, A.S.; Woodhouse, M.R.; Chougule, K.M.; Ou, S.; Liu, J.; Ricci, W.A.; Guo, T.; Olson, A.; Qiu, Y.; et al. De Novo Assembly, Annotation, and Comparative Analysis of 26 Diverse Maize Genomes. Science 2021, 373, 105655–105662. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhao, X.; Laroche, A.; Lu, Z.-X.; Liu, H.; Li, Z. Genotyping-by-Sequencing (GBS), an Ultimate Marker-Assisted Selection (MAS) Tool to Accelerate Plant Breeding. Front. Plant Sci. 2014, 5, 484. [Google Scholar] [CrossRef]
- Shendure, J.; Ji, H. Next-Generation DNA Sequencing. Nat. Biotechnol. 2008, 26, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Nepolean, T.; Kaul, J.; Mukri, G.; Mittal, S. Genomics-Enabled Next-Generation Breeding Approaches for Developing System-Specific Drought Tolerant Hybrids in Maize. Front. Plant Sci. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Savadi, S.; Mangalassery, S.; Sandesh, M.S. Advances in Genomics and Genome Editing for Breeding next Generation of Fruit and Nut Crops. Genomics 2021, 113, 3718–3734. [Google Scholar] [CrossRef] [PubMed]
- EU Regulation of New Plant Breeding Technologies and Their Possible Economic Implications for the EU and Beyond—Purnhagen—2021—Applied Economic Perspectives and Policy—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/aepp.13084 (accessed on 30 March 2022).
- Eckerstorfer, M.F.; Engelhard, M.; Heissenberger, A.; Simon, S.; Teichmann, H. Plants Developed by New Genetic Modification Techniques—Comparison of Existing Regulatory Frameworks in the EU and Non-EU Countries. Front. Bioeng. Biotechnol. 2019, 7, 26. [Google Scholar] [CrossRef]
- Enfissi, E.M.A.; Drapal, M.; Perez-Fons, L.; Nogueira, M.; Berry, H.M.; Almeida, J.; Fraser, P.D. New Plant Breeding Techniques and Their Regulatory Implications: An Opportunity to Advance Metabolomics Approaches. J. Plant Physiol. 2021, 258–259, 153378. [Google Scholar] [CrossRef]
- Aglawe, S.B.; Barbadikar, K.M.; Mangrauthia, S.K.; Madhav, M.S. New Breeding Technique “Genome Editing” for Crop Improvement: Applications, Potentials and Challenges. 3 Biotech 2018, 8, 336. [Google Scholar] [CrossRef]
- El-Mounadi, K.; Morales-Floriano, M.L.; Garcia-Ruiz, H. Principles, Applications, and Biosafety of Plant Genome Editing Using CRISPR-Cas9. Front. Plant Sci. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Petolino, J.F.; Kumar, S. Transgenic Trait Deployment Using Designed Nucleases. Plant Biotechnol. J. 2016, 14, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Wesseler, J.H.H.; Zilberman, D. New Plant Breeding Technologies: An Assessment of the Political Economy of the Regulatory Environment and Implications for Sustainability. Sustainability 2021, 13, 3687. [Google Scholar] [CrossRef]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision Genome Editing in Plants: State-of-the-Art in CRISPR/Cas9-Based Genome Engineering. BMC Plant Biol. 2020, 20, 234. [Google Scholar] [CrossRef]
- Lusser, M.; Parisi, C.; Plan, D.; Rodríguez-Cerezo, E. Deployment of New Biotechnologies in Plant Breeding. Nat. Biotechnol. 2012, 30, 231–239. [Google Scholar] [CrossRef]
- Modrzejewski, D.; Hartung, F.; Sprink, T.; Krause, D.; Kohl, C.; Schiemann, J.; Wilhelm, R. What Is the Available Evidence for the Application of Genome Editing as a New Tool for Plant Trait Modification and the Potential Occurrence of Associated Off-Target Effects: A Systematic Map Protocol. Environ. Evid. 2018, 7, 18. [Google Scholar] [CrossRef]
- Sauer, N.J.; Narváez-Vásquez, J.; Mozoruk, J.; Miller, R.B.; Warburg, Z.J.; Woodward, M.J.; Mihiret, Y.A.; Lincoln, T.A.; Segami, R.E.; Sanders, S.L.; et al. Oligonucleotide-Mediated Genome Editing Provides Precision and Function to Engineered Nucleases and Antibiotics in Plants. Plant Physiol. 2016, 170, 1917–1928. [Google Scholar] [CrossRef]
- Dalakouras, A.; Vlachostergios, D. Epigenetic Approaches to Crop Breeding: Current Status and Perspectives. J. Exp. Bot. 2021, 72, 5356–5371. [Google Scholar] [CrossRef]
- Matzke, A.J.; Matzke, M.A. Position Effects and Epigenetic Silencing of Plant Transgenes. Curr. Opin. Plant Biol. 1998, 1, 142–148. [Google Scholar] [CrossRef]
- Songstad, D.D.; Petolino, J.F.; Voytas, D.F.; Reichert, N.A. Genome Editing of Plants. Crit. Rev. Plant Sci. 2017, 36, 1–23. [Google Scholar] [CrossRef]
- Cisgenesis and Genome Editing: Combining Concepts and Efforts for a Smarter Use of Genetic Resources in Crop Breeding—Cardi—2016—Plant Breeding—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/pbr.12345 (accessed on 30 March 2022).
- Espinoza, C.; Schlechter, R.; Herrera, D.; Torres, E.; Serrano, A.; Medina, C.; Arce-Johnson, P. Cisgenesis and Intragenesis: New Tools for Improving Crops. Biol. Res. 2013, 46, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Atlihan, N.; Lu, Z.-X. New Biotechnology Enhances the Application of Cisgenesis in Plant Breeding. Front. Plant Sci. 2014, 5, 389. [Google Scholar] [CrossRef] [PubMed]
- Holme, I.B.; Wendt, T.; Holm, P.B. Intragenesis and Cisgenesis as Alternatives to Transgenic Crop Development. Plant Biotechnol. J. 2013, 11, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Súnico, V.; Higuera, J.J.; Molina-Hidalgo, F.J.; Blanco-Portales, R.; Moyano, E.; Rodríguez-Franco, A.; Muñoz-Blanco, J.; Caballero, J.L. The Intragenesis and Synthetic Biology Approach towards Accelerating Genetic Gains on Strawberry: Development of New Tools to Improve Fruit Quality and Resistance to Pathogens. Plants 2022, 11, 57. [Google Scholar] [CrossRef]
- Song, G.; Walworth, A.E.; Loescher, W.H. Grafting of Genetically Engineered Plants. J. Am. Soc. Hortic. Sci. 2015, 140, 203–213. [Google Scholar] [CrossRef]
- Kodama, H.; Miyahara, T.; Oguchi, T.; Tsujimoto, T.; Ozeki, Y.; Ogawa, T.; Yamaguchi, Y.; Ohta, D. Effect of Transgenic Rootstock Grafting on the Omics Profiles in Tomato. Food Saf. 2021, 9, 32–47. [Google Scholar] [CrossRef]
- Tsaballa, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Nianiou-Obeidat, I. Vegetable Grafting from a Molecular Point of View: The Involvement of Epigenetics in Rootstock-Scion Interactions. Front. Plant Sci. 2021, 11, 621999. [Google Scholar] [CrossRef]
- Ishino, Y.; Krupovic, M.; Forterre, P. History of CRISPR-Cas from Encounter with a Mysterious Repeated Sequence to Genome Editing Technology. J. Bacteriol. 2018, 200, e00580-17. [Google Scholar] [CrossRef]
- Charpentier, E.; Doudna, J.A. Rewriting a Genome. Nature 2013, 495, 50–51. [Google Scholar] [CrossRef]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 Toolkit for Multiplex Genome Editing in Plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Kouranov, A.; Armstrong, C.; Shrawat, A.; Sidorov, V.; Huesgen, S.; Lemke, B.; Boyle, T.; Gasper, M.; Lawrence, R.; Yang, S. Demonstration of Targeted Crossovers in Hybrid Maize Using CRISPR Technology. Commun. Biol. 2022, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Sreekumar, A.; Prasad, D.; Das, A.V.; Pillai, M.R. Genome Editing of Oncogenes with ZFNs and TALENs: Caveats in Nuclease Design. Cancer Cell Int. 2018, 18, 169. [Google Scholar] [CrossRef]
- Petolino, J.F. Genome Editing in Plants via Designed Zinc Finger Nucleases. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Davies, J.P.; Kumar, S.; Sastry-Dent, L. Use of Zinc-Finger Nucleases for Crop Improvement. Prog. Mol. Biol. Transl. Sci. 2017, 149, 47–63. [Google Scholar] [CrossRef]
- Shukla, V.K.; Doyon, Y.; Miller, J.C.; DeKelver, R.C.; Moehle, E.A.; Worden, S.E.; Mitchell, J.C.; Arnold, N.L.; Gopalan, S.; Meng, X.; et al. Precise Genome Modification in the Crop Species Zea Mays Using Zinc-Finger Nucleases. Nature 2009, 459, 437–441. [Google Scholar] [CrossRef]
- Weinthal, D.; Tovkach, A.; Zeevi, V.; Tzfira, T. Genome Editing in Plant Cells by Zinc Finger Nucleases. Trends Plant Sci. 2010, 15, 308–321. [Google Scholar] [CrossRef] [PubMed]
- ISAAA Inc. Pocket K59 (English). Available online: https://www.isaaa.org/resources/publications/ (accessed on 25 September 2022).
- Malzahn, A.; Lowder, L.; Qi, Y. Plant Genome Editing with TALEN and CRISPR. Cell Biosci. 2017, 7, 21. [Google Scholar] [CrossRef]
- Sauer, N.J.; Mozoruk, J.; Miller, R.B.; Warburg, Z.J.; Walker, K.A.; Beetham, P.R.; Schöpke, C.R.; Gocal, G.F.W. Oligonucleotide-Directed Mutagenesis for Precision Gene Editing. Plant Biotechnol. J. 2016, 14, 496–502. [Google Scholar] [CrossRef]
- Zhu, T.; Mettenburg, K.; Peterson, D.J.; Tagliani, L.; Baszczynski, C.L. Engineering Herbicide-Resistant Maize Using Chimeric RNA/DNA Oligonucleotides. Nat. Biotechnol. 2000, 18, 555–558. [Google Scholar] [CrossRef]
- Weinhold, B. Epigenetics: The Science of Change. Environ. Health Perspect. 2006, 114, A160–A167. [Google Scholar] [CrossRef] [PubMed]
- Kakoulidou, I.; Avramidou, E.V.; Baránek, M.; Brunel-Muguet, S.; Farrona, S.; Johannes, F.; Kaiserli, E.; Lieberman-Lazarovich, M.; Martinelli, F.; Mladenov, V.; et al. Epigenetics for Crop Improvement in Times of Global Change. Biology 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Varotto, S.; Tani, E.; Abraham, E.; Krugman, T.; Kapazoglou, A.; Melzer, R.; Radanović, A.; Miladinović, D. Epigenetics: Possible Applications in Climate-Smart Crop Breeding. J. Exp. Bot. 2020, 71, 5223–5236. [Google Scholar] [CrossRef]
- Chen, Y.; Müller, F.; Rieu, I.; Winter, P. Epigenetic Events in Plant Male Germ Cell Heat Stress Responses. Plant Reprod. 2016, 29, 21–29. [Google Scholar] [CrossRef]
- Regulski, M.; Lu, Z.; Kendall, J.; Donoghue, M.T.A.; Reinders, J.; Llaca, V.; Deschamps, S.; Smith, A.; Levy, D.; McCombie, W.R.; et al. The Maize Methylome Influences MRNA Splice Sites and Reveals Widespread Paramutation-like Switches Guided by Small RNA. Genome Res. 2013, 23, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Telem, R.S.; Wani, S.H.; Singh, N.B.; Nandini, R.; Sadhukhan, R.; Bhattacharya, S.; Mandal, N. Cisgenics—A Sustainable Approach for Crop Improvement. Curr. Genom. 2013, 14, 468–476. [Google Scholar] [CrossRef]
- Jacobsen, E.; Schouten, H.J. Cisgenesis. In Molecular Techniques in Crop Improvement, 2nd ed.; Jain, S.M., Brar, D.S., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 591–611. ISBN 978-90-481-2967-6. [Google Scholar]
- Almeraya, E.V.; Sánchez-de-Jiménez, E. Intragenic Modification of Maize. J. Biotechnol. 2016, 238, 35–41. [Google Scholar] [CrossRef]
- Yadava, P.; Abhishek, A.; Singh, R.; Singh, I.; Kaul, T.; Pattanayak, A.; Agrawal, P.K. Advances in Maize Transformation Technologies and Development of Transgenic Maize. Front. Plant Sci. 2017, 7, 1949. [Google Scholar] [CrossRef]
- Zhao, D.; Song, G. Rootstock-to-Scion Transfer of Transgene-Derived Small Interfering RNAs and Their Effect on Virus Resistance in Nontransgenic Sweet Cherry. Plant Biotechnol. J. 2014, 12, 1319–1328. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, L.; Jittayasothorn, Y.; Kang, Y.; Jiao, C.; Fei, Z.; Zhong, G.-Y. Messenger RNA Exchange between Scions and Rootstocks in Grafted Grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef]
- Gedil, M.; Menkir, A. An Integrated Molecular and Conventional Breeding Scheme for Enhancing Genetic Gain in Maize in Africa. Front. Plant Sci. 2019, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
| Database | Description | Reference | Link |
|---|---|---|---|
| Maize Genetic and Genomic Database (MaizeGBD) | Genomic sequence data Gene expression analysis Phenotypes of plants | [44,45,46,47,48,49] | https://www.maizegdb.org/, accessed on 26 September 2022 |
| Maize Database of Images and Genomes (MaizeDIG) | Genotype/phenotype database for maize | [50,51] | http://maizedig.maizegdb.org/, accessed on 26 September 2022 |
| MaizeSNPDB | Database of maize lines and SNP sites | [52] | https://venyao.xyz/MaizeSNPDB/, accessed on 26 September 2022 |
| ZeaMap | Maize and teosinte comparative genomics Multi-omics data Genetic maps | [53,54,55,56] | http://www.zeamap.com/, accessed on 26 September 2022 |
| Panzea | Molecular and functional diversity of the maize genome | [56,57,58] | https://www.panzea.org/data, accessed on 26 September 2022 |
| Gramene | Comparative genomics of plants | [59,60,61,62] | https://www.gramene.org/, accessed on 26 September 2022 |
| Plant Genome Database (GBD) | Plant genome information, Molecular sequence data | [48,63] | https://www.plantgdb.org/, accessed on 26 September 2022 |
| UniProt | Database of protein sequences | [64,65] | https://www.uniprot.org/, accessed on 26 September 2022 |
| Genbank | Database of nucleic acids sequences | [66,67,68] | https://www.ncbi.nlm.nih.gov/genbank/, accessed on 26 September 2022 |
| European Molecular Biology Laboratory (EMBL) Database | Nucleotide sequence database | [69] | http://www.ebi.ac.uk, accessed on 26 September 2022 |
| Phytozome | Plant comparative genomics | [63,70] | https://phytozome-next.jgi.doe.gov/, accessed on 26 September 2022 |
| EsemblPlants | Genome-scale information | [63,71] | http://plants.ensembl.org/species.html, accessed on 26 September 2022 |
| Gene/Locus | Marker | Target Trait | References |
|---|---|---|---|
| Ht1 | umc150B | resistance to the fungal pathogen Helminthosporium turcicum | [88] |
| o2 | umc1066 | quality, lysine, and tryptophan content | [89,90] |
| crtRB1 | crtRB1 3′TE | ||
| lcyE | lcyE 5′TE, SNP216, SNP2238, lcyE 3′InDel | [90] | |
| qMrdd8 | IDP25K | resistance to rough dwarf disease | [91] |
| QTL | bnlg1063, umc2082, bnlg1621, umc2013, bnlg1740, umc2059, SSR85, STS06 | resistance to Fusarium verticillioides ear root | [92] |
| Zm953, Zm972/Rgsr8.1 | SSR78 | resistance to Gibberella stalk rot | [93] |
| su1 | SuDel6FR, SuDel36FR, SNP2703, SNP2703-CG85/89 | encodes a starch debranching enzyme that influences the sweetness of kernels | [94] |
| RpiX178-1 | umc2047, IDP2347, SSRZ8 | resistance to Pythium stalk rot | [95] |
| RpiX178-2 | bnlg1444, umc2041 | ||
| Zm00001d006675 | bnlg2077 | DNA repair | [96] |
| ZmCNR13 | InDel1413, SNP2305, SNP2337 | ear traits | [97] |
| brd1 | umc2240, SNP M1-8, IDP7806 | brassinosteroid synthesis | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, L.; Ona, A.; Berindean, I.; Racz, I.; Muntean, S. Maize Breeding: From Domestication to Genomic Tools. Agronomy 2022, 12, 2365. https://doi.org/10.3390/agronomy12102365
Muntean L, Ona A, Berindean I, Racz I, Muntean S. Maize Breeding: From Domestication to Genomic Tools. Agronomy. 2022; 12(10):2365. https://doi.org/10.3390/agronomy12102365
Chicago/Turabian StyleMuntean, Leon, Andreea Ona, Ioana Berindean, Ionuț Racz, and Sorin Muntean. 2022. "Maize Breeding: From Domestication to Genomic Tools" Agronomy 12, no. 10: 2365. https://doi.org/10.3390/agronomy12102365
APA StyleMuntean, L., Ona, A., Berindean, I., Racz, I., & Muntean, S. (2022). Maize Breeding: From Domestication to Genomic Tools. Agronomy, 12(10), 2365. https://doi.org/10.3390/agronomy12102365








