Abstract
Flourensia cernua is a bush that grows in the semi-desert regions of Mexico. It has been used in traditional medicine due to its healing properties and currently represents an alternative source of bioactive molecules for different areas of the agri-food and health industries. The objective of this study was to extract and characterize the purified polyphenolic compounds (PPCs) and essential oils (EOs) of F. cernua leaves, determine the total flavonoid content, evaluate the antioxidant activity by three different assays, and determine, for the first time, its inhibitory effect against enzymes involved in the degradation of carbohydrates (α-amylase and α-glucosidase). In addition, the analysis of functional groups (by FTIR-ATR assay) and the identification of the chemical constituents present in both essential oils and phenolic compounds were carried out by GC/MS and UPLC-QToF/MS2, respectively. The results indicate that PPCs and EOs are rich in flavonoid-type compounds. In addition, they showed potential for free-radical scavenging and the inhibition of the lipid oxidation process. The analyzed EOs and PPCs had potential against α-amylase and α-glucosidase enzymes, which are related to high blood sugar levels. FTIR-ATR analysis allowed for the identification of functional groups characteristic of polyphenolic compounds and the chemical constituents of EOs. Finally, compounds such as caryophyllene, caryophyllene oxide, and germacrene-D were identified by GC/MS assay and luteolin 7-O-rutinoside and apigenin-6-C-glucosyl-8-C-arabinoside by UPLC/QToF-MS2. The results indicate that the PPCs and EOs of F. cernua have the potential to be used as antioxidant and enzyme inhibitor agents.
1. Introduction
Flourensia cernua (Figure 1) is a bush that grows in the desert areas of the southern United States and northern Mexico, extending into the central states of Zacatecas and Hidalgo [1]. It is one of the three species of the genus Flourensia along with F. microphylla and F. retinophylla, which are endemic to the state of Coahuila de Zaragoza, Mexico [2]. Infusions of F. cernua leaves have been widely used in traditional medicine, being effective against rheumatism, venereal diseases, herpes, bronchitis, chickenpox, and the common cold, as well as for gastrointestinal diseases such as stomach pain, diarrhea, and dysentery, besides being used as a purgative [3,4]. In addition, the antifungal potential of F. cernua extracts has been demonstrated against Alternaria sp., Rhizoctonia solani, Fusarium oxysporum, Phytophthora capsici, Aspergillus flavus, Botrytis sp., Rhyzopus sp., Colletotrichum gloesporioides, and Penicillum digitatum [2,5,6]. Its inhibitory potential has also been evaluated against medically important bacteria such as multidrug-resistant Mycobacterium tuberculosis [1], and its potential as a cyanobactericidal and thermicidal agent has been addressed [7]. In the food field, the efficiency of F. cernua extract components as bioactive agents in edible coatings to improve the shelf life of tomatoes and Fuji apples has recently been evaluated [8,9].

Figure 1.
Flourensia cernua plant.
Furthermore, it has been reported that species belonging to the genus Flourensia produce essential oils, and although this genus is composed of approximately 42 species, only F. cernua together with F. oolepis, F. thurifera, and F. campestris have been analyzed in terms of profiling their chemical components [10]. However, the evaluation of the functional properties given by the essential oil of F. cernua has not been explored so far, and it represents an opportunity area for novel research and future innovative applications of this oil and the polyphenolic compounds from this species.
Among the wide applications that the phytomolecules in essential oils and phenolic compounds may have, one of the most important is in the health field. Diabetes is a global health problem that mainly affects the metabolism of macromolecules (carbohydrates) and consequently causes the deterioration of other functions [11]. Currently, synthetic drugs such as α-amylase and α-glucosidase enzyme inhibitors are administered to control sugar levels; however, their prolonged use causes a series of side effects [12]. In this regard, bioactive compounds of natural sources are of great interest in the search for safer and more effective antidiabetic agents.
The objective of this study was to obtain and chemically characterize the EOs and PPCs of F. cernua leaves from three different communities and determine the antioxidant activity and inhibitory effect against the enzymes involved in the degradation of carbohydrates (α-amylase and α-glucosidase).
2. Materials and Methods
2.1. Reagents
Ethanol, quercetin, gallic acid, linoleic acid, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)), Tween 20, Amberlite XAD-16, ferrous chloride (FeCl2), ferric chloride (FeCl3), monobasic potassium phosphate (KH2PO4), dibasic potassium phosphate (K2HPO4), potassium ferricyanide (C6N6FeK3), sodium chloride (NaCl), phenol (C6H6O), sodium sulfite (Na2SO3), sodium nitrite (NaNO2), aluminum chloride (AlCl3), sodium carbonate anhydrous (Na2CO3), acetic acid (CH3COOH), sodium hydroxide (NaOH), sodium carbonate (Na2CO3), porcine pancreatic α-amylase, α-Glucosidase from Saccharomyces cerevisiae, starch, dinitrosalicylic acid (DNS) (C7H4N2O7), p-nitrophenyl glucopyranoside (pNPG) (C12H15NO8), acetonitrile and water suitable for UPLC, and formic acid (CH2O2) were all purchased from Sigma-Aldrich.
2.2. Plant Material
The plant material (leaves) was collected in three communities in northern Mexico called San Jerónimo (SJ) in the state of Zacatecas and Estanque de León (EL) and Tortuga (TG) in the state of Coahuila de Zaragoza. Subsequently, the plants were transferred to the laboratory, washed with distilled water, and dried in an oven at 50 °C for 48 h using a Jeio Tech model ON-12G convection oven. The samples were placed into airtight bags and stored at room temperature until use. Figure 2 describes the general process performed in this study.
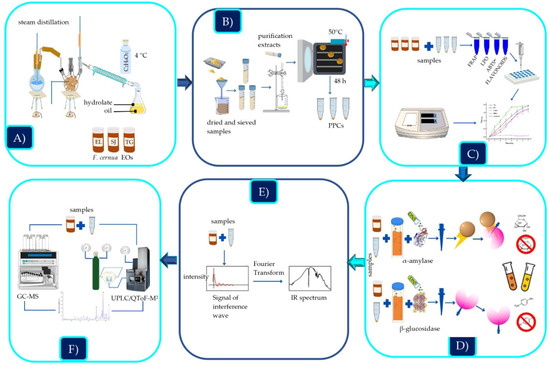
Figure 2.
General experimental process: (A) extraction of EOs, (B) extraction of PPCs, (C) evaluation of antioxidant activity, (D) enzyme inhibition assays, (E) analysis by FTIR-ATR, (F) identification by GC/MS and UPLC/QToF-MS2.
2.3. Essential Oil Extraction by Steam Distillation
A steam distillation system was established for essential oil extraction, consisting of a steam generator, which was made to impinge on 100 g of vegetable sample contained in a 3-mouth ball flask for extracting the essential oil. The vapor was conducted through a connector to a Liebig-type condenser connected to a Polyscience LM6 Benchtop Chiller for refrigeration at 4 °C, and, finally, separation by decantation was performed. The essential oil extraction process was carried out in triplicate.
2.4. Extraction of Polyphenolic Compounds
The extraction was based on the methodology previously reported by Ascacio-Valdes et al. [13], with some modifications. Briefly, 20 g of leaves (the previously ground and sieved samples adjusted to a particle size of 592 µm) were placed in 100 mL of distilled water in amber-colored bottles. Subsequently, the bottles were incubated in a water bath at 60 °C for 30 min. Finally, the obtained extract was filtered through a Whatman #41 filter paper and dried at 50 °C for 24 h. This extraction process was carried out in triplicate.
Purification of Extracts and Sample Preparation
The purification was carried out by a stationary phase (Amberlite XAD-16) and a mobile phase (ethanol). First, 20 mL of the aqueous extract was added to the stationary phase and distilled water was used as the eluent to discard unwanted compounds; then, ethanol was used as the eluent to obtain the purified polyphenolic compounds. The solvent was evaporated (50 °C for 48 h.) and the polyphenols were recovered as a fine powder. The samples of PPCs were prepared at 1000 ppm (dissolved in distilled water) for all the performed analyses. The essential oils were prepared at a 1:100 ratio for the evaluation of the antioxidant activity and at a 1:10 (dissolved in ethanol:water 80:20) ratio for the enzyme inhibition assays.
2.5. Flavonoid Content
The flavonoid content was determined according to the method of De la Rosa et al. [14], with some modifications. A sample (EOs or PPCs) volume (31 μL) was mixed with 9.3 μL of 5% sodium nitrite (w/v) and 9.3 μL of distilled water. The solution was manually mixed and incubated for 3 min at 40 °C. Then, 9.3 μL of 10% aluminum chloride (w/v) was added and incubated for 3 min. Finally, 12.50 μL of 0.5 mol∙L−1 sodium hydroxide was added and incubated for 30 min in the dark. The absorbance was recorded at 510 nm using a Synergy HTX MULTI-MODE READER microplate reader. Finally, the results were reported as the μg equivalent of quercetin per milliliter (EQ μg·mL−1) according to a calibration curve prepared with the same standard.
2.6. Antioxidant Activity Assays
2.6.1. ABTS•+ Radical Scavenging Assay
The inhibition of the ABTS•+ radical was evaluated according to the methodology proposed by Berg et al. [15], with slight modifications. The ABTS•+ radical cation was generated by aqueous ABTS solution (7 mmol∙L−1) with potassium persulfate (2.45 mmol∙L−1) mixed in the dark at room temperature for 12 h before use. Working ABTS•+ solution was adjusted in ethanol to 0.700 ± 0.002 nm of absorbance. A sample volume (5 μL) was mixed to react with 95 μL of the ABTS•+ solution, and after 1 min of reaction, the absorbance was measured at 734 nm. Finally, the results were reported as the μg equivalent of Trolox per milliliter (ET μg·mL−1) according to a calibration curve prepared with the same standard.
2.6.2. Ferric Reducing Antioxidant Power (FRAP)
The ferric reducing antioxidant power was determined according to the methodology proposed by Celik et al. [16], with slight modifications. Samples (5 μL) were mixed with 12 μL of phosphate buffer (pH 7), prepared by mixing dibasic potassium phosphate (61.5 mL, 1 M), monobasic potassium phosphate (38.1 mL, 1 mol∙L−1), and water up to 1000 mL in a volumetric flask. Then, 22 μL of 1% potassium ferricyanide was added to the reaction mix, which was homogenized and incubated at 50 °C for 20 min. Next, 10% trichloroacetic acid (12 μL), distilled H2O (45 μL), and 0.1% ferric chloride (10 μL) were added. The absorbance was recorded at a wavelength of 700 nm. Finally, the results were reported as the μg equivalent of gallic acid per milliliter (EGA μg·mL−1) according to a calibration curve prepared with the same standard.
2.6.3. Lipid Peroxidation Inhibition (LPO) Assay
The assay was performed according to Zou et al. [17], with some modifications. A total of 50 μL of the sample to be analyzed was mixed with 1.50 mL of acetate buffer (0.02 M, pH 4) and 100 μL of a linoleic acid solution which was prepared by mixing 0.6 g of linoleic acid and 1.5 g of Tween 20 dissolved in 8 mL of ethanol. For blanks, 50 μL of distilled water was used in place of the sample. The samples were homogenized and incubated at 37 °C in a water bath for one minute. After that, 750 μL of FeCl2 solution was prepared by mixing 0.01 g FeCl2 and 0.017 g EDTA dissolved in 100 mL of distilled water and incubated for 24 h at 37 °C to induce lipid oxidation. Finally, 250 μL aliquots were taken to analyze oxidation (0 and 24 h). For each sample and control, 1 mL of NaOH (0.1 M) was added to stop the oxidation process and 2.5 mL of ethanol (10 %, v/v) to dilute the sample. The absorbance of the samples was measured at 232 nm.
where Acontrol represents the difference in the absorbance value of the control reagent at 0 and 24 h and Asample corresponds to the difference in the reaction absorbance value according to each sample.
Inhibition (%) = [Acontrol − Asample/Acontrol] × 100
2.7. Enzyme Inhibition Assays
2.7.1. α-Amylase Inhibition Assay
This assay was performed using a modified procedure from McCue and Shetty [18]: 250 μL of the sample and 250 μL of porcine pancreatic α-amylase enzyme (0.5 mg·mL−1) in sodium phosphate buffer (20 mmol∙L−1, pH 6.9) were incubated at 25 °C for 10 min, then 250 μL of a 1% starch solution in sodium phosphate buffer (20 mmol∙L−1, pH 6.9) was added and incubated again at 25 °C for 10 min. The reaction was stopped by adding 500 μL of dinitrosalicylic acid (DNS) solution (which contained 1% dinitrosalicylic acid, 0.2% phenol, 0.05% sodium sulfite, and 1% NaOH). The tubes were then placed in a water bath at 90 °C for 5 min and then cooled to room temperature. The reaction mixture was diluted with 5 mL of distilled water and the absorbance was measured at 540 nm.
where Acontrol represents the absorbance value of the control and Asample the reaction value according to each sample.
Inhibition (%) = [Acontrol − Asample/Acontrol] × 100
2.7.2. α-Glucosidase Inhibition Assay
The effect of bioactive compounds against the α-glucosidase activity was determined according to the method described by Kim et al. [19], using α-glucosidase from Saccharomyces cerevisiae in phosphate buffer (20 mmol∙L−1, pH 6.9). A total of 100 μL of α-glucosidase (1.0 U·mL−1) and 50 μL of the sample were incubated at 37 °C for 10 min. After that, 50 μL of the substrate p-nitrophenyl glucopyranoside (pNPG) 3 mmol∙L−1 dissolved in phosphate buffer (20 mmol∙L−1, pH 6.9) was added to initiate the reaction and incubated again at 37 °C for 20 min. The reaction was stopped by adding 500 µL of sodium carbonate (1 mol∙L−1) and the absorbance was read at 405 nm.
where Acontrol represents the absorbance value of the control and Asample the reaction value according to each sample.
Inhibition (%) = [Acontrol − Asample/Acontrol] × 100
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The analyses were performed using an Agilent FTIR model Cary 630 coupled with the ATR accessory with zinc selenide (ZnSe) crystal. The samples (EOs and PPCs) were analyzed using MicroLab PC software in a spectral range of 4000 to 650 cm−1, with a cycle of 32 scans and a resolution of 2 cm−1. The construction of the graph was performed using OriginPro 8 software v8.0724 (B724), OriginLab (Northampton, MA 01060, USA).
2.9. Gas Chromatography Coupled to Mass Spectrometry (GC–MS) Analysis
The GC–MS analysis used the methodology described by Sethi et al. [20], with slight modifications. Briefly, the analysis was performed using Agilent Technology 7890B Network Series GC System equipment and a 5975C triple-axis mass selective detector, with an HP-5ms19091S-433 capillary column (J & W Scientific, Folsom, CA, USA), 30 m × 250 µm × 0.25 µm. A volume of 2 µL of each sample was injected, and the temperature of the injector and the temperature of the ion source were 250 °C and 220 °C, respectively. The oven temperature was initially maintained at 70 °C for 3 min and then increased to 170 °C; the rate of increase was 10 °C/min. This temperature was maintained for 10 min, and finally, the temperature was increased to 250 °C for 20 min. The mass spectra were recorded at 70 ev with a 0.5 s scan interval and a mass range of 50–650 uma. Compounds were identified using the database of the Agilent 7890B equipment.
2.10. UPLC/QToF-MS2 Analysis
The PPC samples were analyzed using an Acquity ultra-performance liquid chromatography (UPLC) system (Waters, Milford, MA, USA) consisting of an autosampler and a binary pump equipped with a 10 µL loop (partial loop injection mode). A Waters BEH PHENYL (2.1 mm × 100 mm, 1.7 µm; WATERS, Waxford, Ireland) was thermostatted at 40 °C. The solvents used were (A) water + 0.1% formic acid (v/v) and (B) acetonitrile + 0.1% formic acid (v/v) at a constant flow rate of 0.3 mL min−1. The elution gradient (over 24 min) was 95% of A for 12 min, gradually passing to 23.5 min with 12.6 of A and returning to normal conditions 0.5 min later (5% of B) for the re-equilibration of the column, using a 3 µL injection of the sample. MS detection was performed on a quadrupole time-of-flight (Q-TOF™, Waters, Milford, MA, USA) orthogonal acceleration Q-ToF mass spectrometer equipped with an electrospray ionization (ESI) source. The sample acquisition mode was in negative ion polarity, normal sensitivity, and dynamic range mode; in a mass range of 50 to 1200 Da, scanning conditions of 1.5 s−1, and centroid data format; using a capillary voltage of 0.31 kV, a source temperature of 120 °C, and a solvation temperature of 250 °C, with a cone gas flow of 40 (L/h) and desolvation of 600 (L/h). The identification of phenolic compounds from the different samples was performed using the full mass spectra and their mass fragmentation spectra. The comparison of the MS2 spectra was carried out based on the literature and databases.
2.11. Statistical Analysis
All assays were carried out in triplicate. A completely randomized design was used; the comparison of means was performed by means of Tukey’s test (p = 0.05). Statistical analysis was performed by means of the IBM SPSS Statistics 25 program.
3. Results
3.1. Content of Essential Oil and Polyphenolic Compounds
The results showed a significant difference (p = 0.05) in the content of polyphenolic compounds, at least in two communities, and the values ranged from 1.3 to 2.1%, the community of EL being where the highest content was obtained. Regarding the content of essential oils, the results showed a significant difference (p = 0.05), at least in two communities, and the values ranged from 0.10 to 0.31%, the community of TG being where the highest content was obtained.
3.2. Flavonoid Content
The obtained results (Table 1) indicate that the values of the flavonoid-type compounds ranged from 133.77 to 258.22 EQ μg·mL−1 and from 134.88 to 300.88 EQ μg·mL−1 for PPCs and EOs, respectively, proving that F. cernua leaves are rich in these kinds of bioactive compounds and showing a significant difference (p = 0.05) between samples.

Table 1.
PPCs and EOs content, flavonoids, and antioxidant activity.
3.3. Antioxidant Activity
The ABTS•+ radical scavenging assay (Table 1) showed a significant difference (p = 0.05) between the PPCs and EOs in the samples from the three communities. In the case of the PPCs, the inhibition values were recorded between 105.34 and 124.39 ET μg·mL−1, the community of EL presenting the highest radical scavenging activity.
On the other hand, the EOs presented radical scavenging values between 55.67 and 103.3 ET μg·mL−1, the community of EL presenting the higher radical scavenging activity.
The ferric reducing power (FRAP) showed a significant difference (p = 0.05) between PPCs and EOs, at least in samples from two communities. In the case of PPCs, the values ranged from 84.02 to 90.50 EGA μg·mL−1; while for EOs, the reducing power presented values between 82.07 and 94.33 EGA μg·mL−1.
The lipoperoxidation inhibition test (LPO) showed a significant difference (p = 0.05) between PPCs and EOs, at least in samples from two communities. Once again, the sample from EL showed the highest percentage of the inhibition of linoleic acid.
3.4. Enzyme Inhibition
3.4.1. α-Amylase Inhibition Assay
The α-amylase enzyme inhibition assays showed a significant difference (p = 0.05) in only one community (EL). This is an indication that the inhibitory potential between both types of secondary metabolites is very similar (Table 2).

Table 2.
Enzymatic inhibition of the purified polyphenolic compounds (PPCs) and essential oils (EOs) of F. cernua leaves.
3.4.2. α-Glucosidase Inhibition Assay
Finally, the α-glucosidase enzyme inhibition assay showed a significant difference (p = 0.05) between PPCs and EOs, but not between communities (Table 2). In this sense, the oil samples showed a percentage of inhibition of the enzyme activity higher than 90%.
3.5. FTIR Assay
3.5.1. IR Spectra of the EOs
Figure 3a shows at 3300 cm−1 the stretching of a hydroxyl group (O-H), as well as the subsequent signal at 3069 cm−1, showing the presence of a C-H bond from the CH3 functional group belonging to aromatic compounds. The region between 3000 and 2750 cm−1 shows the presence of the C-H bond for a CH2 group that may correspond to aromatic hydrocarbon compounds. The peak at 1642 cm−1 indicates the presence of C=C, which may correspond to the characteristics of aromatic rings. At 1375 cm−1, the presence of an O-H interaction deformation of a tertiary alcohol is observed. The peak at 986 cm−1 indicates the presence of C-C, which may correspond to a branched hydrocarbon with a length of more than 4C. Finally, in the region between 850 and 826 cm−1, the presence of C-H is observed, which may correspond to a cyclic unsaturated hydrocarbon of more than 5C.
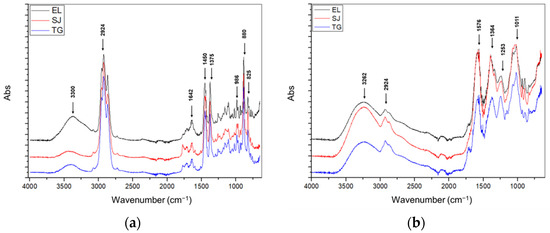
Figure 3.
(a) IR spectra of the essential oils of F. cernua, (b) IR spectra of the purified polyphenolic compounds of F. cernua; Estanque de León (EL), San Jerónimo (SJ), and Tortuga (TG).
3.5.2. IR Spectra of the PPCs
Figure 3b shows, firstly, in the range between 3400 and 3200 cm−1, the presence of the stretching of a hydroxyl group (O-H) of an aromatic ring. Subsequently, in the range between 3000 and 2900 cm−1, the presence of a C-H bond that may belong to the CH2 or CH3 groups of aromatic compounds is observed. The region between 1620 and 1560 cm−1 could be related to the stretching vibration of the C=C bonds of aromatic rings. The band between 1420 and 1380 cm−1 shows the presence of a C-C bond of a carbonyl group. The signal at 1250–1200 cm−1 is attributed to the deformation of an O-H group of polyphenols, while that between 1100 and 1000 cm−1 indicates the presence of the C-O-H functional group of alcohols.
3.6. Compound Identification of the Essential Oil by GC/MS
Since essential oils are mainly composed of volatile compounds, GC/MS can be applied for the separation and identification of low molecular weight and volatile compounds as it has an excellent separation capability, sensitivity, selectivity, and reproducibility [21].
The identification analysis of compounds present in the essential oil of F. cernua (Table 3) showed that the community of SJ presented the highest number of identified compounds, with a total of 26, followed by the community of EL with a total of 14, and finally, the community of TG, with a total of 12, mainly corresponding to the chemical class of monoterpenes and sesquiterpenes as the main components in the three communities.

Table 3.
Identification of essential oil compounds of F. cernua by GC/MS.
3.7. Compound Identification of the Polyphenolic Compounds by UPLC/QToF-MS2
UPLC analysis offers higher resolution and higher quality separation of complex analytes, allowing the detection of a wide range of secondary metabolites from several matrices using porous particles smaller than 2 µm in size [22].
The identification of polyphenolic compounds present in the samples of F. cernua (Table 4) was carried out, and a total of 18 compounds were found among the three communities, with the community of EL presenting the highest number of compounds identified with a total of 14, followed by the communities of SJ and TG with 12 each.

Table 4.
Identification of polyphenolic compounds of F. cernua by UPLC/QToF-M2.
4. Discussion
4.1. Content of Essential Oil and Polyphenolic Compounds
The content of polyphenolic compounds ranged from 1.3 to 2.1%. In a recent study, Alvarez-Perez et al. [26], reported a content of 2.72% of phenolic compounds from dried and ground plant material, which is within the values obtained in the analyzed samples. On the other hand, Zavala et al. [29], reported a content of 10% in the leaf dry extract of F. cernua. This value is higher than those obtained in this study; however, it must be considered that these authors did not carry out a purification process of the bioactive compounds. In addition, there are other factors that could influence the content of phenolic compounds such as the extraction method, solvents used, time of the extraction process, and temperature, among others [26].
On the other hand, reports on the essential oils of F. cernua are limited. The first report corresponds to Tellez et al. [30], who obtained values between 0.28 and 0.34% of essential oil content from the leaves of this plant. In addition, Priotti et al. [31] reported an essential oil content of 0.6% from leaves of F. oolepis. Likewise, in a recent study, Piazza et al. [32] obtained values between 0.24 and 0.88% of essential oil from the leaves of F. campestris processed in different years. Our obtained results are intermediate values with respect to the other species of the same genus reported by these authors.
It has been reported that the drying methods of plant material have a significant effect on the content of essential oils and polyphenolic compounds [33]. However, Hanna et al. [34] described that drying at high temperatures (45 °C) allowed for obtaining a higher yield of essential oils than other methods at lower temperatures (sun and shade). This is accordant with the finding of Álvarez-Pérez et al. [26], who used a drying temperature of 60 °C, which shows that despite the drying temperature, the samples presented high antioxidant power and enzyme inhibitory potential. Thus, the obtained results in this study suggest that a drying temperature of 50 °C for F. cernua leaves is an alternative option to obtain the molecules of several biological actives.
4.2. Flavonoids Content
Studies about the biological potential of flavonoids have intensified in the last two decades. This is due to the current interest of society in healthier foods, as well as the incidence of diseases such as cancer, cardiovascular accidents, and coronary heart diseases [35]. The obtained results (Table 2) agree with previous findings reported, indicating that all species of the genus Flourensia produce flavonoid-type bioactive compounds [36].
According to Wei et al. [37], currently, no relevant studies have been published regarding the presence of flavonoid-type compounds in essential oils, which also are present in the essential oil of Pelargonium graveolens leaves. This is accordant with the previous findings reported by Mohamed et al. [38]. Moreover, it has been reported that some flavonoid-type compounds are present in relatively high concentrations in the same oil glands where the essential oils occur, which also can also act as a good natural solvent to extract these molecules [39]. Then, the leaves of F. cernua can be considered as a source of these phytomolecules that could act as anti-inflammatory agents for the respiratory tract in asthma problems [40].
4.3. Antioxidant Activity
In the ABTS•+ radical scavenging assay, the PPCs from the community of EL presented the highest radical scavenging activity with a value of 124.39 ET μg·mL−1, equivalent to 76.30% of radical inhibition. Our results are close to those obtained by Álvarez-Pérez et al. [26], who obtained an approximate inhibition value of 87% in aqueous extracts from F. cernua leaves (at 1000 ppm). The differences between the values obtained may be due to the extraction time and the conditions of the plant (phenological state, stress conditions, and geographical location).
In the same way, the samples of EOs from the community of EL presented higher radical scavenging activity with a value of 103.3 µg ET μg·ml−1, equivalent to 63.13% of radical inhibition. To the best of our knowledge, at present, there are no reports about the antioxidant potential of F. cernua essential oils; for that reason, the present study is the first to evaluate the antioxidant capacity of this oil. However, the values obtained are higher than those reported by Yang et al. [41] in samples of essential oils from other plant materials such as lavender (Lavendular angustifolia), grapefruit (Citrus paradise), and lemon (Citrus limon) with approximate inhibition values of 25%, 37%, and 22%, respectively.
For the ferric reducing power (FRAP), in the case of the PPCs, the community of EL presented the highest reducing activity with a value of 90.50 EGA μg·mL−1, this value was higher than those reported by Jasso de Rodriguez et al. [42] for aqueous and ethanol extracts of F. cernua leaves, which demonstrates that the use of water is a good alternative for polyphenolic compound recovery from this plant material.
In addition, the reducing power of the samples of EOs from the community of EL presented the highest reducing activity with a value of 94.33 EGA μg·mL−1. The ferric reducing power is an indicator of the capacity of a matrix of bioactive compounds to transfer electrons and therefore act as a reducing agent. Such capacity is related, at the same time, to a high antioxidant capacity [43]. Hitherto, there are no current reports on the ferric reducing antioxidant power of the essential oil of F. cernua.
On the linoleic acid lipoperoxidation inhibition assay, it is observed that EOs presented higher inhibition percentages than PPCs did. For PPCs, inhibition values ranged from 10.91 to 34.89%, the community of EL presenting the highest inhibitory activity. The obtained results are lower than those reported by Álvarez-Pérez et al. [26], who obtained a percentage of lipoperoxidation inhibition near 58%. Thus, phenolics from F. cernua leaves could be applied to reduce cholesterol oxidation (low-density lipoproteins) and thus improve cardiovascular health [44].
The EO inhibition values ranged from 25.09 to 47.16%, with the samples from the community of EL presenting the highest inhibitory activity at 47.16%. As previously mentioned, there are no reports about the inhibitory potential of the lipid oxidation process of the essential oil of F. cernua; however, it has been reported that essential vegetable oils, many of which have been approved by the FDA (USA), represent an effective alternative to synthetic preservatives to prevent the process of lipid oxidation since they act as inhibitors of the reactions that initiate this process and prevent the deterioration of lipids, thus avoiding the loss of food quality and the reduction in the shelf life of food products [45,46]. However, the inhibitory effect of other molecules should be addressed in future studies.
4.4. Enzyme Inhibition
4.4.1. α-Amylase Inhibition Assay
In the case of the PPCs, the percentage of α-amylase inhibition values ranged from 10.14 to 44.93%, with the community of EL presenting the highest inhibitory potential. The mechanism of α-amylase inhibition by polyphenols has been proposed as the result of binding interactions between polyphenols and the active site of the enzyme (competitive inhibition) which is induced by hydrogen bonds and hydrophobic forces [47]. As reported for flavonoid-type compounds, the intensity of binding to α-amylase increases depending on the number of hydroxyl groups in the molecular structure [48].
On the other hand, for EOs, the inhibition values ranged from 8.66 to 15.43%, with the community of EL presenting the highest inhibitory activity. Currently, there are no reports on F. cernua essential oils on α-amylase inhibitory potential; however, Rahali et al. [49] indicated that monoterpenes, commonly found in plant essential oils, have a high potential to inhibit enzymes such as α-amylase, which is related to the development of diseases such as diabetes and obesity.
4.4.2. α-Glucosidase Inhibition Assay
In the case of PPCs, the values of α-glucosidase inhibition ranged from 4.41 to 9.12%, with the community of S, presenting the highest inhibitory activity. Dai et al. [50] reported that polyphenolic compounds strongly inhibit α-glucosidase enzyme activity by spontaneously binding via hydrogen bridges and hydrophobic interactions. In addition, it has also been mentioned that inhibition occurs in a non-competitive manner and the studies about the molecular models suggest that polyphenolic compounds bind to an allosteric site of the enzyme [51].
On the other hand, for the EOs, the inhibition values ranged from 92.57 to 97.07%, with the community of TG presenting the highest inhibitory potential at 97.07%. It was also observed that, in this case, the essential oils showed the highest inhibition values with values near 100%. The inhibition of α-glucosidase by essential oils of different medicinal herbs was reported by Ma et al. [52], who mentioned that this process can occur in a non-reversible competitive manner or in a reversible non-competitive manner, which will depend on the plant species and its own components; however, regardless of the type of inhibition, the authors reported that essential oils presented a higher inhibitory activity than acarbose (a drug used in the control of diabetes II).
4.5. FTIR Assay
4.5.1. IR Spectra of the PPCs
The presence of the hydroxyl group (O-H) in the region between 3400 and 3200 cm−1 in the analyzed samples matches with that reported by Álvarez-Pérez et al. [26], in samples of aqueous extracts of F. cernua leaves (3328.97 cm−1), and Jasso de Rodríguez et al. [2], in ethanolic extracts of F. cernua, Flourensia microphylla, and Flourensia retinophylla (3349 cm−1). Both signals are related to the presence of the hydroxyl groups characteristic of the bioactive compounds present in these species. In addition, the presence of double bonds (C=C) at a wavelength of 1620 cm−1 is related to the presence of alkene groups, which are characteristic of polyphenolic extracts [26].
4.5.2. IR Spectra of the EOs
Dinnen [53] indicates that the signals located in the region between 3600 and 3200 cm−1 (O-H), 3200 and 2800 cm−1 (C-H), and the signal between 1750 and 1600 cm−1 (C=C) are characteristic of β-eudesmol, which is a component found of the essential oil of F. cernua.
4.6. Compound Identification of the Essential Oil by GC/MS
Regarding the identification of compounds present in the essential oil from F. cernua leaves, currently, there is only one report on the identification of compounds present in this oil, which corresponds to Téllez et al. [31], who identified a total of 89 compounds, with α-eudesmol, limonene, γ-eudesmol, myrcene, borneol, and δ-3-carene being the main compounds present in this oil. Although the presence of some of these compounds is consistent with the analyzed samples, other unique compounds such as α-himachalene and α-gurjunene (Figure 4) were also identified in the samples from the three communities. It is important to consider that the chemical composition of essential oils will depend on several biotic and abiotic factors, such as plant age, growth stage, time of collection, stress conditions, and phenology [54].
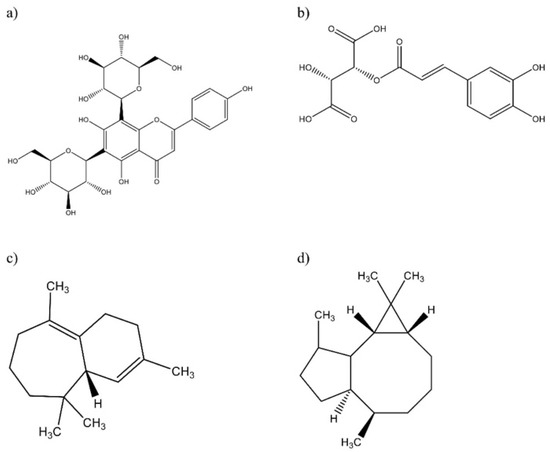
Figure 4.
(a) Vicenin-2 and (b) caftaric acid, identified from polyphenolic compounds (PC) by UPLC/QToF-M2; (c) α-himachalene and (d) α-gurjunene identified from essential oil (EO) by GC/MS.
The identified compounds from the essential oil represent between 99.96 and 100.00% of the total area (Figures S1 and S2). Among the compounds with the highest area (%) are ledol, caryophyllene, and α-himachalene. In this respect, the presence of ledol in essential oils has been related to high antioxidant activity, as well as the inhibition of acetylcholinesterase and butyrylcholinesterase enzymes involved in neuronal deterioration and damage [55]. On the other hand, it has been hypothesized that caryophyllene contributes to the antioxidant power of essential oils; in addition, this compound has been related to a high inhibitory potential of α-amylase, α-glucosidase, and pancreatic lipase enzymes, which are involved in the development of some diseases such as type II diabetes and chronic kidney disease [56]. Finally, essential oils with high content of α-himachalene have been described as a strong inhibitor of pathogenic bacteria and fungi (Pseudomonas aeruginosa, Escherichia coli, and Candida albicans) of medical interest [57]. These compounds could be related to the functional properties of the EOs of F. cernua; however, the contribution of the other compounds by a synergic effect should be addressed.
4.7. Compound Identification of the Polyphenolic Compounds by UPLC/QToF-MS2
It is important to mention that the use of a UPLC/QToF-MS2 system for phytochemical identification gives several advantages over other techniques such as better peak detection, as well as higher sensitivity, resolution, and speed of analysis, which reduces reading times for each analyzed sample [58]. Álvarez-Perez et al. [26] reported a total of 15 compounds, including luteolin 7-O-rutinoside and apigenin-6-C-glucosyl-8-C-arabinoside as the main components present in the F. cernua extracts, which also were identified in the analyzed samples. In addition, the presence of quinic acid and caffeic acid was also detected, which have been reported in the species F. microphylla [23]. Moreover, other bioactive compounds such as caftaric acid and vicenin-2 were identified (Figure 4), which have not been reported in species of the genus Flourensia, but in other members of the Asteraceae family (Echinacea purpurea and Lychnophora sp., respectively) to which F. cernua also belongs [25,28].
The identified polyphenolic compounds represent between 94.99 and 95.97% of the total area (Figures S1 and S2). Among the compounds with the highest area (%) are Apigenin-6-C-glucosyl-8-C-arabinoside and luteolin 7-O-rutinoside. The luteolin 7-O-rutinoside, together with the apigenin glucosides, have been reported as good inhibitors of α-amylase and α-glucosidase enzymes through the creation of complexes with both enzymes [59]. Currently, there is not much information on the functional activities of apigenin-6-C-glucosyl-8-C-arabinoside, however, it has been reported that the presence of flavonoid C-glucosides is related to higher antioxidant potential with respect to flavonoid O-glucosides [60].
5. Conclusions
The present study allowed us to demonstrate the functional properties of the polyphenolic compounds and essential oils from F. cernua leaves. The results indicated that the PPCs and EOs are rich in flavonoid-type compounds with strong antioxidant activity and inhibitory properties against α-amylase and α-glucosidase enzymes, the sample from Estanque de León presenting the highest activities. The data obtained from the FTIR-ATR analysis indicated the presence of O-H, C-H, C=C, and C-C functional groups, related to the bioactive compounds present in the analyzed samples. Finally, GC/MS and UPLC/QToF-MS2 analysis allowed for the identification of compounds such as α-himachalene and α-gurjunene in the essential oil, and caftaric acid and vicenin-2 in the polyphenolic compounds. The results obtained place the phytomolecules obtained from F. cernua as a viable alternative to be applied in the agri-food and health industries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102274/s1, Figure S1: Chromatograms of the EOs obtained by GC/MS assay; Figure S2: Chromatograms of PPCs obtained by UPLC/Q-TOF M2.
Author Contributions
Methodology, investigation, writing—original draft preparation, and formal analysis, N.E.A.-L.; formal analysis, data curation, and methodology, M.D.G.-H.; project administration and supervision, R.R.; methodology and visualization, B.L.-S.; formal analysis and methodology, A.D.P.-G. and G.R.; conceptualization, supervision, project administration, funding acquisition, writing—original draft preparation, and writing—review and editing G.C.G.M.-Á. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by CONAFOR-CONACYT (B-S-131466, B-S-65769) and PAICyT-UANL (CT1525-21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank the National Forestry Commission and the National Council of Science and Technology for the facilities provided for this research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, or in the writing of the manuscript.
References
- Molina-Salinas, G.M.; Ramos-Guerra, M.C.; Vargas-Villarreal, J.; Mata-Cárdenas, B.D.; Becerril-Montes, P.; Said-Fernández, S. Bactericidal activity of organic extracts from Flourensia cernua DC against strains of Mycobacterium tuberculosis. Arch. Med. Res. 2006, 37, 45–49. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Puente-Romero, G.N.; Díaz-Jiménez, L.; Rodríguez-García, R.; Ramírez-Rodríguez, H.; Villarreal-Quintanilla, J.A.; Genisheva, Z.A. In vitro gastrointestinal digestion of microencapsulated extracts of Flourensia cernua, F. microphylla, and F. retinophylla. Ind. Crops Prod. 2019, 138, 111444. [Google Scholar] [CrossRef]
- Ventura, J.; Gutiérrez-Sánchez, G.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal cultures of tar bush and creosote bush for production of two phenolic antioxidants (pyrocatechol and gallic acid). Folia Microbiol. 2009, 54, 199–203. [Google Scholar]
- Jasso de Rodríguez, D.; Hernández-Castillo, D.; Angulo-Sánchez, J.L.; Rodríguez-García, R.; Villarreal Quintanilla, J.A.; Lira-Saldivar, R.H. Antifungal activity in vitro of Flourensia sp. extracts on Alternaria s, Rhizoctonia solani, and Fusarium oxysporum. Ind. Crops Prod. 2007, 25, 111–116. [Google Scholar] [CrossRef]
- Galván, J.V.; Díaz, C.A.G.; Fernández, R.G. Efecto de los extractos acuosos de hojas de plantas de gobernadora (Larrea tridentata), hojasen (Flourensia cernua) y encino (Quercus pungens), sobre el crecimiento micelial in vitro de hongos fitopatógenos. Acta Univ. 2014, 24, 13–19. [Google Scholar]
- Guerrero-Rodríguez, E.; Solís-Gaona, S.; Hernández-Castillo, F.D.; Flores-Olivas, A.; Sandoval-López, V.; Jasso-Cantú, D. Actividad Biológica in vitro de Extractos de Flourensia cernua DC en Patógenos de Postcosecha: Alternaria alternata (Fr.: Fr.) Keissl., Colletotrichum gloeosporioides (Penz.) Penz. y Sacc. y Penicillium digitatum (Pers.: Fr.) Sacc. Rev. Mex. Fitopatol. 2007, 25, 48–53. [Google Scholar]
- Tellez, M.; Estell, R.; Fredrickson, E.D.; Powell, J.; Wedge, D.; Schrader, K.; Kobaisy, M. Extracts of Flourensia cernua (L): Volatile constituents and antifungal, antialgal, and antitermite bioactivities. J. Chem. Ecol. 2001, 27, 2263–2273. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Aguirre-Joya, J.A.; Rojas, R.; Vicente, A.; Aguilar-González, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Candelilla wax edible coating with Flourensia cernua bioactives to prolong the quality of tomato fruits. Foods 2020, 9, 1303. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Pastrana-Castro, L.; Barbosa-Pereira, L.; Rua-Rodríguez, M.L.; Saucedo, S.; Ventura-Sobrevilla, J.M.; Aguilar, C.N. Nanocoating with extract of tarbush to retard Fuji apples senescence. Postharvest Biol. Technol. 2017, 134, 67–75. [Google Scholar] [CrossRef]
- Rios, M.Y. Chemistry and biology of the genus Flourensia (Asteraceae). Chem. Biodivers. 2015, 12, 1595–1634. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Bousta, D. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of Withania frutescens L. Foliar extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Mirza, W. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.; Martínez-Hernández, J.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Euphorbia antisyphilitica residues as a new source of ellagic acid. Chem. Pap. 2010, 64, 528–532. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Vazquez-Flores, A.A.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Medina-Campos, O.N.; Ávila-Nava, A.; Pedraza-Chaverri, J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J. Funct. Foods 2014, 7, 219–228. [Google Scholar] [CrossRef]
- Berg, R.; Haenen, G.R.; van den Berg, H.; Bast, A.A.L.T. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- McCue, P.P.; Shetty, K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar]
- Kim, Y.M.; Wang, M.H.; Rhee, H.I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef]
- Sethi, A.; Prakash, R.; Shukla, D.A.; Bhatia, A.; Singh, R.P. Identification of phytochemical constituents from biologically active pet ether and chloroform extracts of the flowers of Allamanda violacea A. DC (Apocynaceae). Asian J. Plant Sci. Res. 2011, 3, 95–108. [Google Scholar]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; Garcia, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef]
- McShane, A.J.; Farrokhi, V.; Nemati, R.; Li, S.; Yao, X. An Overview of Quantitative Proteomic Approaches. In Comprehensive Analytical Chemistry: Fundamentals of Advanced Omics Technologies: From Genes to Metabolites, 1st ed.; Simó, C., Cifuentes, A., García-Cañas, V., Eds.; Esevier: Amsterdam, The Netherlands, 2014; Volume 63, pp. 111–135. [Google Scholar]
- Jasso de Rodríguez, D.; Carrillo-Lomelí, D.A.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Rodríguez-García, R.; Díaz-Jiménez, M.L.V.; Villarreal-Quintanilla, J.A. Antioxidant, anti-inflammatory and apoptotic effects of Flourensia microphylla on HT-29 colon cancer cells. Ind. Crops Prod. 2017, 107, 472–481. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, P.; Ye, F.Y.; Shi, R.; Ma, Y.M.; Zhong, J.; Jia, Y.Q. Identification and pharmacokinetics of multiple constituents in rat plasma after oral administration of Yinchenzhufu decoction. J. Ethnopharmacol. 2014, 153, 714–724. [Google Scholar] [CrossRef]
- Cech, N.B.; Eleazer, M.S.; Shoffner, L.T.; Crosswhite, M.R.; Davis, A.C.; Mortenson, A.M. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts. J. Chromatogr. A 2006, 1103, 219–228. [Google Scholar] [CrossRef]
- Alvarez-Perez, O.B.; Ventura-Sobrevilla, J.M.; Ascacio-Valdés, J.A.; Rojas, R.; Verma, D.K.; Aguilar, C.N. Valorization of Flourensia cernua DC as source of antioxidants and antifungal bioactives. Ind. Crops Prod. 2020, 152, 112422. [Google Scholar] [CrossRef]
- Zabidi, N.A.; Ishak, N.A.; Hamid, M.; Efliza Ashari, S. Subcritical water extraction of antioxidants from Curculigo latifolia root. J. Chem. 2019, 2019, 8047191. [Google Scholar] [CrossRef]
- Silva, D.B.; Turatti IC, C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in Lychnophora species. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Zavala, D.; Carrillo, M.L.; Alvarado, B.; Ch, A.O.S. Evaluación de la toxicidad aguda de un extracto alcohólico de hojas de hojasén (Flourensia cernua). Rev. Mex. Cienc. Farm. 2010, 41, 50–54. [Google Scholar]
- Tellez, M.R.; Estell, R.E.; Fredrickson, E.L.; Havstad, K.M. Essential Oil of Flourensia cernua DC. J. Essent. Oil Res. 1997, 9, 619–624. [Google Scholar] [CrossRef]
- Priotti, Z.E.; Zygadlo, J.A.; Ariza-Espinar, L. Essential oils of Flourensia oolepis SL Blake. J. Essent. Oil Res. 1997, 9, 345–347. [Google Scholar] [CrossRef]
- Piazza, L.A.; López, D.; Silva, M.P.; Lopez Rivilli, M.J.; Tourn, M.G.; Cantero, J.J.; Scopel, A.L. Volatiles and nonvolatiles in Flourensia campestris Griseb (Asteraceae), how much do capitate glandular trichomes matter? Chem. Biodivers. 2018, 15, e1700511. [Google Scholar] [CrossRef]
- Shanjani, P.S.; Mirza, M.; Calagari, M.; Adams, R.P. Effects drying and harvest season on the essential oil composition from foliage and berries of Juniperus excelsa. Ind. Crops Prod. 2010, 32, 83–87. [Google Scholar] [CrossRef]
- Hanaa, A.M.; Sallam, Y.I.; El-Leithy, A.S.; Aly, S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012, 57, 113–116. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; Estrada-Soto, S.; Flores-Morales, V.; Aguilar, M.I. Chemical constituents from Flourensia resinosa SF Blake (Asteraceae). Biochem. Syst. Ecol. 2013, 51, 240–242. [Google Scholar] [CrossRef]
- Wei, L.; Yang, H.; Li, H.; Zhu, M.; Mi, S.; Lu, Q.; Zu, Y. Comparison of chemical composition and activities of essential oils from fresh leaves of Pelargonium graveolens L. Herit. extracted by hydrodistillation and enzymatic pretreatment combined with a solvent-free microwave extraction method. Ind. Crops Prod. 2022, 186, 115204. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ali, S.I.; El-Baz, F.K. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS ONE 2013, 8, e60269. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Zhao, C.; Tian, G.; Zhang, H.; Xiao, H.; Zheng, J. Chemical mapping of essential oils, flavonoids and carotenoids in citrus peels by Raman microscopy. J. Food Sci. 2017, 82, 2840–2846. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Yang, S.A.; Jeon, S.K.; Lee, E.J.; Shim, C.H.; Lee, I.S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Salas-Méndez ED, J.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez ML, V.; Sáenz-Galindo, A.; Carrillo-Lomelí, D.A. Antifungal activity in vitro of ethanol and aqueous extracts of leaves and branches of Flourensia sp against postharvest fungi. Ind. Crops Prod. 2017, 107, 499–508. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. (Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition—A review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Das, S.; Chaudhari, A.K.; Dubey, N.K. Assessment of Melissa officinalis L. essential oil as an eco-friendly approach against biodeterioration of wheat flour caused by Tribolium castaneum Herbst. Environ. Sci. Pollut. Res. 2019, 26, 14036–14049. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A.; de Melo, N.R. Essential oils for food application: Natural substances with established biological activities. Food Bioprocess Technol. 2018, 11, 43–71. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, F.; Yang, F.; Zhao, Y.; Zhang, C.; Yamamoto, K. Interaction of dietary polyphenols with bovine milk proteins: Molecular structure–affinity relationship and influencing bioactivity aspects. Mol. Nutr. Food Res. 2011, 55, 1637–1645. [Google Scholar] [CrossRef]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Rahali, N.; Mehdi, S.; Younsi, F.; Boussaid, M.; Messaoud, C. Antioxidant, α-amylase, and acetylcholinesterase inhibitory activities of Hertia cheirifolia essential oils: Influence of plant organs and seasonal variation. Int. J. Food Prop. 2017, 20, 1637–1651. [Google Scholar] [CrossRef]
- Dai, T.; Li, T.; He, X.; Li, X.; Liu, C.; Chen, J.; McClements, D.J. Analysis of inhibitory interaction between epigallocatechin gallate and alpha-glucosidase: A spectroscopy and molecular simulation study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118023. [Google Scholar] [CrossRef]
- Özgünseven, A.; Barut, B.; Šoral, M.; Sari, S.; Akaydın, G.; Özel, A.; Şöhretoğlu, D. Alpha-glucosidase and tyrosinase inhibiton of polyphenols isolated from Potentilla speciosa var. speciosa: In vitro and in silico perspectives. Ind. Crops Prod. 2021, 170, 113806. [Google Scholar] [CrossRef]
- Ma, L.; Lin, Q.; Lei, D.; Liu, S.; Wang, X.; Zhao, Y. Alpha-glucosidase inhibitory activities of essential oils extracted from three chinese herbal medicines. Chem. Eng. Trans. 2018, 64, 61–66. [Google Scholar] [CrossRef]
- Dineen, R.D. Gas Chromatographic Identification of Trace Amounts of Organic Compounds. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 1985. [Google Scholar]
- Estell, R.E.; James, D.K.; Fredrickson, E.L.; Anderson, D.M. Within-plant distribution of volatile compounds on the leaf surface of Flourensia cernua. Biochem. Syst. Ecol. 2013, 48, 144–150. [Google Scholar] [CrossRef]
- Ozer, M.S.; Sarikurkcu, C.; Ceylan, O.; Akdeniz, I.; Tepe, B. A comprehensive study on chemical composition, antioxidant and enzyme inhibition activities of the essential oils of Chenopodium botrys collected from three different parts of Turkey. Ind. Crops Prod. 2017, 107, 326–331. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Maharik, N.; Abdallah, S.; Shawahna, R.; Mousa, A.; Qtishat, A. Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Ind. Crops Prod. 2020, 158, 112946. [Google Scholar] [CrossRef]
- Sanad, H.; Belattmania, Z.; Nafis, A.; Hassouani, M.; Mazoir, N.; Reani, A.; Sabour, B. Chemical Composition, and In Vitro Antioxidant and Antimicrobial Activities of the Marine Cyanolichen Lichina pygmaea Volatile Compounds. Mar. Drugs 2022, 20, 169. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, M.; Wang, L.; Ying, X.; Peng, J.; Jiang, M.; Luo, G. Comparative evaluation of different cultivars of Flos Chrysanthemi by an anti-inflammatory-based NF-κB reporter gene assay coupled to UPLC-Q/TOF MS with PCA and ANN. J. Ethnopharmacol. 2015, 174, 387–395. [Google Scholar] [CrossRef]
- Cui, J.; Zeng, S.; Zhang, C. Anti-hyperglycaemic effects of Burdock (Arctium lappa L.) leaf flavonoids through inhibiting α-amylase and α-glucosidase. Int. J. Food Sci. Technol. 2022, 57, 541–551. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, T.; Zhang, C.; Guo, M. Flavonoids of Lotus (Nelumbo nucifera) seed embryos and their antioxidant potential. J. Food Sci. 2017, 82, 1834–1841. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).