Humic Acids Formation during Compositing of Plant Remnants in Presence of Calcium Carbonate and Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Plant Material and Biochar
2.2. Incubation Experiment
2.3. Laboratory Methods
2.4. Statistical Analyses
3. Results and Discussion
3.1. Mineralization
3.2. Humification
3.3. Elemental Composition and 13C-NMR Data of HAs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAs | humic acids |
| FAs | fulvic acids |
| HAs1 | labile HAs |
| HAs2 | stabilized HAs |
| NMR | nuclear magnetic resonance |
References
- Zhang, J.; Lü, F.; Shao, L.; He, P. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ishola, T.M.; Ishola, E.T. Composting and Sustainable Development. In Encyclopedia of Sustainability in Higher Education; Filho, W.L., Ed.; Springer: Cham, Switzerland, 2019; pp. 21–22. [Google Scholar]
- Omar, L.; Ahmed, O.H.; Jalloh, M.B.; Majid, N.M.A. Rice Husk Compost Production and Use in Mitigating Ammonia Volatilization from Urea. Sustainability 2021, 13, 1832. [Google Scholar] [CrossRef]
- Shevtsova, L.K.; Chernikov, V.A.; Sychev, V.G.; Belichenko, M.V.; Rukhovich, O.V.; Ivanova, O.I. Influence of long-term use of fertilizers on the composition, properties and structural characteristics of humic acids in the main types of soils. Agrochemistry 2019, 10, 3–15. [Google Scholar] [CrossRef]
- Boguta, P.; Skic, K.; Sokołowska, Z.; Frąc, M.; Sas-Paszt, L. Chemical Transformation of Humic Acid Molecules under the Influence of Mineral, Fungal and Bacterial Fertilization in the Context of the Agricultural Use of Degraded Soils. Molecules 2021, 26, 4921. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Awasthi, S.K.; Wang, Q.; Wang, Z.; Lahori, A.H.; Ren, X.; Chen, H.; Wang, M.; Zhao, J.; Zhang, Z. Influence of biochar on volatile fatty acids accumulation and microbial community succession during biosolids composting. Bioresour. Technol. 2018, 251, 158–164. [Google Scholar] [CrossRef]
- Meng, L.Q.; Zhang, S.M.; Gong, H.N.; Zhang, X.C.; Wu, C.D.; Li, W.G. Improving sewage sludge composting by addition of spent mushroom substrate and sucrose. Bioresour. Technol. 2018, 253, 197–203. [Google Scholar] [CrossRef]
- Antonangelo, J.A.; Sun, X.; Zhang, H. The roles of co-composted biochar (COMBI) in improving soil quality, crop productivity, and toxic metal amelioration. J. Environ. Manag. 2021, 277, 111443. [Google Scholar] [CrossRef] [PubMed]
- Barthod, J.; Rumpel, C.; Dignac, M.-F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.; Ren, X.; Zhao, J.; Wang, M.; Chen, H.; Zhang, Z. Recent Advances in Composting of Organic and Hazardous Waste: A Road Map to Safer Environment. Biosynthetic Technol. Environ. Chall. 2018, 307–329. [Google Scholar] [CrossRef]
- Toledo, M.; Siles, J.; Guti´errez, M.; Martín, M. Monitoring of the composting process of different agroindustrial waste: Influence of the operational variables on the odorous impact. Waste Manag. 2018, 76, 266–274. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Luo, C.; Shao, L.; He, P. Humification characterization of biochar and its potential as a composting amendment. J. Environ. Sci. 2014, 26, 390–397. [Google Scholar] [CrossRef]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. Rev. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Glaser, B. Synergisms between Compost and Biochar for Sustainable Soil Amelioration. Manag. Org. Waste 2012, 1, 167–198. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Hussain, Q.; Usman, A.R.A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 2017, 29, 2124–2161. [Google Scholar] [CrossRef]
- Videgain-Marco, M.; Marco-Montori, P.; Martí-Dalmau, C.; Jaizme-Vega, M.C.; Manyà-Cervelló, J.J.; García-Ramos, F.J. Effects of Biochar Application in a Sorghum Crop under Greenhouse Conditions: Growth Parameters and Physicochemical Fertility. Agronomy 2020, 10, 104. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Wei, L.; Shutao, W.; Jin, Z.; Tong, X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 2014, 154, 148–154. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Qu, M.; Yin, Y.; Fan, K.; Hu, B.; Zhang, H.; Wei, M.; Ma, C. Effects of biochar on the microbial activity and community structure during sewage sludge composting. Bioresour. Technol. 2019, 272, 171–179. [Google Scholar] [CrossRef]
- Guo, X.-X.; Liu, H.-T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef]
- Qiaoping, T.; Wei-Xiang, W.; Cheng, W.; Da, D. Spectroscopic evidence for biochar amendment promoting humic acid synthesis and intensifying humification during composting. J. Hazard. Mater. 2014, 280, 409–416. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of biochar addition on the humic substances of composting manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef]
- Alexandrova, L. Soil Organic Matter and Its Transformation Processes; Nauka: Leningrad, Russia, 1980; p. 287. [Google Scholar]
- Orlov, D. Soil Humic Acids and General Theory Humification; Moscov State University: Moscow, Russia, 1990; p. 325. [Google Scholar]
- Semenov, V.; Kogut, B.; Zinyakova, N.; Masyutenko, N.; Maslyukova, L.; Lebedeva, T.; Tulina, A. Biologically Active Organic Matter in Soils of European Russia. Eurasian Soil Sci. 2018, 51, 434–447. [Google Scholar] [CrossRef]
- Ponomaryova, V.V.; Plotnikova, T.A. Humus and Soil Formation; Nauka: Leningrad, Russia, 1980; p. 221. [Google Scholar]
- Zvyagintsev, D.; Shapovalov, A.; Putsikin, Y.; Stepanov, A.; Lysak, L.; Bulankina, M. Resistance of humic acids to microbial dedradation. Soil Sci. 2004, 2, 44–47. [Google Scholar]
- Ovchinnikova, M. Features of natural stability and agrogenic transformation of soil humus. Eurasian Soil Sci. 2013, 46, 1150–1163. [Google Scholar] [CrossRef]
- Orlova, N.E.; Plotnikova, T.A.; Bakina, L.G. Interaction of Humic Acids with Calcium and Implications for the Liming of Soils. Eurasian Soil Sci. 1992, 24, 12–16. [Google Scholar]
- Bakina, L.; Nebol’sin, A.; Nebol’sina, Z. Changes in the content and composition of humus in the sandy loamy soddy-podzolic soil in a long-term liming experiment. Eurasian Soil Sci. 2011, 44, 525–533. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef]
- Orlova, N.; Abakumov, E.; Orlova, E.; Yakkonen, K.; Shahnazarova, V. Soil organic matter alteration under biochar amendment: Study in the incubation experiment on the Podzol soils of the Leningrad region (Russia). J. Soils Sediments 2019, 19, 2708–2716. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Matsumoto, K.; Sonoki, T. The efficiency of a low dose of biochar in enhancing the aromaticity of humic-like substance extracted from poultry manure compost. Agronomy 2019, 9, 248. [Google Scholar] [CrossRef]
- Smirnova, K.; Gurtovaya, A. Agroecological assessment of high-dose biochar use during the melioration of agro-podzolic soils. Mater. Study Russ. Soils 2021, 13, 79–83. [Google Scholar]
- Mineev, V. Workshop on Agrochemistry; Publishing House of Moscow State University: Moskow, Russia, 2001; 689p. [Google Scholar]
- Vorobyova, L. Chemical Analysis of Soils: Textbook; Mosckow University Press: Moskow, Russia, 1998; 272p. [Google Scholar]
- Kumada, K. Chemistry of Soil Organic Matter; Japan Scientific Societies Press: Tokyo, Japan; Elsevier: Tokyo, Japan, 1987; Volume 17, p. 240. [Google Scholar]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis; Sparks, D.L., Ed.; Soil Science Society of America Book Series 5 Part 3—Chemical Methods; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1996; pp. 1011–1020. [Google Scholar]
- Klucakova, M.; Kalina, M. Composition, particle size, charge and colloidal stability of pH-fractionated humic acids. J. Soils Sediments 2015, 15, 1900–1908. [Google Scholar] [CrossRef]
- Drichko, V.; Bakina, L.; Orlova, N. Stable and labile components of humus in soddy podzolic soils. Eurasian Soil Sci. 2013, 46, 37–43. [Google Scholar] [CrossRef]
- Lodygin, E.; Beznosikov, V.; Abakumov, E. Humic substances elemental composition of selected taiga and tundra soils from Russian European North-East. Pol. Polar Res. 2017, 38, 125–147. [Google Scholar] [CrossRef][Green Version]
- Kostenko, I.; Abakumov, E. Characterization of Humic Acids in Mountainous Meadow Soils and Burozems of the Crimea Using 13C-NMR. Eurasian Soil Sci. 2018, 51, 1411–1418. [Google Scholar] [CrossRef]
- Orlova, N.; Bakina, L.; Orlova, E. Methods of Studying the Content and Composition of Humus; Publishing House of Saint-Petersburg State University: Saint-Petersburg, Russia, 2007; p. 147. [Google Scholar]
- Bakina, L.; Chugunova, M.; Zaitseva, T.; Nebol’sina, Z. The effect of liming on the complex of soil microorganisms and the humus status of a soddy-podzolic soil in a long-term experiment. Eurasian Soil Sci. 2014, 47, 110–118. [Google Scholar] [CrossRef]
- Lodygin, E.; Beznosikov, V.; Vasilevich, R. Molecular composition of humic substances in tundra soils (13C-NMR spectroscopic study). Eurasian Soil Sci. 2014, 47, 400–406. [Google Scholar] [CrossRef]
- Chukov, S.; Lodygin, E.; Abakumov, E. Application of 13C NMR Spectroscopy to the Study of Soil Organic Matter: A Review of Publications. Eurasian Soil Sci. 2018, 51, 889–900. [Google Scholar] [CrossRef]
- Dergacheva, M. The System of Humic Substances in Soils; Nauka: Novosibirsk, Russia, 1989; p. 110. [Google Scholar]
- Semenov, V.; Kogut, B. Soil Organic Matter; GEOS: Moscow, Russia, 2015; 233p. [Google Scholar]
- Ovchinnikova, M. Changes in the Content, Composition, and Properties of Humic Substances in Particle-Size Fractions of Soddy-Podzolic Soils under the Impact of Long-Term Drainage. Eurasian Soil Sci. 2018, 51, 647–657. [Google Scholar] [CrossRef]
- Chukov, S.; Ejarque, E.; Abakumov, E. Characterization of humic acids from tundrasoils of northern western Siberia by electron paramagnetic resonance spectroscopy. Eurasian Soil Sci. 2017, 50, 30–33. [Google Scholar] [CrossRef]

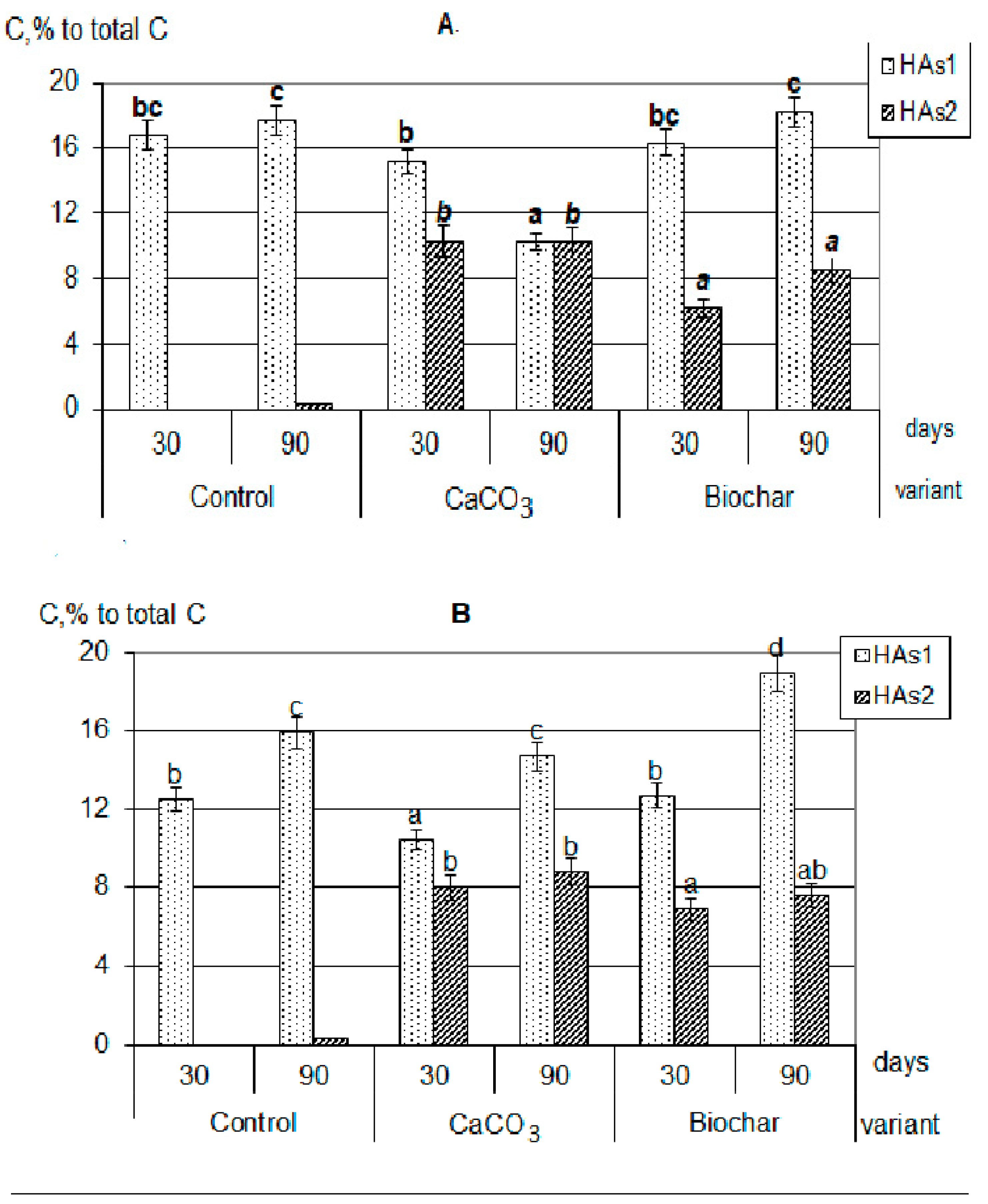

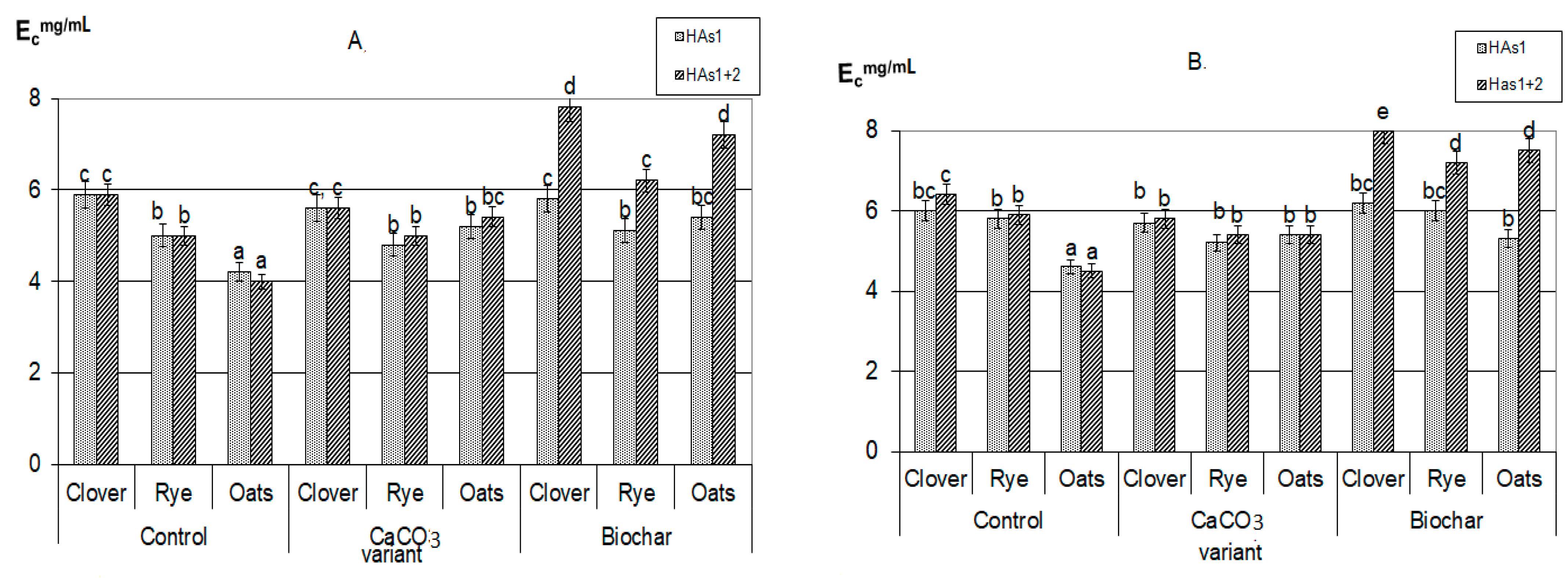

| Plant | C | N | Ash | C/N |
|---|---|---|---|---|
| % | ||||
| Clover | 41.6 ± 0.8 | 1.88 ± 0.02 | 5.34 ± 0.01 | 22.1 |
| Rye | 43.2 ± 1.6 | 1.12 ± 0.05 | 5.88 ± 0.03 | 38.6 |
| Oats | 42.0 ± 1.0 | 0.61 ± 0.08 | 5.72 ± 0.04 | 68.4 |
| Variants | Initial Content | 30 Days of Composting | 90 Days of Composting | |||||
|---|---|---|---|---|---|---|---|---|
| C0, % on Dry Weight, | C, % on Dry Weight | C, % on Initial Content | C, % of the Content in the Control | C, % on Dry Weight | C, % on Initial Content | C, % of the Content in the Control | ||
| Clover | control | 6.94 ± 0.08 | 3.34 ± 0.04 | 48.1 | - | 2.88 ± 0.06 | 41.5 | - |
| CaCO3 | 2.82 ± 0.08 | 40.6 | 84.4 | 2.72 ± 0.04 | 39.2 | 94.4 | ||
| biochar | 2.88 ± 0.09 | 41.5 | 86.3 | 2.36 ± 0.06 | 34.0 | 81.9 | ||
| Rye | control | 7.50 ± 0.12 | 4.07 ± 0.16 | 54.3 | - | 3.39 ± 0.10 | 45.2 | - |
| CaCO3 | 3.74 ± 0.09 | 49.9 | 91.9 | 3.06 ± 0.06 | 40.8 | 90.3 | ||
| biochar | 3.62 ± 0.05 | 48.3 | 89.0 | 2.78 ± 0.14 | 37.1 | 82.1 | ||
| Oats | control | 7.30 ± 0.10 | 4.62 ± 0.06 | 63.3 | - | 3.65 ± 0.12 | 50.0 | - |
| CaCO3 | 4.02 ± 0.10 | 56.4 | 89.1 | 3.30 ± 0.09 | 45.2 | 90.4 | ||
| biochar | 4.00 ± 0.08 | 54.8 | 86.6 | 3.08 ± 0.07 | 42.2 | 84.4 | ||
| Variants | The Sum of Soluble Substances | Sum of Substances Precipitated by 0.5 M of H2SO4 | |||
|---|---|---|---|---|---|
| % on Ctot | ECmg/mL | % on Ctot | ECmg/mL | ||
| Clover | control | 2.1 ± 0.1 | 0.8 ± 0.1 | 2.1 ± 0.1 | 0.8 ± 0.1 |
| CaCO3 | 1.6 ± 0.1 | 0.5 ± 0.0 | 1.3 ± 0.2 | 0.5 ± 0.1 | |
| biochar | 2.0 ± 0.2 | 0.5 ± 0.1 | 2.0 ± 0.1 | 0.6 ± 0.1 | |
| Rye | control | 1.5 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.2 | 0.8 ± 0.1 |
| CaCO3 | 1.8 ± 0.2 | 0.7 ± 0.1 | 1.1 ± 0.2 | 0.7 ± 0.1 | |
| biochar | 1.4 ± 0.1 | 0.7 ± 0.1 | 1.2 ± 0.3 | 0.8 ± 0.1 | |
| Oats | control | 2.0 ± 0.1 | 0.4 ± 0.1 | 1.4 ± 0.1 | 0.5 ± 0.1 |
| CaCO3 | 1.6 ± 0.1 | 0.4 ± 0.0 | 1.3 ± 0.1 | 0.4 ± 0.1 | |
| biochar | 1.7 ± 0.1 | 0.5 ± 0.0 | 1.5 ± 0.1 | 0.6 ± 0.1 | |
| Variant | C | N | H | O | H/C | O/C | O/H | C/N | ω | |
|---|---|---|---|---|---|---|---|---|---|---|
| Content, at % | ||||||||||
| Clover | control | 37.31 | 2.69 | 41.88 | 18.12 | 1.12 | 0.49 | 0.43 | 13.87 | −0.15 |
| CaCO3 | 36.66 | 2.51 | 42.17 | 18.65 | 1.15 | 0.51 | 0.44 | 14.60 | −0.13 | |
| biochar | 38.09 | 2.39 | 41.22 | 18.30 | 1.08 | 0.48 | 0.44 | 15.94 | −0.12 | |
| Rye | control | 34.99 | 1.60 | 46.14 | 17.26 | 1.32 | 0.49 | 0.37 | 21.87 | −0.33 |
| CaCO3 | 35.64 | 1.71 | 45.19 | 17.47 | 1.27 | 0.49 | 0.39 | 20.84 | −0.29 | |
| biochar | 37.23 | 1.84 | 43.23 | 17.69 | 1.16 | 0.48 | 0.41 | 20.23 | −0.21 | |
| Oats | control | 37.54 | 1.62 | 45.66 | 17.26 | 1.22 | 0.46 | 0.38 | 23.17 | −0.30 |
| CaCO3 | 36.08 | 1.56 | 44.95 | 17.42 | 1.24 | 0.48 | 0.39 | 23.13 | −0.28 | |
| biochar | 36.26 | 1.60 | 44.43 | 17.74 | 1.22 | 0.49 | 0.40 | 22.66 | −0.25 | |
| Origin biochar | 67.60 | 0.30 | 35.10 | 3.40 | 0.52 | 0.05 | 0.10 | 225.33 | −0.42 | |
| Variant | Chemical Shift, ppm | Aliphatic Group | Aromatic Group | AR/AL | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0–47 | 47–60 | 60–110 | 110–160 | 160–185 | 185–200 | ||||
| Control | 32 | 7 | 3 | 27 | 24 | 7 | 49 | 51 | 1.04 |
| CaCO3 | 36 | 8 | 2 | 24 | 24 | 6 | 52 | 48 | 0.92 |
| Biochar | 25 | 7 | 18 | 30 | 16 | 4 | 54 | 46 | 0.85 |
| Origin biochar | 15 | 4 | 16 | 55 | 6 | 4 | 39 | 61 | 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, N.; Orlova, E.; Abakumov, E.; Smirnova, K.; Chukov, S. Humic Acids Formation during Compositing of Plant Remnants in Presence of Calcium Carbonate and Biochar. Agronomy 2022, 12, 2275. https://doi.org/10.3390/agronomy12102275
Orlova N, Orlova E, Abakumov E, Smirnova K, Chukov S. Humic Acids Formation during Compositing of Plant Remnants in Presence of Calcium Carbonate and Biochar. Agronomy. 2022; 12(10):2275. https://doi.org/10.3390/agronomy12102275
Chicago/Turabian StyleOrlova, Nataliya, Elena Orlova, Evgeny Abakumov, Kseniia Smirnova, and Serafim Chukov. 2022. "Humic Acids Formation during Compositing of Plant Remnants in Presence of Calcium Carbonate and Biochar" Agronomy 12, no. 10: 2275. https://doi.org/10.3390/agronomy12102275
APA StyleOrlova, N., Orlova, E., Abakumov, E., Smirnova, K., & Chukov, S. (2022). Humic Acids Formation during Compositing of Plant Remnants in Presence of Calcium Carbonate and Biochar. Agronomy, 12(10), 2275. https://doi.org/10.3390/agronomy12102275








