Fractionation of Soil Organic Matter into Labile and Stable Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Used
- A:
- Borová Lada locality at an altitude of 895 m, GPS coordinates: 48.986409, 13.671749; forest soil sample (O-horizon) of medium-heavy cambisols (forest composition: spruce 65%, pine 21%, birch 10%);
- B:
- Zahrádky near Borová Lada locality at an altitude of 880 m, GPS coordinates: 48.977437, 13.689511; forest soil sample (O-horizon) of medium-heavy gleysols (forest composition: spruce 81%, beech 11%, pine 4%);
- C:
- Plavsko near Stráž and Nežárkou locality at an altitude of 464 m, GPS coordinates: 49.088498, 14.894712; forest soil sample (O-horizon) of medium-heavy stagnosols (forest composition: pine 79%, spruce 13%, oak 4%);
- D:
- Opařany locality at an altitude of 465 m, GPS coordinates: 49.405163, 14.478683; agricultural soil sample (A-horizon) of medium-heavy cambisols;
- E:
- Třeboň locality at an altitude of 434 m, GPS coordinates: 49.023263, 14.647633; agricultural soil sample (A-horizon) of medium-heavy luvisols;
- F:
- Modřice u Brna locality at an altitude of 204 m, GPS coordinates: 49.115030, 16.614573; agricultural soil sample (A-horizon) of medium-heavy chernozems.
2.2. Classical Method (CM)
2.3. Modified Method (MM)
2.3.1. Quality of Primary Soil Organic Matter
2.3.2. Calculation of the Amounts of CPSOM and CSOF
2.3.3. Determination of Quality of Stable Organic Fraction
2.4. Statistical Analysis
3. Results
3.1. Fractionation According to the Carbon Content
3.2. Evaluation of Soil Organic Matter Quality
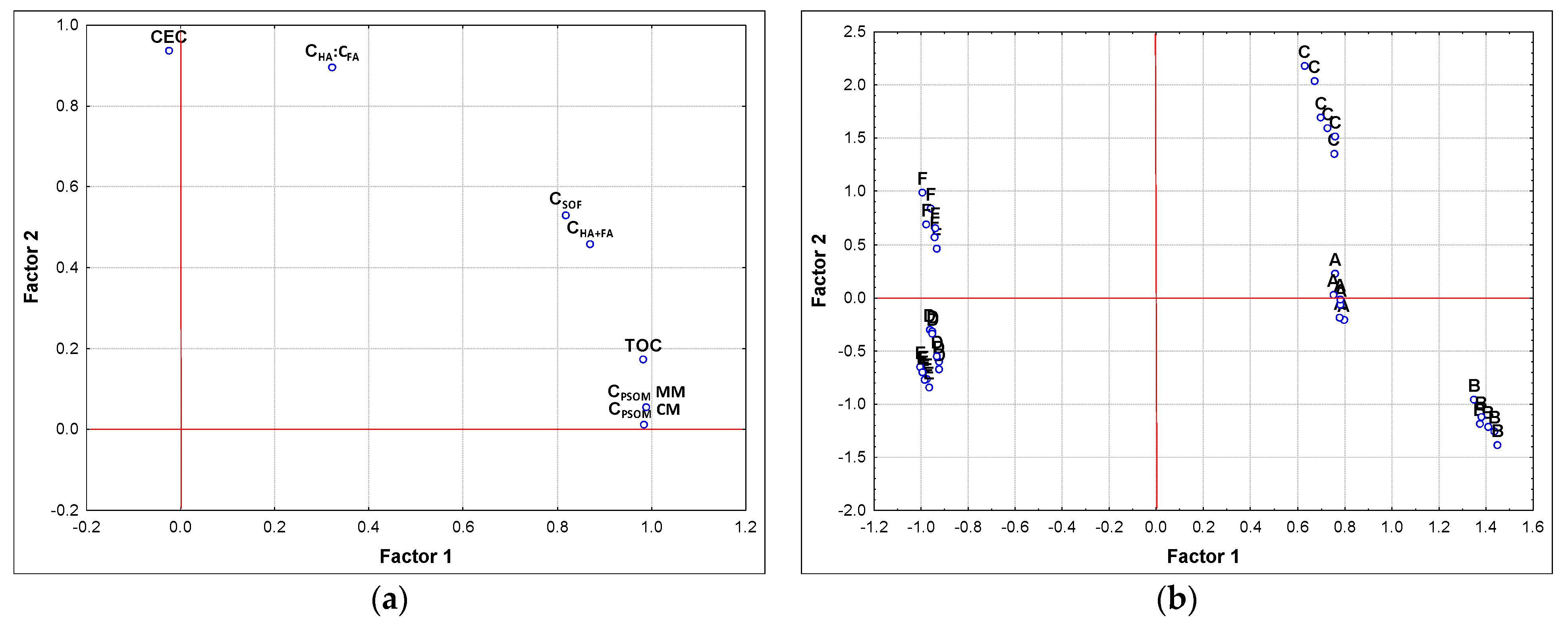
3.3. Evaluation of Results from the Point of View Principal Component Analysis and Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregorich, E.G.; Carter, M.R.; Angers, D.A.; Monreal, C.; Ellert, B. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar] [CrossRef] [Green Version]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean Technol. Environ. Policy 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- Osman, K.T. Soil Degradation, Conservation, and Remediation; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789400775909. [Google Scholar]
- Lal, R. Soil carbon sequestration and aggregation by cover cropping. J. Soil Water Conserv. 2015, 70, 329–339. [Google Scholar] [CrossRef]
- Stehel, V.; Vochozka, M.; Maroušková, A.; Šál, J.; Kolář, L. Charred fermentation residues accelerate methanogenesis and sorb air pollutants. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 301–305. [Google Scholar] [CrossRef]
- Nachshon, U. Soil degradation processes: It’s time to take our head out of the sand. Geosciences 2021, 11, 2. [Google Scholar] [CrossRef]
- Darwish, O.H.; Persaud, N.; Martens, D.C. Effect of long-term application of animal manure on physical properties of three soils. Plant Soil 1995, 176, 289–295. [Google Scholar] [CrossRef]
- Zhang, G.X.; Shen, L.X.; Guo, Y.M. Effect of soil structure on water infiltration under moistube irrigation. J. Irrig. Drain. Eng. 2016, 35, 35–39. [Google Scholar]
- Leroy, B.L.M.; Herath, H.M.S.K.; Sleutel, S.; De Neve, S.; Gabriels, D.; Reheul, D.; Moens, M. The quality of exogenous organic matter: Short-term effects on soil physical properties and soil organic matter fractions. Soil Use Manag. 2008, 24, 139–147. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.P.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; De Courcelles, V.D.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Hutchinson, J.J.; Campbell, C.A.; Desjardins, R.L. Some perspectives on carbon sequestration in agriculture. Agric. For. Meteorol. 2007, 142, 288–302. [Google Scholar] [CrossRef]
- Tian, J.; Lou, Y.; Gao, Y.; Fang, H.; Liu, S.; Xu, M.; Blagodatskaya, E.; Kuzyakov, Y. Response of soil organic matter fractions and composition of the microbial community to long-term organic and mineral fertilization. Biol. Fertil. Soils 2017, 53, 523–532. [Google Scholar] [CrossRef]
- Kalantary, F.; Kahani, M. Optimization of the biological soil improvement procedure. Int. J. Environ. Sci. Technol. 2018, 16, 4231–4240. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Kopecký, M.; Peterka, J.; Kolář, L.; Konvalina, P.; Maroušek, J.; Váchalová, R.; Herout, M.; Strunecký, O.; Batt, J.; Tran, D.K. Influence of selected maize cultivation technologies on changes in the labile fraction of soil organic matter sandy-loam cambisol soil structure. Soil Tillage Res. 2021, 207, 104865. [Google Scholar] [CrossRef]
- Li, X.G.; Jia, B.; Lv, J.; Ma, Q.; Kuzyakov, Y.; Li, F.M. Nitrogen fertilization decreases the decomposition of soil organic matter and plant residues in planted soils. Soil Biol. Biochem. 2017, 112, 47–55. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile Organic Matter Fractions as Central Components of the Quality of Agricultural Soils: An Overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Kolář, L.; Vaněk, V.; Kužel, S.; Peterka, J.; Borová-Batt, J.; Pezlarová, J. Relationships between quality and quantity of soil labile fraction of the soil carbon in Cambisols after liming during 5 years. Plant. Soil Environ. 2011, 57, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Maia, S.M.F.; Xavier, F.A.S.; Oliveira, T.S.; Mendonça, E.S.; Araújo Filho, J.A. Organic carbon pools in a Luvisol under agroforestry and conventional farming systems in the semi-arid region of Ceará, Brazil. Agrofor. Syst. 2007, 71, 127–138. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Novotny, E.H.; Knicker, H.; de Oliveira, R.R. Humic acid composition and soil fertility of soils near an ancient charcoal kiln: Are they similar to Terra Preta de Índios soils? J. Soils Sediments 2019, 19, 1374–1381. [Google Scholar] [CrossRef]
- Mendonça, E.S.; Rowell, D.L. Mineral and Organic Fractions of Two Oxisols and Their Influence on Effective Cation-Exchange Capacity. Soil Sci. Soc. Am. J. 1996, 60, 1888–1892. [Google Scholar] [CrossRef]
- Rahal, N.S.; Alhumairi, B.A.J. Modelling of soil cation exchange capacity for some soils of east gharaf lands from mid-Mesopotamian plain (Wasit province/Iraq). Int. J. Environ. Sci. Technol. 2019, 16, 3183–3192. [Google Scholar] [CrossRef]
- Arthur, E. Rapid estimation of cation exchange capacity from soil water content. Eur. J. Soil Sci. 2017, 68, 365–373. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in an oxic paleustalf under different pasture leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Körschens, M.; Schulz, E.; Behm, R. Heißwasserlöslicher C und N im boden als kriterium für das N-Nachlieferungsvermögen. Zentralbl. Mikrobiol. 1990, 145, 305–311. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G.; Osmond, G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Maroušek, J.; Bartoš, P.; Filip, M.; Kolář, L.; Konvalina, P.; Maroušková, A.; Moudrý, J.; Peterka, J.; Šál, J.; Šoch, M.; et al. Advances in the agrochemical utilization of fermentation residues reduce the cost of purpose-grown phytomass for biogas production. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 00, 1–11. [Google Scholar] [CrossRef]
- IUSS Working Group. WRB World reference base for soil resources 2014. In International Soil Classification System for Naming soils and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; ISBN 9789251083697. [Google Scholar]
- Meloun, M.; Militký, J. Statistical Data Analysis, A Practical Guide with 1250 Exercises and Answer Key on CD; Woodhead Publishing India: New Delhi, India, 2011. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.A. Humin. Soil Sci. 2001, 166, 848–857. [Google Scholar] [CrossRef]
- Burdon, J. Are the traditional concepts of the structures of humic substances realistic? Soil Sci. 2001, 166, 752–769. [Google Scholar] [CrossRef]
- Knicker, H.; Hilscher, A.; González-Vila, F.J.; Almendros, G. A new conceptual model for the structural properties of char produced during vegetation fires. Org. Geochem. 2008, 39, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Heymann, K.; Lehmann, J.; Solomon, D.; Liang, B.; Neves, E.; Wirick, S. Can functional group composition of alkaline isolates from black carbon-rich soils be identified on a sub-100nm scale? Geoderma 2014, 235–236, 163–169. [Google Scholar] [CrossRef]
- Marín-Spiotta, E.; Gruley, K.E.; Crawford, J.; Atkinson, E.E.; Miesel, J.R.; Greene, S.; Cardona-Correa, C.; Spencer, R.G.M. Paradigm shifts in soil organic matter research affect interpretations of aquatic carbon cycling: Transcending disciplinary and ecosystem boundaries. Biogeochemistry 2014, 117, 279–297. [Google Scholar] [CrossRef]
- Blume, H.-P.; Brümmer, G.W.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Scheffer/Schachtschabel: Lehrbuch der Bodenkunde; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010. [Google Scholar]
- Klotzbücher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Kopecký, M.; Kolář, L.; Váchalová, R.; Konvalina, P.; Batt, J.; Mráz, P.; Menšík, L.; Hoang, T.N.; Dumbrovský, M. Black Carbon and Its Effect on Carbon Sequestration in Soil. Agronomy 2021, 11, 2261. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilization, grazing, and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Lalande, R.; Gagnon, B.; Royer, I. Impact of natural or industrial liming materials on soil properties and microbial activity. Can. J. Soil Sci. 2009, 89, 209–222. [Google Scholar] [CrossRef]
- Lovell, R.D.; Jarvis, S.C. Soil microbial biomass and activity in soil from different grassland management treatments stored under controlled conditions. Soil Biol. Biochem. 1998, 30, 2077–2085. [Google Scholar] [CrossRef]
- Campbell, C.A.; Paul, E.A.; Rennie, D.A.; Mccallum, K.J. Applicability of the Carbon-Dating Method of Analysis To Soil Humus Studies. Soil Sci. 1967, 104, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh-Mehrjardi, R. Digital mapping of cation exchange capacity using genetic programming and soil depth functions in Baneh region, Iran. Arch. Agron. Soil Sci. 2016, 62, 109–126. [Google Scholar] [CrossRef]
- Khaledian, Y.; Kiani, F.; Ebrahimi, S.; Brevik, E.C.; Aitkenhead-Peterson, J. Assessment and Monitoring of Soil Degradation during Land Use Change Using Multivariate Analysis. Land Degrad. Dev. 2017, 28, 128–141. [Google Scholar] [CrossRef]
- Moral, F.J.; Rebollo, F.J. Characterization of soil fertility using the Rasch model. J. Soil Sci. Plant Nutr. 2017, 17, 486–498. [Google Scholar] [CrossRef]
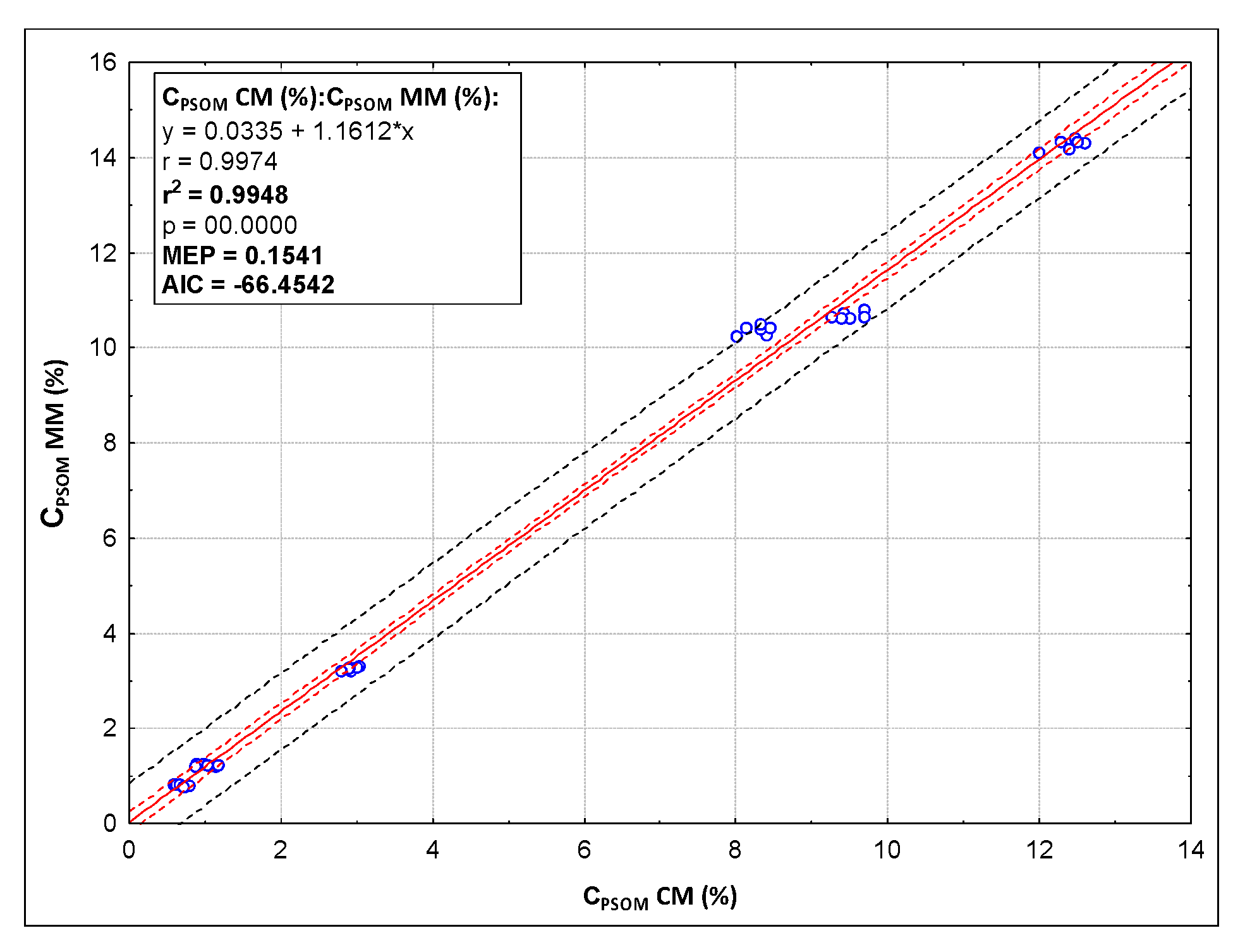
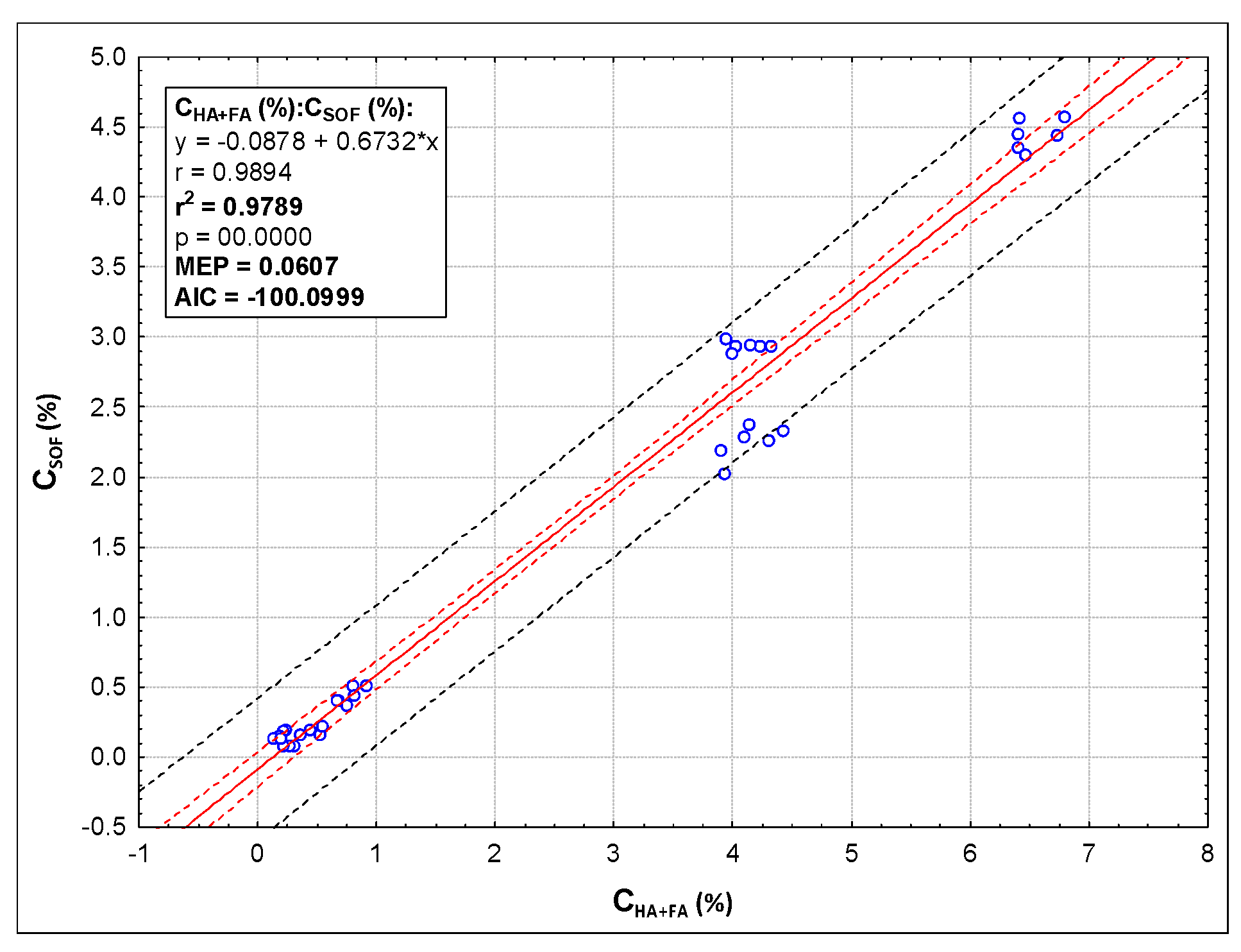
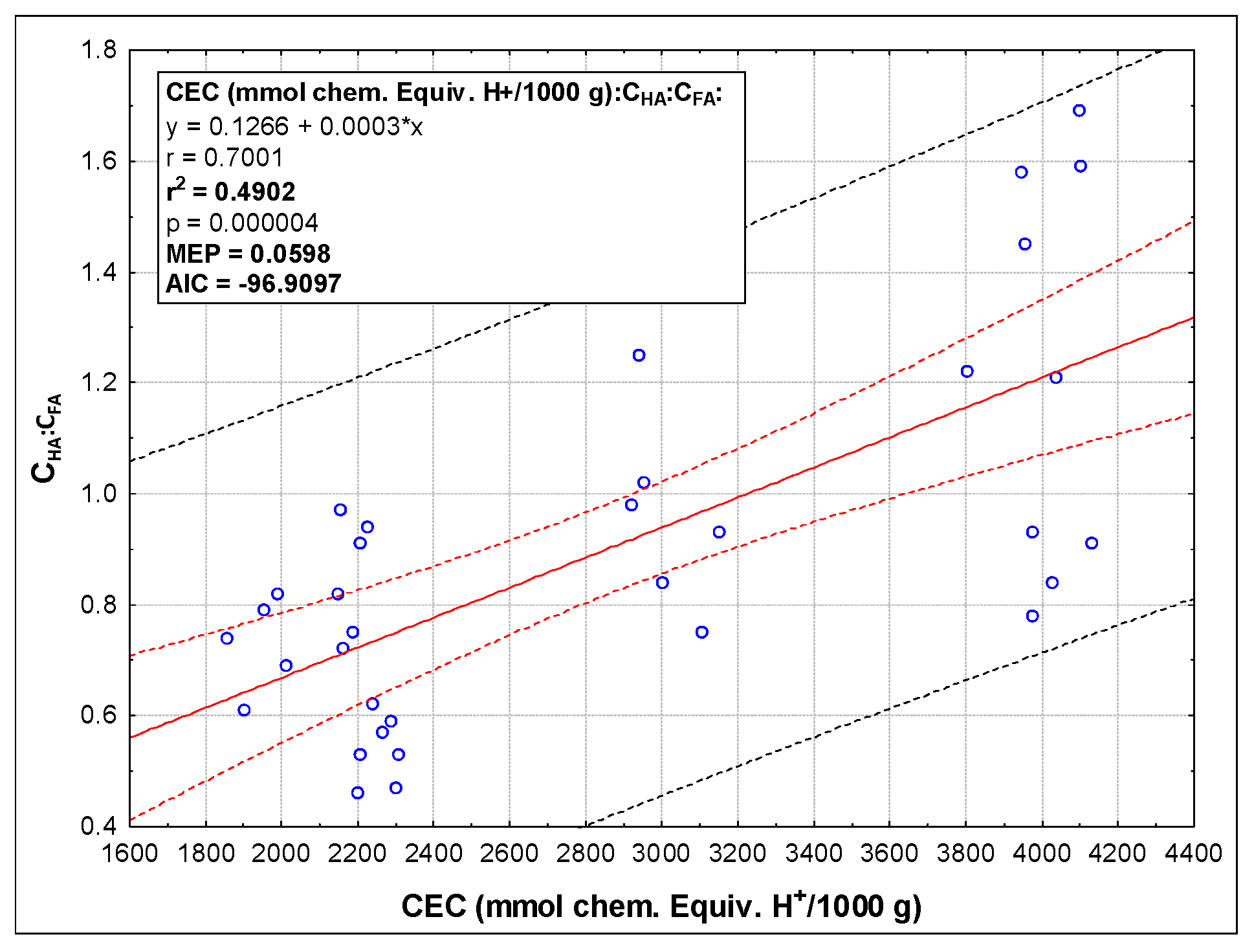
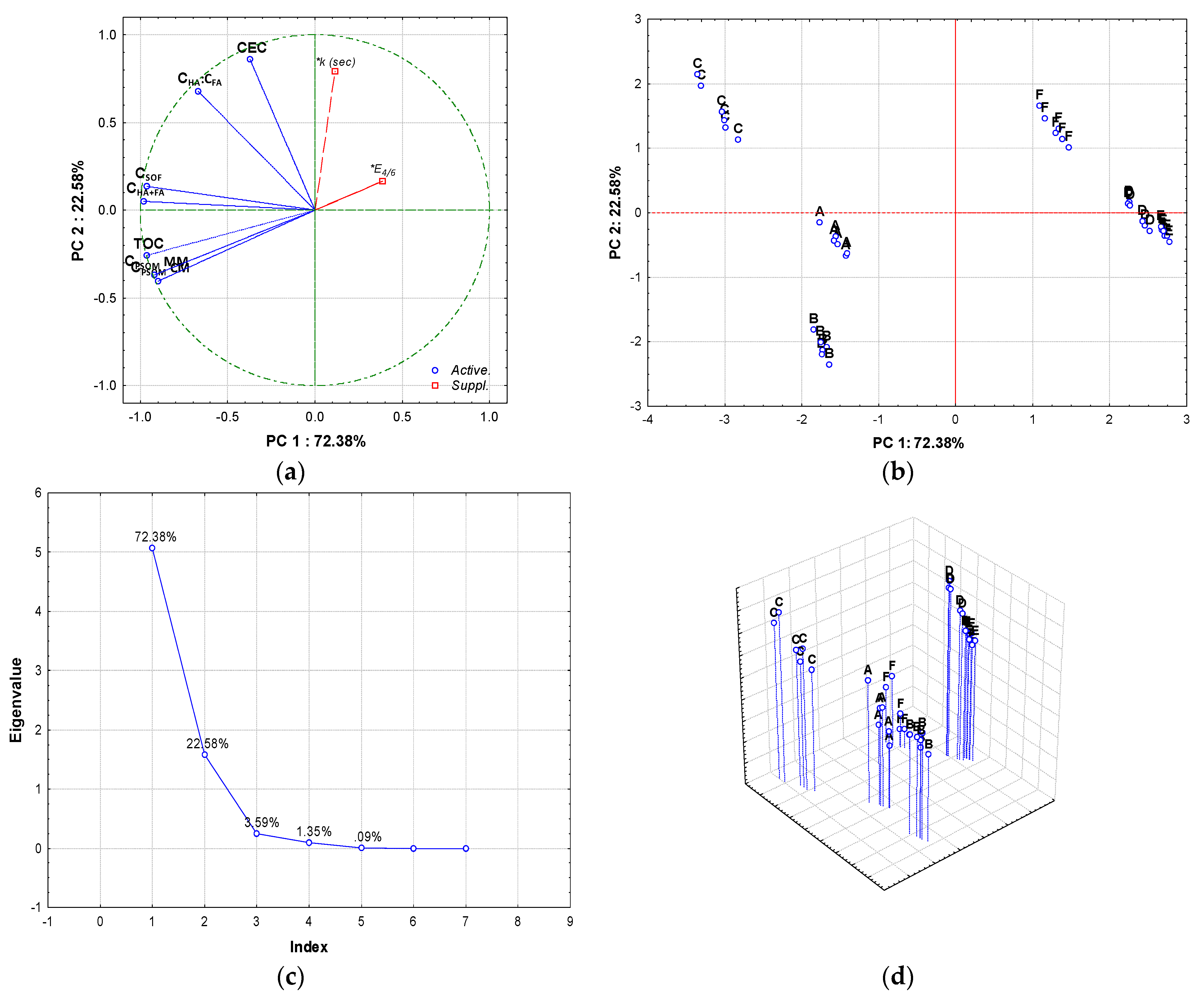
| Parameter | Method | Sample | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| TOC | C-Analyzer | ||||||
| CPSOM | CM | ||||||
| CPSOM | MM | ||||||
| CHA+FA | CM | ||||||
| CSOF | MM | ||||||
| Fraction | Sample | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| 1 | ||||||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| Parameter | Method | Sample | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| CEC | MM | 3012 ± 86c | 1978 ± 93a | 4088 ± 119d | 2196 ± 31b | 2261 ± 43b | 3991 ± 99d |
| CHA:CFA | CM | 0.96 ± 0.16b | 0.75 ± 0.08ab | 1.71 ± 0.19c | 0.82 ± 0.13b | 0.53 ± 0.05a | 0.98 ± 0.17b |
| E4/6 | CM | 14.30 ± 0.57c | 3.55 ± 0.22b | 4.13 ± 0.12b | 2.81 ± 0.26a | 3.59 ± 0.19b | 2.67 ± 0.25a |
| Speed constant k (s) | MM | 3.16 ± 0.08d | 4.49 ± 0.11e | 0.81 ± 0.08a | 2.06 ± 0.12b | 1.88 ± 0.06b | 2.65 ± 0.14c |
| Parameter | Factor Weights | Contributions of a Given Factor to the Communality | |||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 1 | Factor 2 | Communalities | |
| TOC | 0.9832 | 0.1733 | 0.9104 | 0.9511 | 0.9378 |
| CSOF | 0.8182 | 0.5294 | 0.5823 | 0.9100 | 0.9055 |
| CHA+FA | 0.8702 | 0.4587 | 0.6724 | 0.9258 | 0.9220 |
| CPSOM CM | 0.9849 | 0.0110 | 0.9266 | 0.9276 | 0.9213 |
| CPSOM MM | 0.9893 | 0.0545 | 0.9328 | 0.9387 | 0.9286 |
| CEC | –0.0249 | 0.9355 | 0.0000 | 0.5730 | 0.5360 |
| CHA:CFA | 0.3227 | 0.8953 | 0.0775 | 0.8036 | 0.7428 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopecký, M.; Kolář, L.; Perná, K.; Váchalová, R.; Mráz, P.; Konvalina, P.; Murindangabo, Y.T.; Ghorbani, M.; Menšík, L.; Dumbrovský, M. Fractionation of Soil Organic Matter into Labile and Stable Fractions. Agronomy 2022, 12, 73. https://doi.org/10.3390/agronomy12010073
Kopecký M, Kolář L, Perná K, Váchalová R, Mráz P, Konvalina P, Murindangabo YT, Ghorbani M, Menšík L, Dumbrovský M. Fractionation of Soil Organic Matter into Labile and Stable Fractions. Agronomy. 2022; 12(1):73. https://doi.org/10.3390/agronomy12010073
Chicago/Turabian StyleKopecký, Marek, Ladislav Kolář, Kristýna Perná, Radka Váchalová, Petr Mráz, Petr Konvalina, Yves Theoneste Murindangabo, Mohammad Ghorbani, Ladislav Menšík, and Miroslav Dumbrovský. 2022. "Fractionation of Soil Organic Matter into Labile and Stable Fractions" Agronomy 12, no. 1: 73. https://doi.org/10.3390/agronomy12010073
APA StyleKopecký, M., Kolář, L., Perná, K., Váchalová, R., Mráz, P., Konvalina, P., Murindangabo, Y. T., Ghorbani, M., Menšík, L., & Dumbrovský, M. (2022). Fractionation of Soil Organic Matter into Labile and Stable Fractions. Agronomy, 12(1), 73. https://doi.org/10.3390/agronomy12010073











