Control of Substrate Water Availability Using Soil Sensors and Effects of Water Deficit on the Morphology and Physiology of Potted Hebe andersonii
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Culture Conditions
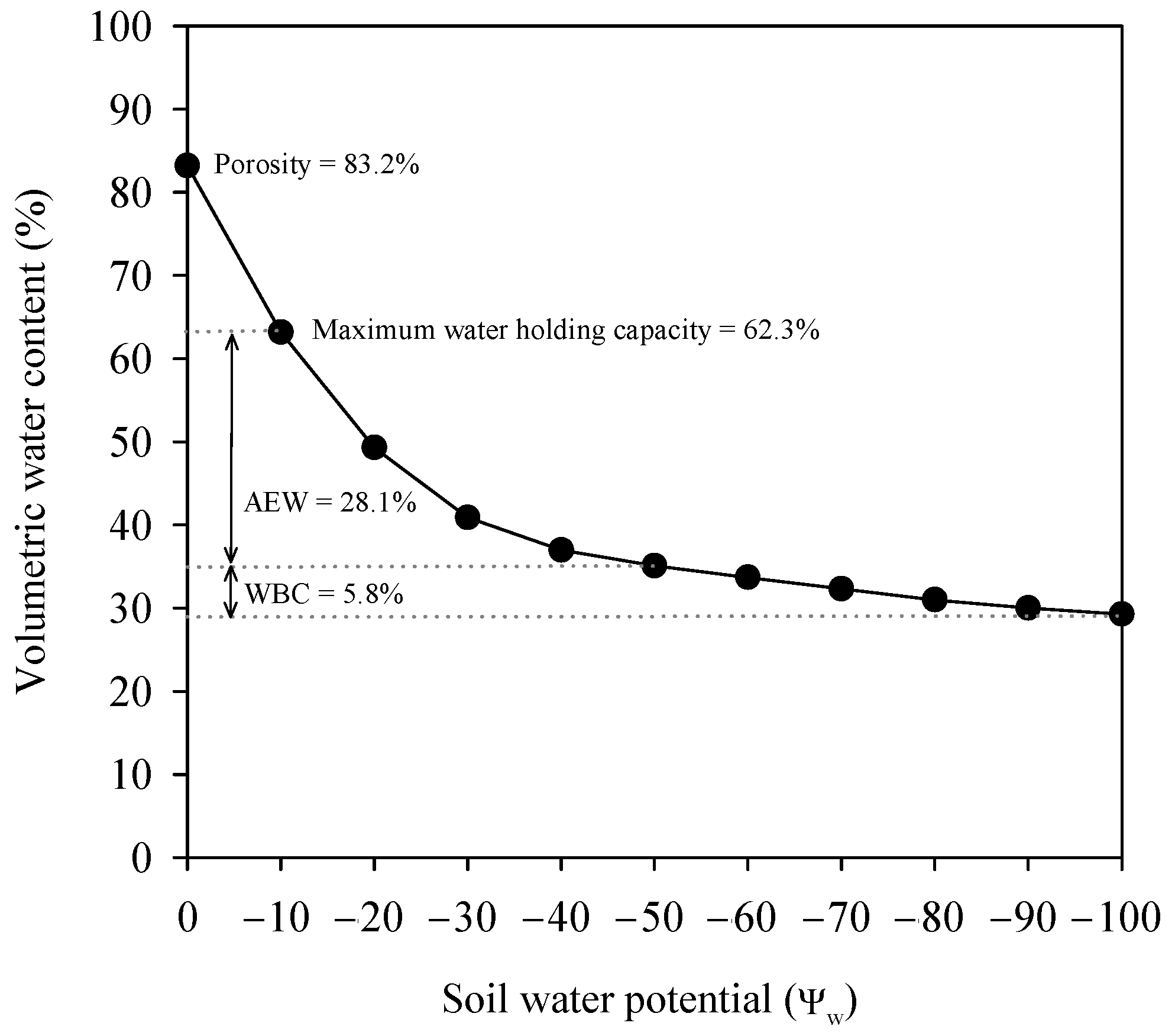
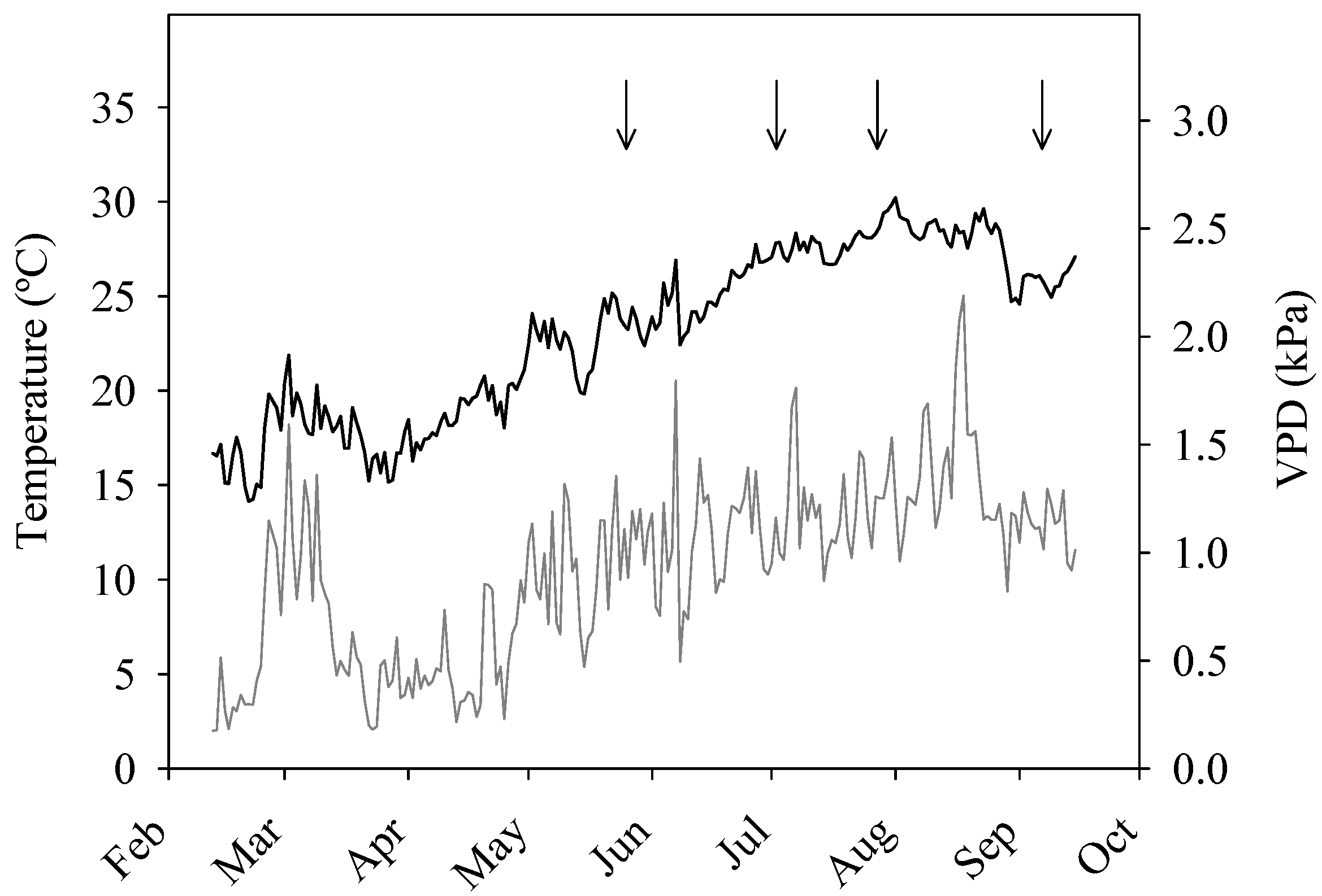
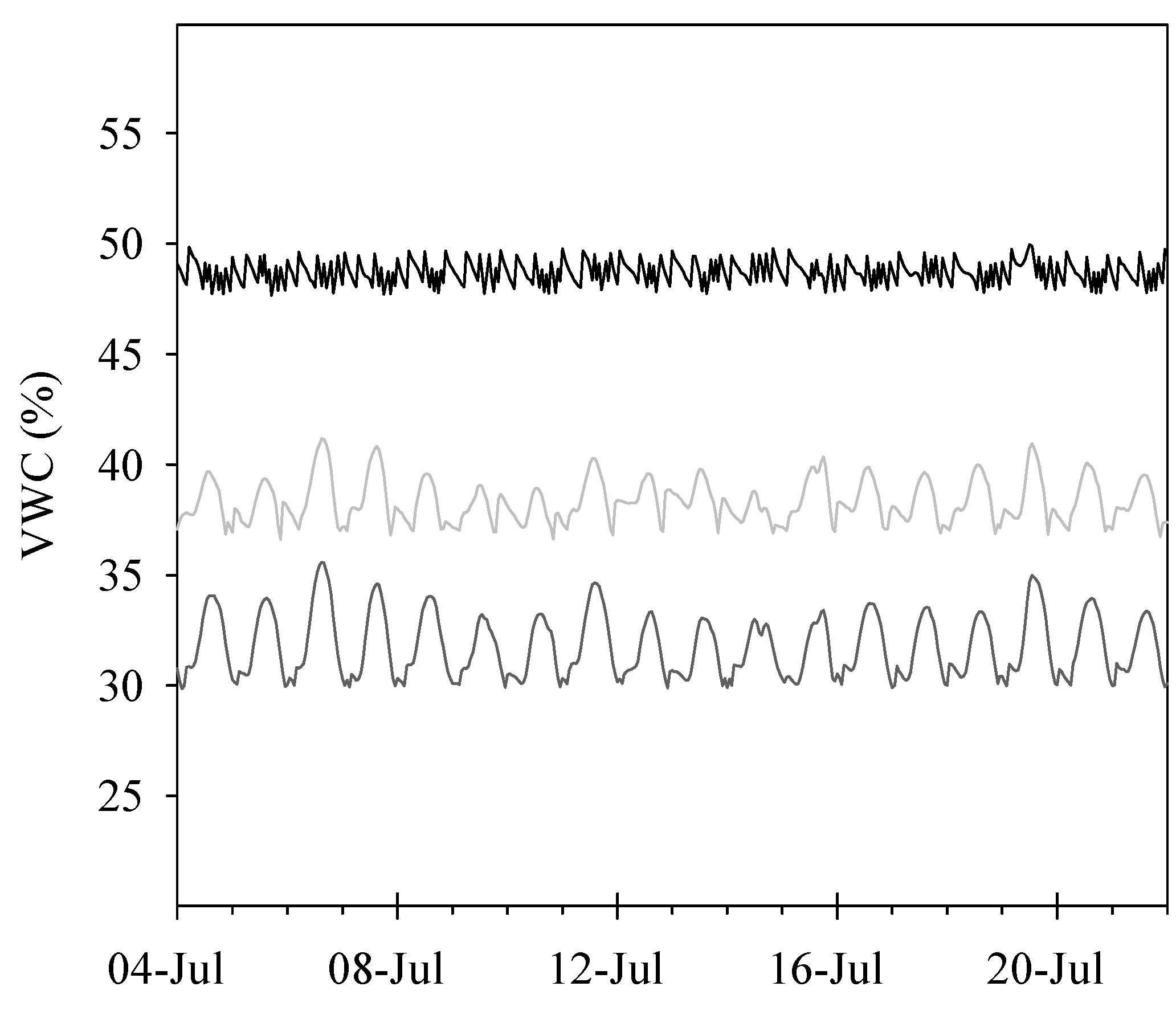
2.2. Irrigation System and Treatments
2.3. Growth, SPAD, and Color
2.4. Substrate EC and Plant Mineral Content
2.5. Plant Water Status
2.6. Gas Exchange and Chlorophyll Fluorescence
2.7. Leaf Cross-Sectional Anatomy
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Water Consumption and Water-Use Efficiency
3.2. Growth and Development
3.3. Leaf Anatomy Changes
3.4. Plant Water Relations
3.5. Leaf Gas Exchange
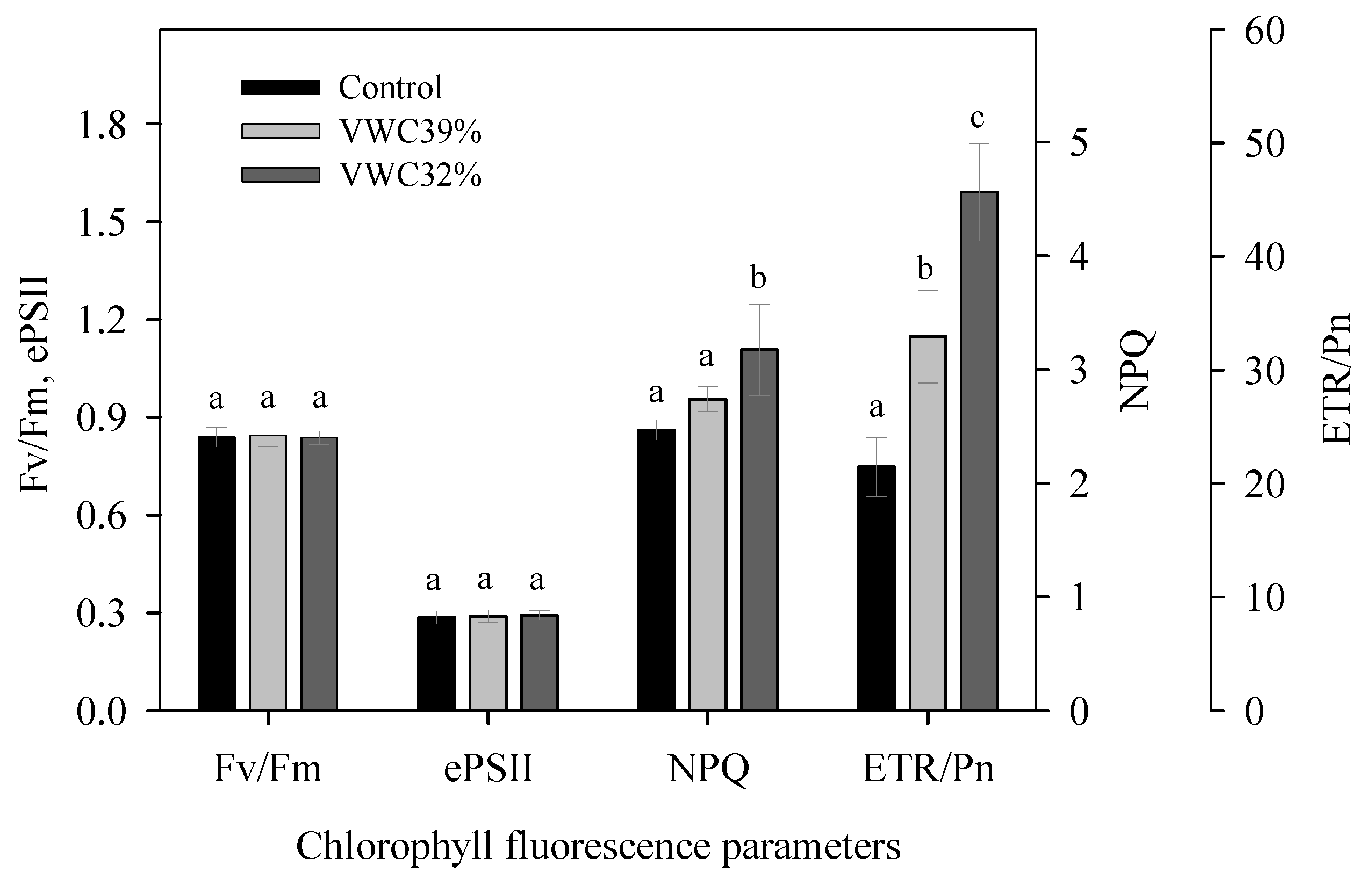
3.6. Chlorophyll Fluorescence
3.7. Substrate EC and Plant Mineral Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhen, S.; Burnett, S.E. Effects of Substrate Volumetric Water Content on English Lavender Morphology and Photosynthesis. HortScience 2015, 50, 909–915. [Google Scholar] [CrossRef]
- Nemali, K.S.; van Iersel, M.W. An Automated System for Controlling Drought Stress and Irrigation in Potted Plants. Sci. Hortic. 2006, 110, 292–297. [Google Scholar] [CrossRef]
- Álvarez, S.; Navarro, A.; Bañón, S.; Sánchez-Blanco, M.J. Regulated Deficit Irrigation in Potted Dianthus Plants: Effects of Severe and Moderate Water Stress on Growth and Physiological Responses. Sci. Hortic. 2009, 122, 579–585. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, Y.; Kumar, P. Diseases of Ornamental Plants and Their Management. In Eco-Friendly Innovative Approaches in Plant Disease Management; International Book Distributors and Publisher: New Delhi, India, 2012; pp. 543–572. ISBN 81-7089-375-5. [Google Scholar]
- Álvarez, S.; Gómez-Bellot, M.J.; Castillo, M.; Bañón, S.; Sánchez-Blanco, M.J. Osmotic and Saline Effect on Growth, Water Relations, and Ion Uptake and Translocation in Phlomis Purpurea Plants. Environ. Exp. Bot. 2012, 78, 138–145. [Google Scholar] [CrossRef]
- Mangiafico, S.S.; Newman, J.; Merhaut, D.J.; Gan, J.; Faber, B.; Wu, L. Nutrients and Pesticides in Stormwater Runoff and Soil Water in Production Nurseries and Citrus and Avocado Groves in California. HortTechnology 2009, 19, 360–367. [Google Scholar] [CrossRef]
- Ucar, Y.; Kazaz, S.; Eraslan, F.; Baydar, H. Effects of Different Irrigation Water and Nitrogen Levels on the Water Use, Rose Flower Yield and Oil Yield of Rosa Damascena. Agric. Water Manag. 2017, 182, 94–102. [Google Scholar] [CrossRef]
- Rouphael, Y.; Raimondi, G.; Caputo, R.; Pascale, S.D. Fertigation Strategies for Improving Water Use Efficiency and Limiting Nutrient Loss in Soilless Hippeastrum Production. HortScience 2016, 51, 684–689. [Google Scholar] [CrossRef]
- Belayneh, B.E.; Lea-Cox, J.D.; Lichtenberg, E. Costs and Benefits of Implementing Sensor-Controlled Irrigation in a Commercial Pot-in-Pot Container Nursery. HortTechnology 2013, 23, 760–769. [Google Scholar] [CrossRef]
- Iersel, M.W.V.; Dove, S.; Kang, J.-G.; Burnett, S.E. Growth and Water Use of Petunia as Affected by Substrate Water Content and Daily Light Integral. HortScience 2010, 45, 277–282. [Google Scholar] [CrossRef]
- Montesano, F.F.; van Iersel, M.W.; Boari, F.; Cantore, V.; D’Amato, G.; Parente, A. Sensor-Based Irrigation Management of Soilless Basil Using a New Smart Irrigation System: Effects of Set-Point on Plant Physiological Responses and Crop Performance. Agric. Water Manag. 2018, 203, 20–29. [Google Scholar] [CrossRef]
- Caser, M.; Lovisolo, C.; Scariot, V. The Influence of Water Stress on Growth, Ecophysiology and Ornamental Quality of Potted Primula Vulgaris ‘Heidy’ Plants. New Insights to Increase Water Use Efficiency in Plant Production. Plant Growth Regul. 2017, 83, 361–373. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ortuño, M.F.; Bañon, S.; Álvarez, S. Deficit Irrigation as a Strategy to Control Growth in Ornamental Plants and Enhance Their Ability to Adapt to Drought Conditions. J. Hortic. Sci. Biotechnol. 2019, 94, 137–150. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Tribulato, A.; Toscano, S.; Di Lorenzo, V.; Romano, D. Effects of Water Stress on Gas Exchange, Water Relations and Leaf Structure in Two Ornamental Shrubs in the Mediterranean Area. Agronomy 2019, 9, 381. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf Physiological and Anatomical Responses of Lantana and Ligustrum Species under Different Water Availability. Plant Physiol. Biochem. 2018, 127, 380–392. [Google Scholar] [CrossRef]
- Rafi, Z.N.; Kazemi, F.; Tehranifar, A. Morpho-Physiological and Biochemical Responses of Four Ornamental Herbaceous Species to Water Stress. Acta Physiol. Plant 2018, 41, 7. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C.; Rouphael, Y. Biochemical, Physiological, and Molecular Aspects of Ornamental Plants Adaptation to Deficit Irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Fascella, G.; Maggiore, P.; Demma Carà, M.; Zizzo, G.V. Growth and Flowering Response of Euphorbia × Lomi Poysean Cultivars under Two Irrigation Regimes. Acta Hortic. 2011, 893, 939–943. [Google Scholar] [CrossRef]
- Álvarez, S.; Gómez-Bellot, M.J.; Acosta-Motos, J.R.; Sánchez-Blanco, M.J. Application of Deficit Irrigation in Phillyrea Angustifolia for Landscaping Purposes. Agric. Water Manag. 2019, 218, 193–202. [Google Scholar] [CrossRef]
- Castillo-Campohermoso, M.A.; Broetto, F.; Rodríguez-Hernández, A.M.; de Abril Alexandra Soriano-Melgar, L.; Mounzer, O.; Sánchez-Blanco, M.J. Plant-Available Water, Stem Diameter Variations, Chlorophyll Fluorescence, and Ion Content in Pistacia Lentiscus under Salinity Stress. Terra Latinoam. 2020, 38, 103–111. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Niu, G.; Deng, C.; Wang, Y.; Gardea-Torresdey, J. Growth, Gas Exchange, and Mineral Nutrients of Ornamental Grasses Irrigated with Saline Water. HortScience 2019, 54, 1840–1846. [Google Scholar] [CrossRef]
- Steudle, E. Water Uptake by Roots: Effects of Water Deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Rengel, Z. Chapter 12—Nutrient Availability in Soils. In Marschner’s Mineral Nutrition of Higher Plants (Third Edition); Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 315–330. ISBN 978-0-12-384905-2. [Google Scholar]
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long Term Responses and Adaptive Strategies of Pistacia Lentiscus under Moderate and Severe Deficit Irrigation and Salinity: Osmotic and Elastic Adjustment, Growth, Ion Uptake and Photosynthetic Activity. Agric. Water Manag. 2018, 202, 253–262. [Google Scholar] [CrossRef]
- Demetriou, G.; Neonaki, C.; Navakoudis, E.; Kotzabasis, K. Salt Stress Impact on the Molecular Structure and Function of the Photosynthetic Apparatus—The Protective Role of Polyamines. Biochim. Biophys. Acta—Bioenerg. 2007, 1767, 272–280. [Google Scholar] [CrossRef]
- Warrington, I.J.; Southward, R.C. Seasonal Frost Tolerance of Hebe Species and Cultivars. N. Z. J. Crop Hortic. Sci. 1995, 23, 437–445. [Google Scholar] [CrossRef][Green Version]
- Kristensen, L.N. The genus Hebe: A botanical report. Tidsskr. Planteavls 1989, 93, 369–437. [Google Scholar]
- de Boodt, M.; Verdonck, O.; Cappaert, I. Determination and Study of the Water Availability of Substrates for Ornamental Plant Growing. Acta Hortic. 1974, 1, 51–58. [Google Scholar] [CrossRef]
- Arguedas Rodríguez, F.R. Calibrating Capacitance Sensors to Estimate Water Content, Matric Potential, and Electrical Conductivity in Soilless Substrates. Master’s Thesis, University of Maryland, College Park, MD, USA, 2009. [Google Scholar]
- Steiner, J.L.; Howell, T.A.; Schneider, A.D. Lysimetric Evaluation of Daily Potential Evapotranspiration Models for Grain Sorghum. Agron. J. 1991, 83, 240–247. [Google Scholar] [CrossRef]
- Gaskin, G.J.; Miller, J.D. Measurement of Soil Water Content Using a Simplified Impedance Measuring Technique. J. Agric. Eng. Res. 1996, 63, 153–159. [Google Scholar] [CrossRef]
- Valdés, R.; Miralles, J.; Ochoa, J.; Sánchez-Blanco, M.J.; Bañón Arias, S. Saline Reclaimed Wastewater Can Be Used to Produce Potted Weeping Fig (Ficus Benjamina L.) with Minimal Effects on Plant Quality. Span. J. Agric. Res. 2012, 10, 1167. [Google Scholar] [CrossRef]
- Miralles, J.; van Iersel, M.W. A Calibrated Time Domain Transmissometry Soil Moisture Sensor Can Be Used for Precise Automated Irrigation of Container-Grown Plants. HortScience 2011, 46, 889–894. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Sonneveld, C.; van den Ende, J. Soil Analysis by Means of a 1:2 Volume Extract. Plant Soil 1971, 35, 505–516. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Savi, T.; Bacaro, G.; Nardini, A. A Simplified Framework for Fast and Reliable Measurement of Leaf Turgor Loss Point. Plant Physiol. Biochem. 2019, 139, 395–399. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Nortes, P.A.; Sánchez-Blanco, M.J.; Ortuño, M.F. Sensitivity of Thermal Imaging and Infrared Thermometry to Detect Water Status Changes in Euonymus Japonica Plants Irrigated with Saline Reclaimed Water. Biosyst. Eng. 2015, 133, 21–32. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of Arbuscular Mycorrhizae on Photosynthesis and Water Status of Maize Plants under Salt Stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 87–131. ISBN 978-81-322-0807-5. [Google Scholar]
- Burnett, S.E.; van Iersel, M.W. Morphology and Irrigation Efficiency of Gaura Lindheimeri Grown with Capacitance Sensor-Controlled Irrigation. HortScience 2008, 43, 1555–1560. [Google Scholar] [CrossRef]
- Garland, K.F.; Burnett, S.E.; Day, M.E.; van Iersel, M.W. Influence of Substrate Water Content and Daily Light Integral on Photosynthesis, Water Use Efficiency, and Morphology of Heuchera Americana. J. Am. Soc. Hort. Sci. 2012, 137, 57–67. [Google Scholar] [CrossRef]
- Paço, T.A.; Ferreira, M.I.; Rosa, R.D.; Paredes, P.; Rodrigues, G.C.; Conceição, N.; Pacheco, C.A.; Pereira, L.S. The Dual Crop Coefficient Approach Using a Density Factor to Simulate the Evapotranspiration of a Peach Orchard: SIMDualKc Model versus Eddy Covariance Measurements. Irrig. Sci. 2012, 30, 115–126. [Google Scholar] [CrossRef]
- Kool, D.; Agam, N.; Lazarovitch, N.; Heitman, J.L.; Sauer, T.J.; Ben-Gal, A. A Review of Approaches for Evapotranspiration Partitioning. Agric. For. Meteorol. 2014, 184, 56–70. [Google Scholar] [CrossRef]
- Navarro, A.; Bañon, S.; Olmos, E.; Sánchez-Blanco, M.J. Effects of Sodium Chloride on Water Potential Components, Hydraulic Conductivity, Gas Exchange and Leaf Ultrastructure of Arbutus Unedo Plants. Plant Sci. 2007, 172, 473–480. [Google Scholar] [CrossRef]
- Waisel, Y.; Eshel, A.; Kafkafi, U. Plant Roots: The Hidden Half, 3rd ed.; Books in soils, plants, and the environment; M. Dekker: New York, NY, USA; Basel, Switzerland, 2002; ISBN 978-0-8247-0631-9. [Google Scholar]
- Chaves, M.M.; Oliveira, M.M. Mechanisms Underlying Plant Resilience to Water Deficits: Prospects for Water-Saving Agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, M.; Warrington, D.N. Growth and Transpiration of Maize and Winter Wheat in Response to Water Deficits in Pots and Plots. Environ. Exp. Bot. 2011, 71, 65–71. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, D.; Chen, X.; Zhang, J. A Simple Method for Estimating Field Crop Evapotranspiration from Pot Experiments. Water 2018, 10, 1823. [Google Scholar] [CrossRef]
- Jones, P.; Jones, J.W.; Allen, L.H., Jr. Seasonal Carbon and Water Balances of Soybeans Grown Under Stress Treatments in Sunlit Chambers. Trans. ASAE 1985, 28, 2021–2028. [Google Scholar] [CrossRef]
- Fernández-García, N.; Olmos, E.; Bardisi, E.; García-De la Garma, J.; López-Berenguer, C.; Rubio-Asensio, J.S. Intrinsic Water Use Efficiency Controls the Adaptation to High Salinity in a Semi-Arid Adapted Plant, Henna (Lawsonia Inermis L.). J. Plant Physiol. 2014, 171, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Sánchez-Blanco, M.J. Changes in Growth Rate, Root Morphology and Water Use Efficiency of Potted Callistemon Citrinus Plants in Response to Different Levels of Water Deficit. Sci. Hortic. 2013, 156, 54–62. [Google Scholar] [CrossRef]
- Liu, F.; Stützel, H. Biomass Partitioning, Specific Leaf Area, and Water Use Efficiency of Vegetable Amaranth (Amaranthus Spp.) in Response to Drought Stress. Sci. Hortic. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- del Amor, F.M.; Marcelis, L.F.M. Differential Effect of Transpiration and Ca Supply on Growth and Ca Concentration of Tomato Plants. Sci. Hortic. 2006, 111, 17–23. [Google Scholar] [CrossRef]
- Dwyer, J.M.; Hobbs, R.J.; Mayfield, M.M. Specific Leaf Area Responses to Environmental Gradients through Space and Time. Ecology 2014, 95, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. (Eds.) Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2010; ISBN 978-0-87893-565-9. [Google Scholar]
- Álvarez, S.; Bañón, S.; Sánchez-Blanco, M.J. Regulated Deficit Irrigation in Different Phenological Stages of Potted Geranium Plants: Water Consumption, Water Relations and Ornamental Quality. Acta Physiol. Plant. 2012, 35, 1257–1267. [Google Scholar] [CrossRef]
- Alem, P.; Thomas, P.A.; van Iersel, M.W. Controlled Water Deficit as an Alternative to Plant Growth Retardants for Regulation of Poinsettia Stem Elongation. HortScience 2015, 50, 565–569. [Google Scholar] [CrossRef]
- Ammar, A.; Ben Aissa, I.; Mars, M.; Gouiaa, M. Comparative Physiological Behavior of Fig (Ficus Carica L.) Cultivars in Response to Water Stress and Recovery. Sci. Hortic. 2020, 260, 108881. [Google Scholar] [CrossRef]
- Giovannelli, A.; Deslauriers, A.; Fragnelli, G.; Scaletti, L.; Castro, G.; Rossi, S.; Crivellaro, A. Evaluation of Drought Response of Two Poplar Clones (Populus x Canadensis Monch “I-214” and P. Deltoides Marsh. ’Dvina’) through High Resolution Analysis of Stem Growth. J. Exp. Bot. 2007, 58, 2673–2683. [Google Scholar] [CrossRef]
- Gu, M.; Robbins, J.A.; Rom, C.R. The Role of Ethylene in Water-Deficit Stress Responses in Betula Papyrifera Marsh. HortScience 2007, 42, 1392–1395. [Google Scholar] [CrossRef]
- McMichael, B.L.; Jordan, W.R.; Powell, R.D. Abscission Processes in Cotton: Induction by Plant Water Deficit1. Agron. J. 1973, 65, 202–204. [Google Scholar] [CrossRef]
- Blanusa, T.; Vysini, E.; Cameron, R.W.F. Growth and Flowering of Petunia and Impatiens: Effects of Competition and Reduced Water Content Within a Container. HortScience 2009, 44, 1302–1307. [Google Scholar] [CrossRef]
- Fitter, A.; Hay, R. Introduction. In Environmental Physiology of Plants; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1–20. [Google Scholar]
- Giuliani, R.; Koteyeva, N.; Voznesenskaya, E.; Evans, M.A.; Cousins, A.B.; Edwards, G.E. Coordination of Leaf Photosynthesis, Transpiration, and Structural Traits in Rice and Wild Relatives (Genus Oryza). Plant Physiol. 2013, 162, 1632–1651. [Google Scholar] [CrossRef]
- Jenks, M.A.; Hasegawa, P.M. (Eds.) Plant Abiotic Stress; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-99411-5. [Google Scholar]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in Pigment Composition, Photosynthetic Rates and Chlorophyll Fluorescence Images of Sun and Shade Leaves of Four Tree Species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Acosta-Motos, J.-R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and Biochemical Mechanisms of the Ornamental Eugenia Myrtifolia L. Plants for Coping with NaCl Stress and Recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-López, A.; Barrera, G.M.; Suzán-Azpiri, H.; Bolaños, M.Q. Chapter 3—Photosynthesis. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Thorston, UK, 2019; pp. 47–72. ISBN 978-0-12-813278-4. [Google Scholar]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Zollinger, N.; Kjelgren, R.; Cerny-Koenig, T.; Kopp, K.; Koenig, R. Drought Responses of Six Ornamental Herbaceous Perennials. Sci. Hortic. 2006, 109, 267–274. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-Analysis: Drivers of Plant Drought Tolerance. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Maroco, J.P.; Pereira, J.S.; Chaves, M.M. Stomatal Responses to Leaf-to-Air Vapour Pressure Deficit in Sahelian Species. Aust. J. Plant Physiol. 1997, 24, 381–387. [Google Scholar] [CrossRef]
- Medrano, H. Regulation of Photosynthesis of C3 Plants in Response to Progressive Drought: Stomatal Conductance as a Reference Parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Steduto, P.; Hsiao, T.C.; Fereres, E. On the Conservative Behavior of Biomass Water Productivity. Irrig. Sci. 2007, 25, 189–207. [Google Scholar] [CrossRef]
- Yu, D.J.; Kim, S.J.; Lee, H.J. Stomatal and Non-Stomatal Limitations to Photosynthesis in Field-Grown Grapevine Cultivars. Biol. Plant 2009, 53, 133–137. [Google Scholar] [CrossRef]
- Silim, S.N.; Ryan, N.; Kubien, D.S. Temperature Responses of Photosynthesis and Respiration in Populus Balsamifera L.: Acclimation versus Adaptation. Photosynth. Res. 2010, 104, 19–30. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of Decreased Photosynthetic Rate and Metabolic Capacity in Water-Deficient Leaf Cells: A Critical Evaluation of Mechanisms and Integration of Processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Singal, H.R.; Sheoran, I.S.; Singh, R. Effect of Water Stress on Photosynthesis and in Vitro Activities of the PCR Cycle Enzymes in Pigeonpea (Cajanus Cajan L.). Photosynth. Res. 1985, 7, 69–76. [Google Scholar] [CrossRef]
- D’Ambrosio, N.; Arena, C.; De Santo, A.V. Temperature Response of Photosynthesis, Excitation Energy Dissipation and Alternative Electron Sinks to Carbon Assimilation in Beta Vulgaris L. Environ. Exp. Bot. 2006, 55, 248–257. [Google Scholar] [CrossRef]
- Bañón, S.; Miralles, J.; Ochoa, J.; Franco, J.A.; Sánchez-Blanco, M.J. Effects of Diluted and Undiluted Treated Wastewater on the Growth, Physiological Aspects and Visual Quality of Potted Lantana and Polygala Plants. Sci. Hortic. 2011, 129, 869–876. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy1. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Bañón, S.; Conejero, W.; Sánchez-Blanco, M.J. Ornamental Characters, Ion Accumulation and Water Status in Arbutus Unedo Seedlings Irrigated with Saline Water and Subsequent Relief and Transplanting. Environ. Exp. Bot. 2008, 62, 364–370. [Google Scholar] [CrossRef]
- Cassaniti, C.; Leonardi, C.; Flowers, T.J. The Effects of Sodium Chloride on Ornamental Shrubs. Sci. Hortic. 2009, 122, 586–593. [Google Scholar] [CrossRef]
- Camberato, D.M.; Lopez, R.G.; Mickelbart, M.V. PH and Electrical Conductivity Measurements in Soilless Substrates; Purdue University Libraries: West Lafayette, IN, USA, 2009; pp. 1–7. [Google Scholar]
- Alam, S.M. Nutrient Uptake by Plants Under Stress Conditions. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 285–313. [Google Scholar]
- Lawlor, D.W.; Lemaire, G.; Gastal, F. Nitrogen, Plant Growth and Crop Yield. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 343–367. [Google Scholar]
- Lester, G.E.; Jifon, J.L.; Makus, D.J. Impact of Potassium Nutrition on Food Quality of Fruits and Vegetables: A Condensed and Concise Review of the Literature. Better Crops 2010, 94, 18–21. [Google Scholar]
- Yermiyahu, U.; Israeli, L.; David, D.R.; Faingold, I.; Elad, Y. Higher Potassium Concentration in Shoots Reduces Gray Mold in Sweet Basil. Phytopathology 2015, 105, 1059–1068. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Álvarez, S.; Castillo, M.; Bañón, S.; Ortuño, M.F.; Sánchez-Blanco, M.J. Water Relations, Nutrient Content and Developmental Responses of Euonymus Plants Irrigated with Water of Different Degrees of Salinity and Quality. J. Plant Res. 2013, 126, 567–576. [Google Scholar] [CrossRef]


| Ion | Concentration (mg L−1) |
|---|---|
| NO3− | 280.23 |
| NH4+ | 1.89 |
| H2PO4− | 17.09 |
| K+ | 56.06 |
| Ca2+ | 125.72 |
| Mg2+ | 57.18 |
| SO42− | 258.99 |
| Cl− | 245.28 |
| Na+ | 161.37 |
| HCO3− | 105.11 |
| B3+ | 0.61 |
| Mn2+ | 0.45 |
| Fe3+ | 0.07 |
| Zn2+ | 0.07 |
| Cu2+ | 0.09 |
| Control | VWC39% | VWC32% | |
|---|---|---|---|
| Water consumption (L pot−1) * | 37.95 | 27.70 | 16.40 |
| ET (mL pot−1 day−1) * | 249.67 | 182.24 | 107.89 |
| WUE (g L−1) | 1.70 a | 1.83 b | 2.47 c |
| Control | VWC39% | VWC32% | |
|---|---|---|---|
| Plant dry weight (g) | 64.42 c | 50.6 b | 40.5 a |
| Leaf dry weight (g) | 26.72 c | 23.3 b | 20.3 a |
| Stem dry weight (g) | 18.27 c | 14.8 b | 9.97 a |
| Root dry weight (g) | 19.43 c | 12.49 b | 10.22 a |
| Root-to-shoot ratio | 0.423 b | 0.328 a | 0.338 a |
| Leaf area (dm2) | 28.32 c | 22.45 b | 13.5 a |
| SLA * (cm2 g−1) | 105.99 c | 96.35 b | 66.5 a |
| SPAD ** | 52.11 b | 47.78 ab | 43.43 a |
| Plant height (cm) | 30.83 b | 31.43 b | 26.33 a |
| Plant width (cm) | 39.83 c | 37.54 b | 33.58 a |
| No. of stems | 10.97 b | 10.03 ab | 9.67 a |
| No. of flowerings stems | 3.33 b | 3.01 b | 1.23 a |
| Control | VWC39% | VWC32% | |
|---|---|---|---|
| Total leaf thickness (µm) | 474.88 a | 486.89 ab | 506.2 b |
| Upper cuticle (%) | 1.67 a | 1.69 a | 1.58 a |
| Upper epidermis (%) | 3.46 a | 3.47 a | 3.53 a |
| Palisade parenchyma (%) | 51.83 a | 52.19 ab | 54.37 b |
| Spongy parenchyma (%) | 40.34 b | 40.09 b | 37.93 a |
| Lower epidermis (%) | 1.17 a | 1.44 b | 1.47 b |
| Lower cuticle (%) | 1.52 b | 1.12 a | 1.12 a |
| Element (mg g−1) | Plant Organ | Control | VWC39% | VWC32% |
|---|---|---|---|---|
| N | Leaf | 19.19 a | 19.74 a | 20.68 a |
| Stem | 13.57 a | 18.37 b | 19.49 b | |
| Root | 11.8 a | 15.23 b | 16.2 b | |
| P | Leaf | 2.03 a | 2.25 a | 2.14 a |
| Stem | 2.19 a | 2.78 b | 3.43 c | |
| Root | 0.78 a | 0.73 a | 0.81 a | |
| K+ | Leaf | 22.09 a | 26.55 b | 28.63 c |
| Stem | 6.77 a | 9.09 b | 16.6 c | |
| Root | 3.72 a | 3.21 a | 5.93 b | |
| Ca2+ | Leaf | 13.45 a | 12.73 a | 12.27 a |
| Stem | 3.4 a | 3.74 a | 4.07 a | |
| Root | 8.02 a | 8.71 a | 9.43 a | |
| Mg2+ | Leaf | 3.28 a | 3.21 a | 3.45 a |
| Stem | 2.74 a | 3.13 a | 3.23 a | |
| Root | 3.36 b | 2.54 a | 2.81 ab | |
| Na+ | Leaf | 2.18 a | 3.31 b | 5.51 c |
| Stem | 1.92 a | 3.81 b | 8.49 c | |
| Root | 12.42 b | 9.01 a | 9.67 a | |
| Cl− | Leaf | 11.01 a | 19.11 b | 30.41 c |
| Stem | 4.47 a | 8.94 b | 18.31 c | |
| Root | 24.69 c | 20.46 b | 17.19 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañón, D.; Lorente, B.; Bañón, S.; Ortuño, M.F.; Sánchez-Blanco, M.J.; Alarcón, J.J. Control of Substrate Water Availability Using Soil Sensors and Effects of Water Deficit on the Morphology and Physiology of Potted Hebe andersonii. Agronomy 2022, 12, 206. https://doi.org/10.3390/agronomy12010206
Bañón D, Lorente B, Bañón S, Ortuño MF, Sánchez-Blanco MJ, Alarcón JJ. Control of Substrate Water Availability Using Soil Sensors and Effects of Water Deficit on the Morphology and Physiology of Potted Hebe andersonii. Agronomy. 2022; 12(1):206. https://doi.org/10.3390/agronomy12010206
Chicago/Turabian StyleBañón, Daniel, Beatriz Lorente, Sebastián Bañón, María Fernanda Ortuño, María Jesús Sánchez-Blanco, and Juan José Alarcón. 2022. "Control of Substrate Water Availability Using Soil Sensors and Effects of Water Deficit on the Morphology and Physiology of Potted Hebe andersonii" Agronomy 12, no. 1: 206. https://doi.org/10.3390/agronomy12010206
APA StyleBañón, D., Lorente, B., Bañón, S., Ortuño, M. F., Sánchez-Blanco, M. J., & Alarcón, J. J. (2022). Control of Substrate Water Availability Using Soil Sensors and Effects of Water Deficit on the Morphology and Physiology of Potted Hebe andersonii. Agronomy, 12(1), 206. https://doi.org/10.3390/agronomy12010206






