Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives
Abstract
1. Introduction
2. Storage of Orthodox Seeds in Gene Banks
3. Germination-Related Physiology
4. Oxidative Stress in Plants
4.1. Biochemical Effects of Ageing and Oxidative Stress in Seeds
4.2. Oxidation of Major Biological Molecules
4.2.1. Lipids
4.2.2. Proteins
- (1)
- (2)
- methionine (Met) to produce methionine sulfoxide. This stage is also reversible, but the final stage of Met oxidation to sulfone seems to be damaging and irreversible [100]; and
- (3)
- most of the other amino acids, especially arginine (Arg), lysine (Lys), proline (Pro), and threonine (Thr) form stable aldehydes or ketones (carbonyls) in an irreversible reaction [75,100,112] that is not particular to any oxidants [113,115]. Thus, the extent of reactive oxidant-induced modification of proteins is generically measured as protein carbonyl [113,116,117].
4.2.3. Carbohydrates
4.2.4. Polynucleotides
4.3. Cellular Generation of Reactive Oxygen Species (ROS)
4.3.1. The Dual Capacity of ROS
4.3.2. ROS Scavenging in Plant Cells
- (1)
- Superoxide dismutases (SODs): These are ubiquitous metalloenzymes involved in essential defence against superoxide [149] via a redox cycle where the active site metal is deoxidised by one O2•− radical and re-oxidised by another [150]. The three (3) forms of identified SOD, defined by the active site metals, are iron-SOD, copper and zinc-SOD, and manganese-SOD [151]. SOD catalyses the dismutation of O2•− to O2 and H2O2 [147], which can then be broken down by other essential enzymes—the catalases.
- (2)
- Catalases (CATs): These are peroxisome-localised, heme-group-containing enzymes [152], though their presence has also been reported in mitochondria [153]. They are involved in the breakdown of H2O2 to H2O and O2 [147]. They are recognised as essential defence enzymes against ROS-induced oxidative stress [154,155]. In addition, they are involved in plant defence and metabolism as well as the perception of cellular signals [156].
- (3)
- Glutathione reductases (GRs): These flavoproteins occur mostly in the chloroplasts but have also been reported in the cytosol, mitochondria, and peroxisomes [157]. They are extremely specific and are involved in the reduction of oxidised glutathione (GSSG) back to the reduced form (GSH) using NADPH as the reductant [147], thereby sustaining a high GSH to GSSG ratio [158]. They sustain the reduced state of GSH through the ascorbate–glutathione cycle and are involved in maintaining the –SH group and act as a substrate for glutathione-S-transferases. In conjunction with superoxide dismutase and ascorbate–glutathione pathway enzymes, GRs constitute an important ROS scavenger [158]. They have been demonstrated to enhance oxidative stress tolerance in transgenic Nicotiana tabacum [157].
- (4)
- Ascorbate peroxidases (APXs): These heme-containing enzymes are also involved in the decomposition of H2O2 using ascorbate as a reductant [159]. Different isoforms have been reported in the cytosol, chloroplast, mitochondria, thylakoid, stroma, and peroxisome [152,159,160]. Increased APX activity has been reported under abiotic stress such as light [161], drought and heat [162], and heavy metal contamination [163].
- (5)
- Glutathione peroxidases (GPXs): These are non-heme-containing antioxidant enzymes [159] using glutathione as a reductant [164]. They are ubiquitous and predicted to be localised in cytosol, chloroplast, endoplasmic reticulum, mitochondria and plastids [165]. They have been demonstrated to play a role in lipid hydroperoxide detoxification, plant defence, and response to biotic [166] and abiotic stresses [164].
- (1)
- Ascorbic acid (AA): AA is known to be abundant and one of the most potent antioxidants involved in ROS (e.g., O2•− [168]) detoxification and prevention [148,149]. This water-soluble antioxidant is found in all cellular compartments and at higher concentrations in photosynthetic cells [46]. AA is mostly present in its reduced form [169]. It is crucial for the maintenance of membrane structure and capable of completely preventing lipid peroxidation initiation, scavenging ROS, such as singlet oxygen, hydroperoxyl radicals, superoxide, and peroxynitrite, and protects other substrates from oxidative impairment [148,149]. In addition, AA has been documented to be involved in ROS scavenging by controlling redox balance in cells [170]. AA has been reported to enhance abiotic stress tolerance [169,171]. AA is involved in the modulation of the synthesis of tocopherol [172] and the regulation of plant defence responses over and above developmental processes [173].
- (2)
- Glutathione (GSH): In addition to AA, GSH is another non-enzymic antioxidant involved in the detoxification of ROS [168]. Both GSH and AA are involved in the ascorbate–glutathione cycle, where ascorbate peroxidase plays a role in the direct removal of H2O2 [174], singlet oxygen [148] and hydroxyl radical [175]. AA is most abundant in its reduced and active form and found in various cellular compartments, including the cytosol, mitochondria, endoplasmic reticulum, vacuole, peroxisomes, apoplast, and chloroplasts [176]. GSH provides a substrate for several reactions forming oxidised glutathione (GSSG). Balanced GSH to GSSG levels are key to maintaining a redox state in cells [177]. A decline in GSH levels during stress often leads to an imbalanced redox state, thereby causing system deterioration [178]. Heightened biosynthesis of GSH in chloroplasts, instead of protecting cells, may cause oxidative impairment, perhaps by adjusting the general redox state of chloroplasts [179]. It has been reported that the ratio of reduced to oxidised antioxidants can signal the modulation of ROS-scavenging mechanisms [46,51]. GSH plays a major role in protection from oxidative attack on biological membranes [149] and participates in various physiological events, including sulphate transport regulation, xenobiotics detoxification, and signal transduction [148]. Heightened GSH level has been linked with plants’ ability to withstand oxidative stress [180].
- (3)
- Tocopherol (vitamin E): This lipophilic phenolic compound exists in eight similarly potent forms as alpha(α)-, beta(β)-, gamma(γ)- and delta(δ)- tocotrienols and tocopherols [181]. It forms part of the biological membrane, playing both non-antioxidant and radical-chain-breaker functions [181]. It is regarded as a potential ROS and lipid radical scavenger [182]. Reduction in tocopherol levels following seed ageing suggests that it is involved in protection against oxidative stress-induced impairments [183], thus making it a useful indicator of seed deterioration [149]. Its synthetic analogue, trolox, has also been reported to be similarly capable of preventing oxidative impairment [184]. Trolox has some advantages in being moderately soluble in water [185]. Unlike α-tocopherol, trolox may be integrated directly into both lipid and water parts of cells [184], thus making it suitable for conducting studies involving both living systems and model systems [185]. The antioxidant power of trolox has been reported to be much more than that of α-tocopherol [184]. Other synthesised analogues include Vitamin E acetate, α-tocopherylphosphate, and α-tocopherylsuccinate [181].
- (4)
- β-carotene: Besides tocopherols, carotenoids play an important role in the photoprotection of phototrophs by eliminating surplus energy as heat, directly scavenging reactive oxidants [148], including 1O2 free radicals, and protecting cells from oxidative impairment by suppressing lipid peroxidation [149]. Their antioxidant property is attributed to their extended conjugated double bond system [186]. Low β-carotene levels have been shown to protect membrane lipids from peroxidative reactions [149].
- (5)
- Gallic acid (GA): In plants, GA is a relatively ubiquitous [187] endogenous polyphenolic compound with several biological activities, including reacting with active oxidants, preventing their formation and accumulation [188]. GA occurs in the free or conjugate (as esterified hydrolysable tannins [56]) form in several plants [189]. Though polyphenols, such as quercetin [190], as well as GA [191], may act as prooxidants depending on concentration and condition [192], GA is primarily used as an antioxidant [193] due to its capacity to scavenge ROS, such as H2O2 [194].
5. Seed Invigoration Treatments
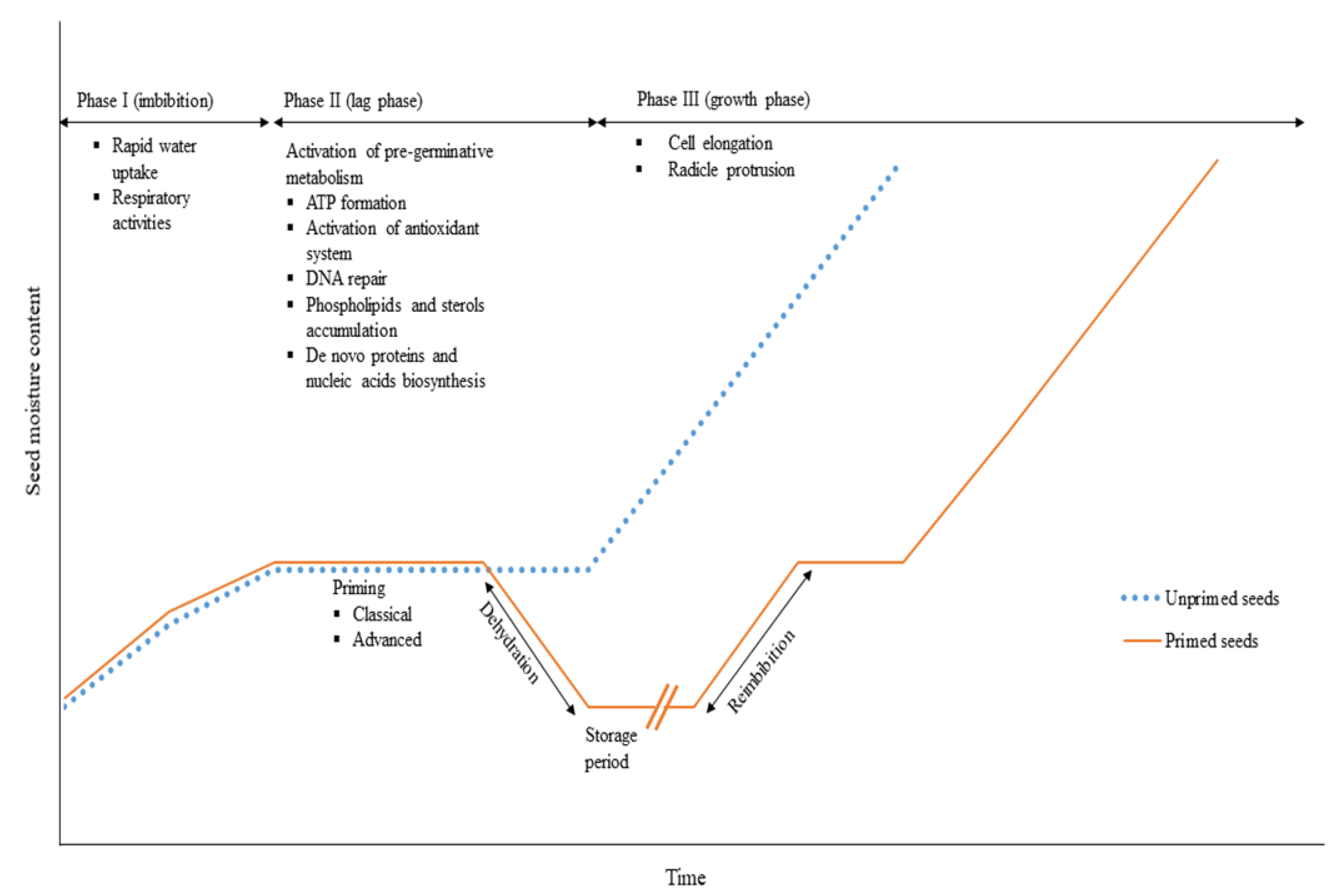
5.1. A Brief History of Seed Pre-Hydration Treatment
5.2. Seed Pre-Hydration and Pre-Germinative Metabolism
5.3. The Seed Priming Technology Overview
5.4. Seed Priming Methods
5.4.1. Classical Seed Priming Techniques
Hydropriming
Osmopriming
Redox Priming
Plant Biostimulant Priming
5.4.2. Advanced Seed Priming Techniques
Nanopriming
Seed Priming with Physical Agents
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoban, S.M.; Hauffe, H.C.; Pérez-Espona, S.; Arntzen, J.W.; Bertorelle, G.; Bryja, J.; Frith, K.; Gaggiotti, O.E.; Galbusera, P.; Godoy, J.A.; et al. Bringing genetic diversity to the forefront of conservation policy and management. Conserv. Genet. Resour. 2013, 5, 593–598. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Sørensen, M.; Pedersen, S.M.; Weiner, J. Feeding the world: Genetically modified crops versus agricultural biodiversity. Agron. Sustain. Dev. 2013, 33, 651–662. [Google Scholar] [CrossRef]
- United Nations World Population Prospects 2019. Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 14 October 2021).
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Khan, M.A.; Tahir, A.; Khurshid, N.; Husnain, M.I.u.; Ahmed, M.; Boughanmi, H. Economic effects of climate change-induced loss of agricultural production by 2050: A case study of Pakistan. Sustainability 2020, 12, 1216. [Google Scholar] [CrossRef]
- Zinyengere, N.; Crespo, O.; Hachigonta, S. Crop response to climate change in southern Africa: A comprehensive review. Glob. Planet. Chang. 2013, 111, 118–126. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Conway, G.; Toenniessen, G. Feeding the world in the twenty-first century. Nature 1999, 402, C55–C58. [Google Scholar] [CrossRef]
- Mann, C.C. Crop scientists seek a new revolution. Science 1999, 283, 310–314. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Thornton, P.K.; Whitbread, A.; Baedeker, T.; Cairns, J.; Claessens, L.; Baethgen, W.; Bunn, C.; Friedmann, M.; Giller, K.E.; Herrero, M.; et al. A framework for priority-setting in climate smart agriculture research. Agric. Syst. 2018, 167, 161–175. [Google Scholar] [CrossRef]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable intensification in agriculture: Premises and policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef]
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef]
- FAO Seeds. Available online: http://www.fao.org/seeds/en/ (accessed on 31 August 2020).
- Solberg, S.Ø.; Yndgaard, F.; Andreasen, C.; von Bothmer, R.; Loskutov, I.G.; Asdal, Å. Long-term storage and longevity of orthodox seeds: A systematic review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Beal, W.J. The vitality of seeds buried in the soil. In Proceedings of the Society for the Promotion of Agricultural Science; 1911; pp. 21–23. [Google Scholar]
- Beal, W.J. The viability of seeds. Bot. Gaz. 1905, 40, 140–143. [Google Scholar] [CrossRef]
- Kivilaan, A.; Bandurski, R.S. The one hundred-year period for Dr. Beal’s seed viability experiment. Am. J. Bot. 1981, 68, 1290–1292. [Google Scholar] [CrossRef]
- Steiner, A.M.; Ruckenbauer, P. Germination of 110-year-old cereal and weed seeds, the Vienna Sample of 1877. Verification of effective ultra-dry storage at ambient temperature. Seed Sci. Res. 1995, 5, 195–199. [Google Scholar] [CrossRef]
- Ruckenbauer, P. Keimfahiger Winterweizen aus dem Jahre 1877.—Beobachtungen und versuche (germinating winter wheat of the year 1877.—Observations and experiments). Die Bodenkultur. 1971, 22, 372–386. [Google Scholar]
- Telewski, F.W.; Zeevaart, J.A.D. The 120-yr period for Dr. Beal’s seed viability experiment. Am. J. Bot. 2002, 89, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.H.; Ellis, R.H. Water and seed survival. Ann. Bot. 1989, 63, 39. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage. Plant Physiol. 1990, 94, 1019–1023. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Seed moisture content, storage, viability and vigour. Seed Sci. Res. 1991, 1, 275–279. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Roberts, E.H. Viability of lettuce seeds: I. Survival in hermetic storage. J. Exp. Bot. 1983, 34, 620–630. [Google Scholar] [CrossRef]
- Still, D.W. The development of seed quality in brassicas. Horttechnology 1999, 9, 335–340. [Google Scholar] [CrossRef]
- Walters, C.; Towill, L. Seeds and pollen. In Agriculture Handbook. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; United States Department of Agriculture: Fort Collins, CO, USA, 2004; pp. 735–743. ISBN 3015046128. [Google Scholar]
- Berjak, P.; Pammenter, N.W. Biotechnological aspects of non-orthodox seeds: An African perspective. S. Afr. J. Bot. 2004, 70, 102–108. [Google Scholar] [CrossRef][Green Version]
- Basra, S.M.A.; Ahmad, N.; Khan, M.M.; Iqbal, N.; Cheema, M.A. Assessment of cottonseed deterioration during accelerated ageing. Seed Sci. Technol. 2003, 31, 531–540. [Google Scholar] [CrossRef]
- Poonguzhali, S. Improving vigour and viability of blackgram cv.co 6 [Vigna mungo (L) Hepper] through seed priming with inorganics. Legum. Res. Int. J. 2016, 39, 820–829. [Google Scholar] [CrossRef]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Chmielarz, P. Cryopreservation of dormant European ash (Fraxinus excelsior) orthodox seeds. Tree Physiol. 2009, 29, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M. Review on physiological aspects of seed deterioration. Int. J. Agric. Crop Sci. 2013, 6, 627–631. [Google Scholar]
- Chmielarz, P. Cryopreservation of orthodox seeds of Alnus glutinosa. CryoLetters 2010, 31, 139–146. [Google Scholar] [PubMed]
- Walters, C.; Wheeler, L.; Stanwood, P.C. Longevity of cryogenically stored seeds. Cryobiology 2004, 48, 229–244. [Google Scholar] [CrossRef]
- Brown, R. Physiology of seed germination. In Differenzierung und Entwicklung/Differentiation and Development. Handbuch der Pflanzenphysiologie/Encyclopedia of Plant Physiology; Lang, A., Ed.; Springer: Berlin/Heidelberg, Germany, 1965; Volume 15, pp. 2541–2555. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Springer US: Boston, MA, USA, 1994; ISBN 978-0-306-44748-8. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Welbaum, G.E.; Bradford, K.J.; Yim, K.-O.; Booth, D.T.; Oluoch, M.O. Biophysical, physiological and biochemical processes regulating seed germination. Seed Sci. Res. 1998, 8, 161–172. [Google Scholar] [CrossRef]
- Pritchard, S.L.; Charlton, W.L.; Baker, A.; Graham, I.A. Germination and storage reserve mobilization are regulated independently in Arabidopsis. Plant J. 2002, 31, 639–647. [Google Scholar] [CrossRef]
- Demidchik, V. Reactive oxygen species and their role in plant oxidative stress. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2017; pp. 64–96. ISBN 9781780647296. [Google Scholar]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Demidchik, V. Reactive oxygen species, oxidative stress and plant ion channels. In Ion Channels and Plant Stress Responses. Signaling and Communication in Plants; Signaling and Communication in Plants; Demidchik, V., Maathuis, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 207–232. [Google Scholar]
- Zhu, J.; Gong, Z.; Zhang, C.; Song, C.P.; Damsz, B.; Inan, G.; Koiwa, H.; Zhu, J.K.; Hasegawa, P.M.; Bressan, R.A. OSM1/SYP61: A syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 2002, 14, 3009–3028. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Varghese, B.; Sershen; Berjak, P.; Varghese, D.; Pammenter, N.W. Differential drying rates of recalcitrant Trichilia dregeana embryonic axes: A study of survival and oxidative stress metabolism. Physiol. Plant. 2011, 142, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, S.; Escobar, C.; Karpinska, B.; Creissen, G.; Mullineaux, P.M. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 1997, 9, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Effects of temperature on oxidative stress defense systems, lipid peroxidation and lipoxygenase activity in Phalaenopsis. Plant Physiol. Biochem. 2005, 43, 213–223. [Google Scholar] [CrossRef]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef]
- Hendry, G.A.F. Oxygen, free radical processes and seed longevity. Seed Sci. Res. 1993, 3, 141–153. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Assosiates Inc.: Sunderland, MA, USA, 2010; ISBN 9780878938667. [Google Scholar]
- Saha, H.; Mitra, M.; Deepa Sankar, P. Oxidative stress and approaches to enhance abiotic stress tolerance in plants. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 724–734. [Google Scholar]
- Bartosz, G. Oxidative stress in plants. Acta Physiol. Plant. 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Mirdad, Z.; Powell, A.A.; Matthews, S. Prediction of germination in artificially aged seeds of Brassica spp. using the bulk conductivity test. Seed Sci. Technol. 2006, 34, 273–286. [Google Scholar] [CrossRef]
- Boniecka, J.; Kotowicz, K.; Skrzypek, E.; Dziurka, K.; Rewers, M.; Jedrzejczyk, I.; Wilmowicz, E.; Berdychowska, J.; Dąbrowska, G.B. Potential biochemical, genetic and molecular markers of deterioration advancement in seeds of oilseed rape (Brassica napus L.). Ind. Crops Prod. 2019, 130, 478–490. [Google Scholar] [CrossRef]
- Golovina, E.A.; Wolkers, W.F.; Hoekstra, F.A. Behaviour of membranes and proteins during natural seed ageing. In Basic and Applied Aspects of Seed Biology: Proceedings of the Fifth International Workshop on Seeds, Reading, 1995; Ellis, R.H., Black, M., Murdoch, A.J., Hong, T.D., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 787–796. ISBN 978-94-011-5716-2. [Google Scholar]
- Golovina, E.A.; Wolkers, W.F.; Hoekstra, F.A. Long-term stability of protein secondary structure in dry seeds. Comp. Biochem. Physiol. Part A Physiol. 1997, 117, 343–348. [Google Scholar] [CrossRef]
- Smith, M.T. Membrane Changes and Lipid Peroxidation during Ageing in Seeds of Lactuca sativa L. Ph.D. Thesis, University of Natal, Durban, South Africa, 1986. [Google Scholar]
- Mira, S.; González-Benito, M.E.; Hill, L.M.; Walters, C. Characterization of volatile production during storage of lettuce (Lactuca sativa) seed. J. Exp. Bot. 2010, 61, 3915–3924. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant Sci. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, G.C.C.; da Silva Binotti, F.F.; Costa, E. Priming effect on the physiological potential of maize seeds under abiotic stress1. Pesqui. Agropecuária Trop. 2017, 47, 328–335. [Google Scholar] [CrossRef][Green Version]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Fu, Y.B.; Wang, H. Arabidopsis seed stored mRNAs are degraded constantly over aging time, as revealed by new quantification methods. Front. Plant Sci. 2020, 10, 1764. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive oxygen species (ROS) generation and detoxifying in plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Mira, S.; Estrelles, E.; González-Benito, M.E.; Corbineau, F. Biochemical changes induced in seeds of Brassicaceae wild species during ageing. Acta Physiol. Plant. 2011, 33, 1803–1809. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef]
- Feng, J.; Shen, Y.; Shi, F.; Li, C. Changes in seed germination ability, lipid peroxidation and antioxidant enzyme activities of Ginkgo biloba seed during desiccation. Forests 2017, 8, 286. [Google Scholar] [CrossRef]
- Sahu, B.; Sahu, A.K.; Thomas, V.; Naithani, S.C. Reactive oxygen species, lipid peroxidation, protein oxidation and antioxidative enzymes in dehydrating Karanj (Pongamia pinnata) seeds during storage. S. Afr. J. Bot. 2017, 112, 383–390. [Google Scholar] [CrossRef]
- Al-maskri, A.; Kharr, M.M.; Ai-mantheriand, O.; Al-habs, K. Effect of accelerated aging on lipid peroxidation, leakage and seedling vigor (RGR) in cucumber (Cucumis sativus L.) seeds. Park. J Agric. Sci. 2002, 39, 330–337. [Google Scholar]
- Alexeyev, M.F. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009, 276, 5768–5787. [Google Scholar] [CrossRef]
- Oenel, A.; Fekete, A.; Krischke, M.; Faul, S.C.; Gresser, G.; Havaux, M.; Mueller, M.J.; Berger, S. Enzymatic and non-enzymatic mechanisms contribute to lipid oxidation during seed aging. Plant Cell Physiol. 2017, 58, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.C. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef]
- Catalá, A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int. J. Biochem. Cell Biol. 2006, 38, 1482–1495. [Google Scholar] [CrossRef]
- Porter, N.A. Chemistry of lipid peroxidation. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 273–282. ISBN 012182005X. [Google Scholar]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57, 715S–725S. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Przybyla, D.; Göbel, C.; Imboden, A.; Hamberg, M.; Feussner, I.; Apel, K. Enzymatic, but not non-enzymatic, 1O2-mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J. 2008, 54, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Gruszka, J.; Kruk, J. Function of plastochromanol and other biological prenyllipids in the inhibition of lipid peroxidation—A comparative study in model systems. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Kristal, B.S.; Park, B.K.; Yu, B.P. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J. Biol. Chem. 1996, 271, 6033–6038. [Google Scholar] [CrossRef]
- Yin, L.; Mano, J.; Wang, S.; Tsuji, W.; Tanaka, K. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 2010, 152, 1406–1417. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Andreou, A.; Feussner, I. Lipoxygenases—Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Yu, Z.; Wang, Y.; Yang, Y.; Liu, Z.; Jiang, J.; Song, M.; Wu, Y. Superior storage stability in low lipoxygenase maize varieties. J. Stored Prod. Res. 2007, 43, 530–534. [Google Scholar] [CrossRef]
- Gayen, D.; Ali, N.; Ganguly, M.; Paul, S.; Datta, K.; Datta, S.K. RNAi mediated silencing of lipoxygenase gene to maintain rice grain quality and viability during storage. Plant Cell. Tissue Organ Cult. 2014, 118, 229–243. [Google Scholar] [CrossRef]
- Song, M.; Wu, Y.; Zhang, Y.; Liu, B.M.; Jiang, J.Y.; Xu, X.; Yu, Z.L. Mutation of rice (Oryza sativa L.) LOX-1/2 near-isogenic lines with ion beam implantation and study of their storability. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 495–500. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Lin, C.; Pan, R.; Ma, W.; Zheng, Y.; Guan, Y.; Hu, J. Suppression of LOX activity enhanced seed vigour and longevity of tobacco (Nicotiana tabacum L.) seeds during storage. Conserv. Physiol. 2018, 6, coy047. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Rinalducci, S.; Murgiano, L.; Zolla, L. Redox proteomics: Basic principles and future perspectives for the detection of protein oxidation in plants. J. Exp. Bot. 2008, 59, 3781–3801. [Google Scholar] [CrossRef] [PubMed]
- Starke-Reed, P.E.; Oliver, C.N. Protein oxidation and proteolysis during aging and oxidative stress. Arch. Biochem. Biophys. 1989, 275, 559–567. [Google Scholar] [CrossRef]
- Stadtman, E. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef]
- Oracz, K.; Bouteau, H.E.M.; Farrant, J.M.; Cooper, K.; Belghazi, M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007, 50, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, A.; Sedlářová, M.; Pospíšil, P. Organic radical imaging in plants: Focus on protein radicals. Free Radic. Biol. Med. 2019, 130, 568–575. [Google Scholar] [CrossRef]
- Johansson, E.; Olsson, O.; Nyström, T. Progression and specificity of protein oxidation in the life cycle of Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 22204–22208. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.V. Molecular targets of oxidative stress. Biochem. J. 2011, 434, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Medicherla, B.; Goldberg, A.L. Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J. Cell Biol. 2008, 182, 663–673. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Ghezzi, P.; Bonetto, V. Redox proteomics: Identification of oxidatively modified proteins. Proteomics 2003, 3, 1145–1153. [Google Scholar] [CrossRef]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef] [PubMed]
- Isbell, H.S.; Frush, H.L.; Martin, E.T. Reactions of carbohydrates with hydroperoxides. Carbohydr. Res. 1973, 26, 287–295. [Google Scholar] [CrossRef]
- Headlam, H.A.; Davies, M.J. Markers of protein oxidation: Different oxidants give rise to variable yields of bound and released carbonyl products. Free Radic. Biol. Med. 2004, 36, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 194, 346–352. [Google Scholar] [CrossRef]
- Morscher, F.; Kranner, I.; Arc, E.; Bailly, C.; Roach, T. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann. Bot. 2015, 116, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Milkovska-Stamenova, S.; Schmidt, R.; Frolov, A.; Birkemeyer, C. GC-MS Method for the quantitation of carbohydrate intermediates in glycation systems. J. Agric. Food Chem. 2015, 63, 5911–5919. [Google Scholar] [CrossRef]
- Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of plant proteins: Regulatory roles and interplay with sugar signalling? Int. J. Mol. Sci. 2019, 20, 2366. [Google Scholar] [CrossRef]
- Lounifi, I.; Arc, E.; Molassiotis, A.; Job, D.; Rajjou, L.; Tanou, G. Interplay between protein carbonylation and nitrosylation in plants. Proteomics 2013, 13, 568–578. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef]
- Tanou, G.; Filippou, P.; Belghazi, M.; Job, D.; Diamantidis, G.; Fotopoulos, V.; Molassiotis, A. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 2012, 72, 585–599. [Google Scholar] [CrossRef]
- Pyngrope, S.; Bhoomika, K.; Dubey, R.S. Oxidative stress, protein carbonylation, proteolysis and antioxidative defense system as a model for depicting water deficit tolerance in Indica rice seedlings. Plant Growth Regul. 2013, 69, 149–165. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Ortega-Villasante, C.; Álvarez-Fernández, A.; del Campo, F.F.; Hernández, L.E. Stress responses of Zea mays to cadmium and mercury. Plant Soil 2006, 279, 41–50. [Google Scholar] [CrossRef]
- Song, H.; Xu, X.; Wang, H.; Tao, Y. Protein carbonylation in barley seedling roots caused by aluminum and proton toxicity is suppressed by salicylic acid. Russ. J. Plant Physiol. 2011, 58, 653–659. [Google Scholar] [CrossRef]
- Xu, X.; Qin, G.; Tian, S. Effect of microbial biocontrol agents on alleviating oxidative damage of peach fruit subjected to fungal pathogen. Int. J. Food Microbiol. 2008, 126, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Rathinasabapathi, B. Transgenic expression of fern Pteris vittata glutaredoxin PvGrx5 in Arabidopsis thaliana increases plant tolerance to high temperature stress and reduces oxidative damage to proteins. Planta 2010, 231, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef]
- Yin, G.; Xin, X.; Fu, S.; An, M.; Wu, S.; Chen, X.; Zhang, J.; He, J.; Whelan, J.; Lu, X. Proteomic and carbonylation profile analysis at the critical node of seed ageing in Oryza sativa. Sci. Rep. 2017, 7, 40611. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Miller, A.R. Oxidation of cell wall polysaccharides by hydrogen peroxide: A potential mechanism for cell wall breakdown in plants. Biochem. Biophys. Res. Commun. 1986, 141, 238–244. [Google Scholar] [CrossRef]
- Schopfer, P.; Liszkay, A.; Bechtold, M.; Frahry, G.; Wagner, A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 2002, 214, 821–828. [Google Scholar] [CrossRef]
- Fry, S.C.; Miller, J.G.; Dumville, J.C. A proposed role for copper ions in cell wall loosening. In Progress in Plant Nutrition: Plenary Lectures of the XIV International Plant Nutrition Colloquium; Springer: Dordrecht, The Netherlands, 2002; Volume 247, pp. 57–67. [Google Scholar]
- Connolly, E.L.; Guerinot, M. Lou Iron stress in plants. Genome Biol. 2002, 3, 1–4. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jouve, L.; Hoffmann, L.; Hausman, J.F. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): Involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004, 6, 74–80. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in plants, fungi, and plant-fungal interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Mazuy, C.; Corbineau, F.; Bailly, C. DNA alteration and programmed cell death during ageing of sunflower seed. J. Exp. Bot. 2011, 62, 5003–5011. [Google Scholar] [CrossRef]
- Roldán-Arjona, T.; Ariza, R.R. Repair and tolerance of oxidative DNA damage in plants. Mutat. Res. 2009, 681, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C. Pyrimido[1,2-a]-purin-10(3H)-one, M1G, is less prone to artifact than base oxidation. Nucleic Acids Res. 2005, 33, 6426–6434. [Google Scholar] [CrossRef]
- Britt, A.B. DNA damage and repair in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 75–100. [Google Scholar] [CrossRef]
- Larsen, N.B.; Rasmussen, M.; Rasmussen, L.J. Nuclear and mitochondrial DNA repair: Similar pathways? Mitochondrion 2005, 5, 89–108. [Google Scholar] [CrossRef]
- Yoshiyama, K.; Sakaguchi, K.; Kimura, S. DNA damage response in plants: Conserved and variable response compared to animals. Biology 2013, 2, 1338–1356. [Google Scholar] [CrossRef]
- Vanderauwera, S.; Suzuki, N.; Miller, G.; van de Cotte, B.; Morsa, S.; Ravanat, J.-L.; Hegie, A.; Triantaphylides, C.; Shulaev, V.; Van Montagu, M.C.E.; et al. Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. USA 2011, 108, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Mullarky, E.; Cantley, L.C. Diverting glycolysis to combat oxidative stress. In Innovative Medicine; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–23. [Google Scholar]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Perl-Treves, R.; Perl, A. Oxidative stress: An introduction. In Oxidative Stress in Plants; Inzé, D., Van Montagu, M., Eds.; Taylor & Francis: London, UK, 2002; pp. 1–32. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Govindaraj, M.; Masilamani, P.; Albert, V.A.; Bhaskaran, M. Role of antioxidant in seed quality—A review. Agric. Rev. 2017, 38, 180–190. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. In Autoxidation in Food and Biological Systems; Springer: Boston, MA, USA, 1981; Volume 110, pp. 250–272. [Google Scholar]
- del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–26. ISBN 9783319750880. [Google Scholar]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase—Representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Shugaev, A.G.; Lashtabega, D.A.; Shugaeva, N.A.; Vyskrebentseva, E.I. Activities of antioxidant enzymes in mitochondria of growing and dormant sugar beet roots. Russ. J. Plant Physiol. 2011, 58, 387–393. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plant. 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Kibinza, S.; Bazin, J.; Bailly, C.; Farrant, J.M.; Corbineau, F.; El-Maarouf-Bouteau, H. Catalase is a key enzyme in seed recovery from ageing during priming. Plant Sci. 2011, 181, 309–315. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Hu, X.; Xing, S.; Xu, L. Cloning and allelic variation of two novel catalase genes (SoCAT-1 and SsCAT-1) in Saccharum officinarum L. and Saccharum spontaneum L. Biotechnol. Biotechnol. Equip. 2015, 29, 431–440. [Google Scholar] [CrossRef]
- Yoshimura, K.; Miyao, K.; Gaber, A.; Takeda, T.; Kanaboshi, H.; Miyasaka, H.; Shigeoka, S. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. Plant J. 2004, 37, 21–33. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Hakeem, K.U.R.; Chandna, R.; Ahmad, P. Role of glutathione reductase in plant abiotic stress. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 149–158. ISBN 978-1-4614-0633-4. [Google Scholar]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front. Plant Sci. 2016, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Chew, O.; Whelan, J.; Millar, A.H. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003, 278, 46869–46877. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, C.; Liu, Q.; Lin, B.; Wang, J. Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol. Plant. 2008, 30, 433–440. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Gill, R.; Hasanuzzaman, M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Tuteja, R.; Tuteja, N. Metal/metalloid stress tolerance in plants: Role of ascorbate, its redox couple, and associated enzymes. Protoplasma 2014, 251, 1265–1283. [Google Scholar] [CrossRef]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Rouhier, N.; Jacquot, J.-P. The plant multigenic family of thiol peroxidases. Free Radic. Biol. Med. 2005, 38, 1413–1421. [Google Scholar] [CrossRef]
- Navrot, N.; Collin, V.; Gualberto, J.; Gelhaye, E.; Hirasawa, M.; Rey, P.; Knaff, D.B.; Issakidis, E.; Jacquot, J.P.; Rouhier, N. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar] [CrossRef]
- Rizhsky, L.; Hallak-Herr, E.; Van Breusegem, F.; Rachmilevitch, S.; Barr, J.E.; Rodermel, S.; Inzé, D.; Mittler, R. Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 2002, 32, 329–342. [Google Scholar] [CrossRef]
- Foyer, C.; Lelandais, M.; Galap, C.; Kunert, K.J. Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol. 1991, 97, 863–872. [Google Scholar] [CrossRef]
- Khan, T.; Mazid, M.; Mohammad, F. A review of ascorbic acid potentialities against oxidative stress induced in plants. J. Agrobiol. 2011, 28, 97–111. [Google Scholar] [CrossRef]
- Tommasi, F.; Paciolla, C.; de Pinto, M.C.; De Gara, L. A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J. Exp. Bot. 2001, 52, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.Y.; Nasir Khan, M.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A.; Al-Rabiah, H.; Mateen, M. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J. Plant Interact. 2018, 13, 409–419. [Google Scholar] [CrossRef]
- Ahmad, I.; Basra, S.M.A.; Wahid, A. Exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide improves the productivity of hybrid maize at low temperature stress. Int. J. Agric. Biol. 2014, 16, 825–830. [Google Scholar]
- Conklin, P.L.; Barth, C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004, 27, 959–970. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Larson, R.A. The antioxidants of higher plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Jiménez, A.; Hernández, J.A.; Pastori, G.; del Río, L.A.; Sevilla, F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998, 118, 1327–1335. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Tausz, M.; Šircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef]
- Creissen, G.; Firmin, J.; Fryer, M.; Kular, B.; Leyland, N.; Reynolds, H.; Pastori, G.; Wellburn, F.; Baker, N.; Wellburn, A.; et al. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 1999, 11, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iannelli, M.A.; Pasqualini, S.; Massacci, A. Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiol. 2003, 133, 829–837. [Google Scholar] [CrossRef]
- Zingg, J.-M.; Azzi, A. Non-antioxidant activities of vitamin E. Curr. Med. Chem. 2004, 11, 1113–1133. [Google Scholar] [CrossRef]
- Holländer-Czytko, H.; Grabowski, J.; Sandorf, I.; Weckermann, K.; Weiler, E.W. Tocopherol content and activities of tyrosine aminotransferase and cystine lyase in Arabidopsis under stress conditions. J. Plant Physiol. 2005, 162, 767–770. [Google Scholar] [CrossRef]
- Senaratna, T.; Gusse, J.F.; McKersie, B.D. Age-induced changes in cellular membranes of imbibed soybean seed axes. Physiol. Plant. 1988, 73, 85–91. [Google Scholar] [CrossRef]
- Hamad, I.; Arda, N.; Pekmez, M.; Karaer, S.; Temizkan, G. Intracellular scavenging activity of Trolox (6-hydroxy-2,5,7,8- tetramethylchromane-2-carboxylic acid) in the fission yeast, Schizosaccharomyces pombe. J. Nat. Sci. Biol. Med. 2010, 1, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, M.; Nunes, C.; Gaspar, D.; Ferreira, H.; Lima, J.L.F.C.; Reis, S. Antioxidant activity of vitamin E and Trolox: Understanding of the factors that govern lipid peroxidation studies in vitro. Food Biophys. 2009, 4, 312–320. [Google Scholar] [CrossRef]
- Collins, A.R. Carotenoids and genomic stability. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2001, 475, 21–28. [Google Scholar] [CrossRef]
- Haslam, E.; Cai, Y. Plant polyphenols (vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41–66. [Google Scholar] [CrossRef]
- Handique, J.G.; Baruah, J.B. Polyphenolic compounds: An overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Ow, Y.-Y.; Stupans, I. Gallic acid and gallic acid derivatives: Effects on drug metabolizing enzymes. Curr. Drug Metab. 2005, 4, 241–248. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Sakagami, H.; Satoh, K. Prooxidant action of two antioxidants: Ascorbic acid and gallic acid. Anticancer Res. 1997, 17, 221–224. [Google Scholar] [PubMed]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Nakatani, N. Natural Antioxidants from Spices. In Phenolic Compounds in Food and Their Effects on Health II: Antioxidants and Cancer Prevention; Huang, M.T., Ho, C.T., Lee, C., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 72–86. [Google Scholar]
- Yen, G.C.; Der Duh, P.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Rakshit, A.; Singh, H.B. Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; ISBN 978-981-13-0031-8. [Google Scholar]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Evenari, M. Seed physiology: Its history from antiquity to the beginning of the 20th century. Bot. Rev. 1984, 50, 119–142. [Google Scholar] [CrossRef]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.; Deeba, F.; Ali, I.; Hussain, H. Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 11–41. ISBN 978-981-13-8624-4. [Google Scholar]
- Darwin, C.R. Effect of salt-water on the germination of seeds. Gardeners’ Chron. Agric. Gazet. 1855, 47, 773. [Google Scholar]
- May, L.H.; Milthorpe, E.J.; Milthorpe, F.L. Pre-sowing hardening of plants to drought. In Field Crop Abstracts; CABI: Wallingford, UK, 1962; pp. 93–98. [Google Scholar]
- Heydecker, W. Germination of an idea: The priming of seeds. Ph.D. Thesis, School of Agriculture Research, University of Nottingham, Nottingham, UK, 1973; pp. 50–67.
- Malnassy, P.G. Physiological and biochemical studies on a treatment hastening the germination of seeds at low temperatures. Ph.D. Thesis, The State University of New Jersey, Rutgers, NJ, USA, 1971. [Google Scholar]
- Sivasubramaniam, K.; Geetha, R.; Sujatha, K.; Raja, K.; Sripunitha, A.; Selvarani, R. Seed Priming: Triumphs and tribulations. Physiology 2011, 98, 197–209. [Google Scholar]
- Rowse, H.R. Methods of priming seed. U.S. Patent 5,119,589, 1992. [Google Scholar]
- Bradford, K.J. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. HortScience 1986, 21, 1105. [Google Scholar]
- Parera, C.A.; Cantliffe, D.J. Presowing Seed Priming. In Horticultural Reviews; John Wiley & Sons, Inc.: Oxford, UK, 1994; Volume 16, pp. 109–141. ISBN 9780470650561. [Google Scholar]
- Singh, V.K.; Singh, R.; Tripathi, S.; Devi, R.S.; Srivastava, P.; Singh, P.; Kumar, A.; Bhadouria, R. Seed priming: State of the art and new perspectives in the era of climate change. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–170. ISBN 9780128180327. [Google Scholar]
- Beckers, G.J.; Conrath, U. Priming for stress resistance: From the lab to the field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Bray, C.M.; West, C.E. The importance of safeguarding genome integrity in germination and seed longevity. J. Exp. Bot. 2015, 66, 3549–3558. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A plant DNA ligase is an important determinant of seed longevity. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef]
- Vonarx, E.J.; Mitchell, H.L.; Karthikeyan, R.; Chatterjee, I.; Kunz, B.A. DNA repair in higher plants. Mutat. Res. 1998, 400, 187–200. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Meimoun, P.; Job, C.; Job, D.; Bailly, C. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant Sci. 2013, 4, 77. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Antioxidant systems in sunflower (Helianthus annuus L.) seeds as affected by priming. Seed Sci. Res. 2000, 10, 35–42. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chen, C.L.; Chen, J.J.; Sung, J.M. Accelerated aging-enhanced lipid peroxidation in bitter gourd seeds and effects of priming and hot water soaking treatments. Sci. Hortic. 2003, 98, 201–212. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Afzal, I.; Khaliq, A. Optimization of hydropriming techniques for rice seed invigoration. Seed Sci. Technol. 2006, 34, 507–512. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Esmaeilpour, B. The Effect of salt priming on the performance of differentially matured cucumber (Cucumis sativus) seeds. Not. Bot. Hort. Agrobot. 2008, 36, 67–70. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Kiss, A.; Erdélyi, É.; Ladányi, M.; Németh, É.Z.; Radácsi, P. The effect of osmopriming on seed germination and early seedling characteristics of Carum carvi L. Agriculture 2020, 10, 94. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus-Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Chandra, J.; Sershen; Varghese, B.; Keshavkant, S. The potential of ROS inhibitors and hydrated storage in improving the storability of recalcitrant Madhuca latifolia seeds. Seed Sci. Technol. 2019, 47, 33–45. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Askari, S.H.; Iqbal, M.; Rasheed, R.; Hussain, I. Recent advances in abiotic stress tolerance of plants through chemical priming: An overview. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 51–79. ISBN 9789811300325. [Google Scholar]
- Varier, A.; Vari, A.K.; Dadlani, M. The subcellular basis of seed priming. Curr. Sci. 2010, 99, 450–456. [Google Scholar]
- Cantliffe, D.J. Priming of lettuce seed for early and uniform emergence under conditions of environmental stress. Acta Hortic. 1981, 29–38. [Google Scholar] [CrossRef]
- Bradford, K.J.; Steiner, J.J.; Trawatha, S.E. Seed priming influence on germination and emergence of pepper seed lots. Crop Sci. 1990, 30, 718–721. [Google Scholar] [CrossRef]
- Khan, A.A.; Peck, N.H.; Samimy, C. Seed osmoconditioning: Physiological and biochemical changes. Isr. J. Bot. 1980, 29, 133–144. [Google Scholar] [CrossRef]
- Taylor, A.G.; Klein, D.E.; Whitlow, T.H. SMP: Solid matrix priming of seeds. Sci. Hortic. 1988, 37, 1–11. [Google Scholar] [CrossRef]
- Mondal, S.; Vijai, P.; Bose, B. Role of seed hardening in rice variety swarna (MTU 7029). Res. J. Seed Sci. 2011, 4, 157–165. [Google Scholar] [CrossRef]
- Matsushima, K.-I.; Sakagami, J.-I. Effects of seed hydropriming on germination and seedling vigour during emergence of rice under different soil moisture conditions. Am. J. Plant Sci. 2013, 4, 1584–1593. [Google Scholar] [CrossRef]
- Forti, C.; Ottobrino, V.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of pre-germinative metabolism in primed eggplant (Solanum melongena L.) seeds. Hortic. Res. 2020, 7, 87. [Google Scholar] [CrossRef]
- Basra, S.M.A.; Farooq, M.; Tabassam, R.; Ahmad, N. Physiological and biochemical aspects of pre-sowing seed treatments in fine rice (Oryza sativa L.). Seed Sci. Technol. 2005, 33, 623–628. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Abid Karim, H.; Afzal, I. Optimization of seed hardening techniques for rice seed invigoration. Emir. J. Food Agric. 2004, 16, 48–58. [Google Scholar] [CrossRef]
- Solaimalai, A.; Subburamu, K. Seed hardening for field crops—A review. Agric. Rev. 2004, 25, 129–140. [Google Scholar]
- Akbar, M. Studies on Seed Priming and Fungicide in Pearl Millet under Dry Land Conditions. Ph.D. Thesis, NWFP Agricultural University, Peshawar, Pakistan, 2008. [Google Scholar]
- Alvarado, A.D.; Bradford, K.J. Priming and storage of tomato (Lycopersicon lycopersicum) seeds. I. Effects of storage temperature on germination rate and viability. Seed Sci. Technol. 1988, 16, 601–612. [Google Scholar]
- Bekendam, J.; van Pijlen, J.G.; Kraak, H.L. The effect of priming on the rate and uniformity of germination of endive seed. Acta Hortic. 1987, 209–218. [Google Scholar] [CrossRef]
- Weges, R. Physiological Analysis of Methods to Relieve Dormancy of Lettuce Seeds. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1987. [Google Scholar]
- Tarquis, A.M.; Bradford, K.J. Prehydration and priming treatments that advance germination also increase the rate of deterioration of lettuce seeds. J. Exp. Bot. 1992, 43, 307–317. [Google Scholar] [CrossRef]
- van Pijlen, J.G.; Groot, S.P.C.; Kraak, H.L.; Bergervoet, J.H.W.; Bino, R.J. Effects of pre-storage hydration treatments on germination performance, moisture content, DNA synthesis and controlled deterioration tolerance of tomato (Lycopersicon esculentum Mill.) seeds. Seed Sci. Res. 1996, 6, 57–63. [Google Scholar] [CrossRef]
- Brancalion, P.H.S.; Novembre, A.D.L.C.; Rodrigues, R.R.; Tay, D. Priming of Mimosa bimucronata seeds—A tropical tree species from Brazil. Acta Hortic. 2008, 782, 163–168. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Araújo, S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef]
- Harris, D.; Raghuwanshi, B.S.; Gangwar, J.S.; Singh, S.C.; Joshi, K.D.; Rashid, A.; Hollington, P.A. Participatory evaluation by farmers of on-farm seed priming in wheat in India, Nepal and Pakistan. Exp. Agric. 2001, 37, 403–415. [Google Scholar] [CrossRef]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.K.; Kaur, N. Effect of osmo- and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. Plant Growth Regul. 2002, 37, 17–22. [Google Scholar] [CrossRef]
- Yan, M. Hydropriming promotes germination of aged napa cabbage seeds. Seed Sci. Technol. 2015, 43, 303–307. [Google Scholar] [CrossRef]
- Bennett, M.A.; Fritz, V.A.; Callan, N.W. Impact of seed treatments on crop stand establishment. Horttechnology 2018, 2, 345–349. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Sershen; Varghese, B.; Pammenter, N.W. Effects of inorganic salt solutions on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. Plants 2020, 9, 1164. [Google Scholar] [CrossRef]
- Singh, A.; Dahiru, R.; Musa, M.; Sani Haliru, B. Effect of osmopriming duration on germination, emergence, and early growth of cowpea (Vigna unguiculata (L.) Walp.) in the sudan savanna of Nigeria. Int. J. Agron. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Fatokun, K.; Beckett, R.P.; Varghese, B.; Cloete, J.; Pammenter, N.W. Influence of cathodic water invigoration on the emergence and subsequent growth of controlled deteriorated pea and pumpkin seeds. Plants 2020, 9, 955. [Google Scholar] [CrossRef]
- Batool, A.; Ziaf, K.; Amjad, M. Effect of halo-priming on germination and vigor index of cabbage (Brassica oleracea var. capitata). J. Environ. Agric. Sci. 2015, 2, 7. [Google Scholar]
- Abdolahi, M.; Andelibi, B.; Zangani, E.; Shekari, F.; Jamaati-E-Somarin, S. Effect of accelerated aging and priming on seed germination of rapeseed (Brassica napus L.) cultivars. Int. Res. J. Appl. Basic Sci. 2012, 3, 499–508. [Google Scholar]
- Carrozzi, L.E.; Creus, C.M.; Barassi, C.A.; Monterubbianesi, G.; Di Benedetto, A. Reparation of aged lettuce (Lactuca sativa) seeds by osmotic priming and Azospirillum brasilense inoculation. Botany 2012, 90, 1093–1102. [Google Scholar] [CrossRef]
- Moradi, A.; Younesi, O. Effects of osmo- and hydro-priming on seed parameters of grain sorghum (Sorghum bicolor L.). Aust. J. Basic Appl. Sci. 2009, 3, 1696–1700. [Google Scholar]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-44080-4. [Google Scholar]
- Kumar, S.R.; Mohanapriya, G.; Sathishkumar, R. Abiotic stress-induced redox changes and programmed cell death in plants—A path to survival or death? In Redox State as a Central Regulator of Plant-Cell Stress Responses; Springer: Cham, Switzerland, 2016; pp. 233–252. ISBN 9783319440811. [Google Scholar]
- Schafer, F.Q.; Buettner, G.R. Redox state and redox environment in biology. In Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 1–14. [Google Scholar]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Draganić, I.; Lekić, S. Seed priming with antioxidants improves sunflower seed germination and seedling growth under unfavorable germination conditions. Turkish J. Agric. For. 2012, 36, 421–428. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Sershen; Varghese, B.; Pammenter, N. Effects of exogenous application of five antioxidants on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. S. Afr. J. Bot. 2021, 137, 85–97. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Sershen; Varghese, B.; Pammenter, N.W. Exogenous antioxidants enhance seedling growth and yield of artificially aged cabbage and lettuce seeds. Horticulturae 2021, 7, 274. [Google Scholar] [CrossRef]
- Burguieres, E.; McCue, P.; Kwon, Y.I.; Shetty, K. Effect of vitamin C and folic acid on seed vigour response and phenolic-linked antioxidant activity. Bioresour. Technol. 2007, 98, 1393–1404. [Google Scholar] [CrossRef]
- Yan, H.-F.; Mao, P.-S.; Sun, Y.; Li, M.-L. Impacts of ascorbic acid on germination, antioxidant enzymes and ultrastructure of embryo cells of aged Elymus sibiricus seeds with different moisture contents. Int. J. Agric. Biol. 2016, 18, 176–183. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.A.; Hameed, A.; Farooq, M. Physiological enhancements for alleviation of salt stress in wheat. Pakistan J. Bot. 2006, 38, 1649–1659. [Google Scholar]
- Bhattacharjee, A.; Gupta, K. Effect of dikegulac-sodium, a growth retardant, on the viability of sunflower seeds. Seed Sci. Technol. 1985, 13, 165–174. [Google Scholar]
- Dey, G.; Mukherjee, R.K. Invigoration of dry seeds with physiologically active chemicals in organic solvents. Seed Sci. Technol. 1988, 16, 145–153. [Google Scholar]
- Bhattacharjee, A.; Bhattacharyya, R.N. Prolongation of seed viability of Oryza sativa L. Seed Sci. Technol. 1989, 17, 309–316. [Google Scholar]
- Ugena, L.; Hýlová, A.; Podlešáková, K.; Humplík, J.F.; Doležal, K.; De Diego, N.; Spíchal, L. Characterization of biostimulant mode of action using novel multi-trait high-throughput screening of arabidopsis germination and rosette growth. Front. Plant Sci. 2018, 9, 1327. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Sreekissoon, A.; Finnie, J.F.; Van Staden, J. Effects of smoke water on germination, seedling vigour and growth of Sceletium tortuosum. S. Afr. J. Bot. 2021, 139, 427–431. [Google Scholar] [CrossRef]
- Gupta, G.P. Role of global climate change in crop yield reductions. In Journal of the Air Pollution Control Association; Saxena, P., Srivastava, A., Eds.; Springer: Singapore, 2020; Volume 13, pp. 87–113. ISBN 9789811534805. [Google Scholar]
- Zhou, J.; Teixeira da Silva, J.; Ma, G. Effects of smoke water and karrikin on seed germination of 13 species growing in China. Open Life Sci. 2014, 9, 1108–1116. [Google Scholar] [CrossRef]
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with seaweed extract and microbial-based commercial biostimulants influences seed germination of five Abelmoschus esculentus genotypes. Plants 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Masondo, N.A.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol. Environ. Saf. 2018, 147, 43–48. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Biostimulant priming in Oryza sativa: A novel approach to reprogram the functional biology under nutrient-deficient soil. Cereal Res. Commun. 2021, 1–8. [Google Scholar] [CrossRef]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Hirota, N.; Nakagawa, J.; Kitazawa, K. Effects of a magnetic field on the germination of plants. J. Appl. Phys. 1999, 85, 5717–5719. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. Influence of stationary magnetic field on lentil seeds. Int. Agrophys. 2010, 24, 321–324. [Google Scholar]
- Teixeira da Silva, J.A.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, D.J.; Katsenios, N.; Efthimiadou, A.; Karkanis, A.; Efthimiadis, P. Investigation of pulsed electromagnetic field as a novel organic pre-sowing method on germination and initial growth stages of cotton. Electromagn. Biol. Med. 2012, 31, 143–150. [Google Scholar] [CrossRef]
- Baby, S.M.; Narayanaswamy, G.K.; Anand, A. Superoxide radical production and performance index of photosystem II in leaves from magnetoprimed soybean seeds. Plant Signal. Behav. 2011, 6, 1635–1637. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Marcu, D.; Cristea, V.; Daraban, L. Dose-dependent effects of gamma radiation on lettuce (Lactuca sativa var. capitata) seedlings. Int. J. Radiat. Biol. 2013, 89, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants: Growth, antioxidant activity and phenolic compounds. Plant Physiol. Biochem. 2014, 83, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Puthur, J.T. Amplification of abiotic stress tolerance potential in rice seedlings with a low dose of UV-B seed priming. Funct. Plant Biol. 2019, 46, 455–466. [Google Scholar] [CrossRef]
- Al-Enezi, N.A.; Al-Bahrany, A.M.; Al-Khayri, J.M. Effect of X-irradiation on date palm seed germination and seedling growth. Emir. J. Food Agric. 2012, 24, 415–424. [Google Scholar]
- De Micco, V.; Paradiso, R.; Aronne, G.; De Pascale, S.; Quarto, M.; Arena, C. Leaf anatomy and photochemical behaviour of Solanum lycopersicum L. Plants from seeds irradiated with low-LET ionising radiation. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Microwave-induced stimulation of L-DOPA, phenolics and antioxidant activity in fava bean (Vicia faba) for Parkinson’s diet. Process Biochem. 2004, 39, 1775–1784. [Google Scholar] [CrossRef]
- Han, F. The effect of microwave treatment on germination, vigour and health of China aster (Callistephus chinensis Nees.) seeds. J. Agric. Sci. 2010, 2, 201–210. [Google Scholar] [CrossRef][Green Version]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; UN: New York, NY, USA, 2015; Report No. A/RES/70/1. [Google Scholar]
| Examples of commonly reported ROS-induced modifications of PUFA [100] | |
| PUFA | Oxidised product |
| Linoleic acid (18:2) | 4-HNE |
| Linolenic acid (18:3) | Cyclic oxylipin, hydroxyoctadecatrieonic acid, MDA |
| Examples of commonly reported ROS-induced modifications of proteins [43,100,112,113] | |
| Amino acid | Oxidised product |
| Cysteine | Cysteic acid (cysteine sulfonic acid) |
| Methionine | Methionine sulfone |
| Arginine, Lysine, Proline, Threonine | Carbonyls (ketones, aldehydes): aminoadipic semialdehyde, pyrrolidone, acrolein, 4-HNE, MDA, glu γ-semialdehyde, 2-amino-3-ketobutyric acid |
| Glutamyl (glutathione, glutamine, glutamate) | Pyruvic acid, oxalic acid |
| Histidine | 2-Oxohistidine, 4-HNE, aspartate, asparagine |
| Phenylalanine | Hydroxyphenylalanines |
| Tryptophan | Kynurenine |
| Tyrosine | 3-Nitrotyrosine |
| Examples of commonly reported ROS-induced modifications of carbohydrates [100,114] | |
| Sugar | Oxidised product |
| Aldohexose, polyol | Aldopentose, formic acid |
| Examples of commonly reported ROS-induced modifications of DNA [100] | |
| DNA | Oxidised product |
| Purines (e.g., guanine) | 8-Hydroxyguanine, FapyGua |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adetunji, A.E.; Adetunji, T.L.; Varghese, B.; Sershen; Pammenter, N.W. Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy 2021, 11, 2369. https://doi.org/10.3390/agronomy11122369
Adetunji AE, Adetunji TL, Varghese B, Sershen, Pammenter NW. Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy. 2021; 11(12):2369. https://doi.org/10.3390/agronomy11122369
Chicago/Turabian StyleAdetunji, Ademola Emmanuel, Tomi Lois Adetunji, Boby Varghese, Sershen, and Norman W. Pammenter. 2021. "Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives" Agronomy 11, no. 12: 2369. https://doi.org/10.3390/agronomy11122369
APA StyleAdetunji, A. E., Adetunji, T. L., Varghese, B., Sershen, & Pammenter, N. W. (2021). Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy, 11(12), 2369. https://doi.org/10.3390/agronomy11122369








