Innovative Pulses for Western European Temperate Regions: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review and Data Collection
2.2. Bibliographic Corpus Analysis
2.3. Descriptive Analysis
2.4. Species Selection for Temperate Regions
3. Results and Discussions
3.1. Descriptive Analysis of the Bibliographic Corpus
3.2. Types of Pulses and Agronomical Needs
3.3. Nutritional Characteristics
3.4. Antinutritional Characteristics
3.5. Potential for Minor Pulse Cultivation in European Temperate Regions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pulses—Common Name | Climate Region | Countries Where Cultivated | Temperature (°C) Min; Max; Optimal | Crop Length a | Water Requirements (mm) | Mineral Requirements | Pest Risk b | Yield (t/Ha) | Kg N Fixed (kg/ha) | Bacteria Associated | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acacia leucophloea | Tropic | Bangladesh, India, Indonesia, Pakistan | 6; 49; - | Y (tree) | 400–1500 | F; I | [88] | ||||
| Adzuki bean | Tropic | China, Japan, Korea, Nepal | 5–10; 34; 15–30 | 60–190 d | 500–1700 | N, P, K | B; F; I | 0.5–3.5 | 100 | Bradyrhizobium spp.; Sinorhizobium fredii | [89,90,91,92,93,94,95,96,97] |
| African locust bean | Tropic | Sudan, Uganda | 0; 45; 21–36 | Y (tree) | 500–1500 | B; F; I | [98,99] | ||||
| African nut tree | Tropic | West and Central Africa, Madagascar | -; -; 18–32 | Y (tree) | 1000 | [39,99] | |||||
| African oil bean | Tropic | Sub-Saharan Africa | 18; -; 25 | Y (tree) | 1000–2000 | I | [39,99] | ||||
| African yam bean | Tropic | Ghana, Nigeria, Togo | 0; -; 19–27 | 150–240 d | 900–2000 | F; I | 3 A * | [90,98,100] | |||
| Bambara groundnut | Tropic | Australia, Cameroon, Nigeria, Sudan | 15; 40; 20–28: no frost | 90–180 d | 600–1200 | N, P, K | F; I; N; V | 0.1–6 | 100 | Bradyrhizobium spp. | [90,101,102,103,104,105,106,107,108,109] |
| Butterfly pea | Tropic | Brazil, Colombia, India, Mexico, Philippines, Venezuela | 5; 35; 20–28 | 120–194 d | 400–1750 | F; I; B | 0.7 | Rhizobium | [110,111,112,113,114] | ||
| Cowpea | Tropic | Burkina Faso, Ghana, Mali, Senegal | 15; 35; 25–35 no frost | 60–340 d | 500–1500 | N, P, K | F; I; P; PW; V | 0.2–7 | 12–50 | Rhizobium spp. NGR234; Bradyrhizobium spp.; Sinorhizobium fredii | [39,62,100,115,116,117,118,119,120,121,122,123,124] |
| Ervil | Temperate | Australia, Iran, Morocco, Turkey | 134–154 d | 200–500 | 0.6–4 | [125,126,127,128,129] | |||||
| Fenugreek | Temperate | Morocco, Tunisia, Spain | −4; -; 18–27 | 90–100 d | 300–400 | N, P, S | Rhizobium spp. NGR234 | [130,131] | |||
| Grass pea | Temperate/subtropic | India, Iran, Italy, Middle East, Spain | 20; -; 10–25 | 90–180 d | 400–650 | N, P | F; I; V | 0.3–105 | 25–50 | [39,63,98,132,133] | |
| Guanacaste | Tropic | Brazil, Costa Rica, Mexico, Venezuela | -; -; 23–28 | Y (tree) | 750–2000 | [134] | |||||
| Horse gram | Tropic | Australia, India, USA | -; 40; 20–32: no frost | 120–150 d | 380–900 | N, P | F; I | 0.5 | Bradyrhizobium | [39,90,100,118,135,136] | |
| Housa groundnut | Tropic | Cameroon, Nigeria, Senegal | 18; 34; 25–32 | 70–180 d | 500–600 | Few | F | 0.5 | 42 | Bradyrhizobium Rhizobium spp. | [39,137,138] |
| Itching bean | Tropic | India | -; -; 19–27 | 400–3000 | 0.2–2 | [139] | |||||
| Jack bean | Tropic | Brazil | -; -; 13–27 | 180–300 d | 800–2000 | N | 4.5 | [100,140,141] | |||
| Kedaung | Tropic | Bangladesh, India, Egypt, Malaysia | N, P, K | F, I | [142] | ||||||
| Kidney bean | Temperate | Brazil, China, EU, India, Mexico, USA | −1; 30;15–20 | 65–105 d | 300–600 | N, P, K, S, Zn | F; I; N; V | 2.2 | 20–44 | Rhizobium | [98,130,132,143] |
| Lablab | Tropic | Cameroon, India, Madagascar | -; -;18–30 | 54–220 d | 650–3000 | Few | F; I; N; V | 1.4–4.5 | 20–140 | Rhizobium spp. NGR234 | [63,90,98,116,144,145,146] |
| Lima bean | Temperate | Brazil, Mexico, Peru | >0; >37; 16–27 | 115–180 d | 900–1500 | N, P, K, Zn | B; F; I; N; V | 0.4–5 | 40–60 | Bradyrhizobium Rhizobium spp. | [39,90,98,147,148,149] |
| Morama bean | Tropic | India | -; 37; - | Y | 100–900 | [39,137] | |||||
| Moth bean | Tropic | Australia, India, Pakistan, Thailand, USA | 25; 45; 24–32 | 70–90 d | 200–750 | I; N; V | 0.07–2.6 | Bradyrhizobium Rhizobium spp. NGR234 | [39,116,150,151,152] | ||
| Mung bean | Temperate | China, India | 12; 40; 28–30 | 50–120 d | 600–1000 | P, K, Ca, Mg, Zn | B; F; I; V | 0.3–2.2 | 14 | Rhizobium inoculums | [39,92,98,102,153,154,155,156] |
| Navy bean | Temperate/Tropic | Australia, EU, USA | 12; 35; 22–30 | 53–300 d | 400 | N, P, Zn | B; F; I | 0.5–5 | 125 | Rhizobium phaseoli | [39,130,157,158] |
| Narbon bean | Temperate | Australia, Iraq, Italy, Jordan, Portugal, Spain, Turkey | 30; -; - | 170 d | 200–500 | PW; F; I; N; V | 0.5–2 | [30,126,127,159,160,161] | |||
| Pigeon Pea | Tropic | India, Kenya, Malawi, Myanmar, Nepal, Tanzania, Uganda | 0; 40; 18–29: no frost | 3–5 y | 600–1400 | P | I; N | 0.6–5 | 69–134 | Bradyrhizobium spp. | [63,92,98,102,141,162,163,164] |
| Pinto bean | Temperate | USA | -; 36; 21–25 | 90–100 | B; V | 0.5–3.9 | [165,166] | ||||
| Pinto peanut | Tropic | Argentina, Brazil, Colombia, USA | -; -; 21–30 | 1100–1500 | K, Al, Mn | F; N; V | 0.3–3 | [63,167,168,169] | |||
| Purple mucuna | Tropic | India | -; 19–27; - | Y | 400–3000 | ||||||
| Red moneywort | Temperate/Tropic | Australia, Asia, Madagascar | 600–1500 | 3–7.5 | Bradyrhizobium | [170] | |||||
| Rice bean | Temperate-Tropic (Alt≈2000 m) | Bangladesh, China, India, Nepal | 10; 40; 25–35 | 120–150 d | 700–1700 | P | B; F; I | 0.2–2.7 | Rhizobium spp. NGR234 | [39,90,102,118,171,172,173,174] | |
| Scarlet runner bean | Temperate-Tropic (Alt≈2000 m) | Africa, Central & South America, EU | 5; >37; 25 | 120–150 d | 1500 | F | F; | 0.9–12.5 | Rhizobium | [39,149,175] | |
| Sesban | Tropic | Chad, Egypt, Kenya, Uganda | 7; 45; 17–20 | Y (tree) | 500–2000 | P, K | F; I; N; V | Rhizobium leguminosarum, Bradyrhizobium | [98,170,176,177,178] | ||
| Sword bean | Tropic | India | -; -; 25–30 | 150–300 d | 900–1500 | F; I; N | 1.5–5.4 | 75–230 | [39,98] | ||
| Tamarind | Tropic | Australia, Cameroon, China, India, Mexico, Nigeria | 4; 41; 15–28 | Y (tree) | 32–3800 | P | B. F; I; N | [179,180,181,182,183] | |||
| Tarwi | Temperate (Alt≈3000 m) | South America | <0; -; - | 150–330 d | N, P | F; I; V | 0.6–4.8 | 100 | [90,184] | ||
| Tepary bean | Temperate/Tropic | Guatemala, Mexico, USA | 8; >32; 17–25 | 60–120 d | 400–1700 | P | B; F; I; N; V | 0.4–1.7 | Bradyrhizobium, Rhizobium leguminosarum bv. phaseoli | [39,90,130,149,185] | |
| Urad | Tropic | India, Pakistan | -; -; 25–35 no frost | 60–140 d | 600–1000 | P, B | F; I | 0.3–2.5 | 18 | Bradyrhizobium yuanmingense | [62,153,154,186] |
| Velvet bean | Tropic | India | 5; -;19–27 | D or Y | 1200–1500 | I; N | 0.5–3.4 | 60–330 | Bradyrhizobium sp. | [90,98,187,188,189,190,191] | |
| Winged bean | Tropic | India, Indonesia, Philippines, Sri Lanka | -; -;20–30 | 120–180 d | 1000 | F; I | 0.7–1.9 | Rhizobium spp. NGR234 | [116,192] | ||
| Yam bean | Tropic | Costa Rica, India, Mexico, Peru | -; 35–40; - | 2 Y | N, P, K | N; I | A * | 165–215 | Rhizobium spp. NGR234 | [64,116,193,194] | |
| Broad bean | Temperate/Tropic | Australia, China, Ethiopia, EU, Jordan, USA | −12; 30;18–27 | 90–220 d | 700–1200 | N, P, K, Ca | F; I; N; V | 1.1–2.2 | 33–550 | Rhizobium Leguminosarum | [39,92,98,130,132,195,196] |
| Chickpea | Temperate/Tropic | EU, Middle East, South Africa | −11; >32; 10–29 | 90–180 d | 500–1800 | P, Zn, S, B | F; I; N; V | 1–5.5 | 35–140 | Rhizobium cicerii | [39,92,98,130,153,195,197,198,199,200,201] |
| Common bean | Temperate | Australia, Africa, Canada, EU, Middle East, USA | −9; 38; - | 70–110 d | 274–550 | N, P, S, Mo | F; I; V | 0.9–2.6 | 465 | Rhizobium leguminosarum; Rhizobium tropici | [202,203,204,205,206,207,208,209,210,211,212,213] |
| Field pea | Temperate/Tropic | Canada, China, EU, India, Russia | <0; -; 7–30 | 90–180 d | 400–1000 | N, P, K, Mg | F; I; N; V | 1–4 | 30–96 | Rhizobium leguminosarum | [39,92,98,130,132,156,195,214] |
| Lentil | Temperate | Australia, Canada, EU, Middle East | 2; 35; 6–27 | 80–130 d | 300–2400 | N, P, K | F; I; N; V | 0.8–7 | 50 | Rhizobium | [39,92,98,102,130,153,195,215,216] |
| Lupin | Temperate | Australia, EU, Middle East, Ukraine | <0; -;18–24 | 115–330 d | 400–1000 | P, Fe | F; I; V | 0.5–5 | 90–400 | Rhizobium lupini | [39,92,98,132,195,217,218,219,220] |
Appendix B
| Pulses—Common Name | Energy (kcal/100 g) | Protein (%) | Oil (Saturated FA) (%) | Carbohydrate (%) | Fiber (%) | Vitamins | Minerals | Reference |
|---|---|---|---|---|---|---|---|---|
| Acacia leucophloea | 382 | 27 | 5 | 58 | 7 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [88,221] | |
| Adzuki bean | 329 | 19.9 | 0.5 (0.2) | 62.9 | 12.7 | B1, B2, B3, B6, B9, A | Ca, Fe, Mg, P, K, Na, Zn | [38,222] |
| African locust bean | 414 | 24–34 | 19–23 | 67 | 11.7 | C | Ca, Fe, Mg, K, Na, Zn, Cu, Mn | [99,223,224,225,226,227] |
| African nut bean | 649 | 26.3 | 58.1 | 4.6 | 2.7 | Ca, Fe, Mg, P, Zn, Cu | [99] | |
| African oil bean | 206 | 48.5 | 33.4 | 8.9 | 6.3 | C, B1, B2, B3 | Ca, Fe, Mg, P, K, Na, Cu, Mn, Pb | [71,99,226,228] |
| African yam bean | 365 | 20.5 | 1–12.2 | 65–78 | 7–12 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [71,79,229,230] | |
| Bambara groundnut | 408 | 18–30 | 6.2 (2.0) | 33.4–68.5 | 1.9 | C, B1, B2, B3, | Ca, Fe, Mg, P, K, Na, Se | [40,104,231,232,233,234,235] |
| Butterfly pea | - | 25.2 | 3.7 | 19.9 | 9.2 | Ca, P | [236] | |
| Cowpea | 343 | 23.9 | 2.1 (0.5) | 59.6 | 10.7 | C, B1, B2, B3, B6, B9, A | Fe, Mg, Zn, Cu, Mn, Cr, Ni, Al, Pb | [38,40,237,238] |
| Ervil | 324 | 20–28 | 11–16 | 61 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [239,240,241,242,243] | ||
| Fenugreek | 323 | 23.0 | 6.4 | 58.0 | 25.0 | C, B1, B2, B3, B6, B9 | Ca, Fe, Mg, P, K, Na, Zn, Mn | [38,244,245] |
| Grass pea | - | 24.4 | 2.8 (0.8) | 55.94 | 11.4 | C, B1, B2 | Ca, Fe, Mg, P, Zn | [155,221,246] |
| Guanacaste | - | 33.9 | 2.8 (0.1) | 56.8 | 1.3 | Ca, Fe, Mg, K, Na, Zn, Cu | [236,247] | |
| Horse gram | 280 | 22 | 0.6 | 37.5 | 5.7 | Ca, Fe, Mg, P, Zn, Mn, Cu | [248,249,250] | |
| Housa groundnut | 367 | 19–21 | 1.1 | 67–74 | 5.5 | C, B1, B2, B3 | Ca, Fe, P, K | [38,40] |
| Itching bean | 382 | 27–37 | 6.6–8.8 | 46–53 | 6–10 | C, B3 | Ca, Fe, Mg, P, K, Na, Cu, Mn | [28,251,252] |
| Jack bean | 389 | 21–27 | 3.5 (0.3) | 60.6 | 2–8 | - | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn, Pb | [40,253,254] |
| Kedaung | 20.1 | 20 (13) | 0.98 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [255,256] | |||
| Kidney bean | 333 | 23.6 | 0.8 (0.1) | 30.0 | 24.0 | C, B1, B2, B3, B9, K, E | Ca, Fe, Mg, P, K | [38,237,257] |
| Lablab | 344 | 21–29 | 1.7 (0.3) | 60.74 | 25.6 | B1, B2, B3, B6, B9 | Ca, Fe, Mg, P, K, Na, Zn | [38,40,258,259] |
| Lima bean | 338 | 21.5 | 0.7 (0.2) | 63.4 | 19.0 | B1, B2, B3, B6, B9, K, E | Ca, Fe, Mg, P, K, Na, Zn | [38,230] |
| Morama bean | 635 | 29–38 | 32–42 (10) | 18.9 | 19–27 | E | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [260,261] |
| Moth bean | 343 | 23–26 | 1.6 (0.4) | 61.5 | 5 | C, B1, B2, B3, B6, B9, A | Ca, Fe, Mg, P, K, Na, Zn | [38,40] |
| Mung bean | 347 | 15–28 | 1.2 (0.4) | 62.6 | 16.3 | C, B1, B2, B3, B6, B9, A | Ca, Fe, Mg, P, K, Na, Zn | [38,40,235,262,263] |
| Narbon bean | 271 | 26.9 | 10–15 | 52–53 | Ca, Fe, Mg, P, K, Zn, Cu, Mn, S | [241,264,265] | ||
| Navy bean | 337 | 22.3 | 1.5 (0.2) | 60.8 | 15.3 | B1, B2, B3, B6, B9 | Ca, Fe, Mg, P, K, Na, Zn | [38,266] |
| Pigeon pea | 343 | 13–26 | 1.5 (0.3) | 62.8 | 15 | B1, B2, B3, B6, B9, A | Ca, Fe, Mg, P, K, Na, Zn | [38,40] |
| Pinto bean | 347 | 21.4 | 1.2 (0.2) | 62.6 | 15.5 | C, B9, K, E | Ca, Fe, Mg, P, K, Na, Zn | [38,266] |
| Pinto peanut | 27.1 | 49.7 (9.4) | 21.4 | [267] | ||||
| Protein pea | 352 | 23.8 | 1.2 (0.2) | 63.7 | 25 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Purple mucuna | 417 | 23.9 | 13.3 (5.3) | 51.7 | 8.1 | C, B3 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [268] |
| Red moneywort | 439 | 16–27 | 14.0 (2.8) | 54.6 | 4.25 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [269,270] | |
| Rice bean | 338 | 18–19 | 0.5 (0.3) | 59.1 | 7.1 | C, B2 | Ca, Fe, P, Zn, Cu, Mn | [38,40,75,172] |
| Scarlet runner bean | 338 | 20.3 | 1.8 (-) | 62.0 | 4.8 | C, B1, B2, B3 | Ca, Fe, P | [231] |
| Sesban | 459 | 30–40 | 5–6 (1–2) | 45–47 | 11–16 | [83,271] | ||
| Sword bean | 361 | 24–30 | 2.6–9.8 (-) | 41–59 | 7–13 | C, B1, B2, | Ca, Fe, Mg, P, K, Na, Zn, Cu, Pb, Hg | [40,253,272,273] |
| Tamarind | 239 | 24–25 | 8–12.5 | 10–19 | 3–4 | C, B1, B2, B3 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [179,274,275,276] |
| Tarwi | 440 | 41–51 | 14–24 (19) | 28.2 | 7.1 | [40,184,277] | ||
| Tepary bean | 353 | 19–24 | 1.2 (-) | 67.8 | 4.8 | B1, B2, B3 | Ca, Fe, P, K, Na | [40,231,262,278] |
| Urad | 341 | 25.2 | 1.6 (0.1) | 59.0 | 18.3 | B1, B2, B3, B6, B9, A | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Velvet bean | 373 | 20–29 | 6–7 | 50–61 | 9–11 | Ca, Fe, Mg, P, K, Na, Zn, Cu, Mn | [38,39,279,280,281,282,283,284,285] | |
| Winged bean | 428 | 30–35 | 16.3 (2.3) | 41.7 | 11–26 | B1, B2, B3, B6, B9 | Ca, Fe, Mg, P, K, Na, Zn | [38,40,281] |
| Yam bean | 390 | 10–32 | 24–26 | 31–33 | 7–8 | C, B1, B2, B3, B6 | Ca, Fe, Mg, P, K, Na, Zn, Mn, Se | [72,286,287] |
| Broad bean | 341 | 26.1 | 1.53 (0.3) | 58.3 | 25 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38,288] |
| Chickpea | 378 | 11–31 | 6.0 (0.6) | 63.0 | 12.2 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38,40,289,290,291] |
| Common bean | 20–24 | 0.8 (0.6) | 75.5 | B9, A | Ca, Fe, Mg, K, P, Na, Zn, Mn, Se, S, B | [292,293,294] | ||
| Field pea | 352 | 23.8 | 1.2 (0.2) | 63.7 | 25 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Lentil | 352 | 24.6 | 1.1 (0.2) | 63.4 | 10.7 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn, Se | [38,295,296,297] |
| Lupin | 371 | 36.2 | 9.7 (1.2) | 40.37 | 18.9 | C, B1, B2, B3, B6, B9 | Ca, Fe, Mg, P, K, Na, Zn | [38,298] |
Appendix C
| Other Crops—Common Name | Latin Name | Energy (Kcal) | Protein (%) | Oil (Saturated FA) (%) | Carbohydrate (%) | Fiber (%) | Vitamins | Minerals | References |
|---|---|---|---|---|---|---|---|---|---|
| African walnut | Tetracarpidium conophorum | 30.1 | 43.4 | 16.9 | 2.6 | Ca, Fe, Mg, K, Mn, Ni, Pb, Na, Cu | [299,300] | ||
| Almond | Prunus dulcis | 579 | 21.2 | 49.9 (3.8) | 21.6 | 12.5 | B1, B2, B3, B6, B9, E | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Cashew | Anacardium occidentale | 552 | 18.2 | 43.9 (7.8) | 30.2 | 3.3 | C, B1, B2, B3, B6, B9, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Castor bean | Ricinus communis | 579 | 20.2 | 45.0 | 3.1 | Ca, Fe, Mg, P, K, Na, Zn | [226] | ||
| Chia | Salvia hispanica | 486 | 16.5 | 30.7 (3.3) | 42.1 | 34.4 | C, B1, B2, B3, A, E | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Conophor nut | Tetracarpidium conophorum | 590 | 22.8 | 4902 | 5.5 | Ca, Fe, Mg, P, K, Na, Zn | [226] | ||
| Cotton | Gossypium hirsutum | 26–46 | 30–38 | 17.3 | Ca, Mg, P, K, S | [301,302,303] | |||
| Cram Cram | Cenchrus Biflorus | 370 | 17.8 | 8.5 | 62.3 | B1, B2, B3 | Ca, Fe, P | [231] | |
| Egusi melon | Citrullus colocynthis | 537 | 31.4 | 43.9 | 6.6 | B1, B2, B3 | Ca, Fe, Mg, P, K, Na, Zn, S | [226,304] | |
| Flaxseed | Linum usitatissimum | 376 | 24.4 | 30.9 (2.9) | 0 | 38.6 | B1, B2, B3, K | Ca, Fe, Mg, P, K, Na, Zn, Mn | [305] |
| Groundnut a | Arachis hypogaea | 570 | 25.1 | 47.6 | 20.9 | 8.7 | B1, B2, B3, B6, B9 | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Hazelnut | Corylus avellana | 628 | 15.0 | 60.8 (4.4) | 19.2 | 11.2 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Hemp | Cannavis sativa | 553 | 31.6 | 48.8 (4.6) | 8.7 | 4.0 | C, B1, B2, B3, B6, B9, A, E | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Kalahari white bautinia | Bautinia Petersiana | 371 | 22.9 | 13.1(3.0) | 40.2 | 13.0 | B1, B2, B3 | Ca, Fe, P | [39] |
| Linseed | Linum usitatissimum | 534 | 18.3 | 42.2 (3.6) | 28.9 | 27.3 | B1, B2, B3, B5, B6, B9, B12, E, K | Ca, Fe, Ni, P, K, Na, Zn, Mn, Se, Co, Cu, Cr | [38] |
| Millet | Pennisetum glaucum | 378 | 11.0 | 4.2 (0.7) | 72.9 | 8.5 | B1, B2, B3, B6, B9, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Pistachio | Pistacia Vera | 560 | 20.2 | 45.3 (5.9) | 27.2 | 10.6 | C, B1, B2, B3, B6, B9, A, E | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Quinoa | Chenopodium quinoa | 368 | 14.1 | 6.1 (0.7) | 64.2 | 7.0 | B1, B2, B3, B6, B9, A, E | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Rapeseed | Brassica napus | 18–20 | 43–45 | 23–27 | [306,307] | ||||
| Sesame | Sesamun indicum | 573 | 17.7 | 49.7 (7.0) | 23.5 | 11.8 | B1, B2, B3, B6, B9, A, E | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Soybean | Glycine Mac Merrill | 446 | 36.5 | 19.9 (2.9) | 30.2 | 9.3 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Spirulina (dried) | Arthrospira maxima | 290 | 57.5 | 7.7 (2.7) | 23.9 | 3.6 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
| Sunflower (kernel) | Helianthus annuus | 584 | 18–21 | 51.0 (4.4) | 20 | 8.6 | C, B1, B2, B3, B6, B9, A, E | Ca, Fe, Mg, P, K, Na, Zn, Se | [38] |
| Walnut | Juglans spp. | 654 | 15.2 | 65.2 (6.1) | 13.7 | 6.7 | C, B1, B2, B3, B6, B9, A, E, K | Ca, Fe, Mg, P, K, Na, Zn | [38] |
Appendix D
| Crops—Common Name | Phytic Acid (mg/g) | Tannins Total (mg/g) | Saponins () | Trypsin Inhibitors (mg/100 g) | Lectins (mg/100 g) | Others a | References |
|---|---|---|---|---|---|---|---|
| Acacia leucophloea | Presence | 0.01 | Presence | [221] | |||
| Adzuki bean | 2.9 | [308] | |||||
| African locust bean | 0.6 | 0.81 | 19.4 | HCN; OX | [224,225,226] | ||
| African nut bean | Presence | 0.07–0.3 | OX | [309] | |||
| African oil bean | 41 | 7.9 | 17.8 | HCN | [99,226] | ||
| African yam bean | 4.3–14.9 | 18.1 | 1.2 | 6.7 | HCN; OX | [79,310] | |
| Bambara groundnut | 0.5–14.8 | tr-5.0 | 1.4 | 6.7 | OX | [232,311,312,313] | |
| Butterfly pea | 11.5 | 8.7 | HCN; L-DOPA i | [236] | |||
| Castor bean | 0.89 | 0.11 | OX; A | [226] | |||
| Conophor nut | 2.1 | 0.21 | OX | [226] | |||
| Cotton | 0.1 | GO | [302] | ||||
| Cowpea | 1.4–3.8 | 1.4–10.2 | 0.3 | 26.4 b | HCN; OX | [82,311,314,315] | |
| Egusi melon | 4.1 | 0.8 | OX | [226] | |||
| Ervil | Presence | Presence | PA | [239,241] | |||
| Fenugreek | 0.1–0.9 | [244,245] | |||||
| Grass pea | 3.0 | 0.2–0.8 | 19.64 b | β-ODAP; CTI | [246,316,317,318,319] | ||
| Groundnut h | 4.18 | 0.04 | 80.8 b | OX; HCN; AL; AI | [87] | ||
| Guanacaste | 9.5 | 3.7 | HCN; L-DOPA | [236] | |||
| Horse gram | 2 | 865 | [248,250] | ||||
| Housa groundnut | Presence | Presence | Presence | [320] | |||
| Itching bean | 4.7–56.8 | 1.8–3.3 | 1.2–1.3 | 43.2–43.7 b | HCN | [28,251,311] | |
| Jack bean | 12.0–13.7 | Tr-0.7 | 1.8 | 16.4 b | HCN; CV; OX | [230,311,314,321,322] | |
| Kedaung | 98.3 | 0.3 | 6.94 b | [81,142] | |||
| Kidney bean | 17.3–24.1 | 5.4–28.8 | 0.9–23 | 4.6–29.3 b | 1.92–9.98 c | HCN; OX | [82,323] |
| Lablab | 6.1–15.7 | 0.2–0.4 | 1.3 | 19.7 b | OX | [230,258,311,314,322] | |
| Lima bean | 13.6 | 6.5–9.1 | 1.2–1.5 | 2.1–17.2 b | HCN; OX | [230,308,311,322] | |
| Linseed | HCN | [324] | |||||
| Morama bean | 0.08 | Presence | [263,325] | ||||
| Moth bean | 3.8–4.2 | 4.8–13 | 33 | 28.3–31.4 b | HCN | [82,311] | |
| Mung bean | 5.8–7.4 | 4.4–8.0 | 2.8–35 | 15.8 b | 2670 d | OX | [82,84,308,326] |
| Narbon bean | Presence | Presence | PA | [239,241,327] | |||
| Navy bean | 12.9–15.8 | 39.9 | 20–160 | 5.9 f | 3.8 c | OX | [82,328,329,330] |
| Pigeon pea | 7.3–16.2 | 3.8–17.1 | 0.04–1.4 | 4.1–19.2 b | HCN; OX; CTI; PA | [40,82,230,311,331] | |
| Pinto bean | 2.6 | 2.3 c | OX | [82,266,308,330] | |||
| Pinto peanut | Presence | ||||||
| Purple mucuna | 3.8–4.5 | 1.8–3.4 | 39.2–44.1 | HCN | [311] | ||
| Rapeseed | Presence | Presence | Glucosinolates | [324] | |||
| Red moneywort | Presence | [269] | |||||
| Rice bean | 3.3–20.3 | 2.4 | 2.3 | 34.3–40.6 | Tr | HCN | [75,172,311,332] |
| Scarlet runner bean | Presence * | Presence * | [333,334] | ||||
| Sesban | 18–51 | 19 | 5.2–14.6 | 50–140 | Presence | PA | [83,272] |
| Sword bean | 3.5–21.4 | 0.01–570 | 1.7–5.2 | 17.4–26.8 b | HCN; OX | [80,230,311,322,335] | |
| Tamarind | Presence | Presence | Presence | [180,336] | |||
| Tarwi | T | AL; HCN | [184] | ||||
| Tepary bean | 1–37 | 11.5–18.0 b | 1.4–18.2 e | [82] | |||
| Urad | 11.2–14.6 | 5.4–11.9 | 0.2–23 | 94.2 g | [82,308,314,337] | ||
| Velvet bean | 5.0–53.6 | 1.8–3.1 | 43.2–52.8 | AL; HCN; L-DOPA | [28,311,321,338,339,340,341,342] | ||
| Winged bean | 7.8–12.3 | 0.3–12.6 | 0.01–0.14 e | 76 e | CTI | [343,344] | |
| Yam bean | Tr | Tr | 0.01 | 0.0003 | HCN | [287] | |
| Broad bean | 6.4 | 0.1–24.1 | 0.4–370 | 1.7–3.3 b | 25–100 d | CTI; L-DOPA | [82,87,311,345] |
| Chickpea | 1.2–12.1 | 08–5.9 | 0.09–600 | 11.9 b | 6.22 d | HCN; OX; PA; CTI | [40,82,311,346] |
| Common bean | 8.2 | 0–4.64 g | 8.57 | CTI; AI | [82,294,347] | ||
| Field pea | 2.2–7.4 | 0.2–13 | 18–110 | 1.5–108 | 5.1–150.6 | OS; PA; AI; HCN; CTI | [82,348] |
| Lentil | 2.4–12.4 | 12.8 | 0.04–0.13 | 2.8 | OX; AI | [349,350] | |
| Lupin | 1.4–3.5 | ABS | ABS | ABS | ABS | PH; AL | [351,352,353] |
| Soybean | 10.0–23.0 | 0.2–5.6 | 0.2–1.12 b | [230,311,354] |
References
- De Boer, J.; Aiking, H. On the merits of plant-based proteins for global food security: Marrying macro and micro perspectives. Ecol. Econ. 2011, 70, 1259–1265. [Google Scholar] [CrossRef]
- Ranalli, P. Improvement of pulse crops in Europe. Eur. J. Agron. 1995, 4, 151–166. [Google Scholar] [CrossRef]
- Voisin, A.-S.; Guéguen, J.; Huyghe, C.; Jeuffroy, M.-H.; Magrini, M.-B.; Meynard, J.-M.; Mougel, C.; Pellerin, S.; Pelzer, E. Legumes for feed, food, biomaterials and bioenergy in Europe: A review. Agron. Sustain. Dev. 2013, 34, 361–380. [Google Scholar] [CrossRef]
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume crops phylogeny and genetic diversity for science and breeding. Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef]
- Ayerdi Gotor, A.; Marraccini, E. Crops providing proteins for food: A review. In Proceedings of the 14th ESA Congress—“Growing Landscapes—Cultivating Innovative Agricultural Systems”, Edinburgh, UK, 5–9 September 2016; pp. 27–28. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 10 January 2021).
- Ahlawat, I.; Sharma, P.; Singh, U. Production, demand and import of pulses in India. Indian J. Agron. 2016, 4, S33–S41. [Google Scholar]
- Watson, C.A.; Reckling, M.; Preissel, S.; Bachinger, J.; Bergkvist, G.; Kuhlman, T.; Lindström, K.; Nemecek, T.; Topp, C.F.E.; Vanhatalo, A.; et al. Chapter Four—Grain legume production and use in European agricultural systems. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 144, pp. 235–303. ISBN 0065-2113. [Google Scholar]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- Schneider, A.V.C. Overview of the market and consumption of puises in Europe. Br. J. Nutr. 2002, 88, 243–250. [Google Scholar] [CrossRef]
- Desriers, M. L’agriculture française depuis cinquante ans: Des petites exploitations familiales aux droits à paiement unique. Agreste Cah. 2007, 2, 3–14. [Google Scholar]
- European Commission Development of Plant Proteins in the European Union. Reportt; Commission to the Council/The European Parliament: Brussels, Belgium, 2018; p. 16. [Google Scholar]
- Wezel, A.; Soboksa, G.; McClelland, S.; Delespesse, F.; Boissau, A. The blurred boundaries of ecological, sustainable, and agroecological intensification: A review. Agron. Sustain. Dev. 2015, 35, 1283–1295. [Google Scholar] [CrossRef]
- Bowen, C.R.; Hollinger, S.E. Geographic screening of potential alternative crops. Renew. Agric. Food Syst. 2004, 19, 141–151. [Google Scholar] [CrossRef]
- Von Richthofen, J.-S.; Pahl, H.; Bouttet, D.; Casta, P.; Cartrysse, C.; Charles, R.; Lafarga, A. What do European farmers think about grain legumes? Grain Legumes 2006, 45, 14–15. [Google Scholar]
- Zander, P.; Amjath-Babu, T.S.; Preissel, S.; Reckling, M.; Bues, A.; Schläfke, N.; Kuhlman, T.; Bachinger, J.; Uthes, S.; Stoddard, F.; et al. Grain legume decline and potential recovery in European agriculture: A review. Agron. Sustain. Dev. 2016, 36, 26. [Google Scholar] [CrossRef]
- Magrini, M.-B.; Anton, M.; Cholez, C.; Corre-Hellou, G.; Duc, G.; Jeuffroy, M.-H.; Meynard, J.-M.; Pelzer, E.; Voisin, A.-S.; Walrand, S. Why are grain-legumes rarely present in cropping systems despite their environmental and nutritional benefits? Analyzing lock-in in the French agrifood system. Ecol. Econ. 2016, 126, 152–162. [Google Scholar] [CrossRef]
- Reckling, M.; Döring, T.; Bergkvist, G.; Stoddard, F.; Watson, C.; Seddig, S.; Chmielewski, F.-M.; Bachinger, J. Grain legume yields are as stable as other spring crops in long-term experiments across northern Europe. Agron. Sustain. Dev. 2018, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chimonyo, V.G.P.; Snapp, S.; Chikowo, R. Grain legumes increase yield stability in maize-based cropping systems. Crop Sci. 2019, 59, 1222–1235. [Google Scholar] [CrossRef]
- Mawois, M.; Vidal, A.; Revoyron, E.; Casagrande, M.; Jeuffroy, M.-H.; Le Bail, M. Transition to legume-based farming systems requires stable outlets, learning, and peer-networking. Agron. Sustain. Dev. 2019, 39, 1–14. [Google Scholar] [CrossRef]
- Chloupek, O.; Hrstkova, P. Adaptation of crops to environment. Theor. Appl. Genet. 2005, 111, 1316–1321. [Google Scholar] [CrossRef]
- Udensi, O.; Umana, E.; Edu, E.; Ikpeme, E. Screening locally grown pulses for proximate, anti-nutritive and mineral compositions: Indices for conservation and improvement. Int. J. Agric. Res. 2011, 6, 504–510. [Google Scholar] [CrossRef]
- Jacobsen, S.-E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef]
- Wiȩcicki, W.; Świȩcicki, W.K.; Wolko, B. Lupinus anatolicus—A new lupin species of the old world. Genet. Resour. Crop Evol. 1996, 43, 109–117. [Google Scholar]
- Spataro, G.; Tiranti, B.; Arcaleni, P.; Bellucci, E.; Attene, G.; Papa, R.; Zeuli, P.S.; Negri, V. Genetic diversity and structure of a worldwide collection of Phaseolus coccineus L. Theor. Appl. Genet. 2011, 122, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.M.; Singh, L.N. A review of the pest complex of Kharif pulses in Uttar Pradesh. PANS 1976, 22, 333–335. [Google Scholar] [CrossRef]
- Tresina, P.S.; Mohan, V.R. Assessment of nutritional and antinutritional potential of underutilized legumes of the Genus mucuna. Trop. Subtrop. Agroecosyst. 2013, 16, 155–169. [Google Scholar]
- Rana, J.C.; Gautam, N.K.; Gayacharan, M.S.; Yadav, R.; Tripathi, K.; Yadav, S.K.; Panwar, N.S.; Bhardwaj, R. Genetic resources of pulse crops in India: An overview. Indian J. Genet. Plant Breed. 2016, 76, 420. [Google Scholar] [CrossRef]
- Thomson, B.; Siddique, K.; Barr, M.; Wilson, J. Grain legume species in low rainfall Mediterranean-type environments I. Phenology and seed yield. Field Crop. Res. 1997, 54, 173–187. [Google Scholar] [CrossRef]
- Saka, J.O.; Ajibade, S.R.; Adeniyan, O.N.; Olowoyo, R.B.; Ogunbodede, B.A. Survey of underutilized grain legume production systems in the southwest agricultural zone of Nigeria. J. Agric. Food Inf. 2004, 6, 93–108. [Google Scholar] [CrossRef]
- Azam-Ali, S. Agricultural diversification: The potential for underutilised crops in Africa’s changing climates. Riv. Biol. Biol. Forum 2007, 1, 27–28. [Google Scholar] [CrossRef]
- Kaoneka, S.R.; Saxena, R.K.; Silim, S.N.; Odeny, D.A.; Rao, N.V.; Shimelis, H.A.; Siambi, M.; Varshney, R.K. Pigeonpea breeding in eastern and southern Africa: Challenges and opportunities. Plant Breed. 2016, 135, 148–154. [Google Scholar] [CrossRef]
- Vadivel, V.; Janardhanan, K. Nutritional and antinutritional characteristics of seven south Indian wild legumes. Plant Foods Hum. Nutr. 2005, 60, 69–75. [Google Scholar] [CrossRef]
- Vadivel, V.; Biesalski, H.K. Bioactive compounds in velvet bean seeds: Effect of certain indigenous processing methods. Int. J. Food Prop. 2012, 15, 1069–1085. [Google Scholar] [CrossRef]
- Pullin, A.S.; Stewart, G.B. Guidelines for systematic review in conservation and environmental management. Conserv. Biol. 2006, 20, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Elsevier. Scopus® Quick Reference Guide; Elsevier: Amsterdam, The Netherlands, 2019; p. 12. [Google Scholar]
- USDA. Food Composition Database. 2018. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 28 October 2018).
- PROTA. Céréales et Légumes Secs; PROTA: Ankara, Turkey, 2006; ISBN 978-90-5782-172-1. [Google Scholar]
- FAO. Utilisation Des Aliments Tropicaux: Légumineuses Tropicales (47/4); Cahiers Techniques de la FAO; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Giraldeli, A.; Gregorio, J.S.; Monquero, P.; Aguillera, M.; Ribeiro, N. Weeds hosts of nematodes in sugarcane culture. Planta Daninha 2017, 35. [Google Scholar] [CrossRef][Green Version]
- Correia, M.V.; Pereira, L.C.; De Almeida, L.; Williams, R.L.; Freach, J.; Nesbitt, H.; Erskine, W. Maize mucuna (Mucuna pruriens (L.) DC) relay intercropping in the lowland tropics of Timor-Leste. Field Crop. Res. 2014, 156, 272–280. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Hameed, A.G.A.-E. Molecular and biochemical markers of some Vicia faba L. genotypes in response to storage insect pests infestation. J. Plant Interact. 2014, 9, 618–626. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Tomás, D.; Silva, M.; Lopes, S.; Viegas, W.; Veloso, M.M. Genetic diversity and population structure in Vicia faba L. landraces and wild related species assessed by nuclear SSRs. PLoS ONE 2016, 11, e0154801. [Google Scholar] [CrossRef]
- Hanly, J.A.; Gregg, P.E.H. Green-manure impacts on nitrogen availability to organic sweetcorn (Zea mays). N. Z. J. Crop. Hortic. Sci. 2004, 32, 295–307. [Google Scholar] [CrossRef]
- Jayanthi, M.; Umarani, R.; Vijayalakshmi, V. Effect of seed fortification with pulse sprout extract on crop growth and seed yield in rice seeds. Asian J. Crop. Sci. 2013, 5, 444–451. [Google Scholar] [CrossRef][Green Version]
- Caracuta, V.; Vardi, J.; Paz, Y.; Boaretto, E. Farming legumes in the pre-pottery Neolithic: New discoveries from the site of Ahihud (Israel). PLoS ONE 2017, 12, e0177859. [Google Scholar] [CrossRef] [PubMed]
- Lykke, A.M.; Mertz, O.; Ganaba, S. Food consumption in rural Burkina Faso. Ecol. Food Nutr. 2002, 41, 119–153. [Google Scholar] [CrossRef]
- Malau-Aduli, B.S.; Eduvie, L.; Lakpini, C.; Malau-Aduli, A.E.O. Crop-residue supplementation of pregnant does influences birth weight and weight gain of kids, daily milk yield but not the progesterone profile of Red Sokoto goats. Reprod. Nutr. Dev. 2004, 44, 111–121. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Girón-Calle, J.; Vioque, J. Antioxidative activity in the seeds of 28 Vicia species from southern Spain. J. Food Biochem. 2011, 35, 1373–1380. [Google Scholar] [CrossRef]
- Cernay, C.; Pelzer, E.; Makowski, D. A global experimental dataset for assessing grain legume production. Sci. Data 2016, 3, 160084. [Google Scholar] [CrossRef]
- Tancoigne, E.; Barbier, M.; Cointet, J.-P.; Richard, G. The place of agricultural sciences in the literature on ecosystem services. Ecosyst. Serv. 2014, 10, 35–48. [Google Scholar] [CrossRef]
- Ruiz-Martinez, I.; Marraccini, E.; Debolini, M.; Bonari, E. Indicators of agricultural intensity and intensification: A review of the literature. Ital. J. Agron. 2015, 10, 74. [Google Scholar] [CrossRef]
- Metzger, M.J.; Bunce, R.G.H.; Jongman, R.H.G.; Mücher, C.A.; Watkins, J.W. A climatic stratification of the environment of Europe. Glob. Ecol. Biogeogr. 2005, 14, 549–563. [Google Scholar] [CrossRef]
- Major, D.J.; Brown, D.M.; Bootsma, A.; Dupuis, G.; Fairey, N.A.; Grant, E.A.; Green, D.G.; Hamilton, R.I.; Langille, J.; Sonmor, L.G.; et al. An evaluation of the corn heat unit system for the short season growing regions across Canada. Can. J. Plant Sci. 1983, 63, 121–130. [Google Scholar] [CrossRef]
- Ray, H.; Bett, K.; Tar’An, B.; Vandenberg, A.; Thavarajah, D.; Warkentin, T. Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Sci. 2014, 54, 1698–1708. [Google Scholar] [CrossRef]
- Ribeiro, H.L.C.; Boiteux, L.S.; Santos, C.A.F. Genetic parameters of earliness and plant architecture traits suitable for mechanical harvesting of cowpea (Vigna unguiculata). Aust. J. Crop Sci. 2014, 8, 1232–1238. [Google Scholar]
- De Oliveira, J.T.; Ribeiro, I.D.S.; Roque, C.G.; Montanari, R.; Gava, R.; Teodoro, P.E. Contribution of morphological traits for grain yield in common bean. Biosci. J. 2018, 34, 951–956. [Google Scholar] [CrossRef]
- Alliprandini, L.F.; Abatti, C.; Bertagnolli, P.F.; Cavassim, J.E.; Gabe, H.L.; Kurek, A.; Matsumoto, M.N.; De Oliveira, M.A.R.; Pitol, C.; Prado, L.C.; et al. Understanding soybean maturity groups in Brazil: Environment, cultivar classification, and stability. Crop. Sci. 2009, 49, 801–808. [Google Scholar] [CrossRef]
- Jia, H.; Jiang, B.; Wu, C.; Lu, W.; Hou, W.; Sun, S.; Yan, H.; Han, T. Maturity group classification and maturity locus genotyping of early-maturing soybean varieties from high-latitude cold regions. PLoS ONE 2014, 9, e94139. [Google Scholar] [CrossRef] [PubMed]
- Appunu, C.; N’Zoue, A.; Moulin, L.; Depret, G.; Laguerre, G. Vigna mungo, V. radiata and V. unguiculata plants sampled in different agronomical–ecological–climatic regions of India are nodulated by Bradyrhizobium yuanmingense. Syst. Appl. Microbiol. 2009, 32, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Bouget, M. Les Légumineuses Vivrières. Le Technicien d’agriculture Tropicale; Editions Maisonneuve et Larose: Paris, France, 1989; 162p. [Google Scholar]
- Sørensen, M.; Heller, J.; Engels, J. Yam Bean. Pachyrhizus DC.–Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1996; Volume 2, ISBN 978-92-9043-282-1. [Google Scholar]
- Klimek-Kopyra, A.; Kulig, B.; Oleksy, A.; Zajac, T. Agronomic performance of naked oat (Avena nuda L.) and faba bean intercropping. Chil. J. Agric. Res. 2015, 75, 168–173. [Google Scholar] [CrossRef]
- Akobundu, I.O.; Udensi, U.E.; Chikoye, D. Velvetbean (Mucuna spp.) suppresses speargrass (Imperata cylindrica (L.) Raeuschel) and increases maize yield. Int. J. Pest Manag. 2000, 46, 103–108. [Google Scholar] [CrossRef]
- Paulo, E.M.; Berton, R.S.; Cavichioli, J.C.; Bulisani, E.A.; Kasai, F.S. Produtividade do cafeeiro Mundo Novo enxertado e submetido à adubação verde antes e após recepa da lavoura. Bragantia 2006, 65, 115–120. [Google Scholar] [CrossRef][Green Version]
- Lusembo, P.; Ebong, C.; Sabiiti, E.N. Integration of cassava tuber and forage legume seed production for sustained soil fertility. Trop. Agric. 1998, 75, 18–20. [Google Scholar]
- Ribeiro, G.M.; Neto, F.B.; De Lima, J.S.S.; Da Silva, M.L.; Júnior, A.P.B.; Dos Santos, E.C. Productive performance of carrot and cowpea intercropping system under different spatial arrangements and population densities. Rev. Caatinga 2018, 31, 19–27. [Google Scholar] [CrossRef]
- Ocimati, W.; Ntamwira, J.; Groot, J.; Taulya, G.; Tittonell, P.; Dhed’A, B.; van Asten, P.; Vanlauwe, B.; Ruhigwa, B.; Blomme, G. Banana leaf pruning to facilitate annual legume intercropping as an intensification strategy in the East African highlands. Eur. J. Agron. 2019, 110, 125923. [Google Scholar] [CrossRef]
- Ameh, G. Proximate and mineral composition of seed and tuber of African yam bean, Sphenostylis stenocarpa (Hoechst. Ex. A. Rich.) harms. ASSET Ser. B 2007, 6, 1–10. [Google Scholar]
- Kisambira, A.; Muyonga, J.H.; Byaruhanga, Y.B.; Tukamuhabwa, P.; Tumwegamire, S.; Grüneberg, W.J. Composition and functional properties of yam bean (Pachyrhizus spp.) seed flour. Food Nutr. Sci. 2015, 06, 736–746. [Google Scholar] [CrossRef]
- Hardarson, G. Methods for enhancing symbiotic nitrogen fixation. Plant Soil 1993, 152, 1–17. [Google Scholar] [CrossRef]
- Liu, J.; You, L.; Amini, M.; Obersteiner, M.; Herrero, M.; Zehnder, A.J.B.; Yang, H. A high-resolution assessment on global nitrogen flows in cropland. Proc. Natl. Acad. Sci. USA 2010, 107, 8035–8040. [Google Scholar] [CrossRef]
- Bepary, R.H.; Wadikar, D.D.; Patki, P.E. Rice bean: Nutritional vibrant bean of Himalayan belt (Northeast India). Nutr. Food Sci. 2016, 46, 412–431. [Google Scholar] [CrossRef]
- Spencer, P.F.; Schaumburg, H.H. Lathyrism: A neurotoxic disease. Neurobehav. Toxicol. Teratol. 1983, 5, 625–629. [Google Scholar] [PubMed]
- Franco, F. Decreto 2484/1967, de 21 de Septiembre; El Código Alimentario Español (CAE): Barcelona, Spain, 1967; Volume 8. [Google Scholar]
- Campbell, C.G.; Mehra, R.B.; Agrawal, S.K.; Chen, Y.Z.; El Moneim, A.M.A.; Khawaja, H.I.T.; Yadov, C.R.; Tay, J.U.; Araya, W.A. Current status and future strategy in breeding grasspea (Lathyrus sativus). Euphytica 1993, 73, 167–175. [Google Scholar] [CrossRef]
- Ndidi, U.S.; Ndidi, C.U.; Olagunju, A.; Muhammad, A.; Billy, F.G.; Okpe, O. Proximate, antinutrients and mineral composition of raw and processed (boiled and roasted) Sphenostylis stenocarpa seeds from southern Kaduna, northwest Nigeria. ISRN Nutr. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abitogun, A.; Oso, G.K. Assessment of processing methods on the chemical composition of sword bean (Canavaliagladiata). IOSR J. Appl. Chem. 2014, 7, 106–112. [Google Scholar] [CrossRef]
- Sala, J.S.; Salam, P.; Pots, K.S.; Kuma, D.B. Effect of processing methods on secondary metabolites and enzyme inhibitors in different developmental stages of Parkia roxburghii G. Don Pods. Am. J. Food Technol. 2014, 9, 89–96. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Hossain, M.; Becker, K. Nutritive value and antinutritional factors in different varieties of Sesbania seeds and their morphological fractions. Food Chem. 2001, 73, 421–431. [Google Scholar] [CrossRef]
- Kataria, A.; Chauhan, B.; Punia, D. Antinutrients and protein digestibility (in vitro) of mungbean as affected by domestic processing and cooking. Food Chem. 1989, 32, 9–17. [Google Scholar] [CrossRef]
- Inuwa, H.M.; Aina, V.O.; Gabi, B.; Aimola, I.; Toyin, A. Comparative determination of antinutritional factos in groundnut oil and palm oil. Adv. J. Food Sci. Technol. 2011, 5, 275–279. [Google Scholar]
- Phillipson, J.D. Natural Toxicants in Feeds and Poisonous Plants; Cheeke, P.R., Shull, L.R., Eds.; AVI Publishing Company: Westport, CT, USA, 1985; 492p, ISBN 0870554824. [Google Scholar]
- El-Hady, E.A.; Habiba, R. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT 2003, 36, 285–293. [Google Scholar] [CrossRef]
- Agroforestry Database 4.0. World Agroforestry Acacia leucophloea; World Agroforestry: Nairobi, Kenya, 2019; p. 5. [Google Scholar]
- Lumpkin, T.A.; McClary, D.C. Azuki Bean: Botany, Production and Uses; CAB International: Wallingford, UK, 1994; ISBN 0-85198-765-6. [Google Scholar]
- Haq, N. Underutilized field legumes. In Biology and Breeding of Food Legumes; Pratap, A., Kumar, J., Eds.; CABI: Oxfordshire, UK, 2011; pp. 329–347. [Google Scholar]
- Han, L.L.; Wang, E.T.; Lu, Y.L.; Zhang, Y.F.; Sui, X.H.; Chen, W.F.; Chen, W.X. Bradyrhizobium spp. and Sinorhizobium fredii are predominant in root nodules of Vigna angularis, a native legume crop in the subtropical region of China. J. Microbiol. 2009, 47, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Jauhar, P.P. Grain legumes. In Genetic Resources, Chromosome Engineering, and Crop Improvement; Taylor & Francis: Boca Raton, FL, USA, 2005; Volume 1, ISBN 0849314305. [Google Scholar]
- Fujihara, S.; Terakado, J.; Takenaka, M.; Yoneyama, T. Specific occurrence of β-phenethylamine in root nodules formed from Bradyrhizobium-legume symbiosis. Plant Soil 2002, 238, 123–132. [Google Scholar] [CrossRef]
- Kang, Y.J.; Lee, J.; Kim, Y.H.; Lee, S.-H. Identification of tissue-specific gene clusters and orthologues of nodulation-related genes in Vigna angularis. Plant Genet. Resour. 2014, 12, S21–S26. [Google Scholar] [CrossRef]
- Kumar, R.; Mittal, R.K.; Pandey, D.P. Genetic variability for yield and growth attributes in adzuki bean. Res. Crops 2012, 13, 562–565. [Google Scholar]
- Wang, S.M.; Redden, R.J.; Jiapeng, J.P.H.; Desborough, P.J.; Lawrence, P.L.; Usher, T. Chinese adzuki bean germplasm: 1. Evaluation of agronomic traits. Aust. J. Agric. Res. 2001, 52, 671–681. [Google Scholar] [CrossRef]
- Van Oers, C.C.C.M. Vigna angularis (Willd.) Ohwi & Ohashi. In Pulses; Plant Resources of South-East, Asia; van der Maesen, L.J.G., Somaatmadja, S., Eds.; PROTA: Bogor, Indonesia, 1989. [Google Scholar]
- Feedipedia. List of Feeds. 2020. Available online: https://www.feedipedia.org/content/feeds?category=13596 (accessed on 26 November 2018).
- Janick, J.; Paull, R.E. The Encyclopedia of Fruit & Nuts. 2008. Available online: http://www.credoreference.com/book/cabifruit (accessed on 5 May 2020).
- Lewin, A.; Rosenberg, C.; Meyer, Z.A.H.; Wong, C.H.; Nelson, L.; Manen, J.-F.; Stanley, J.; Dowling, D.; Denarie, J.; Broughton, W.J. Multiple host-specificity loci of the broad host-range Rhizobium sp. NGR234 selected using the widely compatible legume Vigna unguiculata. Plant Mol. Biol. 1987, 8, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Begemann, F.; Mushonga, J. Bambara Groundnut Vigna subterranea (L.) Verdc: Proceedings of the Workshop on Conservation and Improvement of Bambara Groundnut (Vigna subterranea (L.) Verdc.): Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Harare, Zimbabwe, 1997; ISBN 978-92-9043-299-9. [Google Scholar]
- Verheye, W.H. Soils, Plant Growth and Crop Production; EOLSS Publications: Abu Dhabi, United Arab Emirates, 2010; Volume 3, ISBN 978-1-84826-369-7. [Google Scholar]
- Alake, C.O.; Ayo-Vaughan, M.A.; Ariyo, J.O. Selection criteria for grain yield and stability in Bambara groundnut (Vigna subterranean (L) Verdc) landraces. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 433–447. [Google Scholar] [CrossRef]
- Alake, C.O. Genetic variability, gains from selection and genetic correlations for pod yield and nutritional traits in African landraces of Bambara groundnut. Biol. Agric. Hortic. 2017, 34, 71–87. [Google Scholar] [CrossRef]
- Berchie, J.; Sarkodie-A, J.; Adu-Dapaah, H.; Agyemang, A.; Addy, S.; Asare, E.; Donkor, J. Yield evaluation of three early maturing Bambara groundnut (Vigna subterranea L. Verdc) landraces at the CSIR—Crops Research Institute, Fumesua-Kumasi, Ghana. J. Agron. 2010, 9, 175–179. [Google Scholar] [CrossRef]
- Hasan, M.; Uddin, K.; Mohamed, M.T.M.; Zuan, A.T.K. Nitrogen and phosphorus management for Bambara groundnut (Vigna subterranea) production—A review. Legum. Res. Int. J. 2018, 41, 483–489. [Google Scholar] [CrossRef]
- Wassermann, V.D.; Kruger, A.J.; Heyns, G. The response of Bambara groundnut (Voandzeia subterranea) and pigeon pea (Cajanus cajan) to applications of lime, P and K. S. Afr. J. Plant Soil 1984, 1, 4–8. [Google Scholar] [CrossRef]
- Thottappilly, G.; Rossel, H. Identification and characterization of viruses infecting Bambara groundnut (Vigna subterranea) in Nigeria. Int. J. Pest Manag. 1997, 43, 177–185. [Google Scholar] [CrossRef]
- Kocabas, Z.; Craigon, J.; Azam-Ali, S.N. The germination response of Bambara groundnut (Vigna subterranea (L.) Verdc.) to temperature. Seed Sci. Technol. 1999, 27, 303–313. [Google Scholar]
- Sousa, A.C.B.; Carvalho, M.A.; Ramos, A.K.B.; Campos, T.; Sforça, D.A.; Zucchi, M.I.; Jank, L.; Souza, A.P. Genetic studies in Centrosema pubescens benth, a tropical forage legume: The mating system, genetic variability and genetic relationships between Centrosema species. Euphytica 2011, 181, 223–235. [Google Scholar] [CrossRef]
- Odu, C.T.I.; Fayemi, A.A.; Ogunwale, J.A. Effect of pH on the growth, nodulation and nitrogen fixation of Centrosema pubescens and Stylosanthes gracilis. J. Sci. Food Agric. 1971, 22, 57–59. [Google Scholar] [CrossRef]
- Keller-Grein, G.; Schultze-Kraft, R.; Franco, L.H.; Ramirez, G. Multilocational agronomic evaluation of selected Centrosema pubescens germplasm on acid soils. Trop. Grassl. 2000, 34, 65–77. [Google Scholar]
- Morris, J.B. Characterization of butterfly pea (Clitoria ternatea L.) accessions for morphology, phenology, reproduction and potential nutraceutical, pharmaceutical trait utilization. Genet. Resour. Crop. Evol. 2008, 56, 421–427. [Google Scholar] [CrossRef]
- Staples, I.B. Clitoria ternatea L. In Forages, Plant Resources of South-East Asia; Mannetje, L., Jones, R.M., Eds.; PROSEA Foundation: Bogor, Indonesia, 1992. [Google Scholar]
- Adjei-Nsiah, S.; Alabi, B.; Ahiakpa, J.; Kanampiu, F. Response of grain legumes to phosphorus application in the Guinea savanna agro-ecological zones of Ghana. Agron. J. 2018, 110, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Pueppke, S.G.; Broughton, W.J. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant-Microbe Interact. 1999, 12, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Abaidoo, R.; Dare, M.O.; Killani, S.; Opoku, A. Evaluation of early maturing cowpea (Vigna unguiculata) germplasm for variation in phosphorus use efficiency and biological nitrogen fixation potential with indigenous rhizobial populations. J. Agric. Sci. 2016, 155, 102–116. [Google Scholar] [CrossRef]
- Afutu, E.; Agoyi, E.E.; Amayo, R.; Biruma, M.; Rubaihayo, P.R. Cowpea scab disease (Sphaceloma sp.) in Uganda. Crop. Prot. 2017, 92, 213–220. [Google Scholar] [CrossRef]
- Ayala-Escobar, V.; Gomez-Jaimes, R.; Santiago-Santiago, V.; Madariaga-Navarrete, A.; Castaneda-Vildozola, A.; Nava-Diaz, C. Pseudocercospora cruenta on Vigna unguiculata in Mexico. Australas. Plant Dis. Notes 2013, 8, 115–116. [Google Scholar] [CrossRef]
- Chaturvedi, G.S.; Aggarwal, P.K.; Sinha, S.K. Growth and yield of determinate and indeterminate cowpeas in dryland agriculture. J. Agric. Sci. 1980, 94, 137–144. [Google Scholar] [CrossRef]
- Dakora, F.D.; Aboyinga, R.A.; Mahama, Y.; Apaseku, J. Assessment of N2 fixation in groundnut (Arachis hypogaea L.) and cowpea (Vigna unguiculata L. Walp) and their relative N contribution to a succeeding maize crop in Northern Ghana. World J. Microbiol. Biotechnol. 1987, 3, 389–399. [Google Scholar] [CrossRef]
- Haruna, I.; Usman, A. Agronomic efficiency of cowpea varieties (Vigna unguiculata L. Walp) under varying phosphorus rates in Lafia, Nasarawa state, Nigeria. Asian J. Crop. Sci. 2013, 5, 209–215. [Google Scholar] [CrossRef]
- Wu, X.; Xu, P.; Wang, B.; Lu, Z.; Li, G. Association mapping for Fusarium wilt resistance in Chinese asparagus bean germplasm. Plant Genome 2015, 8, plantgenome2014.11.0082. [Google Scholar] [CrossRef]
- Tripathi, K.; Gore, P.G.; Ahlawat, S.P.; Semwal, D.P.; Gautam, N.K.; Kumar, A. Cowpea genetic resources and its utilization: Indian perspective—A review. Legum. Res. Int. J. 2019, 42, 437–446. [Google Scholar] [CrossRef]
- Russi, L.; Acuti, G.; Trabalza-Marinucci, M.; Porta, R.; Rubini, A.; Damiani, F.; Cristiani, S.; Bosco, A.D.; Martuscelli, G.; Bellucci, M.; et al. Genetic characterisation and agronomic and nutritional value of bitter vetch (Vicia ervilia), an underutilised species suitable for low-input farming systems. Crop. Pasture Sci. 2019, 70, 606–614. [Google Scholar] [CrossRef]
- Berger, J.; Robertson, L.; Cocks, P. Agricultural potential of Mediterranean grain and forage legumes: Key differences between and within Vicia species in terms of phenology, yield, and agronomy give insight into plant adaptation to semi-arid environments. Genet. Resour. Crop. Evol. 2002, 49, 313–325. [Google Scholar] [CrossRef]
- Siddique, K.; Loss, S.P.; Regan, K.L.; Jettner, R.L. Adaptation and seed yield of cool season grain legumes in Mediterranean environments of south-western Australia. Aust. J. Agric. Res. 1999, 50, 375. [Google Scholar] [CrossRef]
- Larbi, A.; El-Moneim, A.A.; Nakkoul, H.; Jammal, B.; Hassan, S. Intra-species variations in yield and quality determinants in Vicia species: 1. Bitter vetch (Vicia ervilia L.). Anim. Feed. Sci. Technol. 2011, 165, 278–287. [Google Scholar] [CrossRef]
- Saoub, H.M.; Akash, M.W. Variations among two vetch landrace species in Jordan. J. Food Agric. Environ. 2012, 10, 763–767. [Google Scholar]
- Government of Saskatchewan Inoculation of Pulse Crops. Soils, Fertility, and Nutrients. 2020. Available online: https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/soils-fertility-and-nutrients/inoculation-of-pulse-crops (accessed on 29 November 2018).
- Branch, S. Fenugreek (Trigonella foenum-graecum L.) as a valuable medicinal plant. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 922–931. [Google Scholar]
- Baldoni, R.; Giardini, L. Coltivazioni Erbacee Cereali e Proteaginose; Patron Editore: Granarolo dell’Emilia, Italy, 2001; ISBN 978-88-555-2541-1. [Google Scholar]
- Patto, M.V.; Rubiales, D. Resistance to rust and powdery mildew in Lathyrus crops. Czech J. Genet. Plant Breed. 2014, 50, 116–122. [Google Scholar] [CrossRef]
- Niembro Rocas, A. Enterolobium cyclocarpum (Jacq.) Griseb. In Tropical Tree Seed Manual; Agricultural Handbook; Vozzo, J.A., Ed.; USDA Forest Service: Washington, DC, USA, 2002; pp. 449–451. [Google Scholar]
- Krishna, K.R. Agroecosystems of South India Nutrient Dynamics, Ecology and Productivity; Brown Walker Press: Boca Raton, FL, USA, 2010; ISBN 1-59942-533-5. [Google Scholar]
- Jansen, P.C.M. Macrotyloma uniflorum (Lam.) Verdc. In Pulses; Plant Resources of South-East, Asia; van der Maesen, L.J.G., Somaatmadja, S., Eds.; PUDOC Publishing: Bogor, Indonesia, 1989. [Google Scholar]
- Mohammed, M.; Sowley, E.; Dakora, F. Variations in N2 fixation of field-grown Kersting’s groundnut (Macrotyloma geocarpum) landraces in response to inoculation with two Bradyrhizobium strains in the northern region of Ghana. S. Afr. J. Bot. 2016, 103, 333. [Google Scholar] [CrossRef]
- PROTA4U Macrotyloma geocarpum (Harms) Marechal. 2020. Available online: https://www.prota4u.org/database/protav8.asp?fr=1&g=pe&p=Macrotyloma+geocarpum+(Harms)+Mar%E9chal+&+Baudet (accessed on 29 November 2018).
- Université de Liège. Fiche Technique du Mucuna; Université de Liège: Liège, Belgium, 2014; Volume 1. [Google Scholar]
- Sheahan, C. Plant Guide for Jack Bean (Canavalia ensiformis); USDA Natural Resources Conservation Service: Washington, DC, USA, 2012; Volume 4.
- Miamoto, A.; Dias-Arieira, C.R.; Cardoso, M.R.; Puerari, H.H. Penetration and reproduction of Meloidogyne javanica on leguminous crops. J. Phytopathol. 2016, 164, 890–895. [Google Scholar] [CrossRef]
- Roy, S.S.; Kumar, S.; Sharma, S.; Devi, A.; Singh, N.; Prakash, N.; Ngachan, S. Tree bean (Parkia roxburghii): A potential multipurpose tree legume of northeast India. In Proceedings of the Souvenir & Abstracts National Symposium on Vegetable Legumes for soil and Human Health, Varanasi, India, 12 February 2016; pp. 201–208. [Google Scholar]
- Long, R.; Temple, S.; Schmierer, J.; Canevari, M.; Meyer, R.D. Common Dry Bean Production in California, 2nd ed.; University of California Press: Oakland, CA, USA, 2010. [Google Scholar]
- Bulyaba, R.; Lenssen, A.W. Influence of Bradyrhizobium inoculation and fungicide seed treatment on development and yield of cowpea, lablab, and soybean. Crop. Forage Turfgrass Manag. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Díaz, M.F.; González, A.; Padilla, C.; Curbelo, F. Performance of forage and grain production of Canavalia ensiformis, Lablab purpureus and Stizolobium niveum plantations sown in September. Cuba. J. Agric. Sci. 2003, 37, 65–71. [Google Scholar]
- Rangaiah, D.V.; Dsouza, M. Hyacinth bean (Lablab purpureus): An adept adaptor to adverse environments. Legume Prespectives 2016, 13, 20. [Google Scholar]
- Long, R.; Temple, S.; Meyer, R.; Schwankl, L.; Godfrey, L.; Canevari, M.; Roberts, P. Lima Bean Production in California; University of California, Agriculture and Natural Resources: Berkley, CA, USA, 2014; ISBN 978-1-60107-860-5. [Google Scholar]
- De Araújo, A.S.F.; Antunes, J.E.L.; de Almeida Lopes, A.C.; Ferreira Gomes, R.L. Rhizobia and Lima Bean Symbiosis: Importance, Occurrence and Diversity. 2015. Available online: https://www.researchgate.net/publication/297766907_Rhizobia_and_lima_bean_symbiosis_Importance_occurrenceand_diversity (accessed on 27 November 2018).
- Marsh, L.E.; Davis, D.W. Influence of high temperature on the performance of some Phaseolus species at different developmental stages. Euphytica 1985, 34, 431–439. [Google Scholar] [CrossRef]
- Brink, M.; Belay, G. Plant Resources of Tropical Africa 1. Cereals Ans Pulses; PROTA Foundation: Wageningen, The Netherlands; Backhuys Publishers: Leiden, The Netherlands; CTA: Wageningen, The Netherlands, 2006; ISBN 978-90-5782-170-7. [Google Scholar]
- Pooniya, V.; Choudhary, A.K.; Dass, A.; Bana, R.S.; Rana, K.S.; Rana, D.S.; Tyagi, V.K.; Puniya, M.M. Improved crop management practices for sustainable pulse production: An Indian perspective. Indian J. Agric. Sci. 2015, 85, 747–758. [Google Scholar]
- Van Oers, C.C.C.M. Vigna aconitifolia (Jacq.) Maréchal. In Pulses, Plant Resources of South-East Asia; van der Maesen, L.J.G., Somaatmadja, S., Eds.; PUDOC Publishing: Bogor, Indonesia, 1989. [Google Scholar]
- Riaz Malik, S.; Imran, M.; Asadullah, M.; Jawad, M.; Sarwar, A. Pulses Program. 2018. Available online: http://www.parc.gov.pk/index.php/en/csi/137-narc/crop-sciences-institue/712-national-coordinated-pulses-programme (accessed on 29 November 2018).
- Tariq, S.; Ali, S.; Ijaz, S.S. Improving nitrogen fixation capacity and yield of mungbean and mashbean by phosphorous management in Pothowar. Sarhad J. Agric. 2007, 23, 6. [Google Scholar]
- Chinnasamy, G.; Bal, A.; McKenzie, D. Fatty acid composition of grass pea (Lathyrus sativus L.) seeds. Lathyrus Lathyrism Newsl. 2005, 4, 2–4. [Google Scholar]
- Akhter, M.; Akanda, A.; Kobayashi, K.; Jain, R.; Mandal, B. Plant virus diseases and their management in Bangladesh. Crop. Prot. 2019, 118, 57–65. [Google Scholar] [CrossRef]
- Hardwick, R.C. Review of recent research on navy beans (Phaseolus vulgaris) in the United Kingdom. Ann. Appl. Biol. 1988, 113, 205–227. [Google Scholar] [CrossRef]
- Gonzalez, D.; Obrador, A.A.; Alvarez, J.M. Behavior of zinc from six organic fertilizers applied to a navy bean crop grown in a calcareous soil. J. Agric. Food Chem. 2007, 55, 7084–7092. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.; Loss, S.; Enneking, D. Narbon bean (Vicia narbonensis L.): A promising grain legume for low rainfall areas of south-western Australia. Aust. J. Exp. Agric. 1996, 36, 53–62. [Google Scholar] [CrossRef]
- Nadal, S.; Moreno, M.T. Behaviour of Narbon bean (Vicia narbonensis L.) under presence-absence of broomrape (Orobanche crenata Forsk.) in rainfed agricultural systems in southern Spain. J. Sustain. Agric. 2007, 30, 133–143. [Google Scholar] [CrossRef]
- Enneking, D.; Maxted, N. Narbon bean (Vicia narbonensis L.). In Evolution of Crop Plants, 2nd ed.; Smartt, J., Simmonds, N.W., Eds.; Longman: London, UK, 1995; pp. 316–321. [Google Scholar]
- Rao, J.V.D.K.K.; Dart, P.J. Nodulation, nitrogen fixation and nitrogen uptake in pigeonpea (Cajanus cajan (L.) Millsp) of different maturity groups. Plant Soil 1987, 99, 255–266. [Google Scholar] [CrossRef]
- Smartt, J. Evolution of grain legumes. II. Old and new world pulses of lesser economic importance. Exp. Agric. 1985, 21, 1–18. [Google Scholar] [CrossRef]
- Ahlawat, I.P.S.; Gangaiah, B.; Singh, I.P. Pigeonpea (Cajanus cajan) research in India—An overview. Indian J. Agric. Sci. 2005, 75, 309–320. [Google Scholar]
- Osorno, J.M.; Wal, A.J.V.; Kloberdanz, M.; Pasche, J.S.; Schroder, S.; Miklas, P.N. A new slow-darkening Pinto bean with improved agronomic performance: Registration of “ND-Palomino”. J. Plant Regist. 2017, 12, 25–30. [Google Scholar] [CrossRef]
- Osorno, J.M.; Grafton, K.F.; Rojas-Cifuentes, G.A.; Gelin, R.; Wal, A.J.V. Registration of “Lariat” and “Stampede” Pinto beans. J. Plant Regist. 2010, 4, 5–11. [Google Scholar] [CrossRef]
- Adjolohoun, S.; Bindelle, J.; Adandedjan, C.C.; Tolebq, S.S.; Houinato, M.R.; Sinsin, A.B. Variety and environmental effects on crude protein concentration and mineral composition of Arachis pintoi (Kaprovickas & Gregory) in Benin (West Africa). J. Appl. Biol. Biotechnol. 2013, 1, 24–28. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Delagarde, R.; Bastianelli, D.; Lebas, F. Pinto peanut (Arachis pintoi); INRA: Paris, France; CIRAD: Montpellier, France; AFZ: Ajman, United Arab Emirates; FAO: Rome, Italy, 2016. [Google Scholar]
- Cook, B.G. Arachis pintoi Krap. & Greg., Nom. Nud. In Forages; Plant Resources of South-East, Asia; Mannetje, L., Jones, R.M., Eds.; PUDOC Publishing: Bogor, Indonesia, 1992. [Google Scholar]
- Doignon-Bourcier, F.; Sy, A.; Willems, A.; Torck, U.; Dreyfus, B.; Gillis, M.; de Lajudie, P. Diversity of Bradyrhizobia from 27 tropical leguminosae species native of Senegal. Syst. Appl. Microbiol. 1999, 22, 647–661. [Google Scholar] [CrossRef]
- Khadka, K.; Acharya, B.D. Cultivation Practices of Ricebean; Local Initiatives for Biodiversity, Research and Development (LI-BIRD): Pokhara, Nepal, 2009; 31p, ISBN 978-9937-8145-1-5. [Google Scholar]
- Dhillon, P.K.; Tanwar, B. Rice bean: A healthy and cost-effective alternative for crop and food diversity. Food Secur. 2018, 10, 525–535. [Google Scholar] [CrossRef]
- Kapoor, C.; Gopi, R.; Karuppaiyan, R. Ricebean: An underutilized pulse crop of Sikkim. Asian Agri-Hist. 2012, 16, 417–421. [Google Scholar]
- Pattanayak, A.; Roy, S.; Sood, S.; Iangrai, B.; Banerjee, A.; Gupta, S.; Joshi, D.C. Rice bean: A lesser-known pulse with well-recognized potential. Planta 2019, 250, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Kimani, P.M.; Njau, S.; Mulanya, M.; Narla, R.D. Breeding runner bean for short-day adaptation, grain yield, and disease resistance in eastern Africa. Food Energy Secur. 2019, 8, e171. [Google Scholar] [CrossRef]
- Sileshi, G.; Maghembe, J.; Rao, M.; Ogol, C.; Sithanantham, S. Insects feeding on Sesbania species in natural stands and agroforestry systems in southern Malawi. Agrofor. Syst. 2000, 49, 41–52. [Google Scholar] [CrossRef]
- Sileshi, G.; Mafongoya, P. Incidence of Mesoplatys ochroptera Stål (Coleoptera: Chrysomelidae) on Sesbania sesban in pure and mixed species fallows in eastern Zambia. Agrofor. Syst. 2002, 56, 225–231. [Google Scholar] [CrossRef]
- Heering, J.H.; Guteridge, R.C. Sesbania sesban (L.) Merrill. In Forages; Plant Resources of South-East, Asia; Mannetje, L., Jones, R.M., Eds.; PUDOC Publishing: Bogor, Indonesia, 1992. [Google Scholar]
- Yusuf, A.; Mofio, B.; Ahmed, A. Proximate and mineral composition of Tamarindus indica linn 1753 seeds. Sci. World J. 2010, 2. [Google Scholar] [CrossRef]
- El-Siddig, K.; Gunasena, H.P.M.; Prasad, B.A.; Pushpakumara, D.K.N.G.; Ramana, K.V.R.; Vijayanand, P.; Williams, J.T. Tamarind: Tamarindus indica L. In Fruits for the Future 1; Williams, J.T., Smith, R.W., Haq, Z., Eds.; Southampton Centre for Underutilised Crops: Southampton, UK, 2006. [Google Scholar]
- Bowe, C.; Haq, N. Quantifying the global environmental niche of an underutilised tropical fruit tree (Tamarindus indica) using herbarium records. Agric. Ecosyst. Environ. 2010, 139, 51–58. [Google Scholar] [CrossRef]
- Cardet, C.; Kandji, T.; Delobel, A.; Danthu, P. Efficiency of neem and groundnut oils in protecting leguminous tree seeds against seed beetles in the Sahel. Agrofor. Syst. 1998, 40, 29–40. [Google Scholar] [CrossRef]
- Castellano, G.; Lugo, Z.; Casassa-Padrón, A.M.; Pérez-Pérez, E.; Núñez-Castellano, K. Plant parasitic nematodes associated to potentials fruit trees, in three areas of Mara, Zulia state, Venezuela. Rev. Fac. Agron. 2014, 31, 414–422. [Google Scholar]
- Urrutia Gutierrez, W. Determinacion de Parametros Optimos de Extraccion Alcalina para la Obtencion de Aislado Proteixo a Partir de Tarwi (Lupinus Mutabilis); Universidad Nacional Micaela Bastidas de Apurímac: Abancay, Peru, 2010. [Google Scholar]
- Mogotsi, K.K. Phaseolus acutifolius . 2018. Available online: https://www.prota4u.org/database/protav8.asp?fr=1&g=pe&p=Phaseolus+acutifolius+A.Gray (accessed on 28 November 2018).
- Mishra, S.; Macedo, M.; Panda, S.; Panigrahi, J. Bruchid pest management in pulses: Past practices, present status and use of modern breeding tools for development of resistant varieties. Ann. Appl. Biol. 2017, 172, 4–19. [Google Scholar] [CrossRef]
- Aeron, A.; Maheshwari, D.K.; Dheeman, S.; Agarwal, M.; Dubey, R.; Bajpai, V.K. Plant growth promotion and suppression of charcoal-rot fungus (Macrophomina phaseolina) in velvet bean (Mucuna pruriens L.) by root nodule bacteria. J. Phytopathol. 2017, 165, 463–478. [Google Scholar] [CrossRef]
- Arora, N.K.; Kang, S.C.; Maheshwari, D.K. Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr. Sci. 2001, 81, 673–677. [Google Scholar]
- Baiswar, P.; Mohapatra, K.P.; Chandra, P.; Ngachan, S.V. Rust resistance in Mucuna pruiens accessions. Indian Phytopathol. 2017, 70, 498–499. [Google Scholar] [CrossRef]
- Wulijarni-Soetjipto, N.; Maligalig, R.F. Mucuna pruriens (L.) DC. Cv. group Utilis. In Auxiliary Plants. Plant Resources of South-East Asia; Faridah Hanum, I., van der Maesen, L.J.G., Eds.; PROSEA Foundation: Bogor, Indonesia, 1997. [Google Scholar]
- Okito, A.; Alves, B.J.R.; Urquiaga, S.; Boddey, R. Nitrogen fixation by groundnut and velvet bean and residual benefit to a subsequent maize crop. Fertilization 2004, 39, 1183–1190. [Google Scholar] [CrossRef]
- National Academies. The Winged Bean: A High Protein Crop for the Tropics. Report of an Ad Hoc Panel of the Advisory Committee on Technology Innovation Board on Science and Technology for International Development; National Academies: Washington, DC, USA, 1975. [Google Scholar]
- Sfrensen, M.; van Hoof, W.C.H. Pachyrhizus erosus (L.) urban. In Plants Yielding Non-Seed Carbohydrates. Plant Resources of South-East Asia; Flach, M., Rumawas, F., Eds.; PROSEA Foundation: Bogor, Indonesia, 1996. [Google Scholar]
- Susan John, K.; George, J.; Shanida Beegum, S.U.; Shivay, Y.S. Soil fertility and nutrient management in tropical tuber crops—An overview. Indian J. Agron. 2016, 61, 263–273. [Google Scholar]
- Unkovich, M.; Pate, J.; Armstrong, E.; Sanford, P. Nitrogen economy of annual crop and pasture legumes in southwest Australia. Soil Biol. Biochem. 1995, 27, 585–588. [Google Scholar] [CrossRef]
- Temesgen, T.; Keneni, G.; Sefera, T.; Jarso, M. Yield stability and relationships among stability parameters in Faba bean (Vicia faba L.) genotypes. Crop. J. 2015, 3, 258–268. [Google Scholar] [CrossRef]
- Ahmed, K.; Awan, M.S. Integrated management of insect pests of chickpea Cicer arietinum (L. Walp) in south Asian countries: Present status and future strategies—A review. Pak. J. Zool. 2013, 45, 1125–1145. [Google Scholar]
- Tiwari, K.; Dwivedi, B.; Pathak, A. Evaluation of iron pyrites as Sulphur fertilizer. Nutr. Cycl. Agroecosyst. 1984, 5, 235–243. [Google Scholar] [CrossRef]
- Thangwana, N.M.; Ogola, J.B.O. Yield and yield components of chickpea (Cicer arietinum): Response to genotype and planting density in summer and winter sowings. J. Food Agric. Environ. 2012, 10, 710–715. [Google Scholar]
- Maqbool, A.; Shafiq, S.; Lake, L. Radiant frost tolerance in pulse crops—A review. Euphytica 2009, 172, 1–12. [Google Scholar] [CrossRef]
- Mola, T.; Alemayehu, S.; Fikre, A.; Ojiewo, C.; Alemu, K.; Abdi, T. Heat tolerance responses of chickpea (Cicer arietinum L.) genotypes in the thermal zone of Ethiopia, a case of Werer station. Ethiop. J. Crop Sci. 2018, 6, 95–118. [Google Scholar]
- Mukoko, O.; Galwey, N.; Allen, D. Developing cultivars of the common bean (Phaseolus vulgaris L.) for southern Africa: Bean common mosaic virus resistance, consumer preferences and agronomic requirements. Field Crop. Res. 1995, 40, 165–177. [Google Scholar] [CrossRef]
- Tigist, S.G.; Melis, R.; Sibiya, J.; Keneni, G. Evaluation of different Ethiopian common bean, Phaseolus vulgaris (Fabaceae) genotypes for host resistance to the Mexican bean weevil, Zabrotes subfasciatus (Coleoptera: Bruchidae). Int. J. Trop. Insect Sci. 2017, 38, 1–15. [Google Scholar] [CrossRef]
- Tigist, S.G.; Melis, R.; Sibiya, J.; Amelework, A.B.; Keneni, G.; Tegene, A. Population structure and genome-wide association analysis of bruchid resistance in Ethiopian common bean genotypes. Crop. Sci. 2019, 59, 1504–1515. [Google Scholar] [CrossRef]
- Swegarden, H.R.; Sheaffer, C.C.; Michaels, T.E. Yield stability of heirloom dry bean (Phaseolus vulgaris L.) cultivars in midwest organic production. HortScience 2016, 51, 8–14. [Google Scholar] [CrossRef]
- Soratto, R.P.; Perez, A.A.G.; Fernandes, A.M. Age of no-till system and nitrogen management on common bean nutrition and yield. Agron. J. 2014, 106, 809–820. [Google Scholar] [CrossRef]
- Maingi, J.M.; Shisanya, C.A.; Gitonga, N.M.; Hornetz, B. Nitrogen fixation by common bean (Phaseolus vulgaris L.) in pure and mixed stands in semi-arid southeast Kenya. Eur. J. Agron. 2001, 14, 1–12. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Mendes, I. Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol. Fertil. Soils 2003, 39, 88–93. [Google Scholar] [CrossRef]
- Hernandez-Armenta, R.; Wien, H.C.; Eaglesham, A.R.J. Carbohydrate partitioning and nodule function in common bean after heat stress. Crop. Sci. 1989, 29, 1292–1297. [Google Scholar] [CrossRef]
- Hernández, G.; Ramírez, M.; Valdés-López, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlereth, A.; Wandrey, M.; Erban, A.; Cheung, F.; et al. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Gutíerrez, A.; Mariot, E.; Cure, J.; Riddle, C.; Ellis, C.; Villacorta, A. A model of bean (Phaseolus vulgaris L.) growth types I–III: Factors affecting yield. Agric. Syst. 1994, 44, 35–63. [Google Scholar] [CrossRef]
- Graham, P.; Ranalli, P. Common bean (Phaseolus vulgaris L.). Field Crop. Res. 1997, 53, 131–146. [Google Scholar] [CrossRef]
- Głowacka, A.; Gruszecki, T.; Szostak, B.; Michałek, S. The response of common bean to sulphur and Molybdenum fertilization. Int. J. Agron. 2019, 2019, 3830712. [Google Scholar] [CrossRef]
- Tedford, E.C.; Inglis, D.A. Evaluation of legumes common to the pacific northwest as hosts for the pea cyst nematode, Heterodera goettingiana. J. Nematol. 1999, 31, 155–163. [Google Scholar]
- Khan, Z.; Gautam, N.K.; Gawade, B.H.; Dubey, S.C. Screening of lentil (Lens culinaris Medik.) germplasm for resistance to root-knot nematode, Meloidogyne incognita. Indian J. Genet. Plant Breed. 2017, 77, 408. [Google Scholar] [CrossRef]
- Huang, J.; Afshar, R.K.; Chen, C. Lentil response to nitrogen application and Rhizobia inoculation. Commun. Soil Sci. Plant Anal. 2016, 47, 2458–2464. [Google Scholar] [CrossRef]
- Clark, S. Plant Guide for White Lupine (Lupinus albus L.); USDA-NRCS Big Flats Plant Materials Center: Corning, NY, USA, 2014.
- Wylie, S.; Wilson, C.; Jones, R.; Jones, M. A polymerase chain reaction assay for cucumber mosaic virus in lupin seeds. Aust. J. Agric. Res. 1993, 44, 41–51. [Google Scholar] [CrossRef]
- Unkovich, M.; Pate, J.; Hamblin, J. The nitrogen economy of broadacre lupin in southwest Australia. Aust. J. Agric. Res. 1994, 45, 149–164. [Google Scholar] [CrossRef]
- Tang, C.; Zheng, S.J.; Qiao, Y.F.; Wang, G.H.; Han, X.Z. Interactions between high pH and iron supply on nodulation and iron nutrition of Lupinus albus L. genotypes differing in sensitivity to iron deficiency. Plant Soil 2006, 279, 153–162. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Siddhuraju, P.; Janardhanan, K. Nutritional assessment and chemical composition of the lesser-known tree legume, Acacia leucophloea (Roxb.) Willd. Food Chem. 1994, 50, 285–288. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, H.; Sato, M. The extent and its source of variation for characteristics related to seed quality of Adzuki beans. III. The water uptake of seeds and hard seededness. Jpn. J. Crop. Sci. 1995, 64, 7–13. [Google Scholar] [CrossRef]
- Koura, K.; Ouidoh, P.; Azokpota, P.; Ganglo, J.C.; Hounhouigan, D.J. Caractérisation physique et composition chimique des graines de Parkia biglobosa (Jacq.) R. Br. en usage au Nord-Bénin. J. Appl. Biosci. 2014, 75, 6239. [Google Scholar] [CrossRef]
- Gernmah, D.I.; Atolagbe, M.O.; Echegwo, C.C. Nutritional composition of the African locust bean (Parkia biglobosa) fruit pulp. Niger. Food J. 2007, 25, 190–196. [Google Scholar] [CrossRef]
- Abdulrahman, B.O.; Osibemhe, M.; Idoko, A.S. The status of mineral and anti-nutritional composition of raw and fermented seeds of African locust bean (Parkia biglobosa). Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3, 1–4. [Google Scholar] [CrossRef]
- Enujiugha, V.N.; Ayodele-Oni, O. Evaluation of nutrients and some anti-nutrients in lesser-known, underutilized oilseeds. Int. J. Food Sci. Technol. 2003, 38, 525–528. [Google Scholar] [CrossRef]
- Ifesan, B.O.T.; Akintade, A.O.; Gabriel-Ajobiewe, R.A.O. Physicochemical and nutritional properties of Mucuna pruriens and Parkia biglobosa subjected to controlled fermentation. Int. Food Res. J. 2017, 24, 2177–2184. [Google Scholar]
- Ikhuoria, E.U. Characteristics and composition of African oil bean seed (Pentaclethra macrophylla Benth). J. Appl. Sci. 2008, 8, 1337–1339. [Google Scholar] [CrossRef][Green Version]
- Adeyeye, E.I.; Oshodi, A.A.; Ipinmoroti, K.O. Functional properties of some varieties of African yam bean (Sphenostylis stenocarpa) flour II. Int. J. Food Sci. Nutr. 1994, 45, 115–126. [Google Scholar] [CrossRef]
- Ajayi, F.T.; Akande, S.R.; Odejide, J.O.; Idowu, B. Nutritive evaluation of some tropical under-utilized grain legume seeds for ruminant’s nutrition. J. Am. Sci. 2010, 7, 1–7. [Google Scholar]
- Leung, W.; Busson, F.; Jardin, C. Food Composition Table for Use in Africa; FAO: Rome, Italy, 1968. [Google Scholar]
- Ijarotimi, O.S.; Esho, T.R. Comparison of nutritional composition and anti-nutrient status of fermented, germinated and roasted bambara groundnut seeds (Vigna subterranea). Br. Food J. 2009, 111, 376–386. [Google Scholar] [CrossRef]
- Akubuo, C.O.; Uguru, M.I. Studies on the nutritive characteristics and fracture resistance to compressive loading of selected Bambara groundnut lines. J. Sci. Food Agric. 1999, 79, 2063–2066. [Google Scholar] [CrossRef]
- Amarteifio, J.O.; Karikari, S.K.; Modise, O.J. The proximate and mineral composition of six landraces of Bambara groundnut. Trop. Sci. 2002, 42, 188–191. [Google Scholar]
- Halimi, R.A.; Barkla, B.J.; Mayes, S.; King, G.J. The potential of the underutilized pulse Bambara groundnut (Vigna subterranea (L.) Verdc.) for nutritional food security. J. Food Compos. Anal. 2018, 77, 47–59. [Google Scholar] [CrossRef]
- Iyayi, E.A.; Kluth, H.; Rodehutscord, M. Chemical composition, antinutritional constituents, precaecal crude protein and amino acid digestibility in three unconventional tropical legumes in broilers. J. Sci. Food Agric. 2006, 86, 2166–2171. [Google Scholar] [CrossRef]
- Baptista, A.; Pinho, O.; Pinto, E.; Casal, S.; Mota, C.; Ferreira, I.M. Characterization of protein and fat composition of seeds from common beans (Phaseolus vulgaris L.), cowpea (Vigna unguiculata L. Walp) and Bambara groundnuts (Vigna subterranea L. Verdc) from Mozambique. J. Food Meas. Charact. 2016, 11, 442–450. [Google Scholar] [CrossRef]
- Da Silva, D.O.M.; Santos, C.A.F.; Boiteux, L.S. Adaptability and stability parameters of total seed yield and protein content in cowpea (Vigna unguiculata) genotypes subjected to semi-arid conditions. Aust. J. Crop Sci. 2016, 10, 1164–1169. [Google Scholar] [CrossRef]
- Berger, J.D.; Siddique, K.H.M.; Loss, S.P. Cool season grain legumes for Mediterranean environments: Species × environment interaction in seed quality traits and anti-nutritional factors in the genus Vicia. Aust. J. Agric. Res. 1999, 50, 389. [Google Scholar] [CrossRef]
- Bakoglu, A.; Bagci, E.; Ciftci, H. Fatty acids, protein contents and metal composition of some feed crops from Turkey. J. Food Agric. Environ. 2009, 7, 343–346. [Google Scholar]
- Martín-Pedrosa, M.; Varela, A.; Guillamón, E.; Cabellos, B.; Burbano, C.; Gomez-Fernandez, J.; de Mercado, E.; Gomez-Izquierdo, E.; Cuadrado, C.; Múzquiz, M. Biochemical characterization of legume seeds as ingredients in animal feed. Span. J. Agric. Res. 2016, 14, e0901. [Google Scholar] [CrossRef]
- Sadeghi, G.; Mohammadi, L.; Ibrahim, S.; Gruber, K. Use of bitter vetch (Vicia ervilia) as a feed ingredient for poultry. World’s Poult. Sci. J. 2009, 65, 51. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Nutritional characteristics of seed proteins in 28 Vicia species (Fabaceae) from southern Spain. J. Food Sci. 2011, 76, C1118–C1124. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Abou-Shleel, S. Effect of air temperature on growth, yield and active ingredients of Fenugreek (Trigonella foenum-graecum). Nat. Sci. 2014, 12, 50–54. [Google Scholar]
- Hanbury, C.; White, C.; Mullan, B.; Siddique, K. A review of the potential of Lathyrus sativus L. and L. cicera L. grain for use as animal feed. Anim. Feed. Sci. Technol. 2000, 87, 1–27. [Google Scholar] [CrossRef]
- Folarin, O.M.; Igbon, O.C. Chemical composition of Enterolobium cyclocarpum (Jacq.) Griseb. seed and physicochemical properties of the oil extract. Hamdard Med. 2010, 53, 21–26. [Google Scholar]
- Ravindran, R.; Sundar, S.T.B. Nutritive value of horse gram (Dolichos biflorus) for egg type chicks and growers. Tamilnadu J. Vet. Anim. Sci. 2009, 5, 125–131. [Google Scholar]
- Bhartiya, A.; Aditya, J.P.; Kant, L. Nutritional and remedial potential of an underutilized food legume horse gram (Macrotyloma uniflorum): A review. J. Anim. Plant Sci. 2015, 25, 908–920. [Google Scholar]
- Prasad, S.K.; Singh, M.K. Horse gram—An underutilized nutraceutical pulse crop: A review. J. Food Sci. Technol. 2014, 52, 2489–2499. [Google Scholar] [CrossRef]
- Kalidass, C.; Mohan, V. Nutritional and antinutritional composition of itching bean (Mucuna pruriens (L.) DC Var. pruriens): An underutilized tribal pulse in western Ghats, Tamil Nadu. Trop. Subtrop. Agroecosyst. 2011, 14, 279–293. [Google Scholar]
- Adebowale, Y.A.; Adeyemi, I.A.; Oshodi, A.A. Functional and physicochemical properties of flours of six Mucuna species. Afr. J. Biotechnol. 2005, 4, 1461–1468. [Google Scholar]
- Abitogun, A.S.; Olasehinde, E.F. Nutritional evaluation of seed and characterization of crude Jack bean (Canavalia ensiformis) oil. IOSR J. Appl. Chem. 2012, 1, 36–40. [Google Scholar] [CrossRef]
- Agbede, J.O. Characterisation of the leaf meals, protein concentrates and residues from some tropical leguminous plants. J. Sci. Food Agric. 2006, 86, 1292–1297. [Google Scholar] [CrossRef]
- Salam, J.; Singh, P.K.; Dutta, B.K.; Sahoo, U. Chemical composition and nutritive indices in Parkia roxburghii g. Don, a leguminous plant of India. Indian J. Agric. Biochem. 2009, 22, 87–93. [Google Scholar]
- Longvah, T.; Deosthale, Y. Nutrient composition and food potential of Parkia roxburghii, a less known tree legume from northeast India. Food Chem. 1998, 62, 477–481. [Google Scholar] [CrossRef]
- Blair, M.W.; Porch, T.; Cichy, K.; Galeano, C.H.; Lariguet, P.; Pankhurst, C.; Broughton, W. Induced mutants in common bean (Phaseolus vulgaris), and their potential use in nutrition quality breeding and gene discovery. Isr. J. Plant Sci. 2007, 55, 191–200. [Google Scholar] [CrossRef]
- Gwanzura, T.; Ng’ambi, J.; Norris, D. Nutrient composition and tannin contents of forage Sorghum, cowpea, lablab and Mucuna hays grown in Limpopo Province of south Africa. Asian J. Anim. Sci. 2012, 6, 256–262. [Google Scholar] [CrossRef]
- Bhardwaj, H.L.; Hamama, A.A. Genotype and environment effects on lablab seed yield and composition. HortScience 2019, 54, 2156–2158. [Google Scholar] [CrossRef]
- Powell, A.M. Marama bean (Tylosema esculentum, Fabaceae) seed crop in Texas. Econ. Bot. 1987, 41, 216–220. [Google Scholar] [CrossRef]
- Holse, M.; Husted, S.; Hansen, S. Chemical composition of Marama bean (Tylosema esculentum)—A wild African bean with unexploited potential. J. Food Compos. Anal. 2010, 23, 648–657. [Google Scholar] [CrossRef]
- Amarteifio, J.; Moholo, D. The chemical composition of four legumes consumed in Botswana. J. Food Compos. Anal. 1998, 11, 329–332. [Google Scholar] [CrossRef]
- Gunathilake, K.T.; Herath, T.; Wansapala, J. Comparison of physicochemical properties of selected locally available legume varieties (mung bean, cowpea and soybean). Potravin. Slovak J. Food Sci. 2016, 10, 424–430. [Google Scholar] [CrossRef]
- Hadjipanayiotou, M.; Economides, S. Chemical composition, in situ degradability and amino acid composition of protein supplements fed to livestock and poultry in Cyprus. Livest. Res. Rural. Dev. 2001, 13, 56. [Google Scholar]
- Brand, T.; Brandt, D.; Cruywagen, C. Chemical composition, true metabolisable energy content and amino acid availability of grain legumes for poultry. S. Afr. J. Anim. Sci. 2004, 34, 116–122. [Google Scholar] [CrossRef][Green Version]
- Anton, A.A.; Ross, K.A.; Beta, T.; Fulcher, R.G.; Arntfield, S.D. Effect of pre-dehulling treatments on some nutritional and physical properties of navy and pinto beans (Phaseolus vulgaris L.). LWT 2008, 41, 771–778. [Google Scholar] [CrossRef]
- Grosso, N.R.; Nepote, V.; Guzmán, C.A. Chemical composition of some wild peanut species (Arachis L.) seeds. J. Agric. Food Chem. 2000, 48, 806–809. [Google Scholar] [CrossRef]
- Fathima, K.R.; Mohan, V. Nutritional and antinutritional assessment of Mucuna atropurpurea DC: An underutilized tribal pulse. Afr. J. Basic Appl. Sci. 2009, 1, 129–136. [Google Scholar]
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. The biochemical composition and nutritional potential of the tribal pulse, Alysicarpus rugosus (Willd.) DC. Food Chem. 1992, 45, 251–255. [Google Scholar] [CrossRef]
- Kallah, M.; Bale, J.; Abdullahi, U.; Muhammad, I.; Lawal, R. Nutrient composition of native forbs of semi-arid and dry sub-humid savannas of Nigeria. Anim. Feed. Sci. Technol. 2000, 84, 137–145. [Google Scholar] [CrossRef]
- Nigussie, Z.; Alemayehu, G. Sesbania sesban (L.) Merrill: Potential uses of an underutilized multipurpose tree in Ethiopia. Afr. J. Plant Sci. 2013, 7, 468–475. [Google Scholar] [CrossRef]
- Rubatzky, V.E.; Yamaguchi, M. World Vegetables: Principles, Production, and Nutritive Values, 2nd ed.; Springer: New York, NY, USA, 1997; ISBN 978-1-4613-7756-6. [Google Scholar]
- Bhat, R.; Karim, A. Exploring the nutritional potential of wild and underutilized legumes. Compr. Rev. Food Sci. Food Saf. 2009, 8, 305–331. [Google Scholar] [CrossRef]
- Ajayi, I.A.; Oderinde, R.A.; Kajogbola, D.O.; Uponi, J.I. Oil content and fatty acid composition of some underutilized legumes from Nigeria. Food Chem. 2006, 99, 115–120. [Google Scholar] [CrossRef]
- Grollier, C.; Debien, C.; Dornier, M.; Reynes, M. Principales caractéristiques et voies de valorisation du tamarin. Fruits 1998, 53, 271–280. [Google Scholar]
- Van Der Stege, C.; Prehsler, S.; Hartl, A.; Vogl, C.R. Tamarind (Tamarindus indica L.) in the traditional West African diet: Not just a famine food. Fruits 2011, 66, 171–185. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and breeding of Lupinus mutabilis: An emerging protein crop. Front. Plant Sci. 2019, 10, 1385. [Google Scholar] [CrossRef]
- Porch, T.G.; Cichy, K.; Wang, W.; Brick, M.; Beaver, J.S.; Santana-Morant, D.; Grusak, M.A. Nutritional composition and cooking characteristics of tepary bean (Phaseolus acutifolius Gray) in comparison with common bean (Phaseolus vulgaris L.). Genet. Resour. Crop. Evol. 2016, 64, 935–953. [Google Scholar] [CrossRef]
- Vadivel, V.; Janardhanan, K. Nutritional and anti-nutritional composition of velvet bean: An under-utilized food legume in south India. Int. J. Food Sci. Nutr. 2000, 51, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Amoo, I.; Atasie, V.; Kolawole, O. Proximate composition, nutritionally valuable minerals, protein functional properties and anti-nutrient contents of Mucuna preta, Mucuna ghana and Mucuna veracruz Mottle. Pak. J. Nutr. 2009, 8, 1204–1208. [Google Scholar] [CrossRef]
- Anugroho, F.; Kitou, M.; Kinjo, K.; Kobashigawa, N. Growth and nutrient accumulation of winged bean and velvet bean as cover crops in a subtropical region. Plant Prod. Sci. 2010, 13, 360–366. [Google Scholar] [CrossRef]
- Arivalagan, M.; Prasad, T.; Singh, H.; Kumar, A. Variability in biochemical and mineral composition of Mucuna pruriens (L.) DC—An underutilized tropical legume. Legum. Res. Int. J. 2014, 37, 483. [Google Scholar] [CrossRef]
- Balogun, I.; Olatidoye, O. Chemical composition and nutritional evaluation of velvet bean seeds (Mucuna utilis) for domestic consumption and industrial utilization in Nigeria. Pak. J. Nutr. 2012, 11, 116–122. [Google Scholar] [CrossRef]
- Bhat, R.; Sridhar, K.; Young, C.-C.; Bhagwath, A.A.; Ganesh, S. Composition and functional properties of raw and electron beam-irradiated Mucuna pruriens seeds. Int. J. Food Sci. Technol. 2008, 43, 1338–1351. [Google Scholar] [CrossRef]
- Chikagwa-Malunga, S.; Adesogan, A.; Sollenberger, L.; Badinga, L.; Szabo, N.; Littell, R. Nutritional characterization of Mucuna pruriens: 1. Effect of maturity on the nutritional quality of botanical fractions and the whole plant. Anim. Feed. Sci. Technol. 2009, 148, 34–50. [Google Scholar] [CrossRef]
- Noman, A.; Hoque, M.; Haque, M.; Pervin, F.; Karim, M. Nutritional and anti-nutritional components in Pachyrhizus erosus L. tuber. Food Chem. 2007, 102, 1112–1118. [Google Scholar] [CrossRef]
- Aremu, C.; Ige, S.A.; Ibirinde, D.; Raji, I.; Abolusoro, S.; Ajiboye, B.; Obaniyi, S.; Adekiya, A.; Asaleye, A. Assessing yield stability in African yam bean (Sphenostylis stenocarpa) performance using year effect. Open Agric. 2020, 5, 202–212. [Google Scholar] [CrossRef]
- Skylas, D.J.; Paull, J.G.; Hughes, D.G.D.; Gogel, B.; Long, H.; Williams, B.; Mundree, S.; Blanchard, C.; Quail, K.J. Nutritional and anti-nutritional seed-quality traits of faba bean (Vicia faba) grown in south Australia. Crop. Pasture Sci. 2019, 70, 463–472. [Google Scholar] [CrossRef]
- Wood, J.A.; Tan, H.-T.; Collins, H.M.; Yap, K.; Khor, S.F.; Lim, W.L.; Xing, X.; Bulone, V.; Burton, R.A.; Fincher, G.B.; et al. Genetic and environmental factors contribute to variation in cell wall composition in mature desi chickpea (Cicer arietinum L.) cotyledons. Plant Cell Environ. 2018, 41, 2195–2208. [Google Scholar] [CrossRef]
- Wood, J.A.; Knights, E.J.; Campbell, G.M.; Choct, M. Differences between easy- and difficult-to-mill chickpea (Cicer arietinum L.) genotypes. Part II: Protein, lipid and mineral composition. J. Sci. Food Agric. 2013, 94, 1446–1453. [Google Scholar] [CrossRef]
- Wood, J.A.; Knights, E.J.; Campbell, G.M.; Choct, M. Differences between easy- and difficult-to-mill chickpea (Cicer arietinum L.) genotypes. Part III: Free sugar and non-starch polysaccharide composition. J. Sci. Food Agric. 2013, 94, 1454–1462. [Google Scholar] [CrossRef]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Paredes, C.M.; Becerra, V.V.; Tay, J.U. Inorganic nutritional composition of common bean (Phaseolus vulgaris L.) genotypes race Chile. Chil. J. Agric. Res. 2009, 69, 486–495. [Google Scholar] [CrossRef]
- Batista, K.A.; Prudêncio, S.H.; Fernandes, K.F. Changes in the functional properties and antinutritional factors of extruded hard-to-cook common beans (Phaseolus vulgaris, L.). J. Food Sci. 2010, 75, C286–C290. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; Sarker, A.; Materne, M.; Vandemark, G.; Shrestha, R.; Idrissi, O.; Hacikamiloglu, O.; Bucak, B.; Vandenberg, A. A global survey of effects of genotype and environment on selenium concentration in lentils (Lens culinaris L.): Implications for nutritional fortification strategies. Food Chem. 2011, 125, 72–76. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; See, C.-T.; Vandenberg, A. Phytic acid and Fe and Zn concentration in lentil (Lens culinaris L.) seeds is influenced by temperature during seed filling period. Food Chem. 2010, 122, 254–259. [Google Scholar] [CrossRef]
- Summerfield, R.J.; Muehlbauer, F.J. Mineral nutrient composition of lentil seeds 1. Commun. Soil Sci. Plant Anal. 1982, 13, 317–333. [Google Scholar] [CrossRef]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Uhunmwangho, E.S.; Omoregie, E.S. Evaluation of nutritive, antinutritive and mineral content of Tetracarpidium conophorum (African walnut) seed oil at different stages of fruit maturation. Saudi J. Life Sci. 2017, 2, 210–216. [Google Scholar]
- Ayodeji, A.E.; Aliyu, N. Tetracarpidium conophorum (African walnut) Hutch. & Dalziel: Ethnomedicinal uses and its therapeutic activities. J. Med. Plants Econ. Dev. 2018, 2, 10. [Google Scholar] [CrossRef]
- Nergiz, C.; Yalcin, H.; Yildiz, H. Some analytical characters of cottonseed varieties grown in Turkey. Grasas Aceites 1997, 48, 411–414. [Google Scholar] [CrossRef]
- Renuka, C.K.; Kumarmath, P.S.; Kadakol, J.C.; Hosamani, S.V. Chemical composition and antinutritional factors in different parts and whole cotton (Gossypium hirsutum) plant. Karnataka J. Agric. Sci. 2005, 18, 114–117. [Google Scholar]
- Bellaloui, N.; Turley, R.; Stetina, S. Cottonseed protein, oil, and minerals in cotton (Gossypium hirsutum L.) lines differing in curly leaf morphology. Plants 2021, 10, 525. [Google Scholar] [CrossRef]
- Akobundu, E.N.T.; Cherry, J.P.; Simmons, J.G. Chemical, functional, and nutritional properties of Egusi (Colocynthis citrullus L.) seed protein products. J. Food Sci. 1982, 47, 829–835. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables; MedPharm Gmbh Scientific Publishing: Boca Raton, FL, USA, 2008; ISBN 978-3-8047-5038-8. [Google Scholar]
- Bell, J.M.; Rakow, G.; Downey, R.K. Mineral composition of oil-free seeds of Brassica napus, B. rapa and B. juncea as affected by location and year. Can. J. Anim. Sci. 1999, 79, 405–408. [Google Scholar] [CrossRef]
- Lajolo, F.M.; Marquez, U.M.L.; Filisetti-Cozzi, T.M.C.C.; McGregor, D.I. Chemical composition and toxic compounds in rapeseed (Brassica napus, L.) cultivars grown in Brazil. J. Agric. Food Chem. 1991, 39, 1933–1937. [Google Scholar] [CrossRef]
- Reddy, N.R.; Pierson, M.D.; Sathe, S.K.; Salunkhe, D.K. Dry bean tannins: A review of nutritional implications. J. Am. Oil Chem. Soc. 1985, 62, 541–549. [Google Scholar] [CrossRef]
- Coulibaly, M.; Kouamé, C.A.; N’Dri, D.Y.; Kouassi, N.K.; Pereko, K.K.A.; Amani, G.N. Effect of post-harvest traditional technologies on the nutrient content and antioxidant compounds of defatted flours from Ricinodendron heudelotti (Baill. Pierre ex Pax) seed kernels. Technologies 2018, 6, 37. [Google Scholar] [CrossRef]
- Nyananyo, B.L.; Nyingifa, A.L. Phytochemical Investigation on the seed of Sphenostylis stenocarpa (Hochst Ex A. Rich.) harms (family Fabaceae). J. Appl. Sci. Environ. Manag. 2011, 15, 419–423. [Google Scholar]
- Ellison, S.L. Carotenoids: Physiology. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 670–675. ISBN 978-0-12-384953-3. [Google Scholar]
- Okafor, J.; Ani, J.C.; Okafor, G.I. Effect of processing methods on qualities of Bambara groundnut (Voandzeia subterranea (L.) Thouars) flour and their acceptability in extruded snacks. Am. J. Food Technol. 2014, 9, 350–359. [Google Scholar] [CrossRef]
- Unigwe, A.; Doria, E.; Adebola, P.; Gerrano, A.S.; Pillay, M. Anti-nutrient analysis of 30 Bambara groundnut (Vigna subterranea) accessions in South Africa. J. Crop Improv. 2017, 32, 208–224. [Google Scholar] [CrossRef]
- Camelo, S.; Torres, V.; Diaz, M.F. Multivariate analysis of the anti-nutritional factors of the seasonal legumes’ grains. Cuba. J. Agric. Sci. 2008, 42, 339–341. [Google Scholar]
- Franco, O.L.; dos Santos, R.C.; Batista, J.A.; Mendes, A.C.M.; de Araújo, M.A.M.; Monnerat, R.G.; Grossi-De-Sá, M.F.; de Freitas, S.M. Effects of black-eyed pea trypsin/chymotrypsin inhibitor on proteolytic activity and on development of Anthonomus grandis. Phytochemistry 2003, 63, 343–349. [Google Scholar] [CrossRef]
- Grela, E.; Studziñski, T.; Matras, J. Antinutritional factors in seeds of Lathyrus sativus cultivated in Poland. Lathyrus Lathyrism Newsl. 2001, 2, 101–104. [Google Scholar]
- Campbell, C.G. Grass Pea: Lathyrus sativus L.: Promoting the Conservation and Use of Underutilized and Neglected Crops; IPGRI: Rome, Italy, 1997; ISBN 978-92-9043-341-5. [Google Scholar]
- Girma, D.; Korbu, L. Genetic improvement of grass pea (Lathyrus sativus) in Ethiopia: An unfulfilled promise. Plant Breed. 2012, 131, 231–236. [Google Scholar] [CrossRef]
- Lambein, F.; Travella, S.; Kuo, Y.-H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Abiola, C.; Oyetayo, V.O. Proximate and anti-nutrient contents of Kersting’s groundnut (Macrotyloma geocarpum) subjected to different fermentation methods. Br. Microbiol. Res. J. 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Betancur-Ancona, D.; Gallegos-Tintoré, S.; Delgado-Herrera, A.; Pérez-Flores, V.; Ruelas, A.C.; Chel-Guerrero, L. Some physicochemical and antinutritional properties of raw flours and protein isolates from Mucuna pruriens (velvet bean) and Canavalia ensiformis (Jack bean). Int. J. Food Sci. Technol. 2008, 43, 816–823. [Google Scholar] [CrossRef]
- Laurena, A.C.; Revilleza, M.R.; Mendoza, E.M.T. Polyphenols, phytate, cyanogenic glycosides, and trypsin inhibitor activity of several Philippine indigenous food legumes. J. Food Compos. Anal. 1994, 7, 194–202. [Google Scholar] [CrossRef]
- Shimelis, E.A.; Rakshit, S.K. Effect of processing on antinutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa. Food Chem. 2007, 103, 161–172. [Google Scholar] [CrossRef]
- Drew, M.D.; Borgeson, T.L.; Thiessen, D.L. A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim. Feed Sci. Technol. 2007, 138, 118–136. [Google Scholar] [CrossRef]
- Nyembwe, P.; Minnaar, A.; Duodu, K.G.; de Kock, H.L. Sensory and physicochemical analyses of roasted Marama beans [Tylosema esculentum (Burchell) A. Schreiber] with specific focus on compounds that may contribute to bitterness. Food Chem. 2015, 178, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005, 89, 489–495. [Google Scholar] [CrossRef]
- Berger, J.; Robertson, L.; Cocks, P. Agricultural potential of Mediterranean grain and forage legumes: 2) Anti-nutritional factor concentrations in the genus Vicia. Genet. Resour. Crop. Evol. 2003, 50, 201–212. [Google Scholar] [CrossRef]
- Lolas, G.M.; Markakis, P. The phytase of navy beans (Phaseolus vulgaris). J. Food Sci. 1977, 42, 1094–1097. [Google Scholar] [CrossRef]
- Richardson, J. The Effects of Pretreatment Conditions and Micronization on the Anti-Nutritional Factors, Cookability, and Microorganisms in Navy Beans (Phaseolus vulgaris L.). Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2003. [Google Scholar]
- Kelkar, S.; Siddiq, M.; Harte, J.; Dolan, K.; Nyombaire, G.; Suniaga, H. Use of low-temperature extrusion for reducing phytohemagglutinin activity (PHA) and oligosaccharides in beans (Phaseolus vulgaris L.) cv. Navy and Pinto. Food Chem. 2012, 133, 1636–1639. [Google Scholar] [CrossRef]
- Saxena, K.B.; Kumar, R.V.; Sultana, R. Quality nutrition through pigeonpea—A review. Health 2010, 2, 1335–1344. [Google Scholar] [CrossRef]
- Kaur, D.; Kapoor, A. Nutrient composition and antinutritional factors of rice bean (Vigna umbellata). Food Chem. 1992, 43, 119–124. [Google Scholar] [CrossRef]
- Hory, H.-D.; Belitz, H.-D. Vergleichende Untersuchungen der trypsinspezifischen reaktiven Zentren verschiedener Phaseolus coccineus und Phaseolus vulgaris-Inhibitoren. Eur. Food Res. Technol. 1976, 162, 341–347. [Google Scholar] [CrossRef]
- Morgan, M.; Manen, J. Lectin variability in Phaseolus coccineus. Phytochemistry 1985, 24, 1981–1985. [Google Scholar] [CrossRef]
- Vadivel, V.; Janardhanan, K. The nutritional and antinutritional attributes of sword bean [Canavalia gladiata (Jacq.) DC.]: An under-utilized tribal pulse from south India. Int. J. Food Sci. Technol. 2004, 39, 917–926. [Google Scholar] [CrossRef]
- De Caluw, E.; Halamov, K.; Van Damme, P. Tamarindus indica L.: A review of traditional uses, phytochemistry and pharmacology. Afr. Focus 2010, 23, 53–83. [Google Scholar] [CrossRef]
- Suneja, Y.; Kaur, S.; Gupta, A.K.; Kaur, N. Levels of nutritional constituents and antinutritional factors in black gram (Vigna mungo L. Hepper). Food Res. Int. 2011, 44, 621–628. [Google Scholar] [CrossRef]
- Misra, L.; Wagner, H. Alkaloidal constituents of Mucuna pruriens seeds. Phytochemistry 2004, 65, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, G.K.A.; Odubena, O.O. Effect of Mucuna pruriens on some haematological and biochemical parameters. J. Med. Plants Res. 2009, 3, 073–076. [Google Scholar]
- Gurumoorthi, P.; Janardhanan, K.; Kalavathy, G. Improving nutritional value of velvet bean, Mucuna pruriens (L.) DC. Var. utilis (Wall.Ex.Wight) L. H. Bailey, an under-utilized pulse, using microwave technology. Indian J. Tradit. Knowl. 2013, 12, 677–681. [Google Scholar]
- Varga, E.; Varga, M. Development and validation of an LC-MS/MS method for the analysis of L-DOPA in oat. Acta Biol. Szeged. 2014, 58, 133–137. [Google Scholar]
- Chandrashekharaiah, K.S. Studies on proteinase inhibitors from the seeds of Mucuna utilis. Res. J. Chem. Environ. 2018, 22, 23–27. [Google Scholar]
- Kotaru, M.; Ikeuchi, T.; Yoshikawa, H.; Ibuki, F. Investigations of antinutritional factors of the winged bean (Psophocarpus tetragonolobus). Food Chem. 1987, 24, 279–286. [Google Scholar] [CrossRef]
- Shet, M.S.; Madaiah, M. Distribution of lectin activity at different stages in the tissues of winged bean (Psophocarpus tetragonolobus (L.) DC). Plant Sci. 1987, 53, 161–165. [Google Scholar] [CrossRef]
- Topal, N.; Bozoğlu, H. Determination of L-Dopa (L-3, 4-Dihydroxyphenylalanine) content of some Faba bean (Vicia faba L.) genotypes. J. Agric. Sci. 2016, 22, 145–151. [Google Scholar]
- El-Adawy, T.A. Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Mater. Veg. 2002, 57, 83–97. [Google Scholar] [CrossRef]
- Sathe, S.K.; Deshpande, S.S.; Reddy, N.R.; Goll, D.E.; Salunkhe, D.K. Effects of germination on proteins, raffinose oligosaccharides, and antinutritional factors in the great northern beans (Phaseolus vulgaris L.). J. Food Sci. 1983, 48, 1796–1800. [Google Scholar] [CrossRef]
- Savage, G.; Deo, S. The nutritional value of peas (Pisum sariurn): A literature review. Nutr. Abstr. Rev. Ser. A 1989, 59, 65–87. [Google Scholar]
- Hefnawy, T. Effect of processing methods on nutritional composition and anti-nutritional factors in lentils (Lens culinaris). Ann. Agric. Sci. 2011, 56, 57–61. [Google Scholar] [CrossRef]
- Thavarajah, P.; Thavarajah, D.; Vandenberg, A. Low phytic acid lentils (Lens culinaris L.): A potential solution for increased micronutrient bioavailability. J. Agric. Food Chem. 2009, 57, 9044–9049. [Google Scholar] [CrossRef]
- Getachew, P.; Umeta, M.; Retta, N.; Bekele, T.; Haki, G.D. Proximate composition and anti-nutritional factors of traditionally processed white lupine (Lupinus albus L.) Fabaceae, grown in Ethiopia. Ethiop. J. Biol. Sci. 2012, 11, 133–146. [Google Scholar]
- Nigussie, Z. Contribution of white lupin (Lupinus albus L.) for food security in northwestern Ethiopia: A review. Asian J. Plant Sci. 2012, 11, 200–205. [Google Scholar] [CrossRef]
- Janßen, G.; Ordon, F.; Jürgens, H.-U. Effects of temperature on the alkaloid content of seeds of Lupinus angustifolius Cultivars. J. Agron. Crop. Sci. 2009, 195, 172–177. [Google Scholar] [CrossRef]
- Boye, J.; Ribereau, S. Assessing compositional differences in soy products and impacts on health claims. In Soybean and Nutrition; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
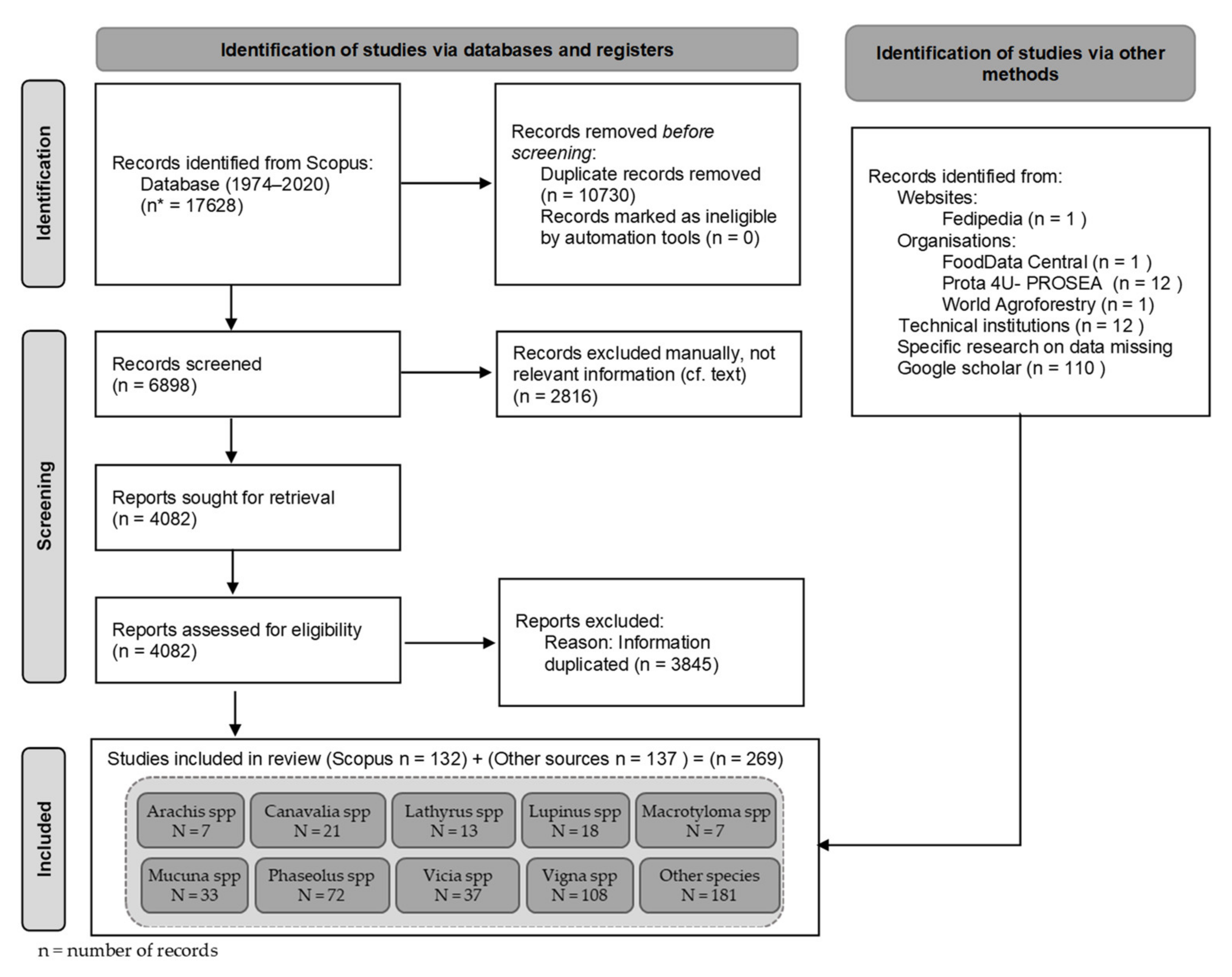
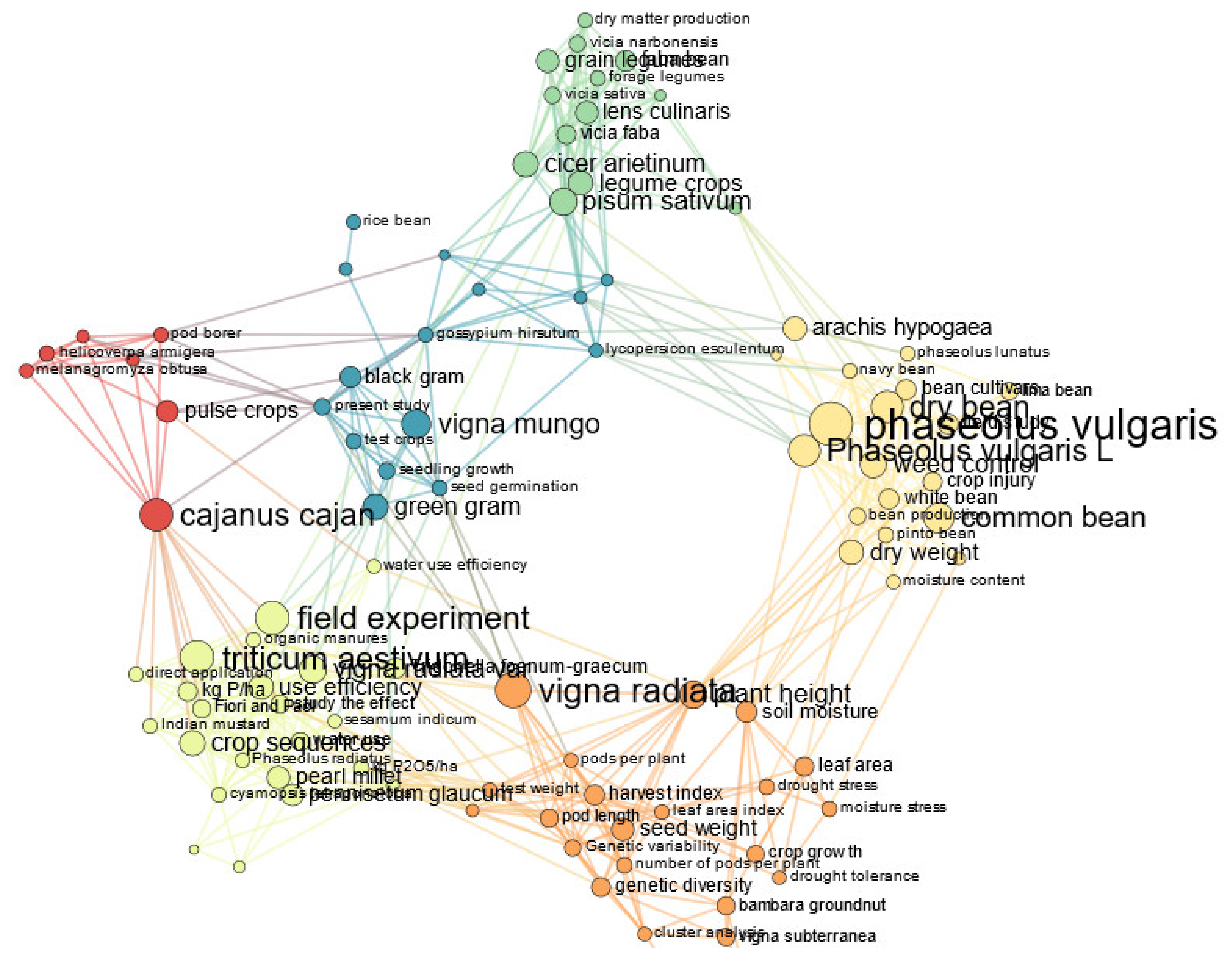
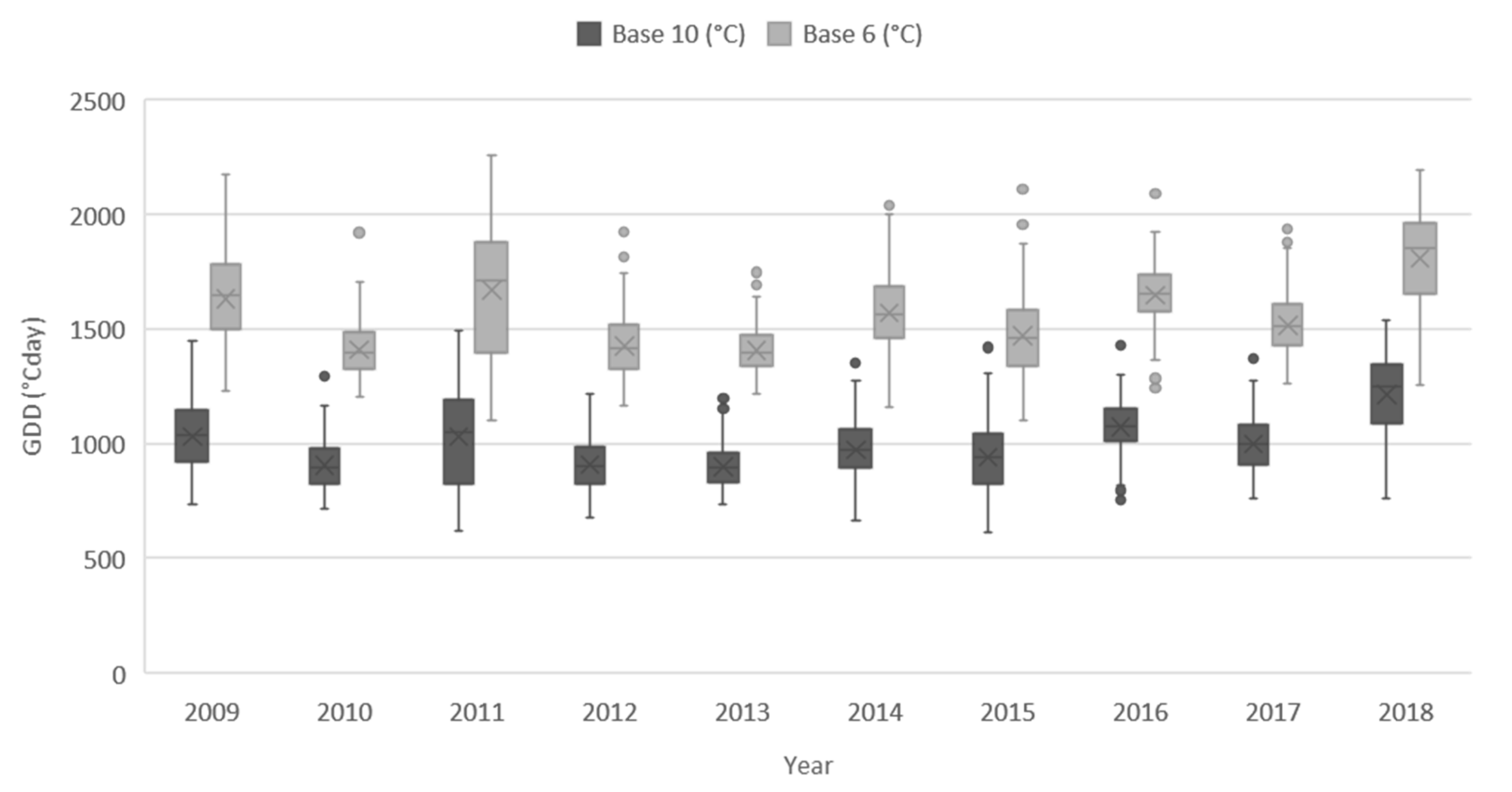
| World (T) | Africa (%) | America (%) | Asia (%) | Europe (%) | Oceania (%) | |
|---|---|---|---|---|---|---|
| Bambara beans | 228,920 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Beans, dry | 28,902,672 | 24.4 | 24.4 | 49.7 | 1.3 | 0.3 |
| Broad beans, horse beans, dry | 5,431,503 | 27.0 | 4.0 | 33.6 | 29.4 | 6.0 |
| Chick peas | 14,246,295 | 4.9 | 6.1 | 83.4 | 3.6 | 2.0 |
| Cow peas, dry | 8,903,329 | 96.8 | 0.7 | 2.2 | 0.3 | 0.0 |
| Lentils | 5,734,201 | 3.3 | 42.7 | 42.5 | 2.2 | 9.3 |
| Lupins | 1,006,842 | 7.5 | 6.3 | 0.0 | 39.0 | 47.1 |
| Peas, dry | 14,184,249 | 3.9 | 39.3 | 18.1 | 37.0 | 1.7 |
| Pigeon peas | 4,425,969 | 15.1 | 1.8 | 83.2 | 0.0 | 0.0 |
| Pulses, others | 4,553,029 | 31.5 | 0.9 | 45.3 | 22.0 | 0.3 |
| Vetches | 762,795 | 42.6 | 12.7 | 12.4 | 31.4 | 0.9 |
| Pulses, total | 88,379,804 | 24.1 | 18.7 | 44.3 | 10.8 | 2.2 |
| Common Name | Other Names | Scientific Name | Retrieved Papers in the Scopus Database (1974–2019) |
|---|---|---|---|
| Acacia leucophloea | Vachellia leucophloea | 4 | |
| Adzuki bean | Azuki bean, aduki bean | Vigna angularis | 102 |
| African locust bean | Parkia biglobosa | 31 | |
| African nut bean | Ricinodendron heudelotti | 1 | |
| African oil bean | Pentaclethra macrophylla | 5 | |
| African yam bean | Sphenostylis stenocarpa | 27 | |
| Bambara groundnut | Earth pea | Vigna subterranea | 99 |
| Butterfly pea | Centrosema pubescens | 16 | |
| Cowpea | Black eye bean, black eye pea | Vigna unguiculata | 1348 |
| Ervil | Bitter vetch | Vicia ervilia | 40 |
| Fenugreek | Trigonela foenum-graecum | 145 | |
| Grass pea | Lathyrus sativus | 131 | |
| Guanacaste | Enterolobium cyclocarpum | 11 | |
| Horsegram | Macrotyloma uniflorum | 43 | |
| Housa groundnut | Hausa groundnut, Kersting groundnut | Macrotyloma geocarpum | 6 |
| Itching bean | Mucuna pruriens var. pruriens | 1 | |
| Jack bean | Canavalia ensiformis | 36 | |
| Kedaung | Tree bean | Parkia roxburghii | 2 |
| Kidney bean | Phaseolus vulgaris | 131 | |
| Lablab | Hyacinth bean | Lablab purpureus | 125 |
| Lima bean | Butter bean, Java bean, Madagascar bean, sugar bean | Phaseolus lunatus | 113 |
| Marama bean | Morama bean | Tylosema esculentum | 8 |
| Moth bean | Mat bean, Turkish gram | Vigna aconitifolia | 63 |
| Mung bean | Golden gram, green gram, Moong bean | Vigna radiata | 811 |
| Navy bean | Pearl haricot, haricot bean | Phaseolus vulgaris | 66 |
| Narbon bean | Narbon vetch | Vicia Narbonensis | 43 |
| Pigeon pea | Red gram | Cajanus cajan | 590 |
| Pinto bean | Phaseolus vulgaris | 58 | |
| Pinto peanut | Arachis pintoi | 70 | |
| Purple mucuna | Mucuna atropurpurea | 0 | |
| Red moneywort | Alysicarpus rugosus | 1 | |
| Rice bean | Vigna umbellata | 55 | |
| Scarlet runner bean | Runner bean | Phaseolus coccineus | 18 |
| Sesban | Sesbania sesban | 46 | |
| Sword bean | Canavalia gladiata | 9 | |
| Tamarind | Tamarindus indica | 45 | |
| Tarwi | Lupinus mutabilis | 30 | |
| Tepary bean | Phaseolus acutifolius | 46 | |
| Urad | Black bean, black lentil, mungo bean | Vigna mungo | 357 |
| Velvet bean | Cowitch | Mucuna pruriens var. utilis | 25 |
| Winged bean | Psophocarpus tetragonolobus | 39 | |
| Yam bean | Pachyrhizus erosus | 45 | |
| Broad bean | Faba bean, horse bean | Vicia faba | 918 |
| Chickpea | Bengal gram | Cicer arietinum | 1795 |
| Common bean | Field bean, bell bean, English bean, Windsor bean, pigeon bean | Phaseolus vulgaris | 1988 |
| Field pea | Pisum sativum | 2014 | |
| Lentil | Lens culinaris | 901 | |
| Lupin | Lupinus spp. | 688 |
| Pulses—Common Name | Temperature (°C) Min; Max; Optimal | Crop Length | Water Requirements (mm) | Yield (t/Ha) | Kg N Fixed (kg/ha) |
|---|---|---|---|---|---|
| Adzuki bean | 5–10; 34; 15–30 | 60–190 d | 500–1700 | 0.5–3.5 | 100 |
| Butterfly pea | 5; 35; 20–28 | 120–194 d | 400–1750 | 0.7 | - |
| Cowpea | 15; 35; 25–35 | 60–340 d | 500–1500 | 0.2–7 | 12–50 |
| Fenugreek | −4; -; 18–27 | 90–100 d | 300–400 | - | - |
| Grass pea | 20; -; 10–25 | 90–180 d | 400–650 | 0.3–10.5 | 25–50 |
| Horse gram | -; 40; 20–32 | 120–150 d | 380–900 | 0.5 | - |
| Itching bean | -; -; 19–27 | - | 400–3000 | 0.2–2 | - |
| Kidney bean | −1; 30;15–20 | 65–105 d | 300–600 | 2.2 | 20–44 |
| Lablab | -; -; 18–30 | 54–220 d | 650–3000 | 1.4–4.5 | 20–140 |
| Lima bean | >0; >37; 16–27 | 115–180 d | 900–1500 | 0.4–5 | 40–60 |
| Moth bean | 25; 45; 24–32 | 70–90 d | 200–750 | 0.07–2.6 | - |
| Navy bean | 12; 35; 22–30 | 53–300 d | 400 | 0.5–5 | 125 |
| Pigeon pea | 0; 40; 18–29 | 3–5 y | 600–1400 | 0.6–5 | 69–134 |
| Pinto bean | -; 36; 21–25 | 90–100 d | - | 0.5–3.9 | - |
| Rice bean | 10; 40; 25–35 | 120–150 d | 700–1700 | 0.2–2.7 | - |
| Sword bean | -; -; 25–30 | 150–300 d | 900–1500 | 1.5–5.4 | 75–230 |
| Tarwi | <0; -; - | 150–330 d | - | 0.6–4.8 | 100 |
| Tepary bean | 8; >32; 17–25 | 60–120 d | 400–1700 | 0.4–1.7 | - |
| Urad | -; -; 25–35 no frost | 60–140 d | 600–1000 | 0.3–2.5 | 18 |
| Velvet bean | 5; -; 19–27 | - | 1200–1500 | 0.5–3.4 | 60–330 |
| Winged bean | -; -; 20–30 | 120–180 d | 1000 | 0.7–1.9 | - |
| Broad bean | −12; 30; 18–27 | 90–220 d | 700–1200 | 1.1–2.2 | 33–550 |
| Chickpea | −11; >32; 10–29 | 90–180 d | 500–1800 | 1–5.5 | 35–140 |
| Common bean | −9; 38; - | 70–110 d | 274–550 | 0.9–2.6 | 465 |
| Field pea | <0; -; 7–30 | 90–180 d | 400–1000 | 1–4 | 30–96 |
| Lentil | 2; 35; 6–27 | 80–130 d | 300–2400 | 0.8–7 | 50 |
| Lupin | <0; -; 18–24 | 115–330 d | 400–1000 | 0.5–5 | 90–400 |
| Pulses—Common Name | Energy (kcal/100 g) | Protein (%) | Oil (Saturated FA) (%) | Carbohydrate (%) | Fiber (%) |
|---|---|---|---|---|---|
| Adzuki bean | 329 | 19.9 | 0.5 (0.2) | 62.9 | 12.7 |
| Butterfly pea | - | 25.2 | 3.7 | 19.9 | 9.2 |
| Cowpea | 343 | 23.9 | 2.1 (0.5) | 59.6 | 10.7 |
| Fenugreek | 323 | 23.0 | 6.4 | 58.0 | 25.0 |
| Grass pea | - | 24.4 | 2.8 (0.8) | 55.94 | 11.4 |
| Horse gram | 280 | 22 | 0.6 | 37.5 | 5.7 |
| Itching bean | 382 | 27–37 | 6.6–8.8 | 46–53 | 6–10 |
| Kidney bean | 333 | 23.6 | 0.8 (0.1) | 30.0 | 24.0 |
| Lablab | 344 | 21–29 | 1.7 (0.3) | 60.74 | 25.6 |
| Lima bean | 338 | 21.5 | 0.7 (0.2) | 63.4 | 19.0 |
| Moth bean | 343 | 23–26 | 1.6 (0.4) | 61.5 | 5 |
| Navy bean | 337 | 22.3 | 1.5 (0.2) | 60.8 | 15.3 |
| Pigeon pea | 343 | 13–26 | 1.5 (0.3) | 62.8 | 15 |
| Pinto bean | 347 | 21.4 | 1.2 (0.2) | 62.6 | 15.5 |
| Rice bean | 338 | 18–19 | 0.5 (0.3) | 59.1 | 7.1 |
| Sword bean | 361 | 24–30 | 2.6–9.8 (-) | 41–59 | 7–13 |
| Tarwi | 440 | 41–51 | 14–24 (19) | 28.2 | 7.1 |
| Tepary bean | 353 | 19–24 | 1.2 (-) | 67.8 | 4.8 |
| Urad | 341 | 25.2 | 1.6 (0.1) | 59.0 | 18.3 |
| Velvet bean | 373 | 20–29 | 6–7 | 50–61 | 9–11 |
| Winged bean | 428 | 30–35 | 16.3 (2.3) | 41.7 | 11–26 |
| Broad bean | 341 | 26.1 | 1.53 (0.3) | 58.3 | 25 |
| Chickpea | 378 | 11–31 | 6.0 (0.6) | 63.0 | 12.2 |
| Common bean | 20–24 | 0.8 (0.6) | 75.5 | - | |
| Field pea | 352 | 23.8 | 1.2 (0.2) | 63.7 | 25 |
| Lentil | 352 | 24.6 | 1.1 (0.2) | 63.4 | 10.7 |
| Lupin | 371 | 36.2 | 9.7 (1.2) | 40.37 | 18.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayerdi Gotor, A.; Marraccini, E. Innovative Pulses for Western European Temperate Regions: A Review. Agronomy 2022, 12, 170. https://doi.org/10.3390/agronomy12010170
Ayerdi Gotor A, Marraccini E. Innovative Pulses for Western European Temperate Regions: A Review. Agronomy. 2022; 12(1):170. https://doi.org/10.3390/agronomy12010170
Chicago/Turabian StyleAyerdi Gotor, Alicia, and Elisa Marraccini. 2022. "Innovative Pulses for Western European Temperate Regions: A Review" Agronomy 12, no. 1: 170. https://doi.org/10.3390/agronomy12010170
APA StyleAyerdi Gotor, A., & Marraccini, E. (2022). Innovative Pulses for Western European Temperate Regions: A Review. Agronomy, 12(1), 170. https://doi.org/10.3390/agronomy12010170







