Abstract
In subtropical regions, we have an incomplete understanding of how long-term tillage, stubble, and nitrogen (N) fertilizer management affects soil biological functioning. We examined a subtropical site managed for 50 years using varying tillage (conventional till (CT) and no-till (NT)), stubble management (stubble burning (SB) and stubble retention (SR)), and N fertilization (0 (N0), 30 (N30), and 90 (N90) kg ha−1 y−1) to assess their impact on soil microbial respiration, easily extractable glomalin-related soil protein (EEGRSP), and N mineralization. A significant three-way tillage × stubble × N fertilizer interaction was observed for soil respiration, with NT+SB+N0 treatments generally releasing the highest amounts of CO2 over the incubation period (1135 mg/kg), and NT+SR+N0 treatments releasing the lowest (528 mg/kg). In contrast, a significant stubble × N interaction was observed for both EEGRSP and N mineralization, with the highest concentrations of both EEGRSP (2.66 ± 0.86 g kg−1) and N mineralization (30.7 mg/kg) observed in SR+N90 treatments. Furthermore, N mineralization was also positively correlated with EEGRSP (R2 = 0.76, p < 0.001), indicating that EEGRSP can potentially be used as an index of soil N availability. Overall, this study has shown that SR and N fertilization have a positive impact on soil biological functioning.
1. Introduction
No-till (NT) systems have been widely reported to maintain agricultural production whilst minimizing disturbance of the soil [1]. The absence of tillage reduces soil physical degradation and the decomposition of organic matter [2]. In addition, the retention of organic residues on the soil surface decreases erosion, increases soil organic carbon (SOC) concentrations, and decreases the flux of CO2 into the atmosphere. In some instances, these increases in SOC concentrations can lead to net sequestration of C [3]. However, we still have an incomplete understanding of how NT systems affect certain aspects of soil biological activity.
Given that tillage physically disrupts fungal hyphae networks, conversion from conventional till (CT) to NT practices often results in an increase in arbuscular mycorrhizal (AM) fungi [4,5,6]. Glomalin (a glycoprotein) is produced by AM fungi, with this compound often associated with improved soil aggregation [7]. Furthermore, glomalin can play an important role in the sequestration of C and N [7]. For example, glomalin contains 37% C and 4% N, and in a tropical rainforest soil from Costa Rica, it was found to account for 3.2% of the total soil C and 5% of the total soil N [8]. Interestingly, it has been reported that easily extractable glomalin-related soil protein (EEGRSP) also serves as a good indicator of N availability in soil [9], indicating that measurement of this fraction may be useful as an indicator of soil N mineralization potential. However, despite the importance of glomalin, comparatively little is known regarding the management practices that influence its concentration.
The retention of stubble and a reduction in tillage are typically associated with increases in SOC and glomalin-related soil protein (GRSP) [7,10,11,12]. Several studies have also shown that the concentration of available N in the soil influences concentrations of GRSP. For example, some researchers have found that the addition of N significantly increased GRSP [13,14], while others have reported that N fertilization did not alter the GRSP concentration in the bulk soil [15] or led to decreased concentrations [16]. These inconsistent results may be related to the initial soil N content [6], as well as to N addition rates and to the duration of fertilization [14,17].
Not only do NT systems impact AM and the production of glomalin, but they also influence soil respiration and microbial activity more broadly. Soil respiration consists of autotrophic root respiration and heterotrophic respiration and plays an important role in global C cycling [18,19]. It is considered that soil respiration is a sensitive indicator of soil metabolic activity and the conversion of SOC to atmospheric CO2 [20]. There are multiple management practices that influence soil microbial respiration in agricultural systems, including tillage, the retention of stubble from crop residues, and N management. However, compared to temperate systems, comparatively little is known about the impact of such management practices on respiration in subtropical systems. Moreover, it remains uncertain how N regulates C cycle–climate feedback, with this being a critical issue in model projections of future states of climate and ecosystems [21].
It is thus the aim of the current study to improve our understanding of how tillage, stubble, and nitrogen management practices affect soil respiration and glomalin concentration in semi-arid subtropical environments to better understand how common agricultural management practices are likely to impact soil biological functioning. To achieve this, we examined a Vertisol at a semi-arid subtropical site that has been managed for 50 years (y) with a combination of different tillage management, NT or CT; stubble management, stubble burning (SB), or stubble retained (SR); and N fertilization at 0 (N0), 30 (N30), and 90 (N90) kg ha−1 y−1. The objective of this study was to determine the effect of NT, SR, and N fertilization on microbial respiration and EEGRSP concentration after 50 years of management. The relationship between EEGRSP and nitrogen mineralization was also examined to test whether EEGRSP measurements would be useful as an index of soil N availability.
2. Materials and Methods
2.1. Experimental Details and Soil Analysis
An experiment was established at Hermitage Research Station (28°12′ S, 152°06′ E), Queensland, Australia, in December 1968 to study the effects of tillage and crop stubble management on soil properties and crop productivity. The soil was developed primarily from basaltic alluvium and contains 65% clay, 24% silt, and 11% sand in the top 0.1 m depth. It is a black self-mulching cracking clay, being classified as a Vertisol, Vertosol, or Ustic Pellustert [22]. The mean annual temperature of the experimental site is 17.5 °C, with mean monthly minimum and maximum temperatures of 2 and 17 °C, respectively, in July and 15 and 30 °C, respectively, in January. Mean annual rainfall at the site is 685 mm, of which 60% is received during the summer months from December to March. Soil pH (1:5 soil:water) in the 0.1 m of soil layer varies from 6.92 (N90) to 7.61 (N0).
The treatments were arranged in a randomized block design consisting of 12 treatments (two tillage practices (CT and NT), two crop stubble management practices (SR and SB), and three N fertilization rates (N0, N30, and N90)), with four replicates comprising 48 plots. The trial was sown with wheat (Triticum aestivum L.) annually, except for a three-year period (1975–1977) in which barley (Hordeum vulgare L. cv. Clipper) was grown [23]. The treatments were arranged in 61.9 m × 6.4 m plots covering a total of 1.9 ha. The CT treatment comprised four or five primary tillage operations with a chisel plow to control weeds during the fallow period each year. The treatments under NT were sprayed with herbicide to control weeds. For the SR treatment, stubble was retained in situ. For the NT treatment, stubble was left on the soil surface, but for the CT treatment, it was incorporated into the top 0.1 m depth using a chisel plow. For the SB treatment, crop stubble was burnt in situ immediately after the crop harvest and before the first tillage operation [23]. The N30 treatment received urea at a rate of 23 kg N ha−1 until 1996 and then 30 kg N ha−1 y−1 thereafter. During the first 8 years of experimentation, the N90 treatment received urea at a rate of 46 kg N ha−1 y−1 until 1975, then 69 kg N ha−1 y−1 until 1996, and then 90 kg N ha−1 y−1 thereafter. Crops could not be grown in 1982, 1991, 1994, and 2004 due to insufficient rainfall at sowing. In all years, crops were mechanically harvested.
Soil samples were taken to 0.1 m depth in May 2018 from five different locations of each plot. This sampling occurred approximately 6 months after the site was harvested and burnt, and the soil received no fresh input of crop residue after the harvest. The samples collected from each plot were bulked and sealed in plastic bags for transport to the laboratory. Soil samples were air-dried before being sieved to <2 mm.
2.2. Measurement of Soil Microbial Respiration
Soil microbial respiration was measured in air-tight plastic jars with a capacity of 270 mL. Of the 12 treatments, microbial respiration was measured for all except the N30 treatments. For the incubation, the moisture content of 50 g of air-dry soil was initially adjusted to 60% of water-holding capacity and was kept at a constant temperature of 25 °C. In the measurement of soil microbial respiration, we excluded the influence of factors such as root respiration and variations in soil water content, which contribute to the high variability found in field estimates. The soil samples were incubated for 98 days, with soil microbial respiration measured after 1, 3, 7, 14, 22, 37, 50, 65, and 98 days. The soil microbial respiration data of the first 7 days were not used to minimize the effect of air-drying and then re-wetting soil on the soil microbial respiration [24]. The concentration of CO2 in the headspace was measured using a CO2 analyzer (WMA-5, PP system, John Morris Scientific, Sydney, Australia).
2.3. Determination of EEGRSP Concentration
The soil protein extraction protocol was based on a neutral sodium citrate buffer solution [25]. In brief, 1 g of soil was placed in a 50 mL polypropylene centrifuge tube (Corning) in which 8 mL of 20 mM sodium citrate at pH 7.0 was added and autoclaved for 30 min (121 °C, 1.4 kg cm−2). Immediately after autoclaving, tubes were centrifuged at 1000× g RCF for 15 min, with the supernatant decanted and stored at 4 °C until analysis. The protein concentration was determined using the Bradford dye-binding assay using bovine serum albumin as the standard [25]. Absorbance of the Bradford reagent was measured with a spectrophotometer (Shimadzu, Kyoto, Japan, UV2600) at 590 nm.
2.4. Determination of N Mineralization Rate
To determine the N mineralization rate, the extractable NO3-N was measured both prior to commencing the incubation (0 d) and after 35 days. The moisture content of 50 g of air-dried soil was adjusted to 60% of water-holding capacity prior to its incubation in a plastic jar at a constant temperature of 25 °C. The NO3-N concentration was measured by taking 3 g of incubated sample (air-dry basis) mixed with 30 mL of 2 M KCl and shaking for 1 h on a rotary shaker. The samples were then centrifuged at 1000× g RCF, and subsamples were drawn using a Swinnex filter holder fitted with a glass microfiber filter for determination of NO3-N by the automated cadmium reduction method [26] using a segmented flow analyzer (SEAL analytical, Norderstedt, Germany). The net NO3-N mineralized (mg kg−1 soil) was calculated as the difference between the values measured at the two time intervals (0 and 35 d). Only the NO3-N concentration was measured, as the NH4-N concentration was ≤2 mg kg−1.
2.5. Statistical Analysis
All statistical analyses were conducted using SPSS version 25. Treatment effects were analyzed using a three-way Analysis of Variance procedure, with a Fisher’s least-significant-difference (LSD) post hoc test used to compare treatment means. Treatment differences were considered significant at p < 0.05. The relationship between mineralization and EEGRSP was assessed using regression analysis.
3. Results
3.1. Microbial Respiration
The soil microbial respiration rate for all treatments ranged between 0.11 and 1.09 mg CO2-C kg−1 h−1 and varied over time, being consistently high during the initial stages of incubation and then gradually decreasing (Figure 1). However, the magnitude of the soil microbial respiration depended upon the treatment, being significantly affected by tillage, stubble, and N fertilization management practices (Figure 1 and Table 1 and Table 2). These treatment effects were particularly evident from examination of the cumulative soil microbial respiration, with a significant three-way tillage × stubble × N fertilization interaction observed after 7 days (Figure 2 and Table 2). Generally, the NT+SB+N0 treatments released the largest amounts of CO2 over the period of the incubation (1140 mg CO2-C/kg), while the NT+SR+N0 treatment released the lowest amount (529 mg CO2-C/kg).
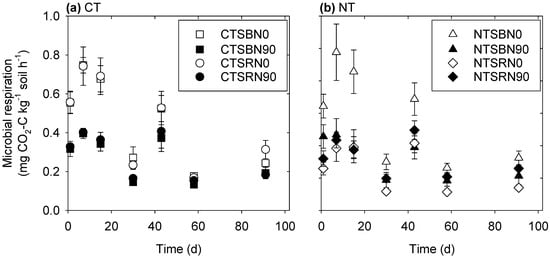
Figure 1.
Effect of tillage, stubble management, and N fertilization on soil microbial respiration rate at different days after incubation (d). Treatments included (a) conventional till (CT) and (b) no-till (NT), with stubble burning (SB), stubble retained (SR), N fertilized at a rate of 0 kg ha−1 (N0), and N fertilized at a rate of 90 kg ha−1 (N90). The standard errors are shown as bar heights.

Table 1.
Significance level (p value) of soil microbial respiration at different stages of incubation for a Vertisol managed for 50 years with different tillage practices, stubble management practices, and N fertilization rates.

Table 2.
Significance level (p value) of soil cumulative microbial respiration at different stages of incubation for a Vertisol managed for 50 years with different tillage practices, stubble management practices, and N fertilization rates.
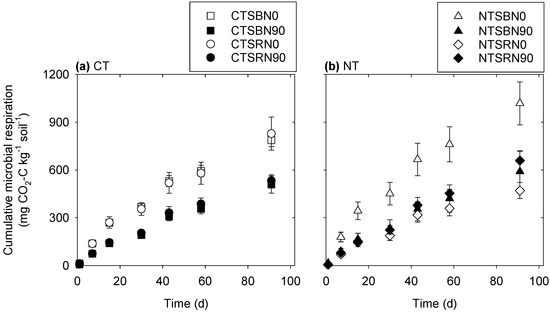
Figure 2.
Effect of tillage, stubble management, and N fertilization on cumulative soil microbial respiration (a,b). Treatments included conventional till (CT), no-till (NT), stubble burning (SB), stubble retained (SR), N fertilized at a rate of 0 kg ha−1 (N0), and N fertilized at a rate of 90 kg ha−1 (N90).
3.2. Easily Extractable Glomalin-Related Soil Protein (EEGRSP)
Treatments were found to cause substantial differences in the EEGRSP concentrations. Specifically, a significant stubble management × fertilizer interaction was observed (Figure 3); while N fertilization significantly increased the EEGRSP concentrations (from 0.68 to 1.69 g/kg when averaged across other treatments), the pattern of this increase was dependent upon stubble management. Specifically, the greatest EEGRSP concentrations were observed when SR was practiced with the N90, SR+N90 treatment (2.66 ± 0.86 g kg−1). Much smaller, or insignificant, increases were observed with other stubble × fertilizer treatment combinations.
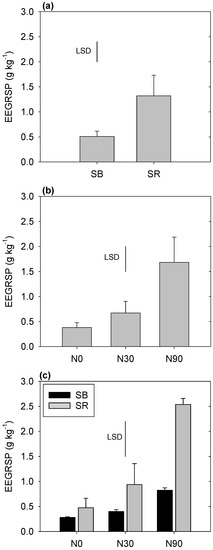
Figure 3.
Effect of stubble management and N fertilization on easily extractable glomalin-related soil protein (EEGRSP) concentration of soil (a–c). Treatments included stubble burning (SB), stubble retained (SR), N fertilized at a rate of 0 kg ha−1 (N0), N30 kg ha−1 (N30), and 90 kg ha−1 (N90). The standard errors are shown as bar heights.
3.3. Net N Mineralization
Nitrogen mineralization was found to be affected by tillage, stubble, and fertilizer management. A significant tillage × stubble interaction was observed, with the SR+NT treatments having greater rates of N mineralization (25.5 mg/kg) than other tillage × stubble combinations (Figure 4). In a similar manner, a significant stubble × fertilizer interaction was also observed, with the highest rates of mineralization in the SR+N90 treatment (30.7 mg/kg) and the lowest in the SR+N0 treatment (13.7 mg/kg). Net N mineralization during the 35 days of incubation was also significantly and positively correlated with the EEGRSP concentration (p = 0.001) (Figure 5).
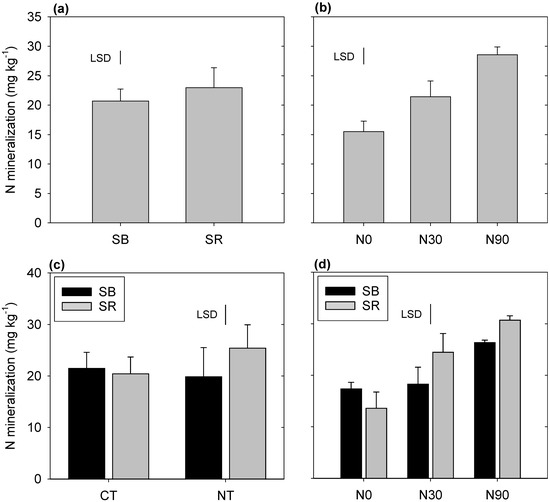
Figure 4.
Effect of tillage, stubble management, and N fertilization on N mineralized after 35 days of incubation. Treatments included (a) stubble management (b) N fertilization (c) tillage × stubble management and (d) N fertilization × stubble management. Standard errors are shown as bar heights.
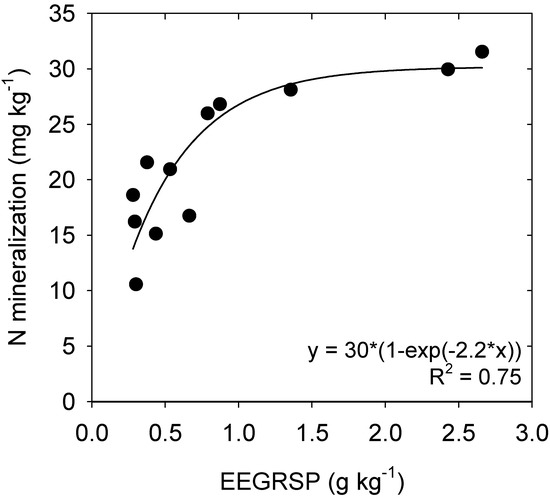
Figure 5.
Relationship between N mineralized within 35 days and easily extractable glomalin-related soil protein (EEGRSP) concentration.
4. Discussion
4.1. Microbial Respiration
After 50 years, differences in tillage practices, stubble management, and N fertilization were all found to impact soil microbial respiration. Microbial respiration rates were higher in the initial phase of the incubation study and decreased progressively with time (Figure 1). This decrease over time is consistent with other studies, where a reduction in the concentration of labile C over the course of a laboratory incubation leads to a decrease in the respiration rate [27,28,29]. When cumulative respiration over the course of the experiment was considered, a consistent three-way tillage × stubble retention × nitrogen addition interaction was observed. The largest release of CO2 from respiration was observed where NT, SB, and N0 treatments were combined, while the lowest rates were observed where NT was combined with SR and N0 treatments.
Tillage, stubble, and N fertilizer management can impact respiration via several mechanisms. No-till management is often reported to decrease C decomposition due to the formation and stabilization of macroaggregates (i.e., >250 µm) [30], which protect soil organic matter (SOM) from decomposition and thus decreases respiration [31]. The soil used in the current study was sieved to <2 mm, and therefore, any impact of macroaggregates >2 mm in NT vs CT treatments would not have been accounted for. Conversely, tillage enhances the oxidation of SOM by disrupting macroaggregates, exposing aggregate-protected SOM to microbial attack [32], and thus increasing the emission of CO2 [33]. For example, one study observed a 14% decrease in the cumulative CO2 flux from soil under NT compared to CT [34]. Similarly, in Canada, a 20–25% reduction in the annual CO2 fluxes from soil under NT compared to CT was reported [35]. However, in the current study, both the greatest and lowest rates of respiration were observed from a treatment combination that included NT (Figure 2), indicating that factors in addition to the aggregate protection of SOM must also have been responsible for the difference in respiration rates between treatments in the current study.
Stubble management can also influence respiration, particularly when it influences the availability or quality of organic substrates. Previously at this study site, higher rates of respiration have been observed under SR treatments, in line with greater concentrations of total SOC and thus substrate availability [29,36]. This is similar to many other results recorded worldwide, where stubble burning is typically observed to reduce soil respiration [37,38,39,40]. However, in the current study, there was no significant difference in the total soil organic C content in SB and SR treatments [37], and the main stubble effect did not have a significant impact on total cumulative respiration. This indicates that there may have been differences in the samples collected at these different times, despite the use of similar sampling protocols and sampling at the same time of year (approximately 6 months after harvest and stubble burning). However, previous work conducted using the same samples as the current study did observe a significant relationship (r = 0.89, p < 0.01) between cumulative soil microbial C respiration and the microbial metabolic quotient (respiration to biomass ratio), which was much higher in SB compared to SR treatments [37]. This indicates that the microbial population in the SB treatment is likely to have been under greater stress and converting a greater proportion of substrates present to CO2 (i.e., reduced carbon use efficiency [CUE]), which may partly account for the greater respiration observed in the NT+SB+N0 versus NT+SR+N0 treatments in the current study (Figure 2).
We also observed that application of N fertilizer (90 kg N ha−1 y−1) typically reduced CO2 flux, except for the NT+SR+N90 treatments, which had a higher respiration rate than the NT+SR+N0 treatments (Figure 2). Reductions in CO2 emissions following the addition of N fertilizer have been observed in numerous studies [28,41,42,43,44,45], including previous studies of the current site [29,36]. This is despite the fact that N addition has been observed to increase total SOC concentrations, and thus substrate supply, in these soils [37]. The decrease in soil microbial respiration for the high N rate (90 kg ha−1) treatment could indicate that there has been a change in the microbial community structure and/or activity that has led to an increase in microbial CUE [46,47,48]. This is supported by previous studies at the site, which have observed significant decreases in the microbial metabolic quotient with N addition, particularly when combined with SB [37]. Several studies have suggested that a reduction in fungi in response to N addition may result in an increase in the number of r-strategist microbes that are relatively more N-demanding (e.g., bacteria) at the expense of N-conservative microbes less efficient in C assimilation [49].
4.2. EEGRSP
In the present study, we observed that EEGRSP was affected by a significant stubble × fertilizer interaction, with the highest concentrations observed when SR was practiced with the N90 treatment (Figure 3). These findings are similar to those of other authors who found that the combined application of N fertilizer and straw retention resulted in a significant increase in the soil EEGRSP [11]. Other studies have also reported that, regardless of tillage practice, SR tended to increase the EEGRSP concentration in soil [10,11]. However, N fertilizer application has also been observed to decrease soil glomalin concentrations, likely due to a decrease in root colonization by AM fungi under conditions of abundant nutrient supply and/or the stimulation of microbial respiration (and SOM/glomalin decomposition) by additional nutrient input [16]. However, in the current study, N addition generally had a negative impact on respiration, which may have reduced glomalin loss via decomposition (Figure 2).
Numerous studies have also reported that tillage has a significant impact on EEGRSP. For example, one study observed substantial increases in the EEGRSP concentrations 3 years after converting from conventional plowing to NT [7]. Similar results were also reported in China, where significantly higher concentrations of EEGRSP were observed in an NT compared to a CT system [12]. The higher EEGRSP concentration in NT systems compared to CT soils suggests either a higher AM fungal density in NT systems or an enhancement of the AM fungal community composition in response to reduced soil disturbance [8,50]. However, interestingly, in the current study, we were unable to observe any significant impact of tillage management on EEGRSP concentration. This may be related to the higher, or similar, rates of respiration (and potentially glomalin decomposition) observed in the NT compared to the CT treatments in the current study (Figure 1 and Figure 2).
4.3. Nitrogen Mineralization
We found that the rate of N mineralization was primarily influenced by N fertilization and stubble management (Figure 4). N mineralization increased significantly with increased application of N fertilizer and was higher when fertilizer application was combined with SR (Figure 4). This is consistent with the greater total N concentrations and lower C:N ratios present in N fertilized treatments [37]. While the total SOC stocks in SB and SR treatments were not significantly different, the C:N ratio was significantly higher under SB management [37], which would have contributed to reduced N mineralization. Previous studies of the site have also observed higher rates of N mineralization in SR+N90 treatments [36]. Similarly, Silgram and Chambers [51] reported that N fertilizer had a significant positive effect on readily mineralizable N in the plow layer at two experimental sites, while Govaerts et al. [52] reported lower N mineralization in treatments where stubble was burnt after the harvest of crops.
A significant tillage × stubble retention interaction was also observed to affect net N mineralization, with significant increases only observed when NT and SR were applied in combination (Figure 4c). This is contrary to previous studies of the site, where NT+SR combinations reduced mineralization rates in comparison to CT+SR [36]. Other authors have also reported that CT along with stubble incorporation can significantly increase N mineralization [53,54]. The reason for the differences observed in the current study is unknown.
In the present study, we also observed a strong relationship between N mineralization capacity and EEGRSP (Figure 5). The N mineralization capacity of the soil increased rapidly with an increase in the concentration of EEGRSP up to ~1 g/kg, after which increases in EEGRSP had a limited impact on N mineralization. This indicates that the glycoprotein glomalin is acting as a readily mineralizable source of N in this soil. Hence, the pool of proteins extracted by this method can be viewed broadly as a soil available N supply indicator that reflects a pool of potentially available organic N in soil [9]. This result is supported by Nannipieri and Eldor [55], who report in their review that the majority of organic-N is proteinaceous in nature. It was also reported by Fine et al. [56] that soil protein is significantly related (r = 0.52, p < 0.01) to total soil N.
5. Conclusions
After 50 years of management, we have found that tillage, stubble management practices, and N fertilization affect soil respiration, EEGRSP, and N mineralization. The impact of the management practices on soil respiration was complex. Specifically, it was observed that cumulative soil microbial respiration was greatest where NT, SB, and N0 treatments were combined, while the lowest rates were observed where NT was practiced with SR and N0. However, N application was generally associated with reduced CO2 flux. It is likely that the impact of management practices on respiration is related to their combined impact on CUE, with treatment combinations that increased CUE resulting in reduced respiration rates, and vice versa. Concentrations of EEGRSP were significantly higher for SR than for SB treatments, and particularly when fertilizer was also applied. Nitrogen mineralization was also found to be highest where fertilizer was applied with SR. Indeed, the amount of mineralizable NO3−N measured after 35 days of incubation was significantly and positively correlated with the EEGRSP concentration, indicating that EEGRSP can potentially be used as an index of N availability in soil. The optimum rate of fertilizer N can be targeted by considering the availability of potential mineralizable N, as well as tillage and stubble management practices.
Author Contributions
P.J.: investigation, data analysis, and writing original draft. K.M.H.: investigation and data analysis. R.C.D. and P.M.K.: study concept, design, editing, and critical review. N.W.M., Y.P.D. and B.A.M.: critical review, editing, and resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported (Recipient ID No. 6510-2018) by the Department of Education, Australian Government.
Acknowledgments
Pramod Jha thanks the Department of Education, Australian Government, for financial support received through Endeavour Research Fellowship (Recipient ID No. 6510-2018). We thank Robert Banks for assistance in the collection of soil samples and Steven Reeves, Department of Environment and Science, for the management of the long-term Hermitage experiment.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Dang, Y.P.; Dalal, R.C.; Menzies, N. No-Till Farming Systems for Sustainable Agriculture: Challenges and Opportunities; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; van Groenigen, K.J.; Lee, J.; Lundy, M.E.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef]
- Lal, R. Sequestering carbon in soils of agro-ecosystems. Food Policy 2011, 36, S33–S39. [Google Scholar] [CrossRef]
- Mäder, P.; Edenhofer, S.; Boller, T.; Wiemken, A.; Niggli, U. Arbuscular mycorrhizae in a long-term field trial comparing low-input (organic, biological) and high-input (conventional) farming systems in a crop rotation. Biol. Fert. Soils 2000, 31, 150–156. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Young, D. Mycorrhizae, crop growth, and crop phosphorus nutrition in maize-soybean rotations given various tillage treatments. Plant Soil 1999, 210, 33–42. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M. Glomalin in Ecosystems. Soil Sci. Soc. Am. J. 2007, 71, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.F.; Starr, J.L.; Paltineanu, I.C. Changes in Aggregate Stability and Concentration of Glomalin during Tillage Management Transition. Soil Sci. Soc. Am. J. 1999, 63, 1825–1828. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Wright, S.F.; Clark, D.A.; Ruess, R.W. Soil Stocks of Glomalin Produced by Arbuscular Mycorrhizal Fungi across a Tropical Rain Forest Landscape. J. Ecol. 2004, 92, 278–287. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Culman, S.W.; Zhao, K. Repeatability and Spatiotemporal Variability of Emerging Soil Health Indicators Relative to Routine Soil Nutrient Tests. Soil Sci. Soc. Am. J. 2018, 82, 939–948. [Google Scholar] [CrossRef]
- Dai, J.; Hu, J.; Zhu, A.; Lin, X.; Nicholson, F. No-tillage with half-amount residue retention enhances microbial functional diversity, enzyme activity and glomalin-related soil protein content within soil aggregates. Soil Use Manag. Manag. 2017, 33, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Zhou, J.-M.; Wang, H.-Y.; Chen, X.-Q.; Du, C.-W. Effect of Long-Term Rice Straw Return on Soil Glomalin, Carbon and Nitrogen. Pedosphere 2007, 17, 295–302. [Google Scholar] [CrossRef]
- Dai, J.; Hu, J.; Zhu, A.; Bai, J.; Wang, J.; Lin, X. No tillage enhances arbuscular mycorrhizal fungal population, glomalin-related soil protein content, and organic carbon accumulation in soil macroaggregates. J. Soils Sediments 2015, 15, 1055–1062. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; He, X.; Liu, J. Glomalin-related soil protein responses to elevated CO2 and nitrogen addition in a subtropical forest: Potential consequences for soil carbon accumulation. Soil Biol. Biochem. 2015, 83, 142–149. [Google Scholar] [CrossRef]
- Garcia, M.O.; Ovasapyan, T.; Greas, M.; Treseder, K.K. Mycorrhizal dynamics under elevated CO2 and nitrogen fertilization in a warm temperate forest. Plant Soil 2008, 303, 301–310. [Google Scholar] [CrossRef]
- Wuest, S.B.; Caesar-TonThat, T.C.; Wright, S.F.; Williams, J.D. Organic matter addition, N, and residue burning effects on infiltration, biological, and physical properties of an intensively tilled silt-loam soil. Soil Tillage Res. 2005, 84, 154–167. [Google Scholar] [CrossRef]
- Cordeiro, C.F.d.S.; Rodrigues, D.R.; Rocha, C.H.; Araujo, F.F.; Echer, F.R. Glomalin and microbial activity affected by cover crops and nitrogen management in sandy soil with cotton cultivation. App. Soil Ecol. 2021, 167, 104026. [Google Scholar] [CrossRef]
- Treseder, K.K. A Meta-Analysis of Mycorrhizal Responses to Nitrogen, Phosphorus, and Atmospheric CO2 in Field Studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, W.H.; Andrews, J.A.; Rustad, L.E.; Huntington, T.G.; Boone, R.D. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Barber, J.J.; Pippen, J.S.; Taneva, L.; Andrews, J.A.; Schlesinger, W.H. Long-Term Effects of Free Air CO2 Enrichment (FACE) on Soil Respiration. Biogeochemistry 2006, 77, 91–116. [Google Scholar] [CrossRef]
- Rochette, P.; Flanagan, L.B. Quantifying rhizosphere respiration in a corn crop under field conditions. Soil Sci. Soc. Am. J. 1997, 61, 466–474. [Google Scholar] [CrossRef]
- Thornton, P.E.; Doney, S.C.; Lindsay, K.; Moore, J.K.; Mahowald, N.; Randerson, J.T.; Fung, I.; Lamarque, J.F.; Feddema, J.J.; Lee, Y.H. Carbon-nitrogen interactions regulate climate-carbon cycle feedbacks: Results from an atmosphere-ocean general circulation model. Biogeosciences 2009, 6, 2099–2120. [Google Scholar] [CrossRef] [Green Version]
- Baillie, I.C. Soil Survey Staff 1999, Soil Taxonomy: A basic system of soil classification for making and interpreting soil surveys, 2nd edition. Agricultural Handbook 436, Natural Resources Conservation. Soil Use Manag. 2001, 17, 57–60. [Google Scholar] [CrossRef]
- Marley, J.M.; Littler, J.W. Winter cereal production on the Darling Downs dash an 11 year study of fallowing practices. Aust. J. Exp. Agric 1989, 29, 807. [Google Scholar] [CrossRef]
- Jones, A.R.; Gupta, V.V.S.R.; Buckley, S.; Brackin, R.; Schmidt, S.; Dalal, R.C. Drying and rewetting effects on organic matter mineralisation of contrasting soils after 36 years of storage. Geoderma 2019, 342, 12–19. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Mfilinge, P.; Atta, N.; Tsuchiya, M. Nutrient dynamics and leaf litter decomposition in a subtropical mangrove forest at Oura Bay, Okinawa, Japan. Trees 2002, 16, 172–180. [Google Scholar] [CrossRef]
- Finn, D.; Page, K.; Catton, K.; Strounina, E.; Kienzle, M.; Robertson, F.; Armstrong, R.; Dalal, R. Effect of added nitrogen on plant litter decomposition depends on initial soil carbon and nitrogen stoichiometry. Soil Biol. Biochem. 2015, 91, 160–168. [Google Scholar] [CrossRef]
- Reeves, S.H.; Somasundaram, J.; Wang, W.J.; Heenan, M.A.; Finn, D.; Dalal, R.C. Effect of soil aggregate size and long-term contrasting tillage, stubble and nitrogen management regimes on CO2 fluxes from a Vertisol. Geoderma 2019, 337, 1086–1096. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter. I, Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Jastrow, J.D. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Beare, M.H.; Hendrix, P.F.; Coleman, D.C. Water-Stable Aggregates and Organic Matter Fractions in Conventional- and No-Tillage Soils. Soil Sci. Soc. Am. J. 1994, 58, 777–786. [Google Scholar] [CrossRef]
- Jacinthe, P.A.; Lal, R. Labile carbon and methane uptake as affected by tillage intensity in a Mollisol. Soil Tillage Res. 2005, 80, 35–45. [Google Scholar] [CrossRef]
- Alluvione, F.; Halvorson, A.D.; Del Grosso, S.J. Nitrogen, Tillage, and Crop Rotation Effects on Carbon Dioxide and Methane Fluxes from Irrigated Cropping Systems. J. Environ. Qual. 2009, 38, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Wang, H.; Selles, F.; McConkey, B.G.; Campbell, C.A. Tillage Effects on Carbon Fluxes in Continuous Wheat and Fallow–Wheat Rotations. Soil Sci. Soc. Am. J. 2000, 64, 2080–2086. [Google Scholar] [CrossRef]
- Thompson, J.P. Soil biotic and biochemical factors in a long-term tillage and stubble management experiment on a vertisol. 2. Nitrogen deficiency with zero tillage and stubble retention. Soil Tillage Res. 1992, 22, 339–361. [Google Scholar] [CrossRef]
- Jha, P.; Hati, K.M.; Dalal, R.C.; Dang, Y.P.; Kopittke, P.M.; Menzies, N.W. Soil carbon and nitrogen dynamics in a Vertisol following 50 years of no-tillage, crop stubble retention and nitrogen fertilization. Geoderma 2020, 358, 113996. [Google Scholar] [CrossRef]
- Wüthrich, C.; Schaub, D.; Weber, M.; Marxer, P.; Conedera, M. Soil respiration and soil microbial biomass after fire in a sweet chestnut forest in southern Switzerland. Catena 2002, 48, 201–215. [Google Scholar] [CrossRef]
- Almendros, G.; Leal, J.A. An evaluation of some oxidative degradation methods of humic substances applied to carbohydrate-derived humic-like polymers. J. Soil Sci. 1990, 41, 51–59. [Google Scholar] [CrossRef]
- Rashid, G.H. Effects of fire on soil carbon and nitrogen in a Mediterranean oak forest of Algeria. Plant Soil 1987, 103, 89–93. [Google Scholar] [CrossRef]
- Fernández, I.; Cabaneiro, A.; Carballas, T. Organic matter changes immediately after a wildfire in an atlantic forest soil and comparison with laboratory soil heating. Soil Biol. Biochem. 1997, 29, 1–11. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Rice, W.A.; Clayton, G.W. Soil microbial diversity and community structure under wheat as influenced by tillage and crop rotation. Soil Biol. Biochem. 1998, 30, 1733–1741. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol. Biochem. 2010, 42, 2336–2338. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Swanston, C.; Homann, P.S.; Caldwell, B.A.; Myrold, D.D.; Ganio, L.; Sollins, P. Long-Term Effects Of Elevated Nitrogen On Forest Soil Organic Matter Stability. Biogeochemistry 2004, 70, 227–250. [Google Scholar] [CrossRef]
- Li, J.; Xie, R.Z.; Wang, K.R.; Ming, B.; Guo, Y.Q.; Zhang, G.Q.; Li, S.K. Variations in Maize Dry Matter, Harvest Index, and Grain Yield with Plant Density. Agron. J. 2015, 107, 829–834. [Google Scholar] [CrossRef]
- Manzoni, S.; Porporato, A. Soil carbon and nitrogen mineralization: Theory and models across scales. Soil Biol. Biochem. 2009, 41, 1355–1379. [Google Scholar] [CrossRef]
- Maaroufi, N.I.; Nordin, A.; Hasselquist, N.J.; Bach, L.H.; Palmqvist, K.; Gundale, M.J. Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Glob. Chang. Biol. 2015, 21, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Silgram, M.; Chambers, B.J. Effects of long-term straw management and fertilizer nitrogen additions on soil nitrogen supply and crop yields at two sites in eastern England. J. Agric. Sci 2002, 139, 115–127. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Ceballos-Ramirez, J.M.; Luna-Guido, M.L.; Limon-Ortega, A.; Deckers, J.; Dendooven, L. Conventionally tilled and permanent raised beds with different crop residue management: Effects on soil C and N dynamics. Plant Soil 2006, 280, 143–155. [Google Scholar] [CrossRef]
- Raiesi, F. Carbon and N mineralization as affected by soil cultivation and crop residue in a calcareous wetland ecosystem in Central Iran. Agric. Ecosyst. Environ. 2006, 112, 13–20. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Dougherty, W.J.; Fang, Y.; Badgery, W.; Hoyle, F.C.; Dalal, R.C.; Cowie, A.L. Impact of agricultural management practices on the nutrient supply potential of soil organic matter under long-term farming systems. Soil Tillage Res. 2018, 175, 71–81. [Google Scholar] [CrossRef]
- Nannipieri, P.; Eldor, P. The chemical and functional characterization of soil N and its biotic components. Soil Biol. Biochem. 2009, 41, 2357–2369. [Google Scholar] [CrossRef]
- Fine, A.K.; Es, H.M.; Schindelbeck, R.R. Statistics, Scoring Functions, and Regional Analysis of a Comprehensive Soil Health Database. Soil Sci. Soc. Am. J. 2017, 81, 589–601. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).