Abstract
Plant biomass in the form of waste materials and by-products from various industries can be a valuable material for the production of composts and growing media for urban gardening. In this study, pulp and paper mill sludge, fruit-vegetable waste, mushroom spent substrate and rye straw were used to produce compost that was further used as a medium component in container cultivation of tomato. The plants were grown in containers with a capacity of 3 dm3 filled with three types of compost-based growing media supplemented with high peat, fen peat, pine bark and wood fiber. The tomato plants grown in 100% peat substrate served as controls. The plants grown in the compost-enriched media had a higher leaf greening index and percentage of ripe fruit, and exhibited an increased content of total polyphenols and flavonoids, potassium, calcium, magnesium and copper in fruit as compared with the control. The tomatoes grown in a medium consisting of 25% compost, 30% high peat, 15% low peat, 20% pine bark and 10% wood fiber reached the highest fresh fruit weight, total polyphenol content and L-ascorbic acid levels. This study demonstrated that the compost produced from natural materials from various sources was a valuable potting medium supplement with positive effects on tomato yield and nutritional value.
1. Introduction
Recently, the idea of urban gardening involving the individual cultivation of vegetables and other crops has gained huge popularity [1]. Growing plants in urban areas transformed into community gardens is a way of spending free time, strengthening social ties and obtaining plants for consumption. Farms, green roofs and walls, and vertical gardens, as well as the production of plants in various types of containers, are becoming more and more popular in urbanized areas [2]. The intensive development of urban gardening may be an impulse for a more comprehensive use of composts and compost-based substrates derived from waste biomass [3,4]. More extensive research is needed to assess the suitability of various biomaterials for the preparation of composts and natural potting media that are the basis for sustainable crop production [5].
The development of waste management technologies aims primarily at preparing the waste for recovery, and particularly recycling. The utilization of waste and by-products by means of composting can be applied everywhere in the world and does not require an advanced infrastructure [6,7,8]. The availability of waste materials and by-products from the paper, food, wood and agricultural industries is currently unlimited [9,10]. Converting waste rich in organic matter and elements into composts is the best method of its disposal, as macro and micronutrients return to the ecosystem [11]. Research shows that the share of compost in the total production of substrates in the EU is only 7.9%. The most often used substrates include composted green waste, composted or aged bark and composted wood waste [12].
Compost is an invaluable organic fertilizer in organic farming. It improves the fertility of substrates and soils, and their physical, chemical and biological properties [13]. Composts are used in the sustainable production of potting media as a source of humus and nutrients [7,11,14]. Their main advantage is their biostimulating effect on the growth and development of plants and increasing crop quality [5]. Previous studies assessed different types of composts prepared from one, two, and seldom more types of nuisance waste materials [15]. Obtaining a new material in the form of compost based on several types of waste materials may increase the potential benefits of the final product. Such a product is no longer classified as waste, as it features completely different properties that positively affect the natural environment [7,16,17].
A potential raw material for the production of compost may be the waste from paper production—pulp and paper mill sludge [18,19,20]. Waste from the pulp and paper industry is characterized by a diversified chemical composition and physical and chemical properties, which depend, for instance, on the technology of the pulp and paper production, the type of pulp, and the type of produced paper [18,21]. The estimated amount of waste generated by the European paper industry is around 11 million tons per year, 70% of which come from paper recycling [22]. Several directives issued in the European Union significantly limited the possibility of landfilling this type of waste. Research shows that pulp and paper mill sludge can supplement the compost with organic matter and minerals and constitute a potential component of horticultural growing media [10,23].
Raw materials for the production of composts should also be sought in the food industry [24]. By 2012, the EU countries produced about 88 million tonnes of food waste [25]. Fruit and vegetable waste can be a rich and sometimes most valuable source of nitrogen in composts. A good example is apple (Malus domestica Borkh.) pomace, containing significant amounts of dietary fiber and polyphenols, of which Poland is the greatest producer [26]. During juice production, these substances usually do not make it into the final product but are retained in the pomace and are often irretrievably lost. The unique composition of by-products from fruit and vegetable processing makes them not a waste, but a highly valuable raw material [27].
Spent mushroom production substrate can be potentially and successfully used in growing media [28]. The production of this waste in Europe reaches over three million tonnes annually [10,29]. Poland, as the largest producer of mushrooms in the EU and third in the world, generates huge amounts of the spent substrate [30]. Composting the spent mushroom substrates and adding them as a component to growing media can solve local ecological problems with the storage of post-production substrate. In Poland, the mushroom substrate is made of winter cereal straw, poultry manure, gypsum and water. Its analyses showed that it is rich in organic matter, nitrogen and other yield promoting elements [31]. Unfortunately, the chemical composition of the substrate is diverse, which is related to the production technology. Research studies demonstrated that the mushroom substrate can be composted with other waste products, and the final material is a valuable organic fertilizer [32].
In most European countries, high peat is the dominant component of soilless substrates. It offers unique physical and chemical properties and is safe in phytosanitary terms, but it is a non-renewable resource. Moreover, the exploitation of peatlands increases CO2 emissions and negatively affects the environment [33,34]. The EU market consumes approximately 34,609 billion m3 of substrates per year, of which peat accounts for 75% [12]. Most of the current research on substrates is aimed at developing low-peat or peat-free compositions [10,12,16]. Substrates not containing high peat are currently the most sought after and the most often developed for environmental and economic purposes [35].
Our study investigated the use of several waste materials, such as pulp and paper mill sludge, fruit-vegetable waste, mushroom spent substrate and rye straw to produce a compost with high fertilizing value. Then, various growing media containing the compost and such components as low peat, high peat, wood fiber, or pine bark were assessed in terms of their suitability for container cultivation of vegetables. Our model plant was a cherry tomato (Lycopersicon esculentum Mill.), as it is one of the most important vegetables and is widely grown in home gardens and balconies for its tasty, healthy and decorative fruit. Tomatoes are easy to grow and serve as a natural source of antioxidants that protect the body against many diseases [36]. This study involved numerous physical and chemical analyses of the compost and the media. It also assessed tomato growth and yield, fruit color and their content of antioxidants and minerals. Our research hypothesis assumed that the yield and biological quality of tomato fruit grown in compost-enriched media are comparable or better than on traditional substrates, such as high peat.
2. Materials and Methods
2.1. Composts Preparation
The composts were prepared by Sobex Sp. z o.o. company (Trzebicz, Poland) between September 2018 and April 2019 from the following waste materials and by-products: pulp and paper mill sludge (Arctic Paper Kostrzyn S.A., Kostrzyn n/O, Poland), fruit-vegetable waste (Tymbark MWS Sp. z o.o., Tymbark, Poland), spent mushroom substrate (mushroom production farm, Rakoniewice, Poland), and rye straw chopped into 5–8 cm-long chaff (local farm). Physical and chemical analyses of the materials used for composting were performed at the Chemical Analysis Laboratory, Institute of Horticulture in Skierniewice (Skierniewice, Poland) and are summarized in Table 1. The methods used for these analyses are presented in Section 2.2.

Table 1.
Physical and chemical properties of waste materials used for composting.
The composition of the initial compost was calculated based on dry weight (DW) analysis, so that the C:N ratio was in the range of 20–30:1 [37]. The compost mass contained 18% (DW) of pulp and paper mill sludge, 40% DW of fruit-vegetable waste, 32% DW of spent mushroom substrate, and 10% DW of rye straw. Each 100 kg of its fresh weight (FW) included 20 kg FW of pulp and paper mill sludge, 54 kg FW of fruit-vegetable waste, 22 kg FW of spent mushroom substrate, and 4 kg FW of rye straw. After mixing, the components were formed into trapezoidal heaps (1.3 m high, 2.4 m wide, 3 m long) and kept under a shelter protecting them from precipitation and sunlight (Figure 1). Every month, the heaps were turned to aerate them. The relative moisture content was measured once a week to maintain a level of 55–65% [38]. The temperature of the heap was measured every week with an agricultural thermometer with a 1.5 m-long probe (Dramiński, Poland). The composting lasted for 16 weeks. At its completion, biological tests required by law were carried out at the Polish Center for Testing and Certification SA (Piła, Poland).

Figure 1.
Compost preparation process: (a) pulp and paper mill sludge; (b) fruit-vegetable waste; (c) mushroom spent substrate; (d) rye straw; (e) compost mixture; (f) compost storage site; (g) final compost.
2.2. Physical and Chemical Analysis of Components, Compost and Growing Media
The pH of the composting materials, final compost and growing media was determined in a suspension of the substrate and distilled water (v:v; 1:2) with the TESTER CP-505 m (Elmetron, Zabrze, Poland), and the electrolytic conductivity (EC) with the CCP-401 conductometer with an EC-60 sensor (Elmetron, Zabrze, Poland) [35]. The moisture content, bulk density, air capacity, water capacity, shrinkage and total porosity were assessed according to European standards [39,40].
Compost samples for physical and chemical tests were collected every month. Each sample was prepared by mixing five collective samples taken from different five spots and spacing, packed in 3 L polyethylene bags and sent on the same day to the Chemical Analysis Laboratory (Institute of Horticulture—PIB, Skierniewice, Poland).
The final compost and the substrates were tested for their fractions of matter particles (% weight). Particle distribution was established using sieves with the following mesh size: >20 mm, 10–20 mm, 5–10 mm, 2–5 mm, 1–2 mm, <1 mm.
A Kjeldahl digestion kit and unit (Vapodest, Gerhardt GmbH, Konigswinter, Germany) were used to determine total nitrogen (N) content by titration. Organic carbon (C) was determined by Dumas’ method with the Carbon Sulfur Determinator CS-530 apparatus (Eltra, GmbH, Neuss, Germany). After wet mineralization of samples in a 65% HNO3 and 75% HClO4 mixture, the content of total forms of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe) manganese (Mn), copper (Cu), boron (B), zinc (Zn) and molybdenum (Mo) was determined by plasma spectrometry (Inductively Coupled Plasma Optical Emission Spectrometry) with the Optima 2000 DV sequential spectrometer (Perkin-Elmer, Boston, MA, USA). The analyses covered the spectra typical of each element in nanometers (nm): P—213.617, K—766.490, Ca—317.933, Mg—285.213, S—181.975, Mn—257.610, Cu—327.393, Zn—206.200, B—249.772, Mo—281.616, sodium (Na)—589.592, iron (Fe)—238.204, nickel (Ni)—231.604, chrome (Cr)—267.716, lead (Pb)—220.353, cadmium (Cd)—228.802 and mercury (Hg)—253.616 [41].
The content of absorbable macronutrients was determined with the universal method [36,42] used in Poland for the analysis of horticultural soils and growing media. The extraction solution included 0.03 N acetic acid (pH 3.2), and the medium ratio was 1:10 (v:v).
2.3. Experimental Design
The experiment was carried out at the Department of Horticulture, West Pomeranian University of Technology in Szczecin from March 24 to July 11, 2019. Thirty-day-old tomato seedlings (Lycopersicon esculentum ‘Maskotka’), produced from seeds in a greenhouse (22 °C/18 °C; day/night) in peat substrate with pH of 5.7 and an average seedling height of 21 cm, were planted on 25 April 2019 into plastic pots with a capacity of 3 dm3 using the following four growing media:
- T0—control (100% peat substrate)
- T1—25% compost, 30% sphagnum peat, 15% fen peat, 20% pine bark, 10% wood fiber;
- T2—30% compost, 20% sphagnum peat, 30% fen peat, 10% pine bark, 10% wood fiber;
- T3—30% compost, 0% sphagnum peat, 30% fen peat, 30% pine bark, 10% woody fiber.
In total, 24 plants were planted for each treatment, with 6 plants per replication. The media were mixed in volumetric proportions (v:v). A commercial 100% peat substrate mixed with the PG Mix fertilizer (2 g dm−3) was used as a control. Sphagnum peat was sourced from Latvian peatlands, and fen peat and pine bark were provided by local Polish companies. Wood fibers were obtained by means of a thermal-mechanical method from Steico sp z o.o. (Czarna Woda, Poland). The chemical properties of the components are presented in Table 2. Based on the chemical analysis of T1, T2 and T3 media, calcium nitrate (15.5% N; 26.5% CaO) was applied at 1.5 g per pot before planting the seedlings.

Table 2.
pH, electrical conductivity (EC), dry matter and nutrient concentrations of growing media components.
The seedlings were placed in a random sub-block system in a high, unheated tunnel covered with two layers of plastic, which was equipped with an automatic ventilation control system. The plants were placed on a nursery mat at 40 × 50 cm spacing (five plants per m2). The average temperature during the growing season ranged from 22 to 28 °C, and the relative humidity was 65–80%. No chemical protection or top dressing was applied, and sticky boards and biological protection (Koppert Biological Systems, Berkel en Rodenrijs, The Netherlands) were used for pest control. Eighty days after seeding, two of the largest leaves from the middle section of a plant from each treatment were harvested for the measurement of the relative chlorophyll content with the Chlorophyll Meter SPAD 502 (Konica, Minolta, Japan). The readings were taken from twelve plants from each treatment at four sites on the leaf blade and averaged. The fresh weight of the aboveground part of the plants was also determined from eight plants from each treatment.
2.4. Tomato Yield and Fruit Characteristics
The fruit yield was assessed once (102 days after seeding), when at least five tomato fruits had ripened per plant. All tomato fruits (marketable and green ones) were harvested separately from each plant, counted and weighed. The percentage of ripe tomato fruits was calculated.
To determine the fruit color, five ripe tomato fruits per treatment were randomly selected. After washing and drying the skins, the color index was determined using a Chroma Meter, model CR-400 (Minolta, Tokyo, Japan). The reading was taken three times for each fruit in its middle part, and then the mean value was calculated. The proportions of red color (red positive/green negative)—a*, yellow color (yellow positive/blue negative)—b* and saturation were analyzed [43].
Biochemical analyses involved 300 g of ripe fruit from each treatment. The tomatoes were ground and then frozen at −20 °C for 12 hrs. Tomato samples were freeze-dried for 24 h in a Beta 2–8 LSC freeze dryer plus (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) and ground to a powder. Tomato methanolic extracts were prepared as described by Grzeszczuk et al. [44].
The total content of polyphenols was determined spectrophotometrically with the Folin–Ciocalteu reagent as described by Łopusiewicz et al. [45]. The absorbance was read with a spectrophotometer (UV-Vis Thermo Scientific Evolution 220) at 765 nm. Gallic acid was used for the preparation of the calibration curve and results were expressed as mg gallic acid equivalents per 100 g of the sample.
The content of flavonoids was determined according to the method of Łopusiewicz et al. [45]. The absorbance was measured at 510 nm. Quercetin was used for the preparation of the calibration curve and the results were expressed as mg quercetin equivalent (QE) per 100 g of the sample.
To determine the content of ascorbic acid, the Tillmans titration method was used, consisting of the reduction of 2,6-dichlorophenolindophenol [46]. This consisted of 2 mL of tomato methanolic extract being mixed with 2 mL of 2% oxalic acid and shaken vigorously. The solution was quickly titrated with 2,6-dichlorophenolindophenol until the pink color held for 30 s. The ascorbic acid content is expressed in milligrams per 100 g of FW.
The antioxidant activity was determined based on 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,2′-azobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) radical scavenging activity according to the protocol provided by Łopusiewicz et al. [47]. In brief, DPPH radical scavenging activity was determined by mixing 1 mL of the methanolic tomato fruit extract with 1 mL of 0.01 mM methanolic solution of DPPH. Absorbance was measured at 517 nm after 30 min incubation in the dark at room temperature. For the ABTS assay, 3 mL of the ABTS solution was mixed with 50 μL of the methanolic tomato fruit extract, and after 6 min incubation in the dark at room temperature, the absorbance was read at 734 nm.
The macro and micronutrients contents in the fruits was determined. The fruits were rinsed three times with distilled water, drained and cut in half. Then, the samples were dried at 70 °C (skin downwards) in a forced air oven, and milled in a Cyclotec TM 1093 laboratory mill (FOSS, Hilleroed, Denmark). Subsequently, the samples were subjected to wet digestion in concentrated nitric acid (0.5 g of the plant material +5 mL of 65% HNO3) in a closed microwave oven (Nova Wave SA, Microwave Tunnel Digestion System). After mineralization, the content of macro- and micronutrients in solutions was determined by the plasma emission method using the ICP-OES Optima 2000 DV inductively coupled plasma spectrometer. A Kjeldahl unit was used to determine the total N content [35].
2.5. Statistical Analysis
The results were statistically analyzed in the Statistica 13.1 program (Tibco Software Inc., Poland) using one-way analysis of variance (ANOVA). Differences between means were assessed with Tukey’s test (p ≤ 0.05).
3. Results and Discussion
3.1. Compost Properties
The compost quality depends on the quality and proportion of its ingredients and the composting process [48]. The signs of completed maturation include the presence of nitrate nitrogen, C:N ratio of 20–30:1, and C:P ratio of 100:1 [49]. The compost contained four types of component, pulp and paper mill sludge, fruit-vegetable waste, spent mushroom substrate, and rye straw, with known parameters (Table 1). The pulp and paper mill sludge had the highest pH (7.1), and was the richest in calcium and micronutrients. The spent mushroom substrate had the highest EC (9.18 mS cm−3) and was the richest in macronutrients. The fruit-vegetable waste exhibited the lowest pH (2.5) and the highest bulk density, while rye straw had the highest dry weight content. The spent mushroom substrate provided the majority of the compost N (2.22%), and the fruit-vegetable waste and rye straw were main sources of organic C.
The determination of dry weight, organic C and total N content in the compost feedstock was necessary to prepare the compost recipe. The compost ingredients are composed correctly if the C:N ratio reaches 20–30:1 [37,38]. In our study, the initial C:N ratio was 32:1 (Table 3), and after composting it dropped to 20.4:1. This decrease in the C:N ratio is due mainly to the total C content loss during composting process. The C:N ratio is one of the most important indicators of compost maturity. The C:N ratio is essential for the development of microorganism during the composting process because it provides the C and N source required for growth [24]. According to Antil et al. [50] and Asquer et al. [51], a C:N ratio of 15–20 is satisfactory, and such a compost is a good source of N for plant growth.

Table 3.
Changes in compost parameters during heap maturation.
The compost had a soil-like texture, was odorless, and its temperature was close to the ambient one. Its pH ranged between 7.4 and 7.7 (Table 3), and largely depended on the pH of the raw materials and composting process.
Compost salinity may limit its use as a substrate component. In our mature compost, the EC was below 3.94 mS cm−3 (Table 3), and was therefore similar to the literature data [52]. During composting, the EC value doubled due to the mineralization of the organic matter and increased concentration of absorbable nutrients. This was to a large extent due to the presence of spent mushroom substrate with high EC and macronutrient content. The dry matter of the compost rose during maturation from 54.1% to 63.8%. Increased dry matter may result from the use of raw materials with a high dry matter (e.g., straw) and which are more difficult to decompose, e.g., materials containing lignin [14]. Its final bulk density reached 0.4 g cm−3 (Table 4), and was, according to Abad et al. [53], favorable for vegetable-growing substrates.

Table 4.
Properties of final compost.
The large-scale use of composts as substrate components in horticulture is hampered by the lack of repeatability of the compost composition, mainly due to the different availability of the raw material and poor control of the composting process. In our study, the components were carefully selected, and their proportions in the compost were precisely calculated and easy to reproduce in subsequent trials.
In Poland, compost quality assurance involves only the final product. The Polish Ministry of Agriculture and Rural Development certifies organic fertilizers based on their chemical properties and pathogen presence, following a positive opinion from a designated institution. According to Polish regulations [54], a compost can be considered an organic fertilizer if it contains at least 0.3% N, 0.2% P2O5, 0.2% K2O, and 30% organic matter. In our compost, these values were higher than the threshold ones presented in Table 4. Our compost was biologically clean and its content of heavy metals (Cd, Pb, Ni, Cr and Hg) did not exceed the levels allowed in Polish regulations [54]. However, all EU countries have their own criteria for compost suitability [55].
An important parameter confirming the favorable chemical properties of compost is the ratio of ammonium and nitrate form of N [50]. In final compost, the level of nitrate form was several times higher than that of ammonium form (Table 4). Moreover, the compost contained considerable amounts of available forms of K, Cl, Na and S.
Figure 2a shows particle size distribution in the final compost. Overall, 35% of the compost had a fraction with a particle size of 1–5 mm. The compost properties depended on particle size [14,15,24].
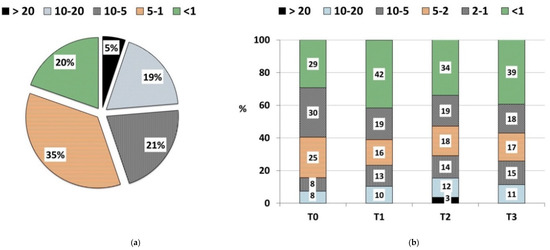
Figure 2.
Particle size (mm) distribution: (a) final compost (% weight); (b) growing media: T0 (control—100% peat substrate); T1 (25% compost, 30% sphagnum peat, 15% fen peat, 20% pine bark, 10% wood fiber); T2 (30% compost, 20% sphagnum peat, 30% fen peat, 10% pine bark, 10% wood fiber); T3 (30% compost, 0% sphagnum peat, 30% fen peat, 30% pine bark, 10% woody fiber) (% weight).
3.2. Media Properties
The tested growing media T1, T2 and T3 were mixtures of the compost, sphagnum peat, fen peat, pine bark, and wood fiber. The components of greater bulk density (compost, fen peat) were balanced with the lighter ones, such as sphagnum peat and wood fiber (Table 2). The T3 medium did not contain high peat.
Figure 2b shows the particle size distribution in the investigated substrates. The size of the substrate particles is an important parameter, as it determines its bulk density, gas exchange, water capacity and thus translates into plant growth [56]. According to Hendreck [57], the optimal particle size of growing media ranges between 0.1 and 0.5 mm. Particles of this size provide the highest available water holding capacity of the substrate. Benito et al. [58] and Jayasinghe et al. [59] claimed that optimal plant growth is ensured by the substrate particles of the size 0.25–2.0 mm, as they guarantee optimal water and air availability. All tested media contained over 50% of particles smaller than 2 mm, with its content decreasing in the following order: T1 > T0 > T3 > T2. Only the T2 growing medium contained the largest particles >20 mm.
Shrinkage is the substrate stability parameter, which is particularly important during long plant growth periods. Excessive shrinkage can damage plant roots [60]. The optimal value of shrinkage ratio should not exceed 30–35% [61]. In our study, the medium shrinkage ratio did not exceed the threshold recommended in the literature (Table 5). The presence of 10% wood fiber in the T1, T2 and T3 media reduced their shrinkage, especially in those also containing high peat. The T0 peat substrate showed the highest percentage of shrinkage, while T3, not containing high peat, showed the least.

Table 5.
Physical properties of tested growing media.
Bulk density reflects the substrate suitability in terms of structural support, movement of water and dissolved substances and aeration. According to Kipp et al. [62], the substrate bulk density in tomato production ranges between 30 and 1400 g dm−3, depending on the substrate and cultivation method. Abad et.al. [53] pointed out that ideal potting media should have bulk density below 0.4 g cm−3, as this provides better air and water availability for plant root development. Moreover, bulk density is an important parameter for the medium distribution on the market, as transport costs increase with the weight of the medium. All growing media in the study met the criteria of an ideal medium in this aspect [53]. The highest bulk density was determined for T3 substrate without high peat, and the smallest T0 control substrate (Table 5).
Total pore space reflects the percentage of free space for water, air and plant roots. The substrate pore space also determines gas exchange in the root zone. The desirable total pore space is considered to be 55–96% [63]. The optimal value of total pore space for tomatoes is 45–99%, depending on the growing method and the substrate. The control medium T0 was characterized by the highest porosity and the largest water-filled pore space, as it contained 100% high peat (Table 5). Cai et al. [63] suggested a water-filled pore space of 36–77% as being acceptable for horticultural crops. In our study, T2 and T3 had the highest percentage of air-filled pore space. The physical properties of the media were consistent with the literature data [62] regarding water content (15–90%) and air content (10–80%) in tomato production growing media.
In our study, the pH and EC of the media (Table 6) was consistent with the recommendations for tomato [42]. In the media T1, T2 and T3 containing N in mainly organic form, the content of assimilable N was supplemented with an initial dose of calcium nitrate. T1, T2 and T3 contained more Cl and S than recommended.

Table 6.
Chemical properties of tested growing media: T0 (control—100% peat substrate); T1 (25% compost, 30% sphagnum peat, 15% fen peat, 20% pine bark, 10% wood fiber); T2 (30% compost, 20% sphagnum peat, 30% fen peat, 10% pine bark, 10% wood fiber); and T3 (30% compost, 0% sphagnum peat, 30% fen peat, 30% pine bark, 10% woody fiber).
3.3. Effect of Tested Growing Media on Tomato Yield and Fruit Charactristics
To assess the effect of the tested media on plant growth, the leaf greening index (SPAD) and the fresh weight of the aboveground part were determined. SPAD is a marker correlated with the content of chlorophyll and nitrogen. It reflects the state of plant nutrition, which determines plant biomass production [64]. SPAD values of the plants grown in all three compost-supplemented media (T1, T2 and T3) were on average 15.8% greater than in the control (T0) (Figure 3b). The plants grown in the T1 and T2 media showed a markedly increased fresh weight of the aboveground part (by 62.2% and 48.6%, respectively, as compared with T0 (Figure 3c)). As this was the first study investigating the effects of adding the compost containing pulp and paper mill sludge, fruit-vegetable waste, mushroom spent substrate and rye straw to the substrates for tomato cultivation, it is difficult to compare our results with those of other authors. Literature data show that plant growth and pigmentation in plants cultivated in compost-enriched media depend mainly on the type of the compost [35,65,66]. Ronga et al. [67] assessed the usefulness of spent coffee ground compost and concluded that its addition to peat substrate significantly increased the SPAD index of tomato plants 30 days after sowing. Contrary to that, enriching the substrate with compost obtained from chestnut waste lowered the chlorophyll content in tomato seedlings [68]. Hashemimajd et al. [69] used compost produced from raw dairy manure, tobacco residue, yard leaf, sewage sludge and rice hulls and reported higher biomass of tomato plants. However, the tomato biomass gain was considerably restricted after supplementing the soil with compost made from pulp and paper sludge [70].
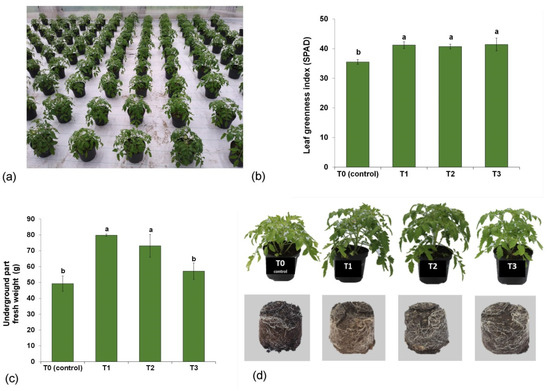
Figure 3.
Effect of compost-enriched media: T0 (control—100% peat substrate); T1 (25% compost, 30% sphagnum peat, 15% fen peat, 20% pine bark, 10% wood fiber); T2 (30% compost, 20% sphagnum peat, 30% fen peat, 10% pine bark, 10% wood fiber); and T3 (30% compost, 0% sphagnum peat, 30% fen peat, 30% pine bark, 10% woody fiber) on the growth of plants in a plastic tunnel: (a) plants after 3 weeks of growing in the substrates; (b) leaf greening index SPAD; (c) biomass of the aboveground part; (d) visual comparison of the aboveground part and the root system. Vertical errors bars indicate the standard error of the mean. Different letters indicate a significant difference at p ≤ 0.05.
Tomato growers are looking for methods that accelerate fruit production and increase fruit yield [65,67]. Tomatoes sold as potted plants are the most attractive when the berries begin to ripen. For this reason, our study assessed the percentage of ripe berries and the number and weight of fruits per plant at this developmental stage.
The tomatoes grown in T1, T2 and T3 substrates, i.e., those containing the compost, produced considerably more ripe berries (40%, 56% and 54%, respectively) than in T0 (23%) (Figure 4b). The number of tomato fruits in the plants grown in T0, T1 and T2 media were similar, and reached, respectively, 18.5, 19.9 and 17.7, while those cultivated in T3 had significantly fewer fruits (13.1) (Figure 4c). The fruit yield expressed as fresh weight was greater in T1 and T2 by 31% and 17% than in T0 (Figure 4d). Verma et al. [71] reported an increased tomato fruit yield in the presence of compost supplemented with half of the recommended dose of a mineral fertilizer. The enhanced accumulation of biomass and raise in the fruit yield of the plants growing in T1 and T2 media probably resulted from the greater availability of nutrients and their more intensive uptake. Moreover, the T1 and T2 media featured a high total pore space and air and organic matter content, which were conducive to the root system development leading to higher yield.
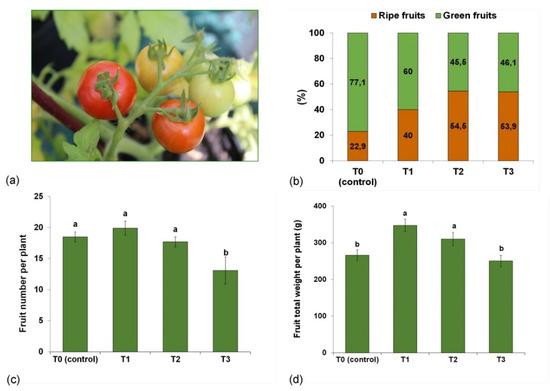
Figure 4.
Effects of compost-enriched media: T0 (control—100% peat substrate); T1 (25% compost, 30% sphagnum peat, 15% fen peat, 20% pine bark, 10% wood fiber); T2 (30% compost, 20% sphagnum peat, 30% fen peat, 10% pine bark, 10% wood fiber); and T3 (30% compost, 0% sphagnum peat, 30% fen peat, 30% pine bark, 10% woody fiber) on the fruit yield: (a) ripening fruit in clusters; (b) percentage (%) of ripe fruits per plant; (c) number of fruits per plant; (d) fresh weight of total fruits per plant. Vertical errors bars indicate the standard error of the mean. Different letters indicate a significant difference at p ≤ 0.05.
Fruit color is one of the most important factors affecting the quality of tomato. The red color is due to the presence of carotenoids, the biosynthesis of which is to a great extent determined by environmental conditions [72]. Tomato cultivars with red fruits should have a high share of red color and a high saturation [73]. These criteria assessed on the basis of parameters a*, b* and saturation were to the greatest extent met by tomato fruits grown in T0 and T1 media (Table 7). The fruits of plants in T0 and T1 were characterized by significantly higher values of red color (parameter a*) and color purity, i.e., saturation, and lower values of yellow color (parameter b*) than in T2 and T3 treatments. A reduced proportion of red and an increased proportion of yellow color in tomatoes grown in T2 and T3 media may indicate an uneven degree of ripeness and/or uneven fruit coloring [74]. Changes in the tomato fruit skin color following soil supplementation with organic additives were reported by Mauromicale et al. [75] and Keabetswe et al. [76].

Table 7.
Influence of growing media on colour parameters of marketable tomato fruits.
Natural antioxidants, primarily those derived from fruits and vegetables, play an important role in the prevention and treatment of many diseases, most often diet-related ones [36]. Therefore, this study determined tomato fruits’ content of phenolic compounds and L-ascorbic acid and assessed their antioxidant potential (Table 8).

Table 8.
Influence of growing media on total polyphenols (TPC), total flavonoids (TFC) and L-ascorbic acid content and antioxidant activity determined by DPPH and ABTS tests of marketable tomato fruits.
In general, fruit from the plants grown in compost-enriched media, i.e., T1, T2 and T3, contained significantly more total polyphenols and total flavonoids than in T0. The fruit of the tomato plants grown in T1 medium had the highest level of total polyphenols, and accumulated more L-ascorbic acid than those from all other treatments. The fruits from T2 and T3 treatments were the richest in total flavonoids. Tomato fruits contain many important bioactive substances with antioxidant properties, the levels of which can be modified by the use of composts. For example, compost supplementation increased the content of lycopene and total polyphenols in tomato, as reported by Verma et al. [71]. Another study by Ribas-Agusti et al. [77] demonstrated an increased content of polyphenols from the group a kaempferol derivative in tomato grown in a substrate enriched with municipal solid waste compost and a mineral fertilizer. Ravindran et al. [78] established that the addition of compost and vermicompost from tannery-fermented products enhanced the lycopene and ascorbic acid content in tomato fruit. In our study, the antioxidant potential of tomato fruit extracts was assessed based on the efficiency of scavenging DPPH and ABTS model free radicals. These are complementary methods of measuring the total antioxidant capacity in vitro that allowed us to determine the reduction power of the investigated extracts. The highest antioxidant potential assessed by the DPPH and ABTS assays was found in tomato extracts from the T1 medium. The extracts from the remaining treatments did not differ in their antioxidant activity. Literature data show that both the antioxidant content and antioxidant activity may be affected by compost supplementation during cultivation [71]. The positive effect of the compost-enriched substrates on the yield potential of tomatoes, and the simultaneous increase in their fruit nutritional value may result from the presence of not only minerals but also other stimulants in the experimental substrates. For example, mushroom waste contains chitin derivatives that exert a biostimulating effect and may enhance the plant content of polyphenols and therefore boost their antioxidant potential [79]. In addition, waste of plant origin subjected to aerobic decomposition during composting can release small-molecule substances such as amino acids, vitamins, enzymes and plant hormones that affect the plant phytochemical composition [15].
The nutritional value of tomato fruit depends on their content of nutrients, and therefore we decided to investigate how the experimental growing media affected fruit levels of macro- and micronutrients (Table 9). The fruit of plants grown in compost-enriched T1, T2 and T3 media had a significantly higher content of potassium, calcium, magnesium and copper than the control ones, but no significant differences were found for the accumulation of N, P, Na, S, Fe, Zn, and B. In the case of Mn, a slight decrease was detected in plants grown in T2 and T3 media. In general, the level of minerals in tomato fruit was similar to that reported earlier [65,80], including the content of trace elements. The biofortification of plants with desired nutrients is a simple and relatively effective method of counteracting the effects of mineral deficiency in humans [81].

Table 9.
Influence of growing media: on mineral and trace element concentrations in marketable tomato fruits.
4. Conclusions
The growing ecological awareness of the producers of fertilizers, media and growers requires pro-ecological efforts aimed at sustainable development. A new philosophy of life based on caring for the planet, the environment and future generations encourages people to save available natural resources, and process and reuse valuable waste. This study showed a positive effect of growing media enriched with compost from waste materials on the yield and quality of tomato fruit. The production of plants in the media containing compost, wood fiber and, most importantly, a reduced amount of high peat than in the commonly used substrates provided a satisfactory fruit yield and nutritional value comparable or higher than in classic peat substrates. This paper presents a good horticultural practice and indicates the possibility of introducing sustainable potting media in the production of healthy food.
Author Contributions
Conceptualization, A.Z.; methodology, A.Z., P.S., J.S.N., W.K. and Ł.Ł.; investigation, A.Z., P.S., J.S.N., W.K., R.P., Ł.Ł. and A.P.; writing, A.Z., P.S.; visualization, A.Z.; project administration, A.Z.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been carried out within the Regional Operational Program—Lubuskie 2020, Project No. RPLB.01.01.00-08-0015/17, founded by the European Union. The APC was funded by the West Pomeranian University of Technology in Szczecin under the UPB grant (Maintaining the Research Potential).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schram-Bijkerk, D.; Otte, P.; Dirven, L.; Breure, A.M. Indicators to support healthy urban gardening in urban management. Sci. Total Environ. 2018, 621, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Tendero, M.; Guyot Phung, C. The revival of urban agriculture: An opportunity for the composting stream. Field Actions Sci. 2019, 20, 40–51. [Google Scholar]
- Ober Allen, J.; Alaimo, K.; Elam, D.; Perry, E. Growing Vegetables and Values: Benefits of Neighborhood-Based Community Gardens for Youth Development and Nutrition. J. Hunger. Environ. Nutr. 2008, 3, 418–439. [Google Scholar] [CrossRef]
- Japakumar, J.; Abdullah, R.; Rosli, N.S.M. Effects of biochar and compost application on soil properties and growth performance of Amaranthus sp. grown at urban community garden. Agrivita 2021, 43, 441–453. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic substrate for transplant production in organic nurseries. A review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef] [Green Version]
- Varma, V.S.; Kalamdhad, A.S. Composting of municipal solid waste (MSW) mixed with cattle manure. Int. J. Environ. Sci. 2013, 3, 2068–2079. [Google Scholar]
- Pergola, M.; Persiani, A.; Palese, A.M.; Di Meo, V.; Pastore, V.; D’Adamo, C.; Celano, G. Composting: The way for a sustainable agriculture. Appl. Soil Ecol. 2018, 123, 744–750. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Sarsaiya, S.; Awasthi, M.K.; Liu, T.; Zhao, J.; Kumar, S.; Zhang, Z. Changes in global trends in food waste composting: Research challenges and opportunities. Bioresour. Technol. 2020, 299, 122555. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhang, S.; Yuan, Z. Adoption of solid organic waste composting products: A critical review. J. Clean. Prod. 2020, 272, 122712. [Google Scholar] [CrossRef]
- Schmilewski, G. Growing media constituents used in the EU in 2013. Acta Hortic. 2017, 1168, 85–92. [Google Scholar] [CrossRef]
- Meena, M.D.; Joshi, P.K.; Jat, H.S.; Chinchmalatpure, A.R.; Narjary, B.; Sheoran, P.; Sharma, D.K. Changes in biological and chemical properties of saline soil amended with municipal solid waste compost and chemical fertilizers in a mustard–pearl millet cropping system. Catena 2016, 140, 1–8. [Google Scholar] [CrossRef]
- Barthod, J.; Rumpel, C.; Dignac, M.-F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17. [Google Scholar] [CrossRef] [Green Version]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems–A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Raviv, M. Can compost improve sustainability of plant production in growing media? Acta Hortic. 2017, 1168, 119–134. [Google Scholar] [CrossRef]
- Zawadzińska, A.; Salachna, P. Ivy pelargonium response to media containing sewage sludge and potato pulp. Plant Soil Environ. 2018, 64, 180–185. [Google Scholar]
- Hazarika, J.; Khwairakpam, M. Evaluation of biodegradation feasibility through rotary drum composting recalcitrant primary paper mill sludge. Waste Manag. 2018, 76, 275–283. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Stavrinides, M.; Moustakas, K.; Tzortzakis, N. Utilization of paper waste as growing media for potted ornamental plants. Clean Technol. Environ. Policy 2019, 21, 1937–1948. [Google Scholar] [CrossRef]
- Kumar, P.; Guatam, S.K.; Kumar, V.; Singh, S.P. Enhancement of optical properties of bagasse pulp by in-situ filler precipitation. BioResources 2009, 4, 1635–1646. [Google Scholar]
- Veluchamy, C.; Kalamdhad, A.S. Influence of pretreatment techniques on anaerobic digestion of pulp and paper mill sludge: A review. Bioresour. Technol. 2017, 245, 1206–1219. [Google Scholar] [CrossRef]
- Monte, M.C.; Fuente, E.; Blanco, A.; Negro, C. Waste management from pulp and paper production in the European Union. Waste Manag. 2009, 29, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Chrysargyris, A.; Tzionis, A.; Xylia, P.; Tzortzakis, N. Effects of salinity on tagetes growth, physiology, and shelf life of edible flowers stored in passive modified atmosphere packaging or treated with ethanol. Front. Plant Sci. 2018, 9, 1765. [Google Scholar] [CrossRef] [Green Version]
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sánchez, A. Composting of food wastes: Status and challenges. Bioresour. Technol. 2018, 248, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenmark, Å.; Jensen, C.; Quested, T.; Moates, G. Estimates of European Food Waste Levels; IVL-Report C; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; Volume 186. [Google Scholar]
- Nosalewicz, A.; Maksim, M.; Brzezińska, M.; Siecińska, J.; Siczek, A.; Nosalewicz, M.; Turski, M.; Frąc, M.; Przysucha, B.; Lipiec, J. The use of apple pomace as a soil amendment enhances the activity of soil microorganisms and nitrogen transformations and affects crop growth. J. Soil Sci. Plant Nutr. 2021, 21, 1831–1841. [Google Scholar] [CrossRef]
- Kruczek, M.; Drygaś, B.; Habryka, C. Pomace in fruit industry and their contemporary potential application. World Sci. News 2016, 48, 259–265. [Google Scholar]
- Hernández, D.; Ros, M.; Carmona, F.; Saez-Tovar, J.A.; Pascual, J.A. Composting spent mushroom substrate from Agaricus bisporus and Pleurotus ostreatus production as a growing media component for baby leaf lettuce cultivation under Pythium irregulare biotic stress. Horticulturae 2021, 7, 13. [Google Scholar] [CrossRef]
- Gea, F.J.; Carrasco, J.; Diánez, F.; Santos, M.; Navarro, M.J. Control of dry bubble disease (Lecanicillium fungicola) in button mushroom (Agaricus bisporus) by spent mushroom substrate tea. Eur. J. Plant Pathol. 2014, 138, 711–720. [Google Scholar] [CrossRef]
- Czop, M.; Pikoń, K. Use of casing soil from spent mushroom compost for energy recovery purposes in Poland. Archit. Civ. Eng. Environ. 2017, 10, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Majchrowska-Safaryan, A.; Pakuła, K.; Becher, M. The influence of spent mushroom substrate fertilization on the selected properties of arable soil. Environ. Prot. Nat. Resour. 2020, 31, 28–34. [Google Scholar] [CrossRef]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in heavy metal bioavailability and speciation from a Pb-Zn mining soil amended with biochars from co-pyrolysis of rice straw and swine manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef]
- Gruda, N. Sustainable peat alternative growing media. Acta Hortic. 2012, 927, 973–980. [Google Scholar] [CrossRef]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media–an option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Zawadzińska, A.; Salachna, P.; Nowak, J.S.; Kowalczyk, W. Response of Interspecific Geraniums to Waste Wood Fiber Substrates and Additional Fertilization. Agriculture 2021, 11, 119. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C., Jr. Chapter three—Current approaches and future trends in compost quality criteria for agronomic, environmental and human health benefits. Adv. Agron. 2017, 144, 143–233. [Google Scholar]
- Xu, L.; Abbaszadeh, P.; Moradkhani, H.; Chen, N.; Zhang, X. Continental drought monitoring using satellite soil moisture, data assimilation and an integrated drought index. Remote Sens. Environ. 2020, 250, 112028. [Google Scholar] [CrossRef]
- EN-13040:2007—Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density; CEN—European Committee for Standardization: Brussels, Belgium, 2007.
- EN 13041:2011—Soil Improvers and Growing Media—Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space; CEN—European Committee for Standardization: Brussels, Belgium, 2011.
- Boss, C.H.; Fredeen, K.J. Concepts, Instrumentation, and Techniques in Inductively Coupled Plasma Optical Emission Spectrometry; Perkin Elmer: Shelton, CT, USA, 2004. [Google Scholar]
- Breś, W.; Komosa, A. Kontrolowane żywienie roślin ogrodniczych. In Żywienie Roślin Ogrodniczych; Podstawy i perspektywy; Komosa, A., Ed.; PWRiL: Poznań, Poland, 2012. (In Polish) [Google Scholar]
- Fraser, B.; Murphy, C.; Bunting, F. Real World Color Management, 2nd ed.; Publisher Pearson Education: New York, NY, USA, 2006; pp. 145–170. [Google Scholar]
- Grzeszczuk, M.; Salachna, P.; Meller, E. Changes in photosynthetic pigments, total phenolic content, and antioxidant activity of Salvia coccinea Buc’hoz Ex Etl. Induced by exogenous salicylic acid and soil salinity. Molecules 2018, 23, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Salachna, P.; Grzeszczuk, M.; Wilas, J. Total phenolic content, photosynthetic pigment concentration and antioxidant activity of leaves and bulbs of selected Eucomis L’Hér. Taxa. Fresen. Environ. Bull. 2015, 24, 4220–4225. [Google Scholar]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of flaxseed oil cake into bioactive camembert-analogue using lactic acid bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef] [PubMed]
- Ceglie, F.G.; Bustamante, M.A.; Ben Amara, M.; Tittarelli, F. The challenge of peat substitution in organic seedling production: Optimization of growing media formulation through mixture design and response surface analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef] [PubMed]
- Krzywy, E. Plant Nutrition; Wydawnictwo Naukowe Akademii Rolniczej: Szczecin, Poland, 2007. (In Polish) [Google Scholar]
- Antil, R.S.; Raj, D.; Narwal, R.P.; Singh, J.P. Evaluation of maturity and stability parameters of composts prepared from organic wastes and their response to wheat. Waste Biomass Valorization 2013, 4, 95–104. [Google Scholar] [CrossRef]
- Asquer, C.; Cappai, G.; De Gioannis, G.; Muntoni, A.; Piredda, M.; Spiga, D. Biomass ash reutilisation as an additive in the composting process of organic fraction of municipal solid waste. Waste Manag. 2017, 69, 127–135. [Google Scholar] [CrossRef]
- Jara-Samaniego, J.; Pérez-Murcia, M.D.; Bustamante, M.A.; Pérez-Espinosa, A.; Paredes, C.; López, M.; López-Lluch, D.B.; Gavilanes-Terán, I.; Moral, R. Composting as sustainable strategy for municipal solid Waste management in the Chimborazo Region, Ecuador: Suitability of the obtained compost for seedling production. J. Clean. Prod. 2017, 141, 1349–1358. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Bures, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development. J. Laws 2008, 119, 765.
- Saveyn, H.; Eder, P. End-of-Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost and Digestate): Technical Proposals; Joint Research Center Scintific and Policy Reports; Publications Office of the European Union: Luxembourg, 2014; p. 352. [Google Scholar]
- Zhang, L.; Sun, X. Effects of rhamnolipid and initial compost particle size on the two-stage composting of green waste. Bioresour. Technol. 2014, 163, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Handreck, K.A. Particle size and the physical properties of growing media for containers. Commun. Soil Sci. Plant Anal. 1983, 14, 209–222. [Google Scholar] [CrossRef]
- Benito, M.; Masaguer, A.; Moliner, A.; De Antonio, R. Chemical and physical properties of pruning waste compost and their seasonal variability. Bioresour. Technol. 2006, 97, 2071–2076. [Google Scholar] [CrossRef]
- Jayasinghe, G.Y.; Arachchi, I.L.; Tokashiki, Y. Evaluation of containerized substrates developed from cattle manure compost and synthetic aggregates for ornamental plant production as a peat alternative. Resour. Conserv. Recycl. 2010, 54, 1412–1418. [Google Scholar] [CrossRef]
- Whitmore, A.P.; Whalley, W.R. Physical effects of soil drying on roots and crop growth. J. Exp. Bot. 2009, 60, 2845–2857. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, S.; Fleige, H.; Horn, R. Shrinkage processes of a drained riparian peatland with subsidence morphology. J. Soils Sediments 2010, 10, 484–493. [Google Scholar] [CrossRef]
- Kipp, J.A.; Wever, G.; de Kreij, C. Substraat. Analyse Eigenschappen Advies; Elsevier Publishers: Amsterdam, The Netherlands, 2000. (In Dutch) [Google Scholar]
- Cai, H.; Chen, T.; Liu, H.; Gao, D.; Zheng, G.; Zhang, J. The effect of salinity and porosity of sewage sludge compost on the growth of vegetable seedlings. Sci. Hortic. 2010, 124, 381–386. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, X. Assessing nitrogen nutritional status, biomass and yield of cotton with ndvi, spad and petiole sap nitrate concentration. Exp. Agric. 2018, 54, 531–548. [Google Scholar] [CrossRef]
- Zaller, J.G. Vermicompost as a substitute for peat in potting media: Effects on germination, biomass allocation, yields and fruit quality of three tomato varieties. Sci. Hortic. 2007, 112, 191–199. [Google Scholar] [CrossRef]
- Aylaj, M.; Lhadi, E.K.; Adani, F. Municipal waste and poultry manure compost affect biomass production, nitrate reductase activity and heavy metals in tomato plants. Compost. Sci. Util. 2019, 27, 11–23. [Google Scholar] [CrossRef]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of spent coffee ground compost in peat-based growing media for the production of basil and tomato potting plants. Commu. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Parillo, R.; Ventorino, V.; Pepe, O.; Rivas, P.C.; Testa, A. Use of compost from chestnut lignocellulosic residues as substrate for tomato growth. Waste Biomass Valorization 2017, 8, 2711–2720. [Google Scholar] [CrossRef]
- Hashemimajd, K.; Kalbasi, M.; Golchin, A.; Shariatmadari, H. Comparison of vermicompost and composts as potting media for growth of tomatoes. J. Plant Nutr. 2004, 27, 1107–1123. [Google Scholar] [CrossRef]
- Campbell, A.G.; Zhang, X.; Tripepi, R.R. Composting and evaluating a pulp and paper sludge for use as a soil amendment/mulch. Compost. Sci. Util. 1995, 3, 84–95. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, A.; Kumar, R.; Kaur, C.; Arora, A.; Shah, R.; Nain, L. Improvement of antioxidant and defense properties of tomato (var. pusa rohini) by application of bioaugmented compost. Saudi J. Biol. Sci. 2015, 22, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Batu, A. Determination of acceptable firmness and colour values of tomatoes. J. Food Eng. 2004, 61, 471–475. [Google Scholar] [CrossRef]
- Zalewska-Korona, M.; Jablonska-Rys, E. Ocena przydatności do przetwórstwa owoców wybranych odmian pomidora gruntowego. Żywność Nauka Technol. Jakość 2012, 2, 77–87. [Google Scholar]
- Mauromicale, G.; Longo, A.M.G.; Monaco, A.L. The effect of organic supplementation of solarized soil on the quality of tomato fruit. Sci. Hortic. 2011, 129, 189–196. [Google Scholar] [CrossRef]
- Keabetswe, L.; Shao, G.C.; Cui, J.; Lu, J.; Stimela, T. A combination of biochar and regulated deficit irrigation improves tomato fruit quality: A comprehensive quality analysis. Folia Hortic. 2019, 31, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Agustí, A.; Seda, M.; Sarraga, C.; Montero, J.I.; Castellari, M.; Muñoz, P. Municipal solid waste composting: Application as a tomato fertilizer and its effect on crop yield, fruit quality and phenolic content. Renew. Agric. Food Syst. 2017, 32, 358–365. [Google Scholar] [CrossRef]
- Ravindran, B.; Lee, S.R.; Chang, S.W.; Nguyen, D.D.; Chung, W.J.; Balasubramanian, B.; Mupambwa, H.A.; Arasu, M.V.; Al-Dhabi, N.A.; Sekaran, G. Positive effects of compost and vermicompost produced from tannery waste-animal fleshing on the growth and yield of commercial crop-tomato (Lycopersicon esculentum L.) plant. J. Environ. Manag. 2019, 234, 154–158. [Google Scholar] [CrossRef]
- Salachna, P.; Łopusiewicz, Ł.; Wesołowska, A.; Meller, E.; Piechocki, R. Mushroom waste biomass alters the yield, total phenolic content, antioxidant activity and essential oil composition of Tagetes patula L. Ind. Crops Prod. 2021, 171, 113961. [Google Scholar] [CrossRef]
- Suárez, M.H.; Rodríguez, E.R.; Romero, C.D. Mineral and trace element concentrations in cultivars of tomatoes. Food Chem. 2007, 104, 489–499. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M.; D’Imperio, M.; Santamaria, P.; Serio, F. Iodine biofortification of four brassica genotypes is effective already at low rates of potassium iodate. Nutrients 2019, 11, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).