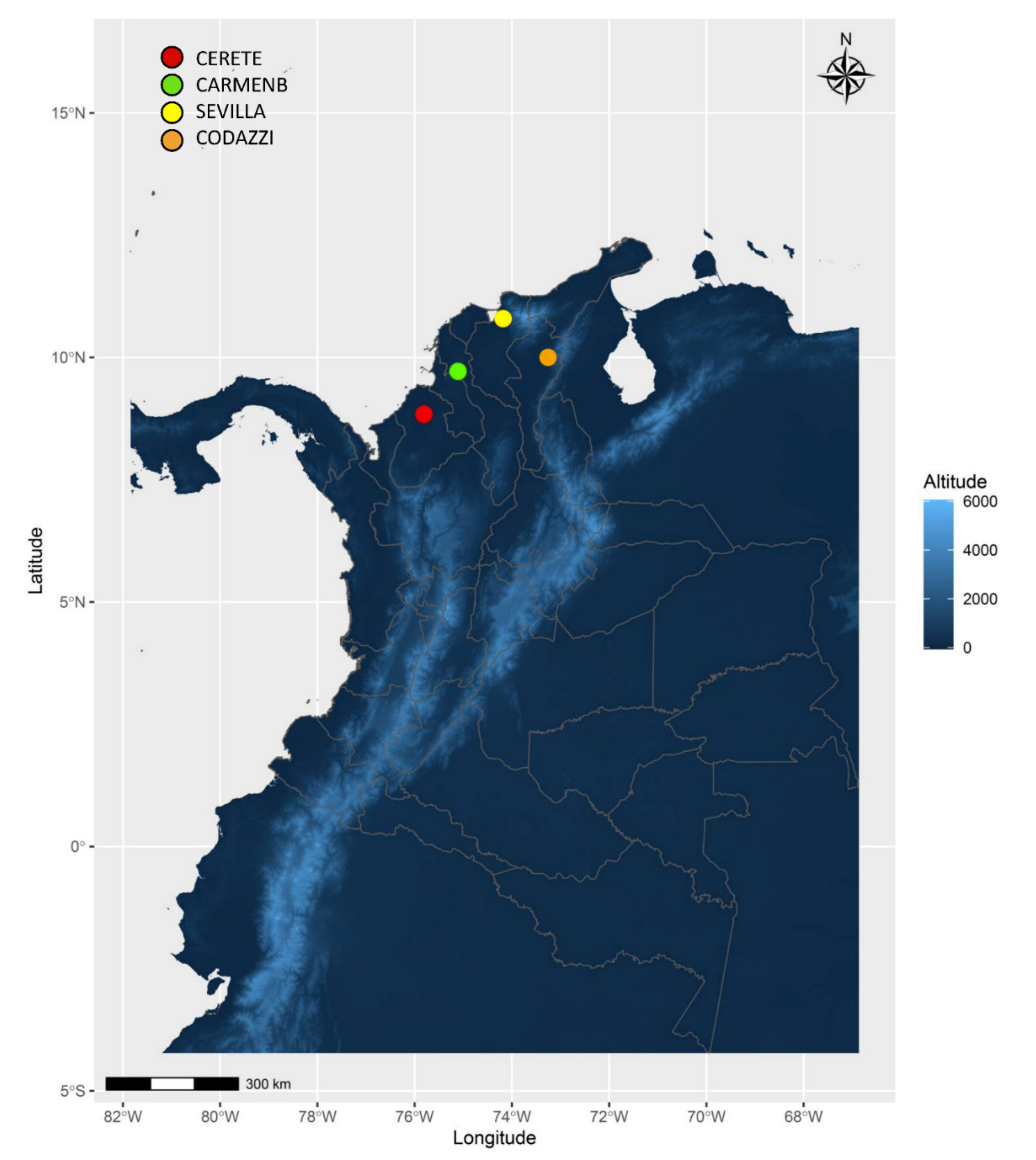
The Caribbean Coast region is characterized by high temperatures. Maximum temperatures ranging between 35–40 °C, minimum between 20–25 °C, and average around 28 °C were recorded (
Table 2). Cassava growth requires in its first vegetative phase at least 300 mm of water, rain fall was sufficient throughout the crop cycle in the different trials. Cereté, Seville, and Codazzi showed an accumulated rainfall above 900 mm, Carmen de Bolívar had the lowest rainfall values, but were still acceptable for cassava (
Table 2). The observed environmental conditions presented in all locations met the expectation for this agroecological region and were suitable for the germplasm evaluated.
3.1. Response of Cassava Genotypes to Different Environmental Conditions
Most of the evaluated genotypes showed adequate sprouting (>80%), except SM1127-8, which exhibited a reduced sprouting. The environmental conditions at Sevilla and Carmen de Bolívar promoted higher sprouting, in comparison with Cereté and Codazzi. However, averages were not below 90%. Plant height was >200 cm in all genotypes (
Figure 2a). Although this trait is not directly included in the selection index, it is an important component of PTS. Excessively high plants are undesirable due to increased vulnerability to lodging [
27,
28]. Interestingly, the tallest genotype (SM1127-8) showed the lowest sprouting, possibly since an excessive plant height increases lodging which, in turn, promotes early sprouting in pre-harvested plants. CM9456-12, CMB8527, GM3790-2, SM2773-32, SM3106-14, SM3386-49 and SM3474-139 showed plant heights comparable to commercial varieties (Corpoica-Caiseli and ICA-Costeña). The environmental conditions at Sevilla and Carmen de Bolívar promoted higher plant height, whereas in Codazzi, plants showed lower average height (
Figure 2b).
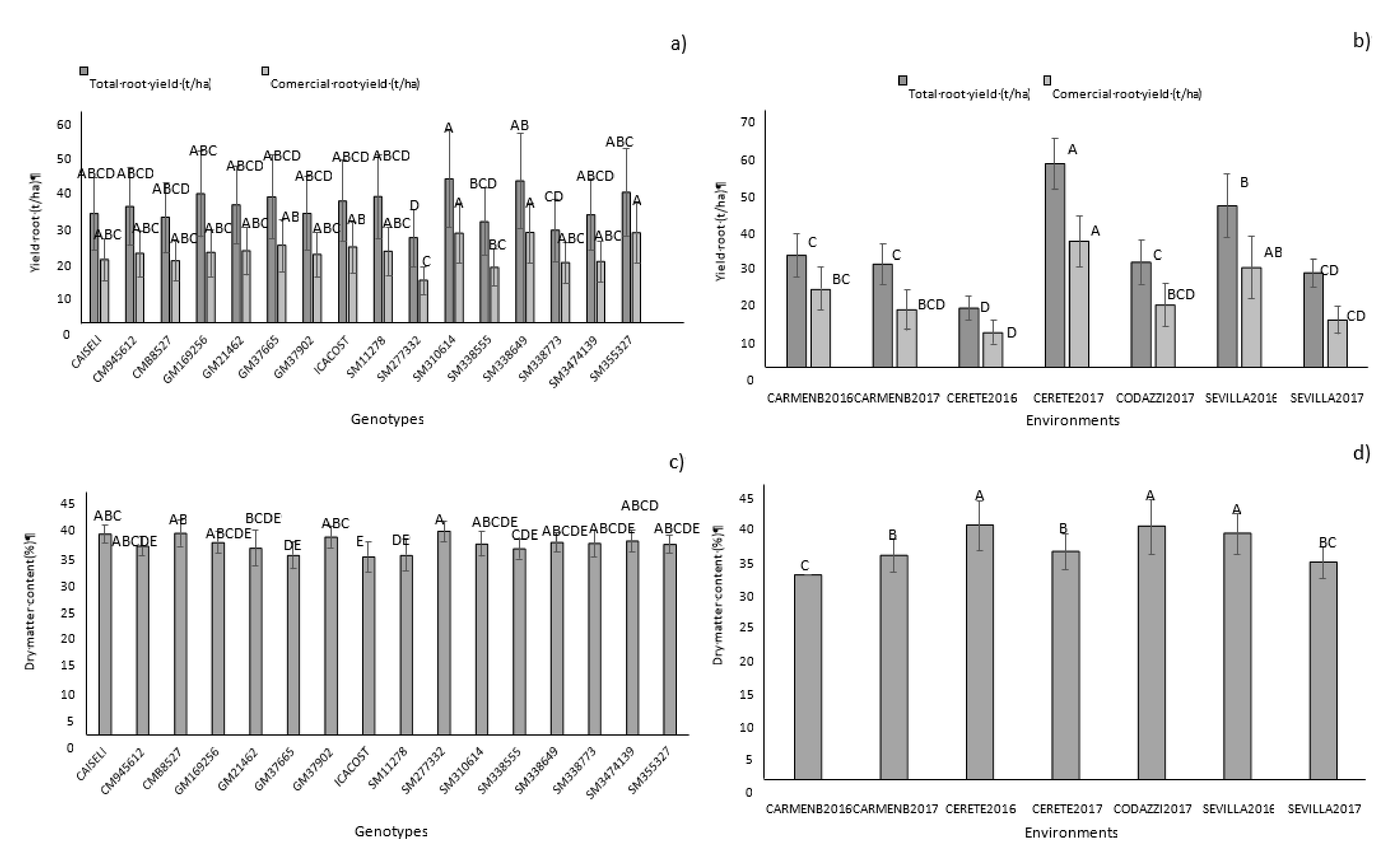
The environment, genotype, and G × E interaction sources of variation were highly significant (
p < 0.01) for total root yield (TRY), CFRY, and DMC (
Table 3). The environmental effect (combination of location and season) had the highest contribution to the variance of the model. A significant proportion of the phenotypic expression of the genotypes, therefore, was influenced by the environmental conditions where they were evaluated. However, the significant effect of genotype on total variation was demonstrated. CM9456-12, GM1692-56, GM214-62, GM3766-5, SM1127-8, SM3106-14, SM3385-55, SM3386-49, and SM 3553-27 showed higher overall averages in total root yield (
Figure 3a,
Table 4). In terms of commercial yield, genotypes SM 3106-14, SM 3553-27, and SM 3386-49 stood out. Significant differences were found between locations (
Table 3 and
Table 4,
Figure 3b). Cereté in the year 2017 showed the highest average for the total and commercial yield of 55.3 and 34.5 t/ha, respectively, followed by Sevilla 2016 (43.9 and 26.4 t/ha, respectively) and Carmen 2016 (30.5 and 21.3 t/ha, respectively).
Differences among crop cycles were found in Cereté and Sevilla (
Table 4). Despite being a vegetative propagated crop, cassava shows high variation among plants stemming from the same clone cultivated in the same plot, which is mainly due to factors such as micro-environmental variation and lack of uniformity in the quality of planting material [
29,
30]. On average, rainfall in the localities mentioned above was higher than 1.000 mm per year (
Table 1). Conditions of low water availability reduce yield especially in annual species [
31,
32]. The experimental clones evaluated had been previously selected for their adaptation to the Caribbean environmental conditions and their superiority in single row trials (SRT), followed by preliminary (PYT) and advanced (AYT) yield trials [
33]. However, some of these genotypes showed average yields for total and commercial roots below the values found in the control genotypes. This illustrates the limitation of selection in early stages. It has been stated that genotypes need to stabilize their phenotypic responses, and this requires several cycles of growth under the target environmental conditions [
33].
Environmental conditions affect the phenotypic expression of characteristics with low heritability. In this case, phenotypic features such as productive parameters (yield) and plant architecture (height) are affected by the environment, for which the appropriate selection of promising genotypes needs to be performed in multi-location evaluations and, preferably, through several growing seasons. In cassava, characteristics such as CFRY, DMC, and plant architecture are highly influenced by the genotype by environment interaction [
19,
33,
34,
35,
36,
37].
DMC averages across environments ranged between 25–40%, which were positive since the minimum established to carry out the selection of promising genotypes was 35.17%. In this sense, 64% of the evaluated genotypes stood out for showing high DMC averages (
Figure 3c,
Table 4). High and stable DMC is one of the objectives pursued by cassava breeding programs. Furthermore, varieties with mealy texture must have dry matter (and starch) content above 33–35%. Starch in fresh cassava roots represents around 85–90% of the DMC. Starch, after water, is the most abundant component in cassava roots [
38]. For fresh consumption, the market requires materials with good culinary quality, low cyanogenic potential and high dry matter content [
30,
39]. However, the results showed significant environmental effects on DMC (
p > 0.05) (
Figure 3d,
Table 3 and
Table 4). Codazzi 2017 presented the highest overall average among environments. Environmental and cultural practices have strong influence on DMC in roots. For example, sprouting before harvest, drastically reduces DMC in roots [
3]. DMC of each genotype in specific environmental conditions represent an expression of G × E interaction. Many studies have identified a differential adaptation for genotypes in diverse environmental conditions and significant G × E effects [
32,
40,
41,
42]. In those cases, a stability analysis must be performed to identify genotypes showing stable performance across seasons and locations.
3.2. Use of Selection Indices and Analysis of Phenotypic Stability to Identify Promising Genotypes
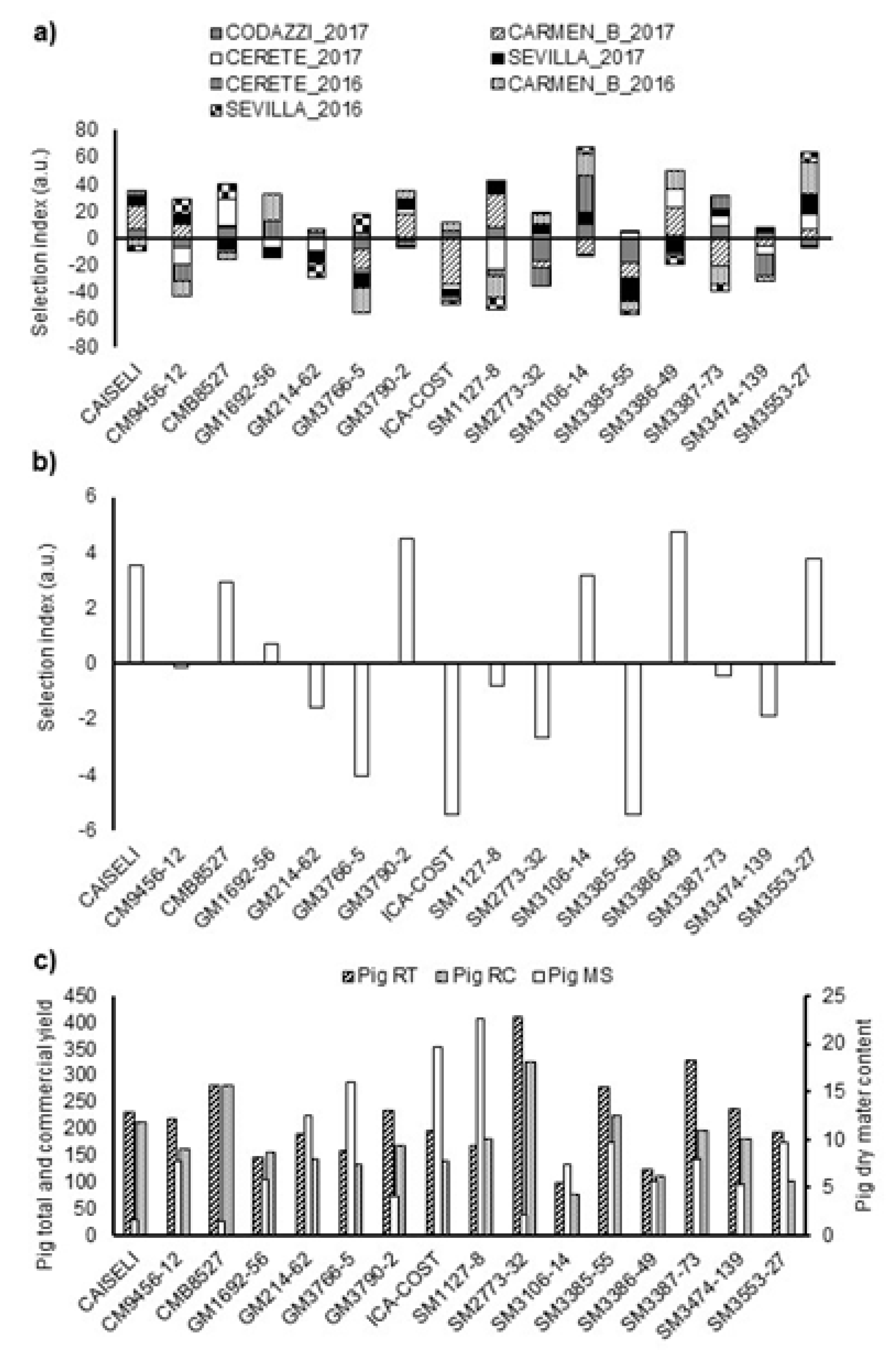
The multi-trait selection index (SIN) considered variables such as CRY, DMC and PTS as previously mentioned. SINs were obtained for both local (individual) and across-locations performances. The results of the analysis by environment (combination of location and season) showed that the genotypes CMB8527, GM1692-56, GM3790-2, SM3106-14, SM3386-49, and SM3553-27 had large positive SIN values in more than three different environments (
Figure 4a. CM9456-12, SM1127-8 and SM3387-73 had desirable performances in at least three environments, but also negative SIN values (of similar magnitude) in at least three environments, indicating that these were genotypes with good adaptation only to particular environments. Finally, genotypes GM214-62, ICA-COSTEÑA, SM2773-32, SM3385-55, and SM3474-139 showed negative values in more than four environments (
Figure 4a).
The analysis combined across locations showed that genotypes with positive SIN, as expected, had good agronomic performance in most environments. However, selecting genotypes based on average SIN across environments may not be adequate since performances of some genotype(s) in some environment(s) may result in undesirable biases. For example, SM 3386-49, SM 3553-27, SM 3106-14, and GM 3790-2 were the genotypes with positive average performances (
Figure 4b,
Table 5), which would indicate their superiority. However, some of those genotypes exhibited a negative performance in some environments, and the average hid this response. The average SIN, for example, may be strongly affected by unrealistically high yields in certain environments. It is important, therefore, to assess the stability and regularity of performance across environments. This is ultimately what defines an outstanding variety and defines if farmers will adopt a variety or not.
The general performance of the genotypes, according to the [
9] index, is defined as the mean square of the distance between the average value of the cultivar and the maximum average response for all locations. Genotypes with lower index values correspond to those with higher phenotypic stability. The most stable genotypes obtained through this analysis mostly coincide with the genotypes considered as stable according to the AMMI analysis [
43]. Although Lin and Binns index is useful for the stability analysis, it can be affected by atypical data, and result in selecting genotypes that adapt very well to a particular environment(s). That is, genotypes SM3386-49, SM3106-14, GM1692-56, and CAISELI were selected for their total root yield (TRY), genotypes CAISELI, SM2773-32, SM3474-139, and GM214-62 for their CRY, and genotypes SM3386-49, SM3553-27, SM3106-14, and CAISELI for their DMC in almost all the environments (
Figure 4c and
Table 5).
The analysis of variance quantified the effects attributable to the genotypes (G), environment (E), and their interaction (G×E) on the expression of CRY and DMC (
Table 6). The AMMI analysis, showed that the first component explained 65.8 and 65% of the variance contained in the G × E interaction for CRY and DMC, while the second component explained 28.9 and 30.9% of them, respectively. Therefore, the selection of a single multiplicative term of the AMMI model was sufficient to explain a large proportion of the relevant data [
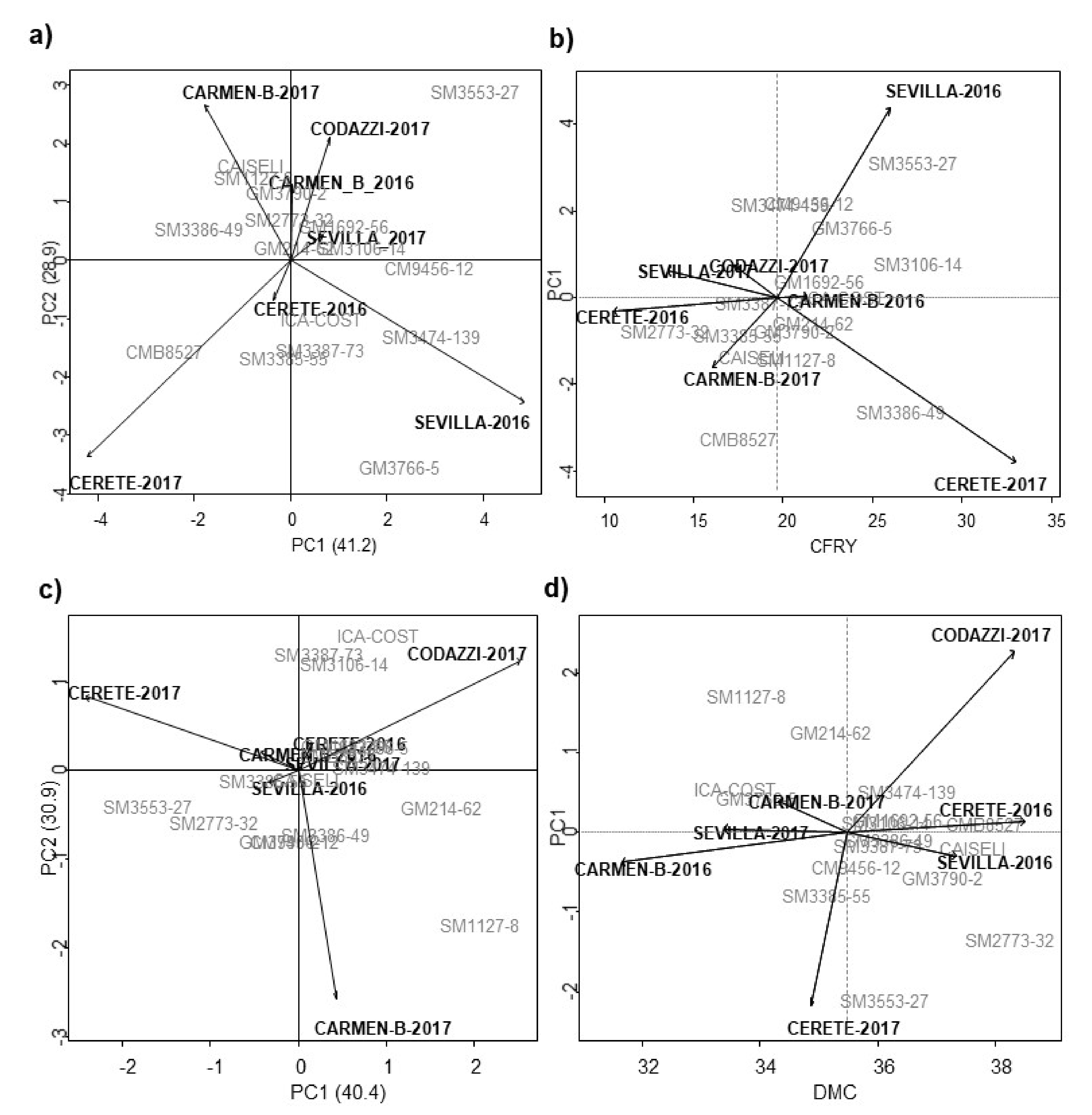
44]. The AMMI biplot obtained from the main genotypes and environments effects, the general mean and the first multiplicative term of the AMMI model (PC1) for CRY and DMC is shown in
Figure 5.
Figure 5a shows that 70% of the sum of squares of the G × E interaction for TRY was decomposed into two main components (PC1 and PC2). The genotypes closest to the origin point were those with little contribution to the interaction effects and, therefore, can be considered more stable. Genotypes GM214-62, SM3106-14, and GM1692-56 were stable. Meanwhile, SM3474-139 and CM9456-12 were located close to the Sevilla environment during 2016 and 2017, SM3387-73, SM3385-55, and CM85-27 showed an interaction with the environment in Cereté during 2016 and 2017, and genotypes SM1127-8 and GM3790-2 in Carmen de Bolívar during 2016. SM3553-27 and GM3766-5 showed the lower stability and high interaction with the environmental conditions found in Codazzi-2017 and Sevilla-2016, respectively (
Figure 5b). Comparing the averaged CRY and PC1), the genotypes GM1692-56, GM214-62, SM3106-14, SM3553-27, and SM3386-49 showed a CRY up to 20 tn/ha and they were closest to the origin point of PC1. The last two genotypes were outstanding for yield variables, and had a higher selection index and were more stable according to Lin and Binns (
Figure 5c).
In terms of DMC, GM3790-2, SM2773-32 and CMB8527 showed values above 35% and comparable with the best commercial control, Caiseli (
Figure 5d); the last two genotypes were also the most stable according to the Lin and Binns index. Cereté-2016, Codazzi-2017, and Sevilla-2016 showed the highest DMC values for most of the genotypes (
Figure 5c).
Several authors have reported significant interactions between the environment and cassava cultivars [
45,
46], representing an opportunity to identify the best discriminating environments and select stable genotypes in different environmental conditions [
47,
48]. Alternatively, it may be desirable to select clones adapted to specific environments [
49]. AMMI allows an adequate selection of stable genotypes across environments as well as genotypes adapted to specific environments [
12,
13,
14,
41,
42,
50]. The AMMI analysis allows a straightforward interpretation of the results using biplot graphics. According to [
12], the AMMI tool is powerful to improve the precision of the genotype by environment interaction. It allows eliminating the error of the estimators of phenotypic stability parameters generated by the effect of some environments in particular genotypes.
Ref. [
51] pointed out that those treatments that exhibit an angle close to 90° are not related to each other, and those that have an angle close to 180° tend to have an opposite behavior, as was the case between the four locations assessed. These were very different from each other, and seven macro environments for the locations of El Carmen de Bolívar, Cereté, Agustín Codazzi, and Sevilla were generated during 2 years (except for Codazzi). Genotypes and environments with high coordinates on PC1 considered in absolute value, contributed more to the G × E interaction. Meanwhile, genotypes and environments with PC1 close to zero had little participation in this effect [
52].
The correlation coefficient between genotypes, environments or the genotype by environment interaction is given approximately by the cosine of the angle formed between the vectors. Thus, if the angle between the vectors is 180° the correlation coefficient is −1, if the angle is 0° the coefficient is +1, and if it is 90° the coefficient is 0. However, the AMMI analysis does not provide a quantitative measure of stability. For this reason, Ref. [
53] proposed an average stability value (ASV) measure to quantify and classify genotypes according to their yield stability in that ASV is the distance of the varieties from point zero of the scatter diagram (PC1 vs. PC2). The genotypes with the lower scores were more stable (
Table 7). According to the ranking using several indexes, SM214-64, GM1692-56, SM2773-32, CM9456-12 and SM3106-14 resulted in the best ASV values. Genotypes that were previously selected as superior, SM3553-27, GM3766-5 and SM3386-49, showed lower ASV values, suggesting lower stability. However, the selection of promising genotypes should consider both their superiority and stability. Therefore, SM3106-14, GM1692-56, CM9456-12 and GM214-62 were recommended for the next evaluation cycle.
3.3. Cooking and Sensory Properties in Selected Genotypes
HCN differs widely in cassava. Non-bitter roots usually have a cyanogenic glucoside concentration < 100 mg HCN equivalents/kg fresh [
54,
55]. All varieties (except Tai 8) can be considered non bitter. SM3474-139, GM3766-5, and SM2773-32 HCN contents close to 100 mg/kg, and the rest showed lower concentration values. Genotypes such as CMB8527, GM3790-2, SM1127-8, SM3385-55, and SM3553-27 showed the HCN content <50 mg/kg, as did the checks Venezolana and Caiseli.
The cooking time for most of the evaluated genotypes was in the range of 20–30 min, however, genotypes such as GM1692-56, GM3766-5, GM3790-2, SM3387-73, SM3553-27, and SM3562-32 showed an extended and undesirable cooking period (
Table 8).
Sensorial description of cassava genotypes from untrained panelists aided to improve the selection of promising genotypes, hedonic scale was used to qualify parameters such color, flavor, texture, and root shape (
Table 9). Frequency analysis of sensorial data showed that CM9456-12, SM1127-8, SM3553-27, and SM3562-32 were preferred by panelists, their color, flavor, texture, and root shape seemed to be superior to commercial varieties. Although, previous reports showed that varieties with mealy texture contain higher contents of dry matter and starch contents. Although larger starch granules were observed in mealy varieties than those of non-mealy varieties [
56], the factors related to flavor and texture remain still poorly understood.
The inclusion of palatability response and quality features determination in cassava genotypes allowed identifying genotypes with higher opportunity to be adopted by farmers as new cassava varieties.