Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice
Abstract
:1. Introduction
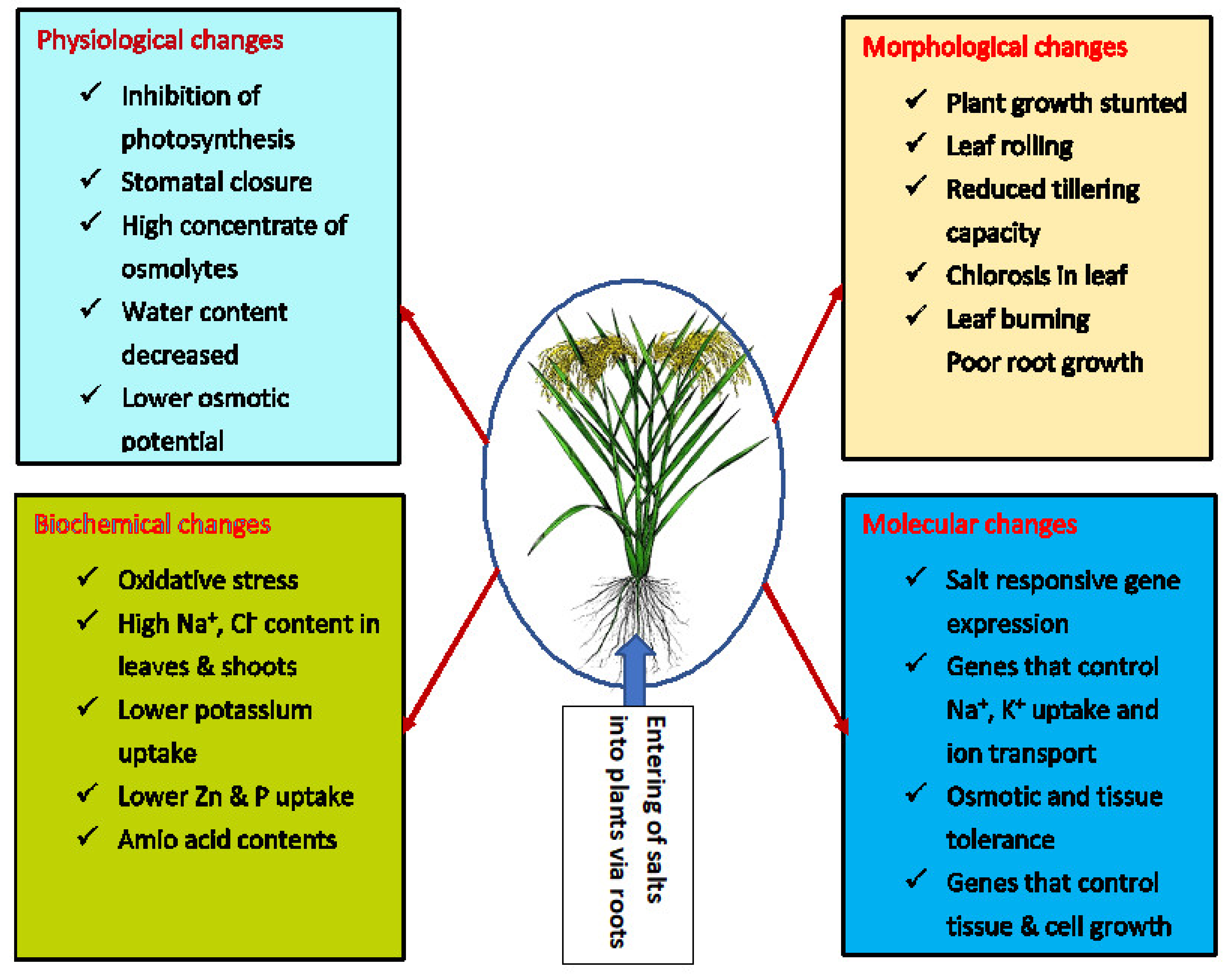
2. Effect Salinity Stress on Rice Growth, Development and Grain Yield
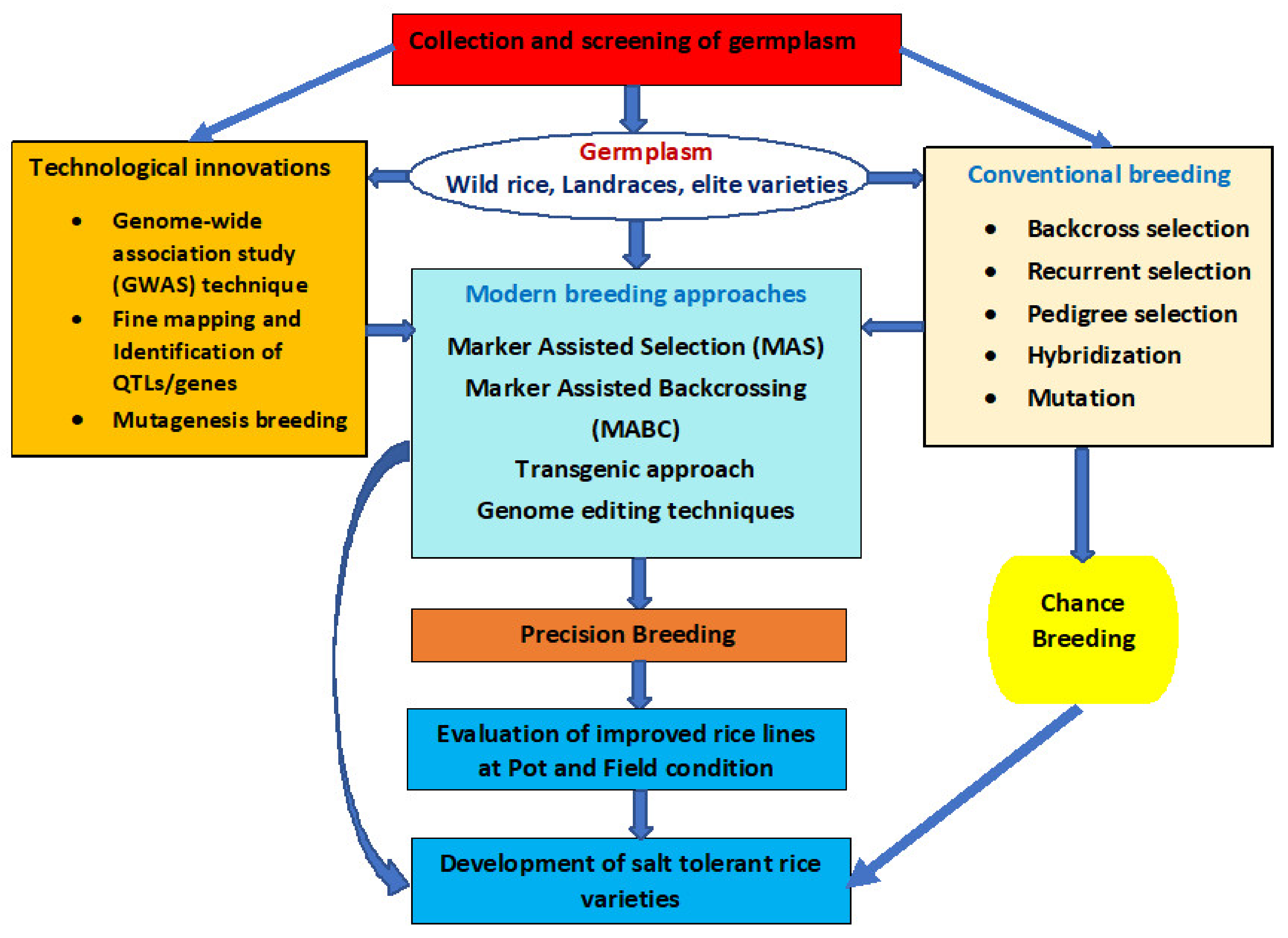
3. Breeding Strategies to Improve Salt Tolerance in Rice
3.1. Mining and Screening of Salt-Tolerance in Rice Germplasm
3.2. QTLs/Genes Mapping to Improve Salt-Tolerant Rice
| Parents | Number of QTLs | Functions | Stage | Reference |
|---|---|---|---|---|
| Dianjingyou × See Rice 86 | 1 | Enhances salinity tolerance at the seedling stage | Seedling | [46] |
| Ahlemi Tarom × Neda | 73 | Enhances salinity tolerance in rice at seedling Stage | Seedling | [56] |
| IR36 × Weiguo | 1 | Enhances salt-tolerant QTL interval at the bud burst stage | Bud burst | [57] |
| Nona Bokra × Koshihikari | 2 | qSNC-7 for shoot Na+ concentration and qSKC-1 for shoot K+ concentration, explained 48.5% and 40.1% of the total phenotypic variance, respectively | Seedling | [58] |
| BRRI dhan29 × Capsule | 16 | Salinity tolerance at the seedling stage | Seedling | [59] |
| Sahel 317 × Madina Koyo | 13 | Resistance to salinity at the early seedling stage | Early seedling | [60] |
| LYP9 × PA64s | 38 | Seedling stage | Seedling | [61] |
| Gharib × Sepidroud | 41 | Growth of rice at the seedling stage under high salinity | Seedling | [62] |
| IR29 × Pokkali | 23 | Long-distant Na+ transport from roots to shoots in rice | Seedlings | [63] |
| Jiucaiqing × IR26 | 16 | Controlling rice seed germination under salt stress at seed germination stage | Germination | [64] |
| Cheniere × Nona Bokra | 32 | Enhanced salt tolerance at seedling stage | Seedling | [65] |
| Indica cultivar, 93-11 × O. rufipogon | 10 | Enhances salinity resistance at the seedling stage | Seedling | [66] |
| Pokkali × Bengal | 50 | Seedling salinity tolerance | [67] | |
| Ilpumbyeo × Moroberekan | 8 | Seedling stage | Seedling | [68] |
| Yiai 1 × Lishuinuo | 13 | Salt or alkaline tolerance at the seedling stage in rice | Seedling | [69] |
| Tarommahali × Khazar | 3 | Salt tolerance in young rice seedlings | Young seedling | [70] |
| Sadri × FL478 | 35 | Yield and yield components under reproductive stage salinity stress | Reproductive | [71] |
| CSR27 × MI48 | 25 | Na+, K+, and CI− ion concentrations in salt-tolerant indica rice | Seedling | [72] |
| IR29 × Hasawi | 34 | Enhances salinity resistance | Seedling | [73] |
| OM7347 × OM5629 | 9 | Enhances salinity tolerance | Seedling, vegetative and reproductive | [74] |
| Kalarata × Azucena | 13 | Associated with salinity stress | Seedling | [75] |
| Wujiaozhan × Nipponbare | 13 | Confers seed germination traits under H2O and salt conditions | Germination | [76] |
| Horkuch × IR29 | 14 | Enhances salinity tolerance | Seedling and reproductive | [77] |
| Target Genes | Gene Function | Expression | Reference |
|---|---|---|---|
| OsTPS1 | Enhances salinity, drought, and cold tolerance in transgenic rice | Overexpression | [18,78] |
| OsSPL10 | SBP-box gene, negatively controls salt tolerance but positively controls trichome formation in rice | Overexpression | [79] |
| OsEXPA7 | Improves salt stress tolerance in rice by regulating ion homeostasis, ROS scavenging, and cell wall-loosening | Overexpression | [53] |
| OsMYB6 | MYB family gene, increases drought and salinity stress tolerance in transgenic rice | Overexpression | [80] |
| OsMADS25 | Transcriptional regulator that regulates the root growth and confers salinity tolerance in rice via the ABA–mediated regulatory pathway and ROS scavenging | Overexpression | [81] |
| OsSOS1 | Regulate ion transport regulation under salt stress | Overexpression | [82] |
| OsDOF15 | Contributes to ethylene-inhibited primary root elongation under salt stress | Overexpression | [83] |
| OsGA2ox5 | A gibberellin metabolism enzyme, GAs homeostasis, development, gravity responses, and stress tolerance in rice | Ectopic expression | [84] |
| OsCDPK7 | Positive regulator involved in both cold and salt/drought tolerance in rice | Overexpression | [85] |
| OsCPK21 | A calcium-dependent protein kinase, confers salinity tolerance in rice | Overexpression | [86] |
| OsCPK12 | Calcium-dependent protein kinase, negatively regulate salt-stress tolerance | Overexpression | [87] |
| OsCam1-1 | ABA biosynthesis and salt tolerance in rice | Overexpression | [88] |
| OsMSR2 | Enhances drought and salt tolerance and increases ABA sensitivity | Overexpression | [89] |
| OsRPK1 | Receptor-like protein kinase genes, negatively regulate salt tolerance | Overexpression | [90] |
3.3. Genome-Wide Association Study (GWAS) Technique
4. Breeding Approaches for Developing Salt-Tolerant Rice
4.1. Conventional/Traditional Breeding
4.2. Marker-Assisted Selection
4.3. Transgenic Approach
4.4. Mutation Breeding
4.5. Genome Editing Techniques
5. Development of Salt-Resistant Rice Varieties
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leridon, H.; Population, I. World population outlook: Explosion or implosion? Popul. Soc. 2020, 573, 1–4. [Google Scholar]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Liu, M.; Pan, T.; Allakhverdiev, S.I.; Yu, M.; Shabala, S. Crop Halophytism: An Environmentally Sustainable Solution for Global Food Security. Trends Plant Sci. 2020, 25, 630–634. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Linh, L.H.; Linh, T.H.; Xuan, T.D.; Ham, L.H.; Ismail, A.M.; Khanh, T.D. Molecular breeding to improve salt tolerance of rice (Oryza sativa L.) in the Red River Delta of Vietnam. Int. J. Plant Genom. 2012, 2012, 949038. [Google Scholar] [CrossRef] [Green Version]
- Izawa, T.; Shimamoto, K. Becoming a model plant: The importance of rice to plant science. Trends Plant Sci. 1996, 1, 95–99. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Kotula, L.; Garcia Caparros, P.; Zörb, C.; Colmer, T.D.; Flowers, T.J. Improving crop salt tolerance using transgenic approaches: An update and physiological analysis. Plant. Cell Environ. 2020, 43, 2932–2956. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, H.; Ouyang, B.; Shi, C.; Demidchik, V.; Hao, Z.; Yu, M.; Shabala, S. NADPH oxidases and the evolution of plant salinity tolerance. Plant Cell Environ. 2020, 43, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Chen, K.-C.; Cheng, T.-S.; Lee, C.; Lin, S.-H.; Tung, C.-W. Chlorophyll fluorescence analysis in diverse rice varieties reveals the positive correlation between the seedlings salt tolerance and photosynthetic efficiency. BMC Plant Biol. 2019, 19, 403. [Google Scholar] [CrossRef] [Green Version]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J. Understanding and Improving Salt Tolerance in Plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Todaka, D.; Nakashima, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Toward understanding transcriptional regulatory networks in abiotic stress responses and tolerance in rice. Rice 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-S.; Choi, W.-Y.; Ko, J.-C.; Kim, T.-S.; Gregorio, G.B. Salinity tolerance of japonica and indica rice (Oryza sativa L.) at the seedling stage. Planta 2003, 216, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Ansari, M.W.; Pareek, A.; Singla-Pareek, S.L. Raising salinity tolerant rice: Recent progress and future perspectives. Physiol. Mol. Biol. Plants 2008, 14, 137–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Lesch, S.M.; Grieve, C.M. Rice growth and yield respond to changes in water depth and salinity stress. Agric. Water Manag. 2003, 59, 67–75. [Google Scholar] [CrossRef]
- Rao, P.S.; Mishra, B.; Gupta, S.R.; Rathore, A. Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed. 2008, 127, 256–261. [Google Scholar] [CrossRef]
- Ren, Z.-H.; Gao, J.-P.; Li, L.-G.; Cai, X.-L.; Huang, W.; Chao, D.-Y.; Zhu, M.-Z.; Wang, Z.-Y.; Luan, S.; Lin, H.-X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef]
- Flowers, T.J.; Flowers, S.A. Why does salinity pose such a difficult problem for plant breeders? Agric. Water Manag. 2005, 78, 15–24. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M. Abiotic Stress Tolerance in Rice (Oryza sativa L.): A Genomics Perspective of Salinity Tolerance. In Rice Crop—Current Developments; InTech: London, UK, 2018; pp. 181–192. [Google Scholar]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef]
- Saleethong, P.; Sanitchon, J.; Kong-ngern, K.; Theerakulpisut, P. Effects of exogenous spermidine (Spd) on yield, yield-related parameters and mineral composition of rice (Oryza sativa L. ssp. indica) grains under salt stress. Aust. J. Crop Sci. 2013, 7, 1293–1301. [Google Scholar]
- Verma, T.S.; Neue, H.U. Effect of soil salinity level and zinc application on growth, yield, and nutrient composition of rice. Plant Soil 1984, 82, 3–14. [Google Scholar] [CrossRef]
- Solis, C.A.; Yong, M.T.; Vinarao, R.; Jena, K.; Holford, P.; Shabala, L.; Zhou, M.; Shabala, S.; Chen, Z.-H. Back to the Wild: On a Quest for Donors Toward Salinity Tolerant Rice. Front. Plant Sci. 2020, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Wungrampha, S.; Kumar, G.; Singla-Pareek, S.L.; Pareek, A. How do rice seedlings of landrace Pokkali survive in saline fields after transplantation? Physiology, biochemistry, and photosynthesis. Photosynth. Res. 2020. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Wu, Z.; Zhang, X.; Wang, M.; Zhang, C.; Zhang, F.; Zhou, Y.; Li, Z. Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorio, G.B.; Senadhira, D.; Mendoza, R.D. Screening Rice for Salinity Tolerance; Discussion Paper Series; International Rice Research Institute: Manila, Philippines, 1997. [Google Scholar]
- Yeo, A.R.; Yeo, M.E.; Flowers, S.A.; Flowers, T.J. Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theor. Appl. Genet. 1990, 79, 377–384. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Brien, C.; Oakey, H.; Berger, B.; Saade, S.; Ho, Y.S.; Schmöckel, S.M.; Tester, M.; Negrão, S. Salinity tolerance loci revealed in rice using high-throughput non-invasive phenotyping. Nat. Commun. 2016, 7, 13342. [Google Scholar] [CrossRef] [Green Version]
- Platten, J.D.; Egdane, J.A.; Ismail, A.M. Salinity tolerance, Na+ exclusion and allele mining of HKT1;5 in Oryza sativa and O. glaberrima: Many sources, many genes, one mechanism? BMC Plant Biol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Singh, B.; Panda, K.; Singh, B.P.; Singh, N.; Misra, P.; Rai, V.; Singh, N.K. Association of SNP Haplotypes of HKT Family Genes with Salt Tolerance in Indian Wild Rice Germplasm. Rice 2016, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.-R.; Fu, C.-Y.; Fan, Z.-L.; Chen, Y.; Chen, W.-F.; Zhang, J.; Jiang, L.-Q.; Lv, S.; Pan, D.-J.; Li, C. Genomic and transcriptomic analysis reveal molecular basis of salinity tolerance in a novel strong salt-tolerant rice landrace Changmaogu. Rice 2019, 12, 99. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Y.; Han, S.; Van Handel, B.; Dong, L.; Li, X.; Xie, X. Whole genome sequencing and comparative transcriptome analysis of a novel seawater adapted, salt-resistant rice cultivar—Sea rice 86. BMC Genom. 2017, 18, 655. [Google Scholar] [CrossRef] [Green Version]
- Prusty, M.R.; Kim, S.-R.; Vinarao, R.; Entila, F.; Egdane, J.; Diaz, M.G.Q.; Jena, K.K. Newly Identified Wild Rice Accessions Conferring High Salt Tolerance Might Use a Tissue Tolerance Mechanism in Leaf. Front. Plant Sci. 2018, 9, 417. [Google Scholar] [CrossRef] [Green Version]
- Rajakani, R.; Sellamuthu, G.; Saravanakumar, V.; Kannappan, S.; Shabala, L.; Meinke, H.; Chen, Z.; Zhou, M.; Parida, A.; Shabala, S.; et al. Microhair on the adaxial leaf surface of salt secreting halophytic Oryza coarctata Roxb. show distinct morphotypes: Isolation for molecular and functional analysis. Plant Sci. 2019, 285, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Majee, M.; Maitra, S.; Dastidar, K.G.; Pattnaik, S.; Chatterjee, A.; Hait, N.C.; Das, K.P.; Majumder, A.L. A Novel Salt-tolerant l-myo-Inositol-1-phosphate Synthase from Porteresia coarctata (Roxb.) Tateoka, a Halophytic Wild Rice. J. Biol. Chem. 2004, 279, 28539–28552. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-Y.; Chao, D.-Y.; Gao, J.-P.; Zhu, M.-Z.; Shi, M.; Lin, H.-X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009, 23, 1805–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaegashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E.; et al. MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat. Biotechnol. 2015, 33, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.X.; Zhu, M.Z.; Yano, M.; Gao, J.P.; Liang, Z.W.; Su, W.A.; Hu, X.H.; Ren, Z.H.; Chao, D.Y. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor. Appl. Genet. 2004, 108, 253–260. [Google Scholar] [CrossRef]
- Thomson, M.J.; de Ocampo, M.; Egdane, J.; Rahman, M.A.; Sajise, A.G.; Adorada, D.L.; Tumimbang-Raiz, E.; Blumwald, E.; Seraj, Z.I.; Singh, R.K.; et al. Characterizing the Saltol Quantitative Trait Locus for Salinity Tolerance in Rice. Rice 2010, 3, 148–160. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Yang, B.; He, Y.; Zhan, C.; Cheng, Y.; Zhang, J.; Zhang, H.; Cheng, J.; Wang, Z. A quantitative trait locus, qSE 3, promotes seed germination and seedling establishment under salinity stress in rice. Plant J. 2019, 97, 1089–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Ahn, J.H.; Cha, Y.S.; Yun, D.W.; Lee, M.C.; Ko, J.C.; Lee, K.S.; Eun, M.Y. Mapping QTLs related to salinity tolerance of rice at the young seedling stage. Plant Breed. 2007, 126, 43–46. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Yu, D.; Xu, P. Identification and Validation a Major QTL from “Sea Rice 86” Seedlings Conferred Salt Tolerance. Agronomy 2020, 10, 410. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.-F.; Li, W.-Q.; Li, W.-Y.; Wu, G.-L.; Zhou, C.-Y.; Chen, K.-M. Characterization of Rice NADPH Oxidase Genes and Their Expression under Various Environmental Conditions. Int. J. Mol. Sci. 2013, 14, 9440–9458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asada, K. The Water-Water Cycle in Chloroplasts: Scavenging of Active Oxygens and Dissipation of Excess Photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; He, S.; Liu, P.; Zhang, W.; Zhang, J.; Chen, S. The role of tocopherol cyclase in salt stress tolerance of rice (Oryza sativa). Sci. China Life Sci. 2011, 54, 181–188. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wu, J.; Zheng, X.; Zheng, S.; Sun, X.; Qiu, Q.; Lu, T. Gene Knockout Study Reveals That Cytosolic Ascorbate Peroxidase 2(OsAPX2) Plays a Critical Role in Growth and Reproduction in Rice under Drought, Salt and Cold Stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef]
- Campbell, M.T.; Bandillo, N.; Al Shiblawi, F.R.A.; Sharma, S.; Liu, K.; Du, Q.; Schmitz, A.J.; Zhang, C.; Véry, A.-A.; Lorenz, A.J.; et al. Allelic variants of OsHKT1;1 underlie the divergence between indica and japonica subspecies of rice (Oryza sativa) for root sodium content. PLoS Genet. 2017, 13, e1006823. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.V.; Mondal, T.K. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Jadamba, C.; Kang, K.; Paek, N.-C.; Lee, S.I.; Yoo, S.-C. Overexpression of Rice Expansin7 (Osexpa7) Confers Enhanced Tolerance to Salt Stress in Rice. Int. J. Mol. Sci. 2020, 21, 454. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, Z.; Xiao, G.; Zhai, M.; Pan, X.; Huang, R.; Zhang, H. OsCYP71D8L as a key regulator involved in growth and stress response by mediating gibberellins homeostasis in rice. J. Exp. Bot. 2019, 71, 1160–1170. [Google Scholar] [CrossRef]
- Wang, J.; Qin, H.; Zhou, S.; Wei, P.; Zhang, H.; Zhou, Y.; Miao, Y.; Huang, R. The Ubiquitin-Binding Protein OsDSK2a Mediates Seedling Growth and Salt Responses by Regulating Gibberellin Metabolism in Rice. Plant Cell 2020, 32, 414–428. [Google Scholar] [CrossRef] [Green Version]
- Sanchouli, S.; Neghab, M.G.; Sabouri, H.; Mehrjerdi, M.Z. Genetic Structure of Salinity Tolerance in Rice at Seedling Stage. J. Genet. Resour. 2019, 5, 22–30. [Google Scholar] [CrossRef]
- Lei, L.; Zheng, H.; Bi, Y.; Yang, L.; Liu, H.; Wang, J.; Sun, J.; Zhao, H.; Li, X.; Li, J.; et al. Identification of a Major QTL and Candidate Gene Analysis of Salt Tolerance at the Bud Burst Stage in Rice (Oryza sativa L.) Using QTL-Seq and RNA-Seq. Rice 2020, 13, 55. [Google Scholar] [CrossRef]
- Rahman, M.A.; Thomson, M.J.; De Ocampo, M.; Egdane, J.A.; Salam, M.A.; Shah-E-Alam, M.; Ismail, A.M. Assessing trait contribution and mapping novel QTL for salinity tolerance using the Bangladeshi rice landrace Capsule. Rice 2019, 12, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoah, N.K.A.; Akromah, R.; Kena, A.W.; Manneh, B.; Dieng, I.; Bimpong, I.K. Mapping QTLs for tolerance to salt stress at the early seedling stage in rice (Oryza sativa L.) using a newly identified donor ‘Madina Koyo’. Euphytica 2020, 216, 156. [Google Scholar] [CrossRef]
- Jahan, N.; Zhang, Y.; Lv, Y.; Song, M.; Zhao, C.; Hu, H.; Cui, Y.; Wang, Z.; Yang, S.; Zhang, A.; et al. QTL analysis for rice salinity tolerance and fine mapping of a candidate locus qSL7 for shoot length under salt stress. Plant Growth Regul. 2020, 90, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Ghomi, K.; Rabiei, B.; Sabouri, H.; Sabouri, A. Mapping QTLs for Traits Related to Salinity Tolerance at Seedling Stage of Rice (Oryza sativa L.): An Agrigenomics Study of an Iranian Rice Population. Omics A J. Integr. Biol. 2013, 17, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhu, Y.; Chen, K.; Shen, C.; Zhao, X.; Shabala, S.; Shabala, L.; Meinke, H.; Venkataraman, G.; Chen, Z.; et al. Identification of new QTL for salt tolerance from rice variety Pokkali. J. Agron. Crop Sci. 2020, 206, 202–213. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Bao, Y.; Wu, Y.; Zhang, H. Quantitative trait loci controlling rice seed germination under salt stress. Euphytica 2011, 178, 297–307. [Google Scholar] [CrossRef]
- Puram, V.R.R.; Ontoy, J.; Subudhi, P.K. Identification of QTLs for Salt Tolerance Traits and Prebreeding Lines with Enhanced Salt Tolerance in an Introgression Line Population of Rice. Plant Mol. Biol. Report. 2018, 36, 695–709. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Ma, X.; Chen, W.; Zhao, J.; Sun, C.; Tan, L.; Liu, F. Integrated RNA Sequencing and QTL Mapping to Identify Candidate Genes from Oryza rufipogon Associated with Salt Tolerance at the Seedling Stage. Front. Plant Sci. 2017, 8, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leon, T.B.; Linscombe, S.; Subudhi, P.K. Identification and validation of QTLs for seedling salinity tolerance in introgression lines of a salt tolerant rice landrace ‘Pokkali’. PLoS ONE 2017, 12, e0175361. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-M.; Ju, H.-G.; Kwon, T.-R.; Oh, C.-S.; Ahn, S.-N. Mapping QTLs for salt tolerance in an introgression line population between Japonica cultivars in rice. J. Crop Sci. Biotechnol. 2009, 12, 121–128. [Google Scholar] [CrossRef]
- Liang, J.; Qu, Y.; Yang, C.; Ma, X.; Cao, G.; Zhao, Z.; Zhang, S.; Zhang, T.; Han, L. Identification of QTLs associated with salt or alkaline tolerance at the seedling stage in rice under salt or alkaline stress. Euphytica 2015, 201, 441–452. [Google Scholar] [CrossRef]
- Sabouri, H.; Rezai, A.M.; Moumeni, A.; Kavousi, A.; Katouzi, M.; Sabouri, A. QTLs mapping of physiological traits related to salt tolerance in young rice seedlings. Biol. Plant. 2009, 53, 657–662. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mendioro, M.S.; Diaz, G.Q.; Gregorio, G.B.; Singh, R.K. Mapping quantitative trait loci associated with yield and yield components under reproductive stage salinity stress in rice (Oryza sativa L.). J. Genet. 2013, 92, 433–443. [Google Scholar] [PubMed]
- Ammar, M.H.M.; Pandit, A.; Singh, R.K.; Sameena, S.; Chauhan, M.S.; Singh, A.K.; Sharma, P.C.; Gaikwad, K.; Sharma, T.R.; Mohapatra, T.; et al. Mapping of QTLs Controlling Na+, K+ and CI− Ion Concentrations in Salt Tolerant Indica Rice Variety CSR27. J. Plant Biochem. Biotechnol. 2009, 18, 139–150. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bimpong, I.K.; Bizimana, J.B.; Pascual, E.D.; Arceta, M.; Swamy, B.P.M.; Diaw, F.; Rahman, M.S.; Singh, R.K. Mapping QTLs using a novel source of salinity tolerance from Hasawi and their interaction with environments in rice. Rice 2017, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.T.; Phuoc, N.T.; Ha, P.T.T.; Buu, B.C. Identifying QTLs Associated and Marker-Assisted Selection for Salinity Tolerance at the Seedling, Vegetative and Reproductive Stages in Rice (Oryza sativa L.). Int. J. Environ. Agric. Biotechnol. 2017, 2, 2927–2935. [Google Scholar] [CrossRef]
- Ocampo, M.; The, H.V.; Thomson, M.; Mitsuya, S.; Yamauchi, A.; Ismail, A. QTL mapping and candidate gene identification in rice using a Kalarata-Azucena population under salt stress. Res. Squre 2020, 1–36. [Google Scholar] [CrossRef]
- Zeng, P.; Zhu, P.; Qian, L.; Qian, X.; Mi, Y.; Lin, Z.; Dong, S.; Aronsson, H.; Zhang, H.; Cheng, J. Identification and fine mapping of qGR6.2, a novel locus controlling rice seed germination under salt stress. BMC Plant Biol. 2021, 21, 36. [Google Scholar] [CrossRef]
- Haque, T.; Elias, S.M.; Razzaque, S.; Biswas, S.; Khan, S.F.; Jewel, G.M.N.A.; Rahman, S.; Juenger, T.E.; Seraj, Z.I. Natural Variation in Growth and Physiology under Salt Stress in Rice: QTL Mapping in a Horkuch × IR29 Mapping Population at Seedling and Reproductive Stages. bioRxiv 2020, 2507. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-W.; Zang, B.-S.; Deng, X.-W.; Wang, X.-P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Zheng, Y.; Su, Z.; Yu, S.; Song, H.; Zheng, X.; Lin, G.; Wu, W. OsSPL10, a SBP-Box Gene, Plays a Dual Role in Salt Tolerance and Trichome Formation in Rice (Oryza sativa L.). G3 Genes Genomes Genet. 2019, 9, 4107–4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Chu, Y.; Chen, H.; Li, X.; Wu, Q.; Jin, L.; Wang, G.; Huang, J. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA–mediated regulatory pathway and ROS scavenging. PLoS Genet. 2018, 14, e1007662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. Na+/K+ Balance and Transport Regulatory Mechanisms in Weedy and Cultivated Rice (Oryza sativa L.) Under Salt Stress. BMC Plant Biol. 2018, 18, 375. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, J.; Chen, X.; Wang, F.; Peng, P.; Zhou, Y.; Miao, Y.; Zhang, Y.; Gao, Y.; Qi, Y.; et al. Rice OsDOF15 contributes to ethylene-inhibited primary root elongation under salt stress. New Phytol. 2019, 223, 798–813. [Google Scholar] [CrossRef]
- Shan, C.; Mei, Z.; Duan, J.; Chen, H.; Feng, H.; Cai, W. OsGA2ox5, a Gibberellin Metabolism Enzyme, Is Involved in Plant Growth, the Root Gravity Response and Salt Stress. PLoS ONE 2014, 9, e87110. [Google Scholar] [CrossRef]
- Saijo, Y.; Hata, S.; Kyozuka, J.; Shimamoto, K.; Izui, K. Over-expression of a single Ca 2+ -dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000, 23, 319–327. [Google Scholar] [CrossRef]
- Asano, T.; Hakata, M.; Nakamura, H.; Aoki, N.; Komatsu, S.; Ichikawa, H.; Hirochika, H.; Ohsugi, R. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant Mol. Biol. 2011, 75, 179–191. [Google Scholar] [CrossRef]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S.; et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012, 69, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Saeng-ngam, S.; Takpirom, W.; Buaboocha, T.; Chadchawan, S. The role of the OsCam1-1 salt stress sensor in ABA accumulation and salt tolerance in rice. J. Plant Biol. 2012, 55, 198–208. [Google Scholar] [CrossRef]
- Xu, G.-Y.; Rocha, P.S.C.F.; Wang, M.-L.; Xu, M.-L.; Cui, Y.-C.; Li, L.-Y.; Zhu, Y.-X.; Xia, X. A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 2011, 234, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-C.; Feng, C.-C.; Yang, M.-M.; Li, J.-L.; Li, X.-X.; Zhao, B.-C.; Huang, Z.-J.; Ge, R.-C. Overexpression of the receptor-like protein kinase genes AtRPK1 and OsRPK1 reduces the salt tolerance of Arabidopsis thaliana. Plant Sci. 2014, 217–218, 63–70. [Google Scholar] [CrossRef]
- McCouch, S.R.; Wright, M.H.; Tung, C.-W.; Maron, L.G.; McNally, K.L.; Fitzgerald, M.; Singh, N.; DeClerck, G.; Agosto-Perez, F.; Korniliev, P.; et al. Open access resources for genome-wide association mapping in rice. Nat. Commun. 2016, 7, 10532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Zhang, M.; Niu, X.; Wang, S.; Xu, Q.; Feng, Y.; Wang, C.; Deng, H.; Yuan, X.; Yu, H.; et al. Genetic variation and association mapping for 12 agronomic traits in indica rice. BMC Genom. 2015, 16, 1067. [Google Scholar] [CrossRef] [Green Version]
- Burghardt, L.T.; Young, N.D.; Tiffin, P. A Guide to Genome-Wide Association Mapping in Plants. Curr. Protoc. Plant Biol. 2017, 2, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, B. Natural Variations and Genome-Wide Association Studies in Crop Plants. Annu. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef]
- Yadav, A.K.; Kumar, A.; Grover, N.; Ellur, R.K.; Bollinedi, H.; Krishnan, S.G.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Singh, A.K. Genome-Wide Association Study Reveals Marker–Trait Associations for Early Vegetative Stage Salinity Tolerance in Rice. Plants 2021, 10, 559. [Google Scholar] [CrossRef]
- Naveed, S.A.; Zhang, F.; Zhang, J.; Zheng, T.-Q.; Meng, L.-J.; Pang, Y.-L.; Xu, J.-L.; Li, Z.-K. Identification of QTN and candidate genes for Salinity Tolerance at the Germination and Seedling Stages in Rice by Genome-Wide Association Analyses. Sci. Rep. 2018, 8, 6505. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Zhang, F.; Zhou, Y. The Application of Multi-Locus GWAS for the Detection of Salt-Tolerance Loci in Rice. Front. Plant Sci. 2018, 9, 1464. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, K.; Zhao, X.; Wang, X.; Shen, C.; Zhu, Y.; Dai, M.; Qiu, X.; Yang, R.; Xing, D.; et al. Identification of genes for salt tolerance and yield-related traits in rice plants grown hydroponically and under saline field conditions by genome-wide association study. Rice 2019, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Norton, G.J.; Price, A.H. Genome-Wide Association Mapping for Salt Tolerance of Rice Seedlings Grown in Hydroponic and Soil Systems Using the Bengal and Assam Aus Panel. Front. Plant Sci. 2020, 11, 1633. [Google Scholar] [CrossRef]
- Rahman, M.A.; Thomson, M.J.; Shah-E-Alam, M.; De Ocampo, M.; Egdane, J.; Ismail, A.M. Exploring novel genetic sources of salinity tolerance in rice through molecular and physiological characterization. Ann. Bot. 2016, 117, 1083–1097. [Google Scholar] [CrossRef]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Oladosu, Y.; Kolapo, K.; Musa, I.; Halidu, J.; Muhammad, I.; Ahmed, M. Marker-Assisted Introgression of Multiple Resistance Genes Confers Broad Spectrum Resistance against Bacterial Leaf Blight and Blast Diseases in PUTRA-1 Rice Variety. Agronomy 2019, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.; Dreccer, F.; Trethowan, R. Drought-adaptive traits derived from wheat wild relatives and landraces. J. Exp. Bot. 2006, 58, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, N.N.; Krishnan, S.G.; Vinod, K.K.; Krishnamurthy, S.L.; Singh, V.K.; Singh, M.P.; Singh, R.; Ellur, R.K.; Rai, V.; Bollinedi, H.; et al. Marker aided incorporation of saltol, a major QTL associated with seedling stage salt tolerance, into Oryza sativa ‘pusa basmati 1121’. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Oladosu, Y.; Okporie, E.; Onyishi, G.; Utobo, E.; Ekwu, L.; Swaray, S.; et al. Marker-assisted selection and gene pyramiding for resistance to bacterial leaf blight disease of rice (Oryza sativa L.). Biotechnol. Biotechnol. Equip. 2019, 33, 440–455. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Sidhu, J.S.; Huang, N.; Vikal, Y.; Li, Z.; Brar, D.S.; Dhaliwal, H.S.; Khush, G.S. A Critical Review of Submergence Tolerance Breeding Beyond Sub 1 Gene to Mega Varieties in the Context of Climate Change. Int. J. Adv. Sci. Res. Eng. 2018, 102, 1011–1015. [Google Scholar] [CrossRef]
- Singh, R.; Singh, Y.; Xalaxo, S.; Verulkar, S.; Yadav, N.; Singh, S.; Singh, N.; Prasad, K.S.N.; Kondayya, K.; Rao, P.V.R.; et al. From QTL to variety-harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Sci. 2016, 242, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.R.; Kumar, B.M.D.; Krishnamurthy, S.L. Genetic variability and diversity of rice landraces of South Western India based on morphological traits. ORYZA 2014, 51, 261–266. [Google Scholar]
- Khan, M.S.K.; Saeed, M.; Iqbal, J. Quantitative trait locus mapping for salt tolerance at maturity stage in indica rice using replicated F2 population. Rev. Bras. Bot. 2016, 39, 641–650. [Google Scholar] [CrossRef]
- Khan, M.S.K.; Saeed, M.; Iqbal, J. Identification of quantitative trait loci for Na+, K+ and Ca++ accumulation traits in rice grown under saline conditions using F2 mapping population. Braz. J. Bot. 2015, 38, 555–565. [Google Scholar] [CrossRef]
- Koyama, M.L.; Levesley, A.; Koebner, R.M.D.; Flowers, T.J.; Yeo, A.R. Quantitative Trait Loci for Component Physiological Traits Determining Salt Tolerance in Rice. Plant Physiol. 2001, 125, 406–422. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, A.; Mithra, S.V.A.; Krishnamurthy, S.L.; Parida, S.K.; Jain, S.; Tiwari, K.K.; Kumar, P.; Rao, A.R.; Sharma, S.K.; et al. Genome-wide association mapping of salinity tolerance in rice (Oryza sativa). DNA Res. 2015, 22, 133–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurokawa, Y.; Noda, T.; Yamagata, Y.; Angeles-Shim, R.; Sunohara, H.; Uehara, K.; Furuta, T.; Nagai, K.; Jena, K.K.; Yasui, H.; et al. Construction of a versatile SNP array for pyramiding useful genes of rice. Plant Sci. 2016, 242, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molla, K.A.; Debnath, A.B.; Ganie, S.A.; Mondal, T.K. Identification and analysis of novel salt responsive candidate gene based SSRs (cgSSRs) from rice (Oryza sativa L.). BMC Plant Biol. 2015, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; SL, K.; Kumar, V.; Singh, B.; Rao, A.; Mithra SV, A.; Rai, V.; Singh, A.K.; Singh, N.K. Mapping QTLs for Salt Tolerance in Rice (Oryza sativa L.) by Bulked Segregant Analysis of Recombinant Inbred Lines Using 50K SNP Chip. PLoS ONE 2016, 11, e0153610. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Haque, T.; Rahman, M.S.; Seraj, Z. Salt Tolerant BR11 and Salt Tolerant BR28 through Marker Assisted Backcrossing (MAB). In Proceedings of the International Scientific Conference on “50 Years of Biochemis-Crop Breeding for Salt Tolerance in the Era of Molecular Markers 19 Try in Bangladesh: Successes and Prospects”; Bangladesh Society for Biochemistrsy and Molecular Biology: Dhaka, Banglades, 2008; Volume 50. [Google Scholar]
- Huyen, L.T.N.; Cuc, L.M.; Ismail, A.M.; Ham, L.H. Introgression the Salinity Tolerance QTLs Saltol into AS996, the Elite Rice Variety of Vietnam. Am. J. Plant Sci. 2012, 3, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Bimpong, I.K.; Manneh, B.; Sock, M.; Diaw, F.; Amoah, N.K.A.; Ismail, A.M.; Gregorio, G.; Singh, R.K.; Wopereis, M. Improving salt tolerance of lowland rice cultivar ‘Rassi’ through marker-aided backcross breeding in West Africa. Plant Sci. 2016, 242, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Gopalakrishnan, S.; Singh, V.P.; Prabhu, K.V.; Mohapatra, T.; Singh, N.K.; Sharma, T.R.; Nagarajan, M.; Ellur, R.K.; Singh, A.; et al. Marker assisted selection: A paradigm shift in Basmati breeding. Indian J. Genet. Plant Breed. 2011, 71, 120. [Google Scholar]
- Geetha, S.; Vasuki, A.; Selvam, P.J.; Saraswathi, R.; Krishnamurthy, S.L.; Dhasarathan, M.; Thamodharan, G.; Baskar, M. Development of sodicity tolerant rice varieties through marker assisted backcross breeding. Electron. J. Plant Breed. 2017, 8, 1013. [Google Scholar] [CrossRef]
- Shailani, A.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Stacking for future: Pyramiding genes to improve drought and salinity tolerance in rice. Physiol. Plant. 2021, 172, 1352–1362. [Google Scholar] [CrossRef]
- Muthu, V.; Abbai, R.; Nallathambi, J.; Rahman, H.; Ramasamy, S.; Kambale, R.; Thulasinathan, T.; Ayyenar, B.; Muthurajan, R. Pyramiding QTLs controlling tolerance against drought, salinity, and submergence in rice through marker assisted breeding. PLoS ONE 2020, 15, e0227421. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Ma, T.; Zhang, A.; Ong, K.H.; Li, Z.; Yang, J.; Yin, Z. Marker-assisted breeding of the rice restorer line Wanhui 6725 for disease resistance, submergence tolerance and aromatic fragrance. Rice 2016, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.K.; Nayak, D.K.; Mohanty, S.; Behera, L.; Barik, S.R. Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deepwater rice variety, Jalmagna. Rice 2015, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Transgenic approaches for abiotic stress tolerance in plants: Retrospect and prospects. Plant Cell Rep. 2008, 27, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-D.; Lin, K.-H.; Chen, C.-C.; Chiang, C.-M. Oryza sativa protein phosphatase 1a (OsPP1a) involved in salt stress tolerance in transgenic rice. Mol. Breed. 2016, 36, 22. [Google Scholar] [CrossRef]
- Amin, U.S.M.; Biswas, S.; Elias, S.M.; Razzaque, S.; Haque, T.; Malo, R.; Seraj, Z.I. Enhanced Salt Tolerance Conferred by the Complete 2.3 kb cDNA of the Rice Vacuolar Na+/H+ Antiporter Gene Compared to 1.9 kb Coding Region with 5′ UTR in Transgenic Lines of Rice. Front. Plant Sci. 2016, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Chen, Q.-J.; Niu, X.-G.; Zhang, R.; Lin, H.-Q.; Xu, C.-Y.; Wang, X.-C.; Wang, G.-Y.; Chen, J. Expression of OsNHX1 gene in maize confers salt tolerance and promotes plant growth in the field. Plant Soil Environ. 2008, 53, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-S.; Zhao, X.-Q.; Li, M.; Huang, L.-Y.; Xu, J.-L.; Zhang, F.; Cui, Y.-R.; Fu, B.-Y.; Li, Z.-K. Complex molecular mechanisms underlying seedling salt tolerance in rice revealed by comparative transcriptome and metabolomic profiling. J. Exp. Bot. 2016, 67, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Guo, L.; Guo, C.; Wang, L.; Chen, L. Over-expression of a DUF1644 protein gene, SIDP361, enhances tolerance to salt stress in transgenic rice. J. Plant Biol. 2016, 59, 62–73. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Ansari, M.W.; Tuteja, R.; Tuteja, N. OsSUV3 transgenic rice maintains higher endogenous levels of plant hormones that mitigates adverse effects of salinity and sustains crop productivity. Rice 2014, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Nath, M.; Yadav, S.; Kumar Sahoo, R.; Passricha, N.; Tuteja, R.; Tuteja, N. PDH45 transgenic rice maintain cell viability through lower accumulation of Na+, ROS and calcium homeostasis in roots under salinity stress. J. Plant Physiol. 2016, 191, 1–11. [Google Scholar] [CrossRef]
- Das, P.; Mishra, M.; Lakra, N.; Singla-Pareek, S.L.; Pareek, A. Mutation Breeding: A Powerful Approach for Obtaining Abiotic Stress Tolerant Crops and Upgrading Food Security for Human Nutrition. Mutagenesis: Exploring Novel Genes and Pathways; Wageningen Academic Publisher: Wageningen, The Netherlands, 2014. [Google Scholar]
- Hoang, T.; Tran, T.; Nguyen, T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Pental, D. When scientists turn against science: Exceptionally flawed analysis of plant breeding technologies. Curr. Sci. 2019, 117, 932–939. [Google Scholar] [CrossRef]
- Pythoud, F. The Cartagena protocol and GMOs. Nat. Biotechnol. 2004, 22, 1347–1348. [Google Scholar] [CrossRef]
- Fraiture, M.-A.; Roosens, N.H.C.; Taverniers, I.; De Loose, M.; Deforce, D.; Herman, P. Biotech rice: Current developments and future detection challenges in food and feed chain. Trends Food Sci. Technol. 2016, 52, 66–79. [Google Scholar] [CrossRef] [Green Version]
- Moin, M.; Bakshi, A.; Saha, A.; Dutta, M.; Kirti, P.B. Gain-of-function mutagenesis approaches in rice for functional genomics and improvement of crop productivity. Brief. Funct. Genom. 2017, 16, 238–247. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Da Luz, V.K.; da Silveira, S.F.; da Fonseca, G.M.; Groli, E.L.; Figueiredo, R.G.; Baretta, D.; Kopp, M.M.; de Magalhães Junior, A.M.; da Maia, L.C.; de Oliveira, A.C. Identification of variability for agronomically important traits in rice mutant families. Bragantia 2016, 75, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Serrat, X.; Esteban, R.; Guibourt, N.; Moysset, L.; Nogués, S.; Lalanne, E. EMS mutagenesis in mature seed-derived rice calli as a new method for rapidly obtaining TILLING mutant populations. Plant Methods 2014, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Parry, M.A.J.; Madgwick, P.J.; Bayon, C.; Tearall, K.; Hernandez-Lopez, A.; Baudo, M.; Rakszegi, M.; Hamada, W.; Al-Yassin, A.; Ouabbou, H.; et al. Mutation discovery for crop improvement. J. Exp. Bot. 2009, 60, 2817–2825. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-L.; Wu, C.; Lei, C.; Baraoidan, M.; Bordeos, A.; Madamba, M.R.S.; Ramos-Pamplona, M.; Mauleon, R.; Portugal, A.; Ulat, V.J.; et al. Chemical- and Irradiation-induced Mutants of Indica Rice IR64 for Forward and Reverse Genetics. Plant Mol. Biol. 2005, 59, 85–97. [Google Scholar] [CrossRef]
- Kharkwal, M.C.; Shu, Q.Y. The role of induced mutations in world food security. In Induced Plant Mutations in the Genomics Era; FAO: Rome, Italy, 2009; pp. 33–38. [Google Scholar]
- Ahloowalia, B.S.; Maluszynski, M. Induced mutations in plant breeding. Euphytica 2001, 118, 167–173. [Google Scholar] [CrossRef]
- Ahloowalia, B.S.; Maluszynski, M.; Nichterlein, K. Global impact of mutation-derived varieties. Euphytica 2004, 135, 187–204. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takehisa, H.; Kazama, Y.; Ichida, H.; Ryuto, H.; Fukunishi, N.; Abe, T.; Kamba, C.; Sato, T. Effects of ion beam irradiation on mutation induction in rice. In Proceedings of the 18th International Conference on Cyclotrons and Their Applications (CYCLOTRONS 2007), Messina, Italy, 1–5 October 2007; pp. 237–239. [Google Scholar]
- Nakhoda, B.; Leung, H.; Mendioro, M.S.; Mohammadi-nejad, G.; Ismail, A.M. Isolation, characterization, and field evaluation of rice (Oryza sativa L., Var. IR64) mutants with altered responses to salt stress. Field Crop. Res. 2012, 127, 191–202. [Google Scholar] [CrossRef]
- Sathish, P.; Gamborg, O.L.; Nabors, M.W. Establishment of stable NaCl-resistant rice plant lines from anther culture: Distribution pattern of K+/Na+ in callus and plant cells. Theor. Appl. Genet. 1997, 95, 1203–1209. [Google Scholar] [CrossRef]
- Lin, K.-C.; Jwo, W.-S.; Chandrika, N.N.P.; Wu, T.-M.; Lai, M.-H.; Wang, C.-S.; Hong, C.-Y. A rice mutant defective in antioxidant-defense system and sodium homeostasis possesses increased sensitivity to salt stress. Biol. Plant. 2016, 60, 86–94. [Google Scholar] [CrossRef]
- Novak, F.J.; Brunner, H. Plant breeding: Induced mutation technology for crop improvement. IAEA Bull. 1992, 4, 25–33. [Google Scholar]
- Song, J.Y.; Kim, D.S.; Lee, M.C.; Lee, K.J.; Kim, J.B.; Kim, S.H.; Ha, B.K.; Yun, S.J.; Kang, S.Y. Physiological characterization of gamma-ray induced salt tolerant rice mutants. Aust. J. Crop Sci. 2012, 6, 421–429. [Google Scholar]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Understanding salinity responses and adopting ‘omics-based’ approaches to generate salinity tolerant cultivars of rice. Front. Plant Sci. 2015, 6, 712. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Gautam, R.K.; Singh, R.K.; Krishnamurthy, S.L.; Ali, S.; Sakthivel, K.; Iquebal, M.A.; Rai, A.; Kumar, D. Harmonizing technological advances in phenomics and genomics for enhanced salt tolerance in rice from a practical perspective. Rice 2019, 12, 89. [Google Scholar] [CrossRef]
- Huang, L.; Wu, D.; Zhang, G. Advances in studies on ion transporters involved in salt tolerance and breeding crop cultivars with high salt tolerance. J. Zhejiang Univ. B 2020, 21, 426–441. [Google Scholar] [CrossRef]
- Kamburova, V.S.; Nikitina, E.V.; Shermatov, S.E.; Buriev, Z.T.; Kumpatla, S.P.; Emani, C.; Abdurakhmonov, I.Y. Genome Editing in Plants: An Overview of Tools and Applications. Int. J. Agron. 2017, 2017, 7315351. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Gao, C.; Qiu, J.-L. Progress and prospects in plant genome editing. Nat. Plants 2017, 3, 17107. [Google Scholar] [CrossRef] [PubMed]
- Zischewski, J.; Fischer, R.; Bortesi, L. Detection of on-target and off-target mutations generated by CRISPR/Cas9 and other sequence-specific nucleases. Biotechnol. Adv. 2017, 35, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Deng, Z.; Sun, Y. An insight into the protospacer adjacent motif of Streptococcus pyogenes Cas9 with artificially stimulated RNA-guided-Cas9 DNA cleavage flexibility. RSC Adv. 2016, 6, 33514–33522. [Google Scholar] [CrossRef]
- Fiaz, S.; Ahmad, S.; Noor, M.; Wang, X.; Younas, A.; Riaz, A.; Riaz, A.; Ali, F. Applications of the CRISPR/Cas9 System for Rice Grain Quality Improvement: Perspectives and Opportunities. Int. J. Mol. Sci. 2019, 20, 888. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Masafumi, M.; Kaya, H.; Toki, S. Efficient targeted mutagenesis of rice and tobacco genomes using Cpf1 from Francisella novicida. Sci. Rep. 2016, 6, 38169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Chen, K.; Wang, X.; Wang, W.; Xu, J.; Ali, J.; Li, Z.; Roy, S.J. Simultaneous Improvement and Genetic Dissection of Salt Tolerance of Rice (Oryza sativa L.) by Designed QTL Pyramiding. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef]
- Marcos, M.; Sharifi, H.; Grattan, S.R.; Linquist, B.A. Spatio-temporal salinity dynamics and yield response of rice in water-seeded rice fields. Agric. Water Manag. 2018, 195, 37–46. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.-L.; Wang, D.; Gao, C. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-N.; Shi, Y.-F.; Zhang, X.-B.; Song, L.-X.; Feng, B.-H.; Wang, H.-M.; Xu, X.; Li, X.-H.; Guo, D.; Wu, J.-L. Single base substitution in OsCDC48 is responsible for premature senescence and death phenotype in rice. J. Integr. Plant Biol. 2016, 58, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Sun, Y.; Du, J.; Zhao, Y.; Xia, L. Generation of Targeted Point Mutations in Rice by a Modified CRISPR/Cas9 System. Mol. Plant 2017, 10, 526–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.; Joshi, R.K.; Zhao, K. Genome Editing in Rice: Recent Advances, Challenges, and Future Implications. Front. Plant Sci. 2018, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Tan Khang, D. Potential application and current achievements of CRISPR/Cas in rice. Ann. Biotechnol. 2018, 1, 1003. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X.; Du, W.; Zhao, Y.; Xia, L. Engineering Herbicide-Resistant Rice Plants through CRISPR/Cas9-Mediated Homologous Recombination of Acetolactate Synthase. Mol. Plant 2016, 9, 628–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; He, M.; Li, J.; Chen, L.; Huang, Z.; Zheng, S.; Zhu, L.; Ni, E.; Jiang, D.; Zhao, B.; et al. Development of Commercial Thermo-sensitive Genic Male Sterile Rice Accelerates Hybrid Rice Breeding Using the CRISPR/Cas9-mediated TMS5 Editing System. Sci. Rep. 2016, 6, 37395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieves-Cordones, M.; Mohamed, S.; Tanoi, K.; Kobayashi, N.I.; Takagi, K.; Vernet, A.; Guiderdoni, E.; Périn, C.; Sentenac, H.; Véry, A. Production of low-Cs + rice plants by inactivation of the K + transporter Os HAK 1 with the CRISPR-Cas system. Plant J. 2017, 92, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Shao, G.; Xie, L.; Jiao, G.; Wei, X.; Sheng, Z.; Tang, S.; Hu, P. CRISPR/CAS9-mediated editing of the fragrant gene Badh2 in rice. Chin. J. Rice Sci. 2017, 3, 216–222. [Google Scholar]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Wen, J.; Zhao, W.; Wang, Q.; Huang, W. Rational Improvement of Rice Yield and Cold Tolerance by Editing the Three Genes OsPIN5b, GS3, and OsMYB30 With the CRISPR–Cas9 System. Front. Plant Sci. 2020, 10, 1663. [Google Scholar] [CrossRef] [Green Version]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Tak, Y.G.; Farnham, P.J. Making sense of GWAS: Using epigenomics and genome engineering to understand the functional relevance of SNPs in non-coding regions of the human genome. Epigenet. Chromatin 2015, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druka, A.; Potokina, E.; Luo, Z.; Jiang, N.; Chen, X.; Kearsey, M.; Waugh, R. Expression quantitative trait loci analysis in plants. Plant Biotechnol. J. 2010, 8, 10–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, L.H. The performance of salt resistant paddy, Pokkali in Ceylon. Trop. Agric. 1949, 105, 124–126. [Google Scholar]
- Qin, H.; Li, Y.; Huang, R. Advances and Challenges in the Breeding of Salt-Tolerant Rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.U.; Hernandez, R.; Naher, F. Adoption of Stress-Tolerant Rice Varieties in Bangladesh. In Technological and Institutional Innovations for Marginalized Smallholders in Agricultural Development; Springer International Publishing: Cham, Switzerland, 2016; pp. 241–255. [Google Scholar]
- Sun, M.F.; Yan, G.H.; Wang, A.M.; Zhu, G.Y.; Tang, H.S.; He, C.X.; Ren, Z.L.; Liu, K.; Zhang, G.Y.; Shi, W.; et al. Research progress on the breeding of salt-tolerant rice varieties. Barley Cereal. Sci. 2017, 34, 1–9. [Google Scholar]
- Das, G.; Rao, G.J.N.; Varier, M.; Prakash, A.; Prasad, D. Improved Tapaswini having four BB resistance genes pyramided with six genes/QTLs, resistance/tolerance to biotic and abiotic stresses in rice. Sci. Rep. 2018, 8, 2413. [Google Scholar] [CrossRef] [Green Version]
- Thanasilungura, K.; Kranto, S.; Monkham, T.; Chankaew, S.; Sanitchon, J. Improvement of a RD6 Rice Variety for Blast Resistance and Salt Tolerance through Marker-Assisted Backcrossing. Agronomy 2020, 10, 1118. [Google Scholar] [CrossRef]
- Ho, V.T.; Thomson, M.J.; Ismail, A.M. Development of salt tolerant IR64 near isogenic lines through marker-assisted breeding. J. Crop Sci. Biotechnol. 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Yadav, A.K.; Kumar, A.; Grover, N.; Ellur, R.K.; Krishnan, S.G.; Bollinedi, H.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Krishnamurthy, S.L.; et al. Marker aided introgression of ‘Saltol’, a major QTL for seedling stage salinity tolerance into an elite Basmati rice variety ‘Pusa Basmati 1509’. Sci. Rep. 2020, 10, 13877. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, B.D.; Kumar, A.; Maurya, S.; Krishnan, S.G.; Vinod, K.K.; Singh, M.P.; Ellur, R.K.; Bhowmick, P.K.; Singh, A.K. Marker-Assisted Introgression of Saltol QTL Enhances Seedling Stage Salt Tolerance in the Rice Variety “Pusa Basmati 1”. Int. J. Genom. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamurthy, S.L.; Pundir, P.; Warraich, A.S.; Rathor, S.; Lokeshkumar, B.M.; Singh, N.K.; Sharma, P.C. Introgressed Saltol QTL Lines Improves the Salinity Tolerance in Rice at Seedling Stage. Front. Plant Sci. 2020, 11, 833. [Google Scholar] [CrossRef]
- Nair, M.M.; Shylaraj, K.S. Introgression of dual abiotic stress tolerance QTLs (Saltol QTL and Sub1 gene) into Rice (Oryza sativa L.) variety Aiswarya through marker assisted backcross breeding. Physiol. Mol. Biol. Plants 2021, 27, 497–514. [Google Scholar] [CrossRef]
- Valarmathi, M.; Sasikala, R.; Rahman, H.; Jagadeeshselvam, N.; Kambale, R.; Raveendran, M. Development of salinity tolerant version of a popular rice variety improved white ponni through marker assisted back cross breeding. Plant Physiol. Rep. 2019, 24, 262–271. [Google Scholar] [CrossRef]
- Mukherjee, R.; Mukherjee, A.; Bandyopadhyay, S.; Mukherjee, S.; Sengupta, S.; Ray, S.; Majumder, A.L. Selective manipulation of the inositol metabolic pathway for induction of salt-tolerance in indica rice variety. Sci. Rep. 2019, 9, 5358. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.W.; Rahman, Z.A.; Goh, H.H.; Hwang, D.J.; Ismail, I.; Zainal, Z. Production of transgenic rice (indica cv. MR219) overexpressing ABP57 gene through Agrobacterium-mediated transformation. Sains Malays 2017, 46, 703–711. [Google Scholar] [CrossRef]
| Improved Rice Genotype | Resistance Genes/QTLs/Traits | Breeding Methods | Country | References |
|---|---|---|---|---|
| Pokkali | Salt resistance | Conventional breeding | Sri Lanka | [179] |
| Nona Bokra, Chin13, Kala Rata, 349 Jhuma | Salinity tolerance | Conventional breeding | Philippines | [180] |
| BRI, BR203-26-2, BRRI dhan47, 53, 54, 55, and 61, Binadhan-8, Binadhan-10 | Salinity tolerance | Conventional breeding | Bangladesh | [100,181] |
| FL530 | Salinity tolerance | Conventional breeding | Thailand | [182] |
| Lansheng, Chikushiqing, Mantaro rice, Hama Minoru, Kanto 51 | Salinity tolerance | Conventional breeding | Japan | [180] |
| Dongjinbyeo, Ganchukbyeo, Gyehwabyeo, Ilpumbyeo, Seomjimbyeo, Nonganbyeo | Salinity tolerance | Conventional breeding | South Korea | [180] |
| VNIIR8207 and Fontan | Salinity tolerance | Conventional breeding | Russia | [180] |
| Changbai No. 6, 7, 9, 13, Jigeng No. 84, Sea Rice 86, Changmaogu | Salinity tolerance | Conventional breeding | China | [183] |
| Improved Tapaswini | Sub1, Saltol, Pi2, Pi9, Gm1, Gm4 | MABC * | India | [184] |
| RD6 Rice Variety | (qBl 1, 2, 11, and 12), Saltol | MABC | India | [185] |
| Improved IR64 | Saltol | MABC | India | [186] |
| Improved Pusa Basmati 1509 | Saltol | MABC | India | [187] |
| Improved Pusa Basmati 1 | Saltol | MABC | India | [188] |
| Improved Pusa44 and Sarjoo 52 | Saltol | MABC | India | [189] |
| Improved Aiswarya | Saltol, Sub1 | MABC | India | [190] |
| Improved PB1121 | Saltol | MABC | India | [103] |
| Improved bengali | Saltol or qSKC1 | MABC | India | [67] |
| Improved white ponni (IWP) | Saltol | MABC | India | [191] |
| Improved IWP | Saltol or qSKC1, qDTY1.1, qDTY2.1) | MABC | India | [121] |
| 7PcINO1, 4PcINO1 and 3PcINO1-lines | PcINO1, PcIMT1 | Transgenic approach | India | [192] |
| OsPP1a-2, OsPP1a-3, OsPP1a-6 transgenic lines | OsPP1a | Transgenic approach | India | [125] |
| Abp57-transgenic rice | Abp57 | Transgenic approach | Malaysia | [193] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice. Agronomy 2021, 11, 1631. https://doi.org/10.3390/agronomy11081631
Haque MA, Rafii MY, Yusoff MM, Ali NS, Yusuff O, Datta DR, Anisuzzaman M, Ikbal MF. Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice. Agronomy. 2021; 11(8):1631. https://doi.org/10.3390/agronomy11081631
Chicago/Turabian StyleHaque, Md Azadul, Mohd Y. Rafii, Martini Mohammad Yusoff, Nusaibah Syd Ali, Oladosu Yusuff, Debi Rani Datta, Mohammad Anisuzzaman, and Mohammad Ferdous Ikbal. 2021. "Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice" Agronomy 11, no. 8: 1631. https://doi.org/10.3390/agronomy11081631
APA StyleHaque, M. A., Rafii, M. Y., Yusoff, M. M., Ali, N. S., Yusuff, O., Datta, D. R., Anisuzzaman, M., & Ikbal, M. F. (2021). Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice. Agronomy, 11(8), 1631. https://doi.org/10.3390/agronomy11081631





