Purification Performance of Filtration Process for Pig Slurry Using Marine Sands, Silty Loam Soils, Fly Ash and Zeolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Experimental Device
2.2. Pig Slurry Sample Collection and Analysis
2.3. Characterization of the Filtration Materials
3. Results and Discussion
3.1. Physical Chemical Characterization of Raw Pig Slurry
3.2. Micronutrient Results in Raw and Filtered Pig Slurry
3.3. Heavy Metal Reduction
3.4. Macronutrient Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rieu, M. Économie et Avenir de la Filière Porcine. Prod. Anim. 2003, 16, 341–348. [Google Scholar]
- Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31991L0676 (accessed on 1 August 2021).
- EUROSTAT Livestock Population in Numbers. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20200923-1 (accessed on 10 August 2021).
- Provolo, G.; Manuli, G.; Finzi, A.; Lucchini, G.; Riva, E.; Sacchi, G.A. Effect of Pig and Cattle Slurry Application on Heavy Metal Composition of Maize Grown on Different Soils. Sustainability 2018, 10, 2684. [Google Scholar] [CrossRef]
- Terrero, M.A.; Muñoz, M.; Faz, Á.; Gómez-López, M.D.; Acosta, J.A. Efficiency of an Integrated Purification System for Pig Slurry Treatment under Mediterranean Climate. Agronomy 2020, 10, 208. [Google Scholar] [CrossRef]
- Iordache, M.; Iordache, A.M.; Sandru, C.; Voica, C.; Zgavarogea, R.; Miricioiu, M.G.; Ionete, R.E. Assessment of Heavy Metals Pollution in Sediments from Reservoirs of the Olt River as Tool for Environmental Risk Management. Rev. Chim. 2020, 70, 4153–4162. [Google Scholar] [CrossRef]
- Petersen, S.; Sommer, S.; Béline, F.; Burton, C.; Dach, J.; Dourmad, J.; Leip, A.; Misselbrook, T.; Nicholson, F.; Poulsen, H.; et al. Recycling of livestock manure in a whole-farm perspective. Livest. Sci. 2007, 112, 180–191. [Google Scholar] [CrossRef]
- Lecomte, T.; Ferrería de la Fuente, J.F.; Neuwahl, F.; Canova, M.; Pinasseau, A.; Jankov, I.; Brinkmann Serge Roudier, T.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for Large Combustion Plants; Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); EUR 28836 EN; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-74303-0. [Google Scholar] [CrossRef]
- Huang, X.-F.; Ye, G.-Y.; Yi, N.-K.; Lu, L.-J.; Zhang, L.; Yang, L.-Y.; Xiao, L.; Liu, J. Effect of plant physiological characteristics on the removal of conventional and emerging pollutants from aquaculture wastewater by constructed wetlands. Ecol. Eng. 2019, 135, 45–53. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Niculescu, V.-C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Miricioiu, M.; Niculescu, V.-C.; Filote, C.; Raboaca, M.; Nechifor, G. Coal Fly Ash Derived Silica Nanomaterial for MMMs—Application in CO2/CH4 Separation. Membranes 2021, 11, 78. [Google Scholar] [CrossRef]
- De Matos, M.P.; Von Sperling, M.; Matos, A. Clogging in horizontal subsurface flow constructed wetlands: Influencing factors, research methods and remediation techniques. Rev. Environ. Sci. Biotechnol. 2018, 17, 87–107. [Google Scholar] [CrossRef]
- Vymazal, J. Does clogging affect long-term removal of organics and suspended solids in gravel-based horizontal subsurface flow constructed wetlands? Chem. Eng. J. 2018, 331, 663–674. [Google Scholar] [CrossRef]
- Verma, S.; Daverey, A.; Sharma, A. Slow sand filtration for water and wastewater treatment—A review. Environ. Technol. Rev. 2017, 6, 47–58. [Google Scholar] [CrossRef]
- Sanft, R.; Walter, A. Differential Equations: Model Formulation, Nonlinear Regression, and Model Selection. In Exploring Mathematical Modeling in Biology through Case Studies and Experimental Activities; Sanft, R., Walter, A., Eds.; Elsevier: Berkeley, CA, USA, 2020; pp. 87–146. ISBN 9780128195956. [Google Scholar]
- Duchaufour, P. Precis de Pedologie; Masson: Paris, France, 1970. [Google Scholar]
- Ncube, P.; Pidou, M.; Stephenson, T.; Jefferson, B.; Jarvis, P. Consequences of pH change on wastewater depth filtration using a multimedia filter. Water Res. 2017, 128, 111–119. [Google Scholar] [CrossRef]
- Available online: https://lafontaine.groupe-esa.com/index.php?lvl=notice_display&id=134773 (accessed on 1 August 2021).
- Kim, K.H.; Yoon, S.Y.; Park, H.C. Recycling of Coal Fly Ash for the Fabrication of Porous Mullite/Alumina Composites. Materials 2014, 7, 5982–5991. [Google Scholar] [CrossRef] [PubMed]
- ASTM American Society for Testing and Materials (ASTM) International Standars. Available online: https://www.astm.org/Standards/water-testing-standards.html (accessed on 10 August 2021).
- Rinaldi, R. Mineralogy of Natural Zeolites: Present Status—Zeolite Mineralogy. In Proceedings of the Sixth International Zeolite Conference; Elsevier: Reno, NV, USA, 1983; pp. 570–583. [Google Scholar]
- Available online: https://www.sciencedirect.com/science/article/pii/B9780080977744005064 (accessed on 1 August 2021).
- Wolinskagrabczyk, A.; Jankowski, A. Membranes for vapour permeation. Pervaporation Vap. Permeat. Membr. Distill. 2015, 10, 145–175. [Google Scholar] [CrossRef]
- Boursier, H. Piggery wastewater characterisation for biological nitrogen removal process design. Bioresour. Technol. 2005, 96, 351–358. [Google Scholar] [CrossRef]
- Antezana, W.; Calvet, S.; Beccaccia, A.; Ferrer, P.; De Blas, C.; García-Rebollar, P.; Cerisuelo, A. Effects of nutrition on digestion efficiency and gaseous emissions from slurry in growing pigs: III. Influence of varying the dietary level of calcium soap of palm fatty acids distillate with or without orange pulp smentation. Anim. Feed. Sci. Technol. 2015, 209, 128–136. [Google Scholar] [CrossRef]
- Provolo, G.; Martínez-Suller, L. In situ determination of slurry nutrient content by electrical conductivity. Bioresour. Technol. 2007, 98, 3235–3242. [Google Scholar] [CrossRef]
- Yagüe, M.R.; Quilez, D. On-farm Measurement of Electrical Conductivity for the Estimation of Ammonium Nitrogen Concentration in Pig Slurry. J. Environ. Qual. 2012, 41, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; González, J.L. The fertilizer value of pig slurry. I. Values depending on the type of operation. Bioresour. Technol. 2005, 40, 1117–1123. [Google Scholar] [CrossRef]
- Villamar, C.A.; Cañuta, T.; Belmonte, M.; Vidal, G. Characterization of Swine Wastewater by Toxicity Identification Evaluation Methodology (TIE). Water Air Soil Pollut. 2012, 223, 363–369. [Google Scholar] [CrossRef]
- Sehar, S.; Naz, I. Role of the Biofilms in Wastewater Treatment. In Microbial Biofilms—Importance and Applications; Elsevier: Berkeley, CA, USA, 2016; pp. 122–140. [Google Scholar]
- El Haouti, R.; Azougarh, Y.; Et-Taleb, S.; Aba-Aaki, R.; Abbaz, M.; Lhanafi, S.; Ez-Zahery, M.; El Alem, N. Treatment of organic pollution of domestic wastewater by infiltration-percolation process on two types of titaniferous sands: Gross and sieved|Traitement de la pollution organique des eaux us?es domestiques par le proc?d? d’infiltration-percolation sur. J. Mater. Environ. Sci. 2016, 7, 59–66. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Alkaline activation of different aluminosilicates as an alternative to Portland cement: Alkali activated cements or geopolymers. Rev. Ing. Construcción 2017, 32, 5–12. [Google Scholar] [CrossRef]
- Available online: https://www.scirp.org/reference/referencespapers.aspx?referenceid=2160443 (accessed on 1 August 2021).
- Available online: https://manualzz.com/doc/5136279/etude-des-lixiviats-de-d%C3%A9charges (accessed on 1 August 2021).
- Noyes, A.A.; Melcher, A.C.; Cooper, H.C.; Eastman, G.W.; Kato, Y. The conductivity and ionization of salts, acids, and bases in aqueous solutions at high temperatures. J. Am. Chem. Soc. 1908, 30, 335–353. [Google Scholar] [CrossRef][Green Version]
- Hery, S.; Endro, Y. Rancang bangun alat penjernih air limbah cair laundry dengan menggunakan media penyaring kombinasi pasir-arang aktif. J. Neutrino 2014, 6, 84–90. [Google Scholar]
- Achak, M.; Ouazzani, N.; Mandi, L. Organic pollutants removal from olive mill wastewater by a combined system of a sand filter and an aquatic plant system. Rev. des Sci. l´eau 2010, 35–51. [Google Scholar] [CrossRef]
- Ahmad, J.; El-Dessouky, H. Design of a modified low cost treatment system for the recycling and reuse of laundry waste water. Resour. Conserv. Recycl. 2008, 52, 973–978. [Google Scholar] [CrossRef]
- Available online: http://www.fndae.fr/documentation/PDF/fndae25.pdf (accessed on 1 August 2021).
- Biod, C.D.E.L.A.; Limites, G.; La, F.; Biologique, D.; Chimique, D.; Biod, V.D.E.L.A.; Limitant, F.; Biod, L.A. La Biodégradabilité des Effluents Urbains. Available online: http://gls.fr/wp-content/uploads/Memotec_19.pdf (accessed on 10 August 2021).
- EPA United States Environmental Protection Agency. Available online: https://iaspub.epa.gov/tdb/pages/treatment/treatmentOverview.do?treatmentProcessId=1934681921 (accessed on 10 August 2021).
- CPW Climate Policy Watcher. Available online: https://www.climate-policy-watcher.org/nitrogen-removal/the-influence-of-ph-on-the-nitrification-rate.html (accessed on 10 August 2021).
- Deschamps, T.; Benzaazoua, M.; Bussière, B.; Belem, T.; Mbonimpa, M. Mécanismes de rétention des métaux lourds en phase solide: Cas de la stabilisation des sols contaminés et des déchets industriels. VertigO 2006. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Son, D.-H.; Ahn, D.-H. Nitrogen and organics removal from industrial wastewater using natural zeolite media. Water Sci. Technol. 2000, 42, 127–134. [Google Scholar] [CrossRef]
- Bourg, A.C. Chemistry and Biology of Solid Waste; Salomons, W., Förstner, U., Eds.; Springer: New York, NY, USA, 1988. [Google Scholar]
- Pitcher, S.; Slade, R.; Ward, N. Heavy metal removal from motorway stormwater using zeolites. Sci. Total Environ. 2004, 334–335, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Bureau d’Etudes Industrielles Energies Renouvelables et Environnement. Available online: http://hmf.enseeiht.fr/travaux/bei/beiere/content/2015/dephosphatation-chimique (accessed on 10 August 2021).
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Guzman, L.; Gunawan, G.; Viswanathan, T. A Comprehensive Review of Phosphorus Removal Technologies and Processes. J. Macromol. Sci. Part A 2014, 51, 538–545. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Oram, B. Hard Water Hardness Calcium Magnesium Water Corrosion Mineral Scale. Available online: https://water-research.net/index.php/water-treatment/tools/hard-water-hardness (accessed on 10 August 2021).
- White, C.; Santangelo, N. Can I Apply Potash in Winter? Peen State Extension. Available online: https://extension.psu.edu/can-i-apply-potash-in-winter#:~:text=Highorganic%2Cmucksoilsand,tightlyandcanbestored (accessed on 10 August 2021).
- Sabadash, V.; Gumnitsky, J.; Hyvlyud, A. Mechanism of Phosphates Sorption by Zeolites Depending on Degree of their Substitution for Potassium Ions. Chem. Chem. Technol. 2016, 10, 235–240. [Google Scholar] [CrossRef]
- Zhao, H.; Vance, G.F.; Ganjegunte, G.K.; Urynowicz, M.A. Use of zeolites for treating natural gas co-produced waters in Wyoming, USA. Desalination 2008, 228, 263–276. [Google Scholar] [CrossRef]
- Santiago, O.; Walsh, K.; Kele, B.; Gardner, E.; Chapman, J. Novel pre-treatment of zeolite materials for the removal of sodium ions: Potential materials for coal seam gas co-produced wastewater. SpringerPlus 2016, 5, 571. [Google Scholar] [CrossRef]
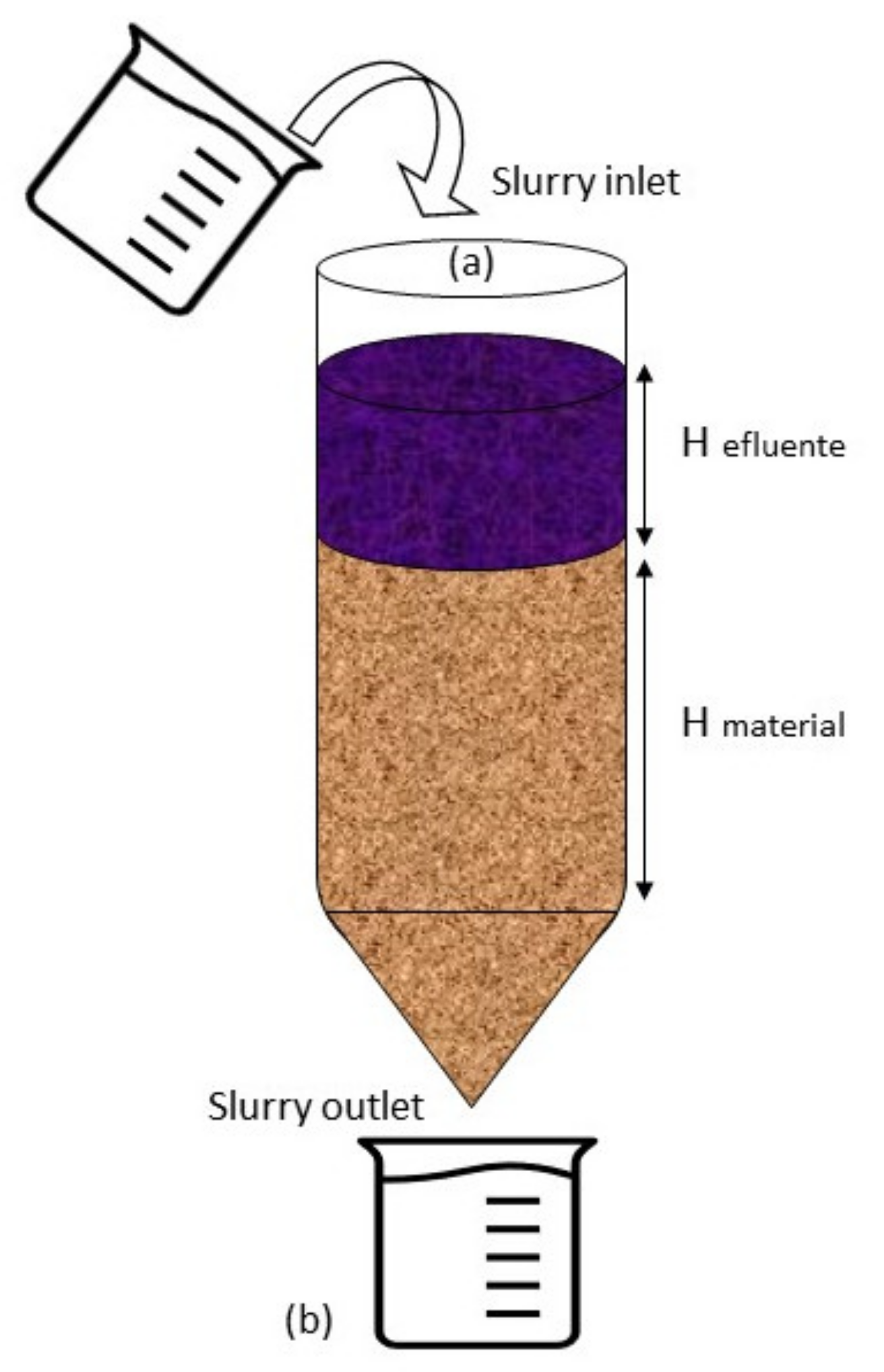

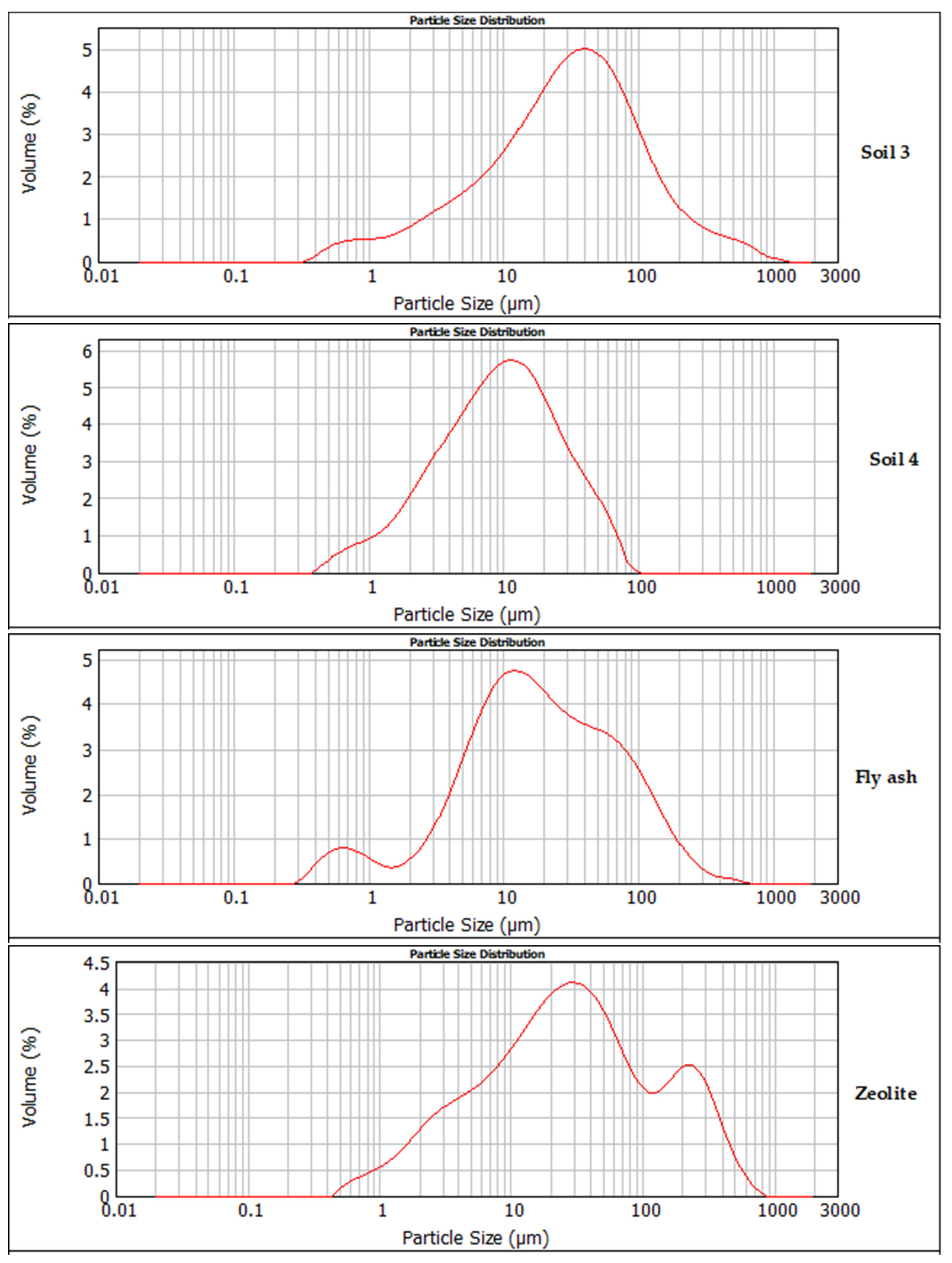
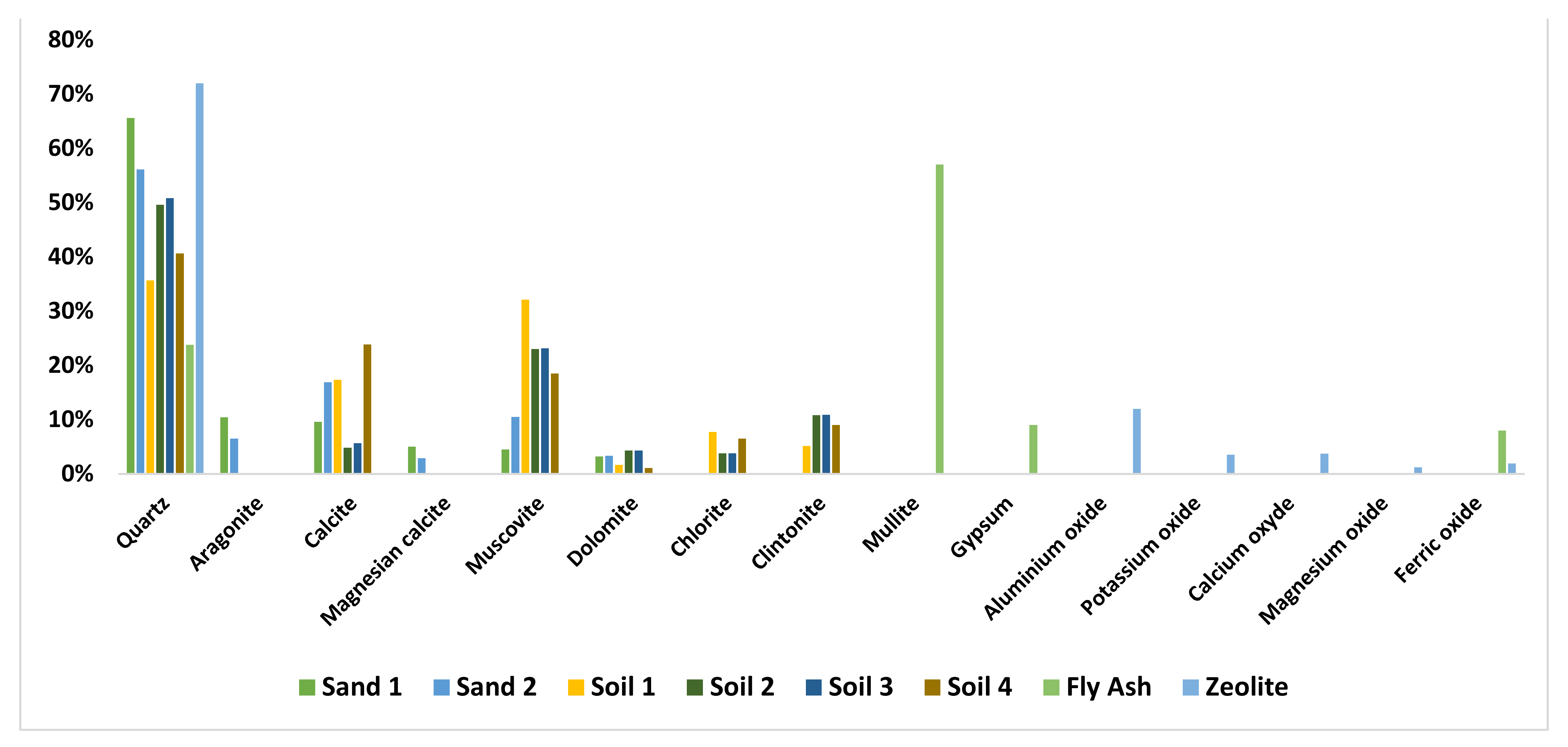
| Material | Sand 1 | Sand 2 | Soil 1 | Soil 2 | Soil 3 | Soil 4 | Fly Ash | Zeolite |
|---|---|---|---|---|---|---|---|---|
| pH | 9.69 | 9.52 | 8.60 | 9.10 | 8.46 | 8.42 | 12.10 | 9.02 |
| EC (ms cm−1) | 0.10 | 0.35 | 1.61 | 0.46 | 2.29 | 0.39 | 6.30 | 0.30 |
| Compounds | Sand 1 | Sand 2 | Soil 1 | Soil 2 | Soil 3 | Soil 4 | Fly Ash | Zeolite |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 34.5 | 32.9 | 37.3 | 48.9 | 48.6 | 41.1 | 40.9 | 64.7 |
| Al2O3 | 1.36 | 2.34 | 12.5 | 17.5 | 18.1 | 14.9 | 26.5 | 12 |
| Fe2O3 | 2.79 | 9.18 | 5.23 | 8.55 | 8.27 | 6.68 | 18.9 | 1.43 |
| CaO | 34.5 | 31.2 | 19.8 | 5.89 | 5.69 | 14.5 | 6.01 | 4.12 |
| MgO | 1.64 | 1.27 | 2.72 | 2.59 | 2.81 | 1.47 | 0.94 | 0.94 |
| SO3 | 0.23 | 0.22 | 0.14 | 0.24 | 0.40 | 0.25 | 1.74 | 0.04 |
| K2O | 0.30 | 0.52 | 2.81 | 0.06 | 5.16 | 3.10 | 2.04 | 2.65 |
| pH | EC (ms cm−1) | TSS (g L−1) | NK (g L−1) | N−NH4 + (g L−1) | Turbidity (NTU) | COD (g L−1) | BOD5 (g L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS | 7.46–7.65 | 7.75 | ±0.14 | 81 | ±1.93 | 0.62 | ±0.11 | 0.52 | ±0.07 | 2000 | ±75.5 | 5.19 | ±0.47 | 4.09 | ±0.38 |
| Sand 1 | 8.29–9.28 | 7.18 | ±0.11 | 3 | ±0.14 | 0.47 | ±0.02 | 0.42 | ±0.02 | 300 | ±8.66 | 1.3 | ±0.15 | 0.8 | ±0.05 |
| % reduction | 7% | 97% | 25% | 19% | 85% | 74% | 80% | ||||||||
| Sand 2 | 8.85–9.10 | 5.2 | ±0.25 | 6 | ±0.08 | 0.47 | ±0.08 | 0.28 | ±0.03 | 350 | ±22.27 | 1.09 | ±0.09 | 1.04 | ±0.20 |
| % reduction | 33% | 93% | 25% | 46% | 83% | 80% | 75% | ||||||||
| Soil 1 | 7.60–8.20 | 3.69 | ±0.06 | 2 | ±0.05 | 0.07 | ±0.00 | 0.05 | ±0.00 | 180 | ±4.63 | 0.98 | ±0.05 | 0.81 | ±0.03 |
| % reduction | 52% | 98% | 89% | 90% | 91% | 80% | 80% | ||||||||
| Soil 2 | 8.10–8.80 | 16.09 | ±0.11 | 5 | ±0.14 | 0.063 | ±0.01 | 0.03 | ±0.00 | 175 | ±3.06 | 1.07 | ±0.13 | 0.52 | ±0.04 |
| % reduction | −108% | 94% | 90% | 94% | 91% | 80% | 88% | ||||||||
| Soil 3 | 8.11–8.81 | 6.95 | ±0.03 | 7 | ±0.15 | 0.05 | ±0.00 | 0.04 | ±0.00 | 160 | ±8.62 | 1.42 | ±0.08 | 0.64 | ±0.05 |
| % reduction | 10% | 91% | 91% | 93% | 92% | 72% | 85% | ||||||||
| Soil 4 | 8.00–8.67 | 9.44 | ±0.25 | 10 | ±0.15 | 0.06 | ±0.01 | 0.02 | ±0.00 | 195 | ±3.79 | 1.44 | ±0.20 | 0.71 | ±0.05 |
| % reduction | −22% | 88% | 90% | 96% | 90% | 72% | 82% | ||||||||
| Fly Ash | 11.60–12.40 | 9.44 | ±0.18 | 0 | ±0.00 | 0.42 | ±0.02 | 0.2 | ±0.01 | 100 | ±11.55 | 1.06 | ±0.06 | 0.11 | ±0.01 |
| % reduction | −22% | 99.99% | 33% | 61% | 95% | 80% | 98% | ||||||||
| Zeolite | 7.40–7.80 | 2.73 | ±0.07 | 0 | ±0.00 | 0.01 | ±0.00 | 0 | ±0.00 | 120 | ±11.02 | 0.52 | ±0.05 | 0.1 | ±0.00 |
| % reduction | 65% | 99.99% | 98% | 99.99% | 94% | 90% | 99.99% | ||||||||
| CW | 7.38–7.5 | 6.65 | ±0.03 | 7 | ±0.12 | 0.4 | ±0.06 | 0.25 | ±0.03 | 400 | ±11.55 | 1.01 | ±0.06 | 1.51 | ±0.15 |
| % reduction | 14% | 91% | 36% | 52% | 80% | 80% | 63% | ||||||||
| Mn (μg L−1) | Cu (μg L−1) | Zn (μg L−1) | Fe (μg L−1) | |||||
|---|---|---|---|---|---|---|---|---|
| PS | 190 | ±0.00 | 106 | ±0.01 | 451 | ±0.01 | 783 | ±0.01 |
| Sand 1 | 23 | ±0.00 | 56 | ±0.01 | BDL * | ±0.00 | BDL * | ±0.00 |
| % reduction | 87.74% | 46.60% | 99.99% | 99.99% | ||||
| Sand 2 | 28 | ±0.00 | 333 | ±0.12 | BDL * | ±0.00 | BDL * | ±0.00 |
| % reduction | 85.11% | -214.15% | 99.99% | 99.99% | ||||
| Soil 1 | 31 | ±0.00 | 63 | ±0.01 | BDL * | ±0.00 | BDL * | ±0.00 |
| % reduction | 84.21% | 40.28% | 99.99% | 99.99% | ||||
| Soil 2 | 9 | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 |
| % reduction | 95.26% | 99.99% | 99.99% | 99.99% | ||||
| Soil 3 | 173 | ±0.00 | 13 | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 |
| % reduction | 8.95% | 87.45% | 99.99% | 99.99% | ||||
| Soil 4 | 171 | ±0.02 | BDL * | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 |
| % reduction | 10.53% | 99.99% | 99.99% | 99.99% | ||||
| Fly Ash | 11 | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 | 213 | ±0.00 |
| % reduction | 94.74% | 99.99% | 99.99% | 72.80% | ||||
| Zeolite | BDL * | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 | 9 | ±0.00 |
| % reduction | 99.99% | 99.99% | 99.99% | 98.85% | ||||
| CW | 601 | ±0.02 | 40 | ±0.00 | 11 | ±0.00 | 11 | ±0.00 |
| % reduction | −215.79% | 62.26% | 97.78% | 98.72% | ||||
| Pb (μg L−1) | Co (μg L−1) | Ni (μg L−1) | Cr (μg L−1) | Al (μg L−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PS | 1 | ±0.00 | 1 | ±0.00 | 11 | ±0.00 | 0.8 | ±0.00 | 38 | ±0.00 |
| Sand 1 | 9 | ±0.00 | 10 | ±0.00 | BDL * | ±0.00 | 0.9 | ±0.00 | BDL * | ±0.00 |
| % reduction | −727% | −550% | 99.99% | −13% | 99.99% | |||||
| Sand 2 | 9 | ±0.00 | 9 | ±0.00 | 60 | ±0.00 | 0.9 | ±0.00 | BDL * | ±0.00 |
| % reduction | −718% | −542% | −440% | −12.50% | 99.99% | |||||
| Soil 1 | BDL * | ±0.00 | BDL * | ±0.00 | 14 | ±0.00 | 1.8 | ±0.00 | BDL * | ±0.00 |
| % reduction | 99.99% | 99.99% | −26.13% | −125% | 99.99% | |||||
| Soil 2 | BDL * | ±0.00 | BDL * | ±0.00 | 4 | ±0.00 | 1.1 | ±0.00 | BDL * | ±0.00 |
| % reduction | 99.99% | 99.99% | 65.77% | −37.50% | 99.99% | |||||
| Soil 3 | BDL * | ±0.00 | BDL * | ±0.00 | 7 | ±0.00 | 0.9 | ±0.00 | BDL * | ±0.00 |
| % reduction | 99.99% | 99.99% | 32.16% | −12.50% | 99.99% | |||||
| Soil 4 | BDL * | ±0.00 | BDL * | ±0.00 | 20 | ±0.00 | 1.8 | ±0.00 | BDL * | ±0.00 |
| % reduction | 99.99% | 99.99% | −87.39% | −125% | 99.99% | |||||
| Fly Ash | BDL * | ±0.00 | BDL * | 0.00 | BDL * | ±0.00 | 4.8 | ±0.00 | BDL * | ±0.00 |
| % reduction | 99.99% | 99.99% | 99.99% | −500% | 99.99% | |||||
| Zeolite | BDL * | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 | BDL * | ±0.00 |
| % reduction | 99.99% | 99.99% | 99.99% | 99.99% | 99.99% | |||||
| CW | BDL * | ±0.00 | BDL * | ±0.00 | 40 | ±0.00 | 3 | ±0.00 | 10 | ±0.00 |
| % reduction | 99.99% | 99.99% | −266% | −325% | 73.75% | |||||
| N (mg L−1) | Na+ (mg L−1) | K + (mg L−1) | Ca2+ (mg L−1) | Mg2+ (mg L−1) | P (mg L−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS | 150 | ±0.6 | 120 | ±0.3 | 125 | ±1.1 | 105 | ±1.7 | 104 | ±1.2 | 20.11 | ±0.0 |
| Sand 1 | 136 | ±0.3 | 120 | ±0.3 | 119 | ±0.6 | 40.5 | ±1.6 | 105 | ±0.1 | 1.25 | ±0.0 |
| % reduction | 9% | −1% | 5% | 61% | −1% | 94% | ||||||
| Sand 2 | 136 | ±0.6 | 120 | ±0.6 | 120 | ±1.7 | 33.67 | ±1.2 | 105 | ±0.1 | 1.73 | ±0.0 |
| % reduction | 9% | 0% | 4% | 67.94% | −1% | 91% | ||||||
| Soil 1 | 74 | ±0.6 | 106 | ±0.6 | 98 | ±2.7 | 129 | ±2.0 | 120 | ±0.1 | 0.31 | ±0.0 |
| % reduction | 51% | 11% | 21.33% | −22.86% | −15% | 98% | ||||||
| Soil 2 | 74 | ±0.3 | 195 | ±0.6 | 85 | ±1.1 | 120 | ±0.0 | 102 | ±0.7 | 0 | ±0.0 |
| % reduction | 64% | −63% | 32.05% | −14.29% | 2% | 99.99% | ||||||
| Soil 3 | 64 | ±0.3 | 260 | ±0.5 | 108 | ±1.5 | 180 | ±0.3 | 173 | ±0.2 | 0 | ±0.0 |
| % reduction | 58% | −117% | 13.60% | −71.43% | −66% | 99.99% | ||||||
| Soil 4 | 58 | ±0.2 | 290 | ±0.6 | 107 | ±1.1 | 183 | ±0.1 | 225 | ±0.0 | 0.27 | ±0.0 |
| % reduction | 61% | −142% | 14.40% | −74.29% | −116% | 99% | ||||||
| Fly Ash | 92 | ±0.0 | 150 | ±0.0 | 170 | ±1.1 | 175 | ±0.5 | 11 | ±0.0 | 0 | ±0.0 |
| % reduction | 39% | −25% | −36% | −66.98% | 89% | 99.99% | ||||||
| Zeolite | 38 | ±0.3 | 70 | ±1.1 | 40 | ±0.9 | 132 | ±0.3 | 101 | ±0.3 | 0.17 | ±0.0 |
| % reduction | 75% | 42% | 67.73% | −26.35% | 3% | 99% | ||||||
| CW | 105 | ±0.6 | 134 | ±0.3 | 132 | ±0.9 | 24 | ±0.1 | 30.03 | ±0.1 | 1.08 | ±0.0 |
| % reduction | 30% | −12% | −6.13% | 77.02% | 71% | 95% | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El bied, O.; García-Valero, A.; Fechtali, T.; Faz, Á.; Acosta, J.A. Purification Performance of Filtration Process for Pig Slurry Using Marine Sands, Silty Loam Soils, Fly Ash and Zeolite. Agronomy 2021, 11, 1608. https://doi.org/10.3390/agronomy11081608
El bied O, García-Valero A, Fechtali T, Faz Á, Acosta JA. Purification Performance of Filtration Process for Pig Slurry Using Marine Sands, Silty Loam Soils, Fly Ash and Zeolite. Agronomy. 2021; 11(8):1608. https://doi.org/10.3390/agronomy11081608
Chicago/Turabian StyleEl bied, Oumaima, Amalia García-Valero, Taoufiq Fechtali, Ángel Faz, and José A. Acosta. 2021. "Purification Performance of Filtration Process for Pig Slurry Using Marine Sands, Silty Loam Soils, Fly Ash and Zeolite" Agronomy 11, no. 8: 1608. https://doi.org/10.3390/agronomy11081608
APA StyleEl bied, O., García-Valero, A., Fechtali, T., Faz, Á., & Acosta, J. A. (2021). Purification Performance of Filtration Process for Pig Slurry Using Marine Sands, Silty Loam Soils, Fly Ash and Zeolite. Agronomy, 11(8), 1608. https://doi.org/10.3390/agronomy11081608








