Microbial and Plant Assisted Synthesis of Cobalt Oxide Nanoparticles and Their Antimicrobial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of Bacterial Strains
2.2. Extraction, Purification and Sequencing of Bacterial DNA
2.3. Characterization of Phytochemical by Thin Layer Chromatography
2.4. Synthesis of Nanoparticles
2.4.1. Synthesis from Plant
2.4.2. Synthesis from Bacterial Strains
2.4.3. Synthesis from Fungus
2.5. Characterization of Nanoparticles
3. Results
3.1. Isolation and Biochemical Characterization of Microbial Isolates
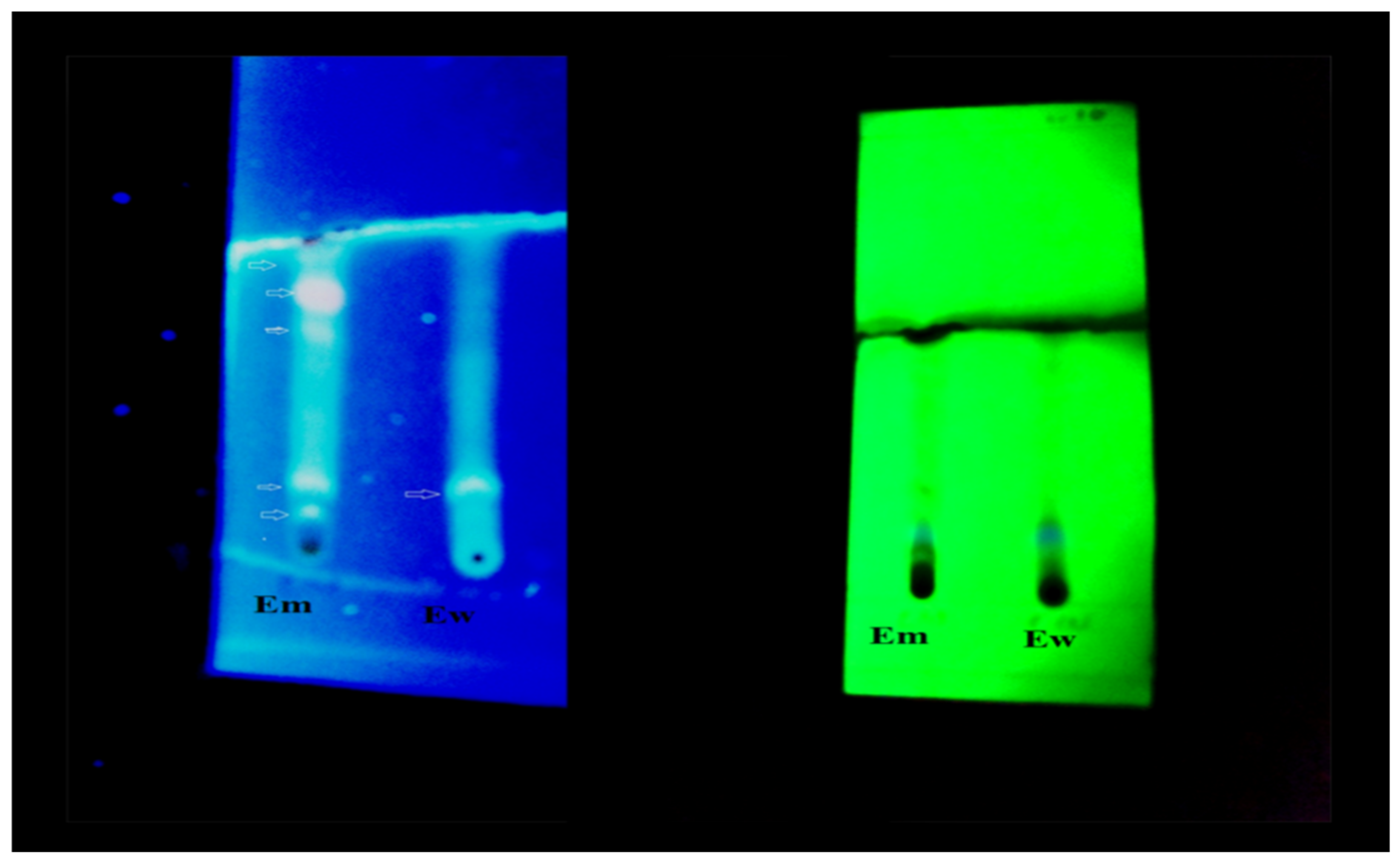
3.2. Thin Layer Chromatography
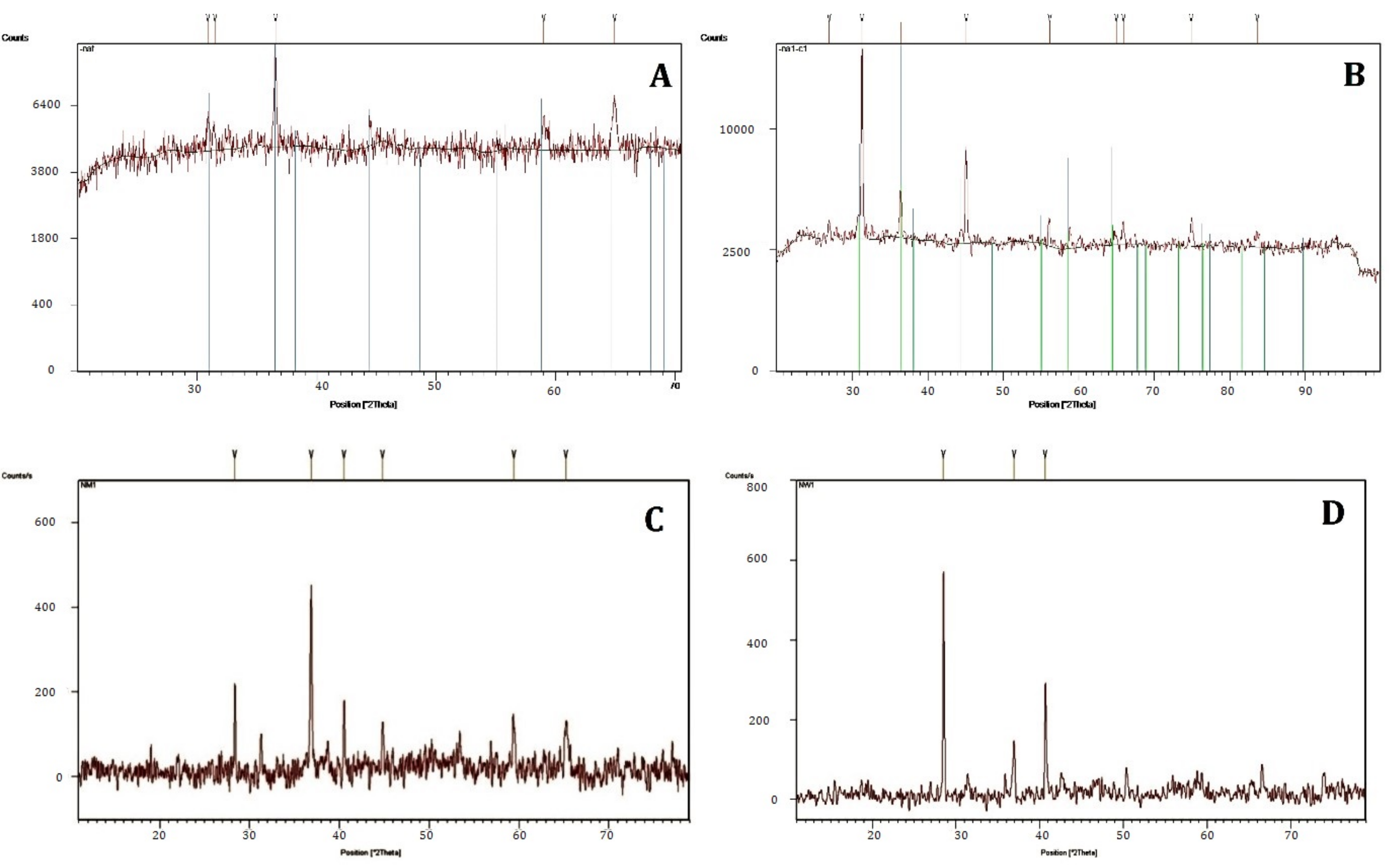
3.3. Characterization of Nanoparticle
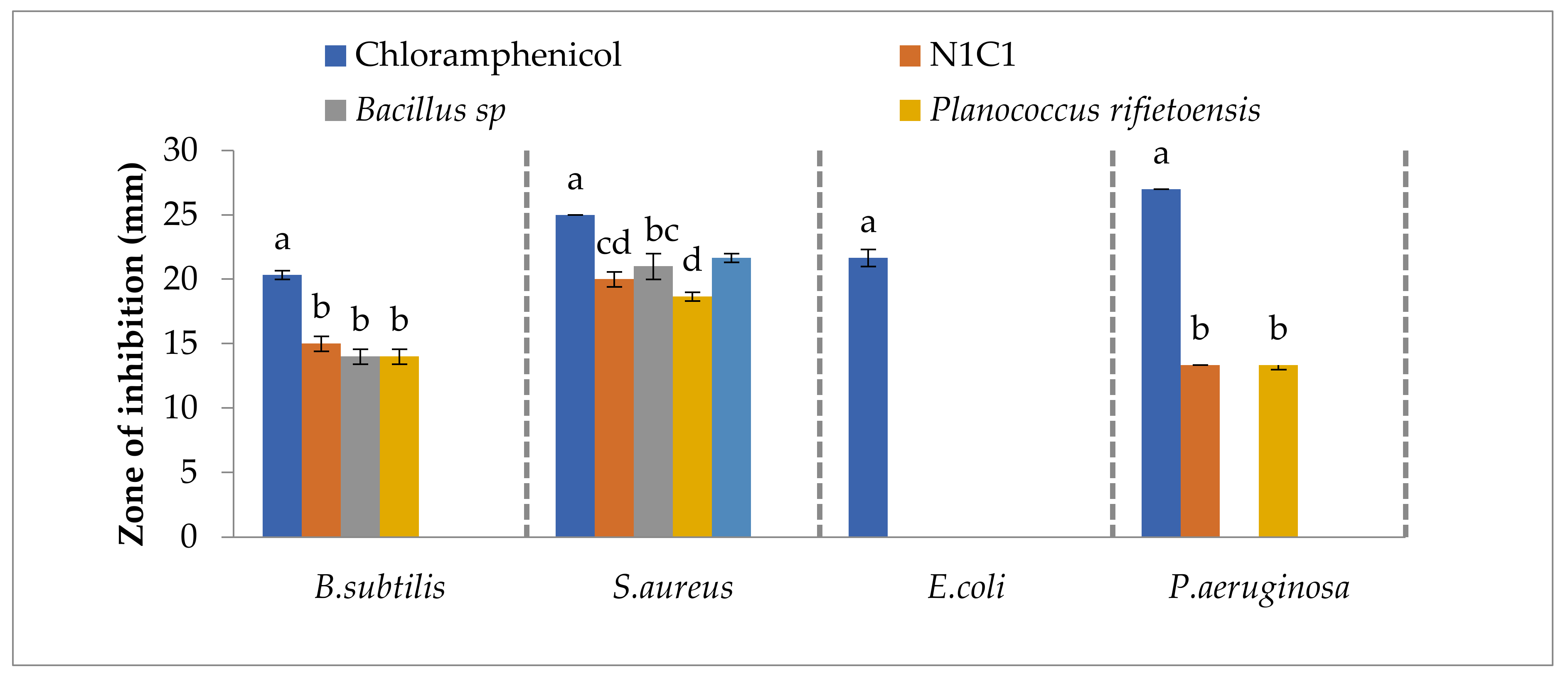
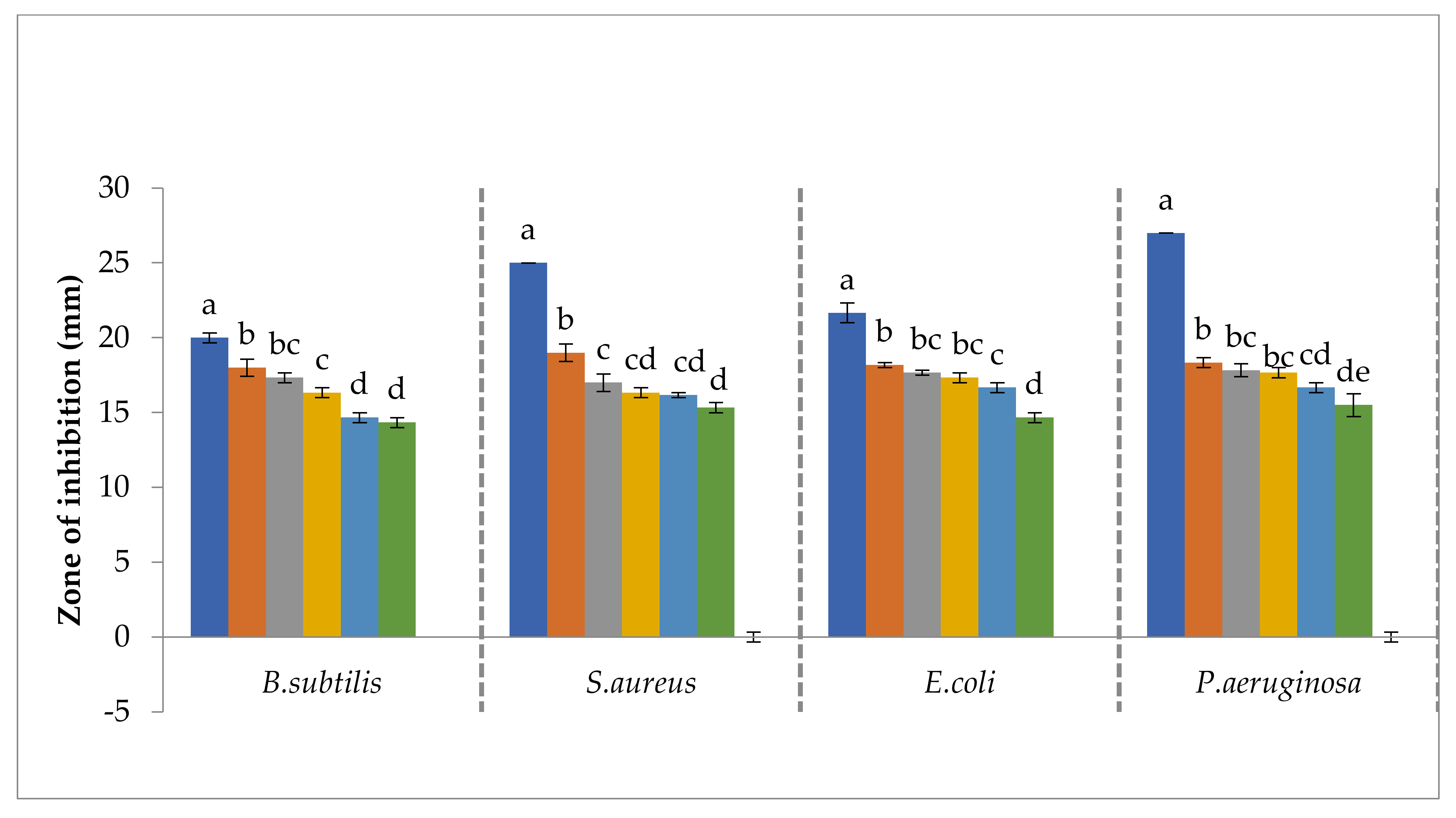
3.4. Antibacterial Activities of Bacterial Isolates, Plant Extract and Synthesized Nanoparticles
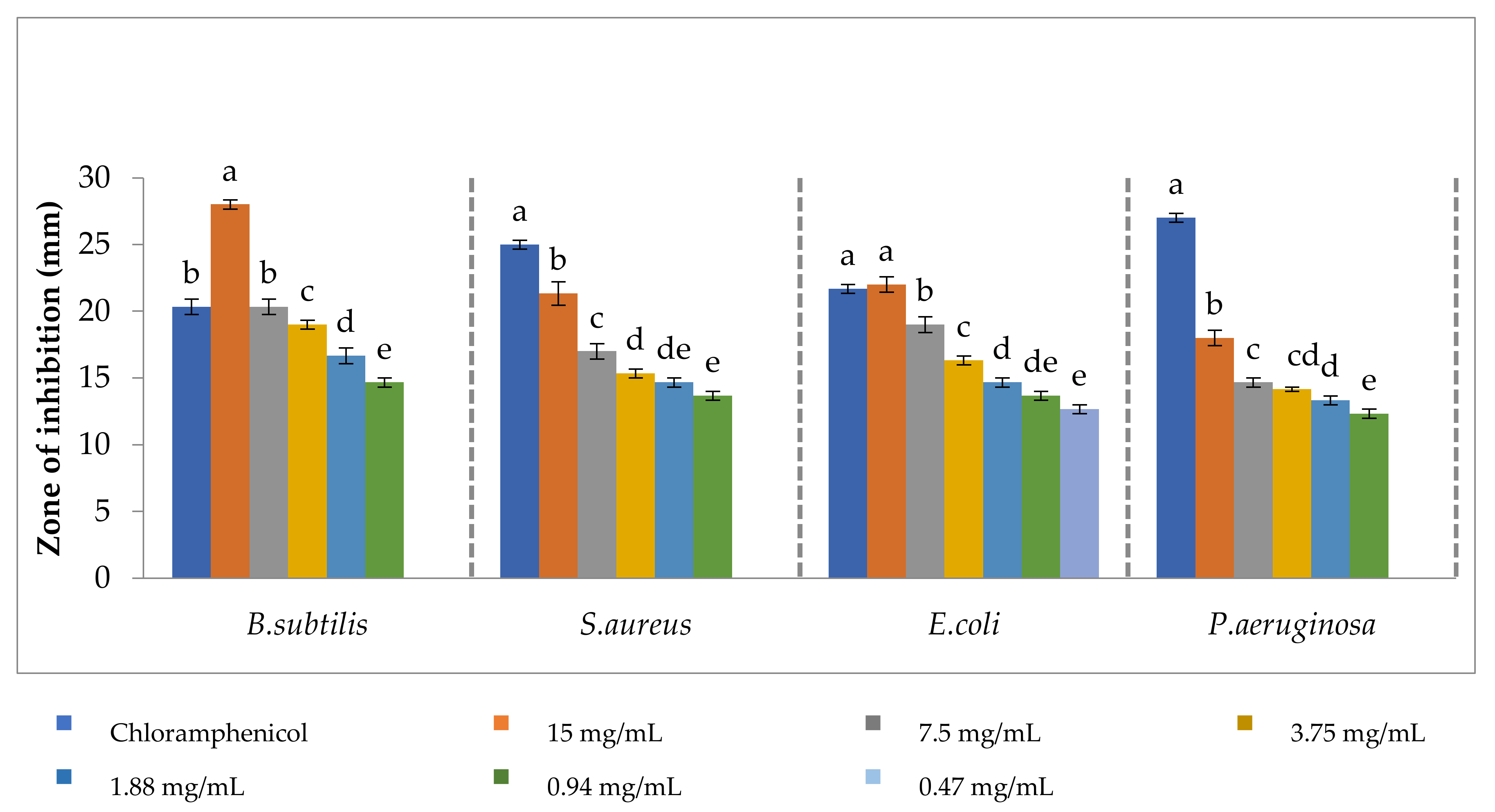
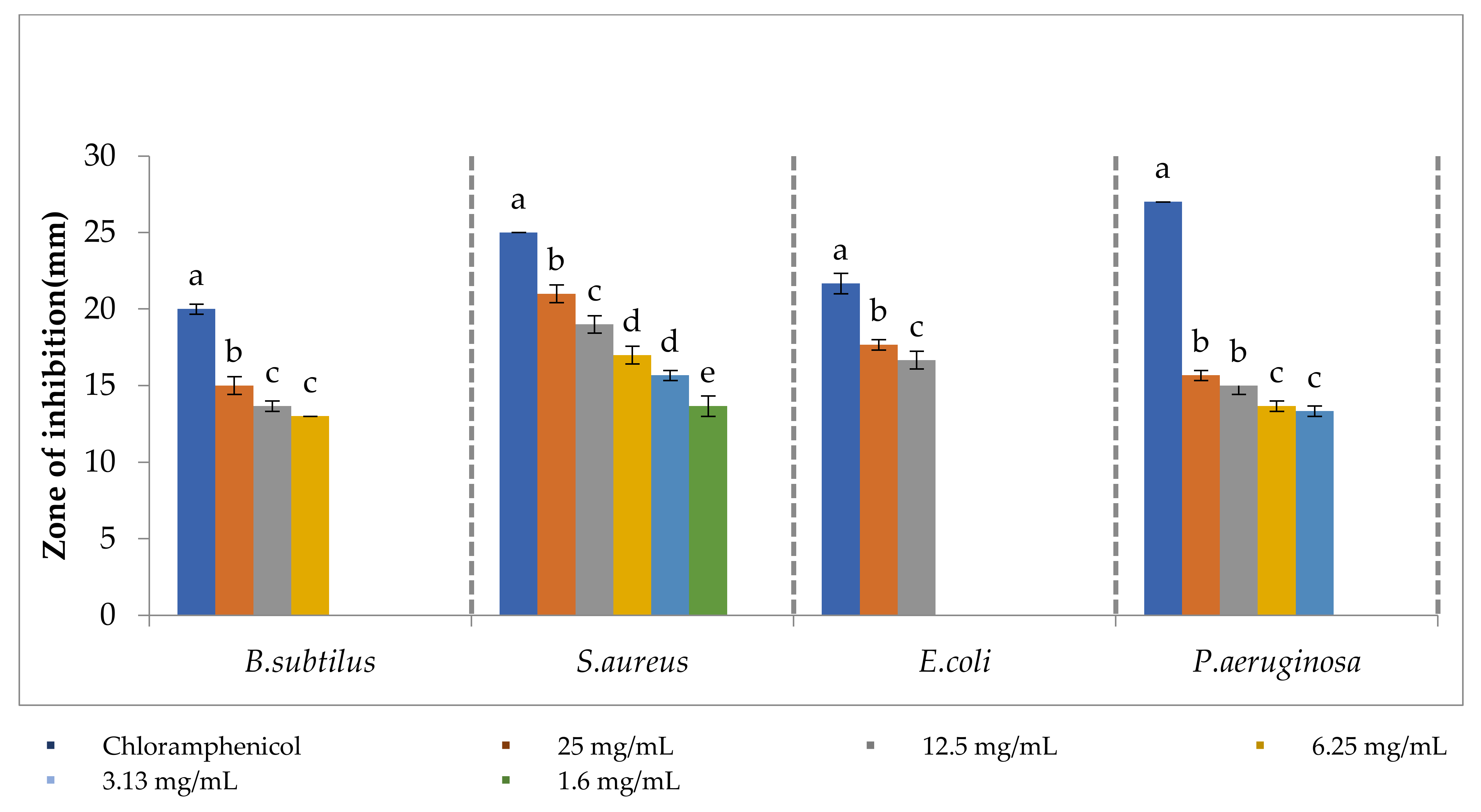
3.5. Antibacterial Activity of Cobalt Oxide Nanoparticle
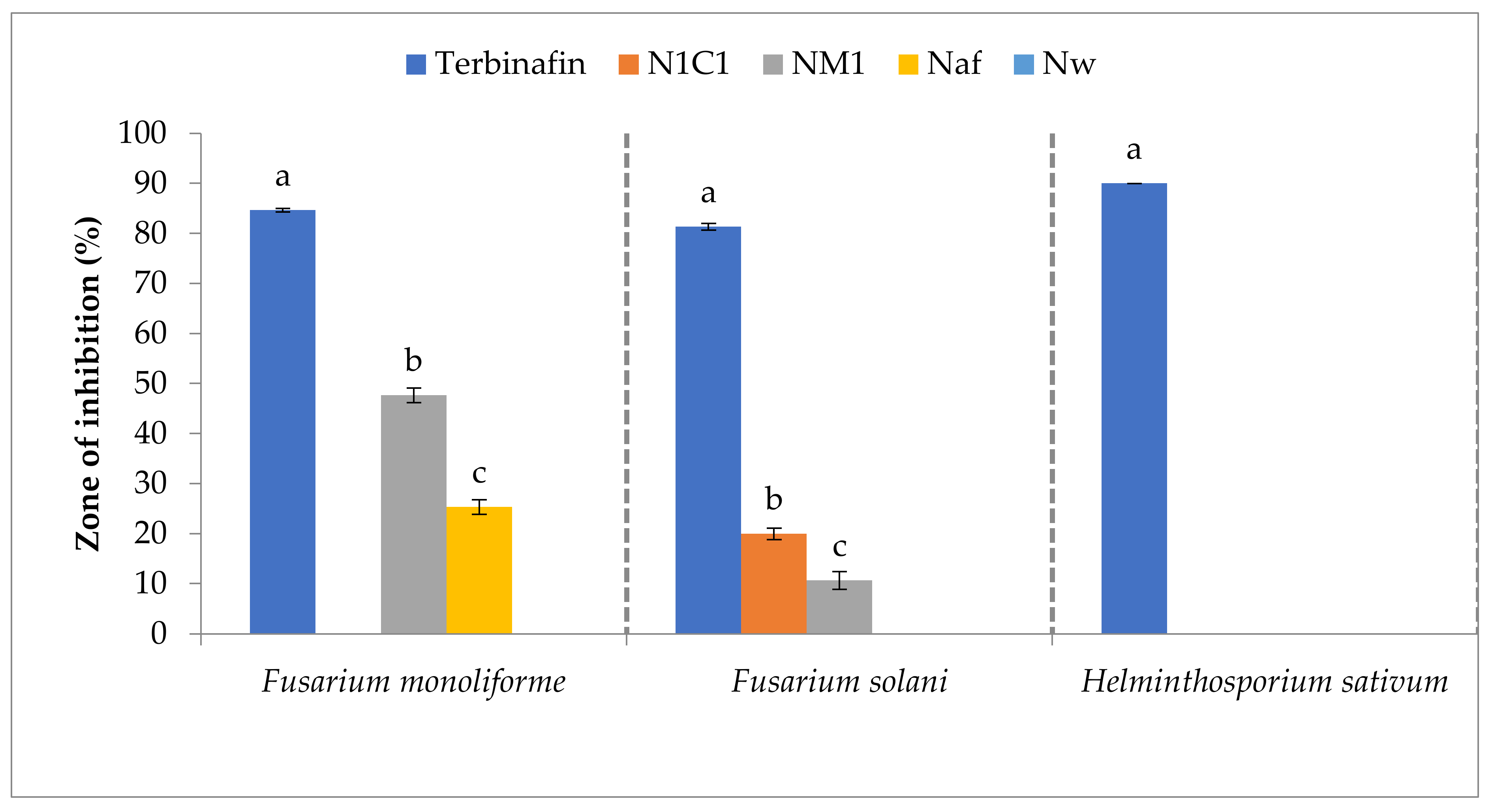
3.6. Antifungal Activities of Cobalt Oxide Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, A.; Mukherjee, P.; Senapati, P.; Mandal, D.; Khan, M.I.; Umar, R.K.; Santry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Amany, A.; Kheshen, E.; Sanaa, F.; Rab, G.E. Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. J. Der Pharm. Chem. 2012, 4, 53–65. [Google Scholar]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe vera Plant Extract. J. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions. J. Colloid Interface Sci. 2007, 309, 126–134. [Google Scholar] [CrossRef]
- Joerger, R.; Klaus, T.; Granqvist, C.G. Biologically produced silver-carbon composite materials for optically functional thin-film coatings. J. Adv. Mater. 2000, 12, 407–409. [Google Scholar] [CrossRef]
- Dimkpa, C.; Wein, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stresses conditions. Plant Cell Environ. 1997, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Prathna, T.C.; Lazar, M.N.; Chandrasekaran, R.M.; Mukherjee, A. Biomimetic Synthesis of Nanoparticles: Science, Technology & Applicability; Mukherjee, A., Ed.; InTechOpen: London, UK, 2010. [Google Scholar]
- Khan, N.; Bano, A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int. J. Phytorem. 2016, 18, 211–221. [Google Scholar] [CrossRef]
- Gu, H.; Ho, E.; Tong, L.; Wang, X. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. J. Nano 2003, 9, 1261–1263. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Ruoho, A.R. Oxidation of thiols to the corresponding symmetric disulfides with benzyltriphenylphosphonium peroxodisulfate (btppd) under nonaqueous conditions. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 1277–1281. [Google Scholar] [CrossRef]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R. Biosynthesis of silver nanoparticles using Alternantherasessilis (Linn.) extract and their antimicrobial, antioxidant activities. J. Colloids Surf. Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Rane, P.M. Psidium guajava leaves assisted green synthesis of metallic nanoparticles: A review. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 60. [Google Scholar] [CrossRef]
- Hennebel, T.; Gusseme, B.D.; Boon, N.; Verstraete, W. Biogenic metals in advanced water treatment. J. Trends Biotechnol. 2009, 27, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Alsamhary, K.I. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Sci. World J. 2013, 2013, 796018. [Google Scholar] [CrossRef] [Green Version]
- Naimi-Shamel, N.; Pourali, P.; Dolatabadi, S. Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J. Mycol. Med. 2019, 29, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Gade, A.; Pierrat, S.; Sonnichsen, C.; Rai, M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. J. Curr. Nano Sci. 2008, 4, 141–144. [Google Scholar] [CrossRef]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Alginate nanoparticles as antituberculosis drug carriers: Formulation de-velopment, pharmacokinetics and therapeutic potential. Ind. J. Chest Dis. Allied Sci. 2005, 48, 171–176. [Google Scholar]
- Das, S.K.; Das, A.R.; Guha, A.K. Gold Nanoparticles: Microbial Synthesis and Application in Water Hygiene Management. J. Langmuir 2009, 12, 9337–11468. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Pphytorem. 2018, 20, 405–414. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. Magnetic nanocomposites with mesoporous structures, synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. J. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olssonand, E.; Granqvist, C.G. Silver based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gade, A.; Ingle, A.; Whiteley, C.; Rai, M. Mycogenic metal nanoparticles: Progress and applications. Biotechnol. Lett. 2010, 32, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M.; Wang, D. Green synthesis of silver nanoparticles–graphene oxide nanocomposite and its application in electrochemical sensing oftryptophan. Biosens. Bioelectron. 2013, 42, 198–206. [Google Scholar] [CrossRef]
- Popescu, M.; Velea, A.; Lőrinczi, A. Biogenic production of nanoparticles. Dig. J. Nanomater. Biostruct. (DJNB) 2010, 5, 1035–1040. [Google Scholar]
- Man, Y.; Wang, B.; Wang, J.; Slaný, M.; Yan, H.; Li, P.; El-Naggar, A.; Shaheen, S.M.; Rinklebe, J.; Feng, X. Use of biochar to reduce mercury accumulation in Oryza sativa L: A trial for sustainable management of historically polluted farmlands. Environ. Int. 2021, 153, 106527. [Google Scholar] [CrossRef]
- Rastogi, A.; Singh, P.; Haraz, F.A.; Barhoum, A. Biological synthesis of nanoparticles: An environmentally benign approach. In Fundamentals of Nanoparticles; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 571–604. [Google Scholar]
- Kumari, M.; Pandey, S.; Giri, V.P.; Bhattacharya, A.; Shukla, R.; Mishra, A.; Nautiyal, C. Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb. Pathog. 2017, 105, 346–355. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Yang, X.; Lu, L.; Wang, X. Preparation of NiO nanoparticles and their catalytic activity in the thermal decomposition of ammonium perchlorate. Thermochim. Acta 2005, 437, 106–109. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Maaza, M. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.). Arab. J. Chem. 2020, 13, 606–619. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Dash, S.K.; Tripathy, S.; Das, B.; Mandal, D.; Pramanik, P.; Roy, S. Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem. Biol. Interact. 2015, 226, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Garg, A.; Pandit, S.; Mokkapati, V.R.S.S.; Mijakovic, I. Antimicrobial Effects of Biogenic Nanoparticles. Nanomaterials 2018, 8, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kousar, B.; Bano, A.; Khan, N. PGPR Modulation of Secondary Metabolites in Tomato Infested with Spodoptera litura. Agronomy 2020, 10, 778. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Vahabi, K.; Mansoori, G.A.; Karimi, S. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei. Insci. J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Khan, N.T.; Khan, M.J.; Jameel, J.; Jameel, N.; Rheman, S.U.A. An overview: Biological organisms that serves as nanofactories for metallic nanoparticles synthesis and fungi being the most appropriate. Bioceram Dev. Appl. 2017, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Boroumand Moghaddam, A.; Namvar, F.; Moniri, M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Vishwakarma, K.; Ramawat, N.; Rai, P.; Singh, V.K.; Mishra, R.K.; Kumar, V.; Tripathi, D.K.; Sharma, S. Nanomaterials and microbes’ interactions: A contemporary overview. 3 Biotech 2019, 9, 68. [Google Scholar] [CrossRef]
- Rajkumari, J.; Magdalane, C.M.; Siddhardha, B.; Madhavan, J.; Ramalingam, G.; Al-Dhabi, N.A.; Arasu, M.V.; Ghilan, A.K.M.; Duraipandiayan, V.; Kaviyarasu, K. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B Biol. 2019, 201, 111667. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, B.; Santra, K.; Pattnaik, G.; Ghosh, S. Preparation, characterization and in-vitro evaluation of sustained release pro-tein-loaded nanoparticles based on biodegradable polymers. Int. J. Nanomed. 2008, 3, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Bano, A.; Zandi, P. Effects of exogenously applied plant growth regulators in combination with PGPR on the physiology and root growth of chickpea (Cicer arietinum) and their role in drought tolerance. J. Plant Interact. 2018, 13, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Steel, K.J. The oxidase reaction as a toxic tool. Microbiology 1961, 25, 297–306. [Google Scholar]
- Choudhary, M.I.; Parveen, Z.; Jabbar, A.; Ali, I. Antifungal steroidal lactones from Withania coagulance. Phytochemistry 1995, 40, 1243–1246. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-C.; Bhat, S.M.; Lee, C.-W.; Shiea, J. Thin layer chromatography combined with electrospray ionization mass spectrometry for characterizing herbal compounds. Int. J. Mass Spectrom. 2018, 434, 264–271. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Biochemical test for identification of Medicinal Bacteria. Macro and micronutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1980, 8, 195–207. [Google Scholar]
- Karumi, Y.; Onyeyili, P.A.; Ogugbuaja, V.O. Identification of Active Principles of M. balsamina (Balsam Apple) Leaf Extract. J. Med. Sci. 2004, 4, 179–182. [Google Scholar]
- Shaw, P.D.; Ping, G.; Daly, S.L.; Cha, C.; Cronan, J.E.; Rinehart, K.L.; Farrand, S.K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 1997, 94, 6036–6041. [Google Scholar] [CrossRef] [Green Version]
- Lou, X.W.; Deng, D.; Lee, J.Y.; Feng, J.; Archer, L.A. Self-supported formation of needlelike Co3O4 nanotubes and their application as lithium-ion battery electrodes. J. Adv. Mater. 2008, 20, 258–262. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature; Freeman: San Francisco, CA, USA, 1982. [Google Scholar]
- Matsushita, M. Experimental observations of aggregations. In The Fractal Approach to Heterogeneous Chemistry: Surfaces, Colloids, Polymers; Avnir, D., Ed.; John Wiley, Sons: New York, NY, USA, 1989; pp. 161–179. [Google Scholar]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles, technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- He, S.; Guo, Y.; Zhang, S.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonascapsulata. J. Mater. 2007, 61, 3984–3987. [Google Scholar]
- Pant, G.; Nayak, N.; Prasuna, R.G. Enhancement of antidandruff activity of shampoo by biosynthesized silver nanoparticles from Solanum trilobatum plant leaf. Appl. Nano Sci. 2012, 3, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Parikh, R.Y.; Singh, S.; Prasad, B.L.V.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp. towards understanding biochemical synthesis mechanism. ChemBioChem 2008, 9, 1415–1422. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticle by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, R.; Bhatti, R.G. Folklore uses of amaranthaceae family from Nara desert, Pakistan. Pak. J. Bot. 2009, 41, 1565–1572. [Google Scholar]
- Khan, N.; Bano, A. Modulation of phytoremediation and plant growth by the treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int. J. Phytoremediation 2016, 18, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.; Reddy, V.M. Antimicrobial studies on the leaves of Aerva javanica. J. Pharm. Res. 2009, 2, 1259–1261. [Google Scholar]
- Retchkiman-Schabes, P.S.; Canizal, G.; Becerra-Herrera, R.; Zorrilla, R.C.; Liu, H.B.; Ascencio, J.A. Biosynthesis and characteri-zation of Ti/Ni bimetallic nanoparticles. J. Opt. Mater. 2006, 29, 95–99. [Google Scholar] [CrossRef]
- Richardson, A.; Chan, B.C.; Crouch, R.D.; Janiec, A.; Chan, B.C.; Crouch, R.D. Synthesis of silver nanoparticles: An undergraduate laboratory using green approach. Chem. Educ. 2006, 11, 331–333. [Google Scholar]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. J. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Yang, X.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.-K. Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering. Appl. Sci. 2021, 11, 4520. [Google Scholar] [CrossRef]
- Windless, C.E. Current status of Fusarium Taxonomy. Phytopathology 1991, 81, 1048–1051. [Google Scholar]
- Armstrong, G.M.; Armostrong, J.K.A. Formae Speciales and Races of Fusarium Oxysporum Causing Wilt Diseases; Pennsylvania State University Press: University Park, PA, USA, 1981. [Google Scholar]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Svanström, Å.; Boveri, S.; Boström, E.; Melin, P. The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Res. Notes 2013, 6, 464. [Google Scholar] [CrossRef] [Green Version]
- Wahyudi, A.T.; Astuti, R.P.; Widyawati, A.; Mery, A.; Nawangsih, A.A. Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J. Microbiol. Antimicrob. 2011, 3, 34–40. [Google Scholar]
- Joshi, S.; Bharucha, C.; Desai, A.J. Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour. Technol. 2008, 99, 4603–4608. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.A.; Gruenert, R.; Bednarski, P.J.; Lindequist, U. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Die Pharm. 2009, 64, 260–268. [Google Scholar]





| Reactions | Tests | Soil Samples | |||
|---|---|---|---|---|---|
| Bacillus sp. (Na13C3) | Planococcus rifietoensis (Na13C4) | Arthrobacter sp. (Na7C4) | Bacillus subtilis (N1C1) | ||
| CS | Colony shape/color | large round/white | Round/orange | Round/yellow | Small round/white |
| CES | Cell shape | Round | Round | Rod | Rod |
| GS | Gram staining | + | + | − | − |
| Oxid | Oxidase | + | + | + | + |
| Cat | Catalase | + | + | + | + |
| CFU | Colony forming unit | 4.5 × 107 | 9 × 103 | 14 × 103 | 5.8 × 107 |
| QTS | |||||
| ONPG | Ortho nitrophenyl β-D-galactopyranoside | − | + | + | − |
| CIT | Sodium citrate | + | + | + | + |
| MALO | Sodium malonate | + | + | + | + |
| LDC | Lysine decarboxylase | + | + | + | + |
| ADH | Arginine dihydrolase | + | + | + | + |
| ODC | Ornithine decarboxylase | + | + | + | + |
| H2S | H2S production | − | − | − | + |
| URE | Urea hydrolysis | + | + | + | + |
| TDA | Tryptophan deaminase | + | + | + | + |
| IND | Indole | − | − | − | − |
| VP | (Vogesproskauer) Acetion | − | − | − | − |
| GEL | Gelatin hydrolysis | − | − | − | − |
| GLU | Acidic from glucose | + | + | + | + |
| MALT | Acid from maltose | + | − | + | + |
| NO3/N2 | + | + | + | + | |
| SUC | Acid from sucrose | − | − | − | + |
| MANN | Acid from mannitol | − | − | − | + |
| ARA | Acid from arabinose | + | + | + | + |
| RHAM | Acid from Rhamnose | + | − | + | + |
| SOR | Acid from sorbitol | − | − | − | + |
| INOS | Acid from inositol | − | − | − | + |
| ADO | Acid from adonitol | − | − | − | + |
| MEL | Acid from Melibiose | − | − | − | − |
| RAF | Acid from raffinose | − | − | − | − |
| Extracts | Rf Value | Class of Compound | Reported Compounds | Color of Compound | References |
|---|---|---|---|---|---|
| Ew | 0.15 | ||||
| 0.68 | Tannins | Bluish gray | [56] | ||
| 0.511 | Flavonoid | Tectochrysin | Dark blue | - | |
| Em | 0.444 | Flavonoids | Genkwanin | Bluish | - |
| 0.57 | Flavonoids | Morin | Bluish | - | |
| 0.668 | Tannins | Bluish gray | - | ||
| 0.8 | Flavonoids (365 nm) | Kaempferol | Light green | [57] |
| Source | Pos. (°2Th.) | Height (cts) | Full-Width Half-Maximum (°2Th.) | d-Spacing (Å) | Tip Width (°2Th.) | Matched by |
|---|---|---|---|---|---|---|
| Fusarium oxysporum | 31.4981 | 508.56 | 0.2460 | 2.84033 | 0.2952 | 01-080-1543 |
| 36.5364 | 5426.86 | 0.1968 | 2.45940 | 0.2362 | 01-080-1543 | |
| 44.821 | 27.25 | 0.1771 | 2.02110 | 0.2125 | 01-080-1532 | |
| 59.0089 | 1526.73 | 0.1968 | 1.56538 | 0.2362 | 01-080-1543 | |
| 64.9460 | 1814.11 | 0.3000 | 1.43471 | 0.3600 | 01-080-1543 | |
| Bacterial species | 31.3115 | 15,451.40 | 0.1968 | 2.85684 | ||
| 36.4362 | 2852.11 | 0.2460 | 2.46593 | |||
| 45.0464 | 5803.07 | 0.2460 | 2.01259 | 01-080-1545 | ||
| 56.1684 | 1340.33 | 0.3444 | 1.63761 | |||
| 64.9833 | 356.50 | 0.6888 | 1.43516 | |||
| 65.9249 | 943.18 | 0.3936 | 1.41693 | |||
| 74.8941 | 1376.67 | 0.1968 | 1.26793 | |||
| 83.6190 | 557.20 | 0.7200 | 1.15547 | |||
| Methanolic extract of Aerva javanica | 31.2819 | 28.77 | 0.1771 | 2.85948 | 0.2125 | 01-080-1532 |
| 36.8551 | 118.44 | 0.1476 | 2.43886 | 0.1771 | 01-080-1532 | |
| 44.8465 | 27.25 | 0.1771 | 2.02110 | 0.2125 | 01-080-1532 | |
| 59.4016 | 33.83 | 0.2952 | 1.55597 | 0.3542 | 01-080-1532 | |
| 65.1555 | 20.28 | 0.4320 | 1.43060 | 0.5184 | 01-080-1532 | |
| Water extract of Aerva javanica | 28.5287 | 159.95 | 0.1033 | 3.12886 | ||
| 36.9674 | 35.99 | 0.2362 | 2.43171 | 00-001-1152 | ||
| 40.6646 | 79.63 | 0.0886 | 2.21875 | |||
| 69.3668 | 8.34 | 0.2657 | 1.35480 | |||
| 73.9683 | 14.87 | 0.4320 | 1.28043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubraiz, N.; Bano, A.; Mahmood, T.; Khan, N. Microbial and Plant Assisted Synthesis of Cobalt Oxide Nanoparticles and Their Antimicrobial Activities. Agronomy 2021, 11, 1607. https://doi.org/10.3390/agronomy11081607
Mubraiz N, Bano A, Mahmood T, Khan N. Microbial and Plant Assisted Synthesis of Cobalt Oxide Nanoparticles and Their Antimicrobial Activities. Agronomy. 2021; 11(8):1607. https://doi.org/10.3390/agronomy11081607
Chicago/Turabian StyleMubraiz, Nadia, Asghari Bano, Tariq Mahmood, and Naeem Khan. 2021. "Microbial and Plant Assisted Synthesis of Cobalt Oxide Nanoparticles and Their Antimicrobial Activities" Agronomy 11, no. 8: 1607. https://doi.org/10.3390/agronomy11081607
APA StyleMubraiz, N., Bano, A., Mahmood, T., & Khan, N. (2021). Microbial and Plant Assisted Synthesis of Cobalt Oxide Nanoparticles and Their Antimicrobial Activities. Agronomy, 11(8), 1607. https://doi.org/10.3390/agronomy11081607







