Abstract
Barley (Hordeum vulgare L.) is an important cereal crop, but its sustainable production is significantly hampered due to the presence of various edaphic stresses. Understanding the variability in root morphological traits among diverse barley genotypes is critical for selecting those with suitable root traits for breeding new cultivars better adapted to stress environments. Root morphological traits in an early growth stage (30 days after transplanting) in a panel of 189 barley genotypes (mostly advanced breeding lines) were assessed using a semi-hydroponic phenotyping platform followed by a validation experiment of eight genotypes with contrasting root systems in two soils. The phenotyping experiment showed large variation (coefficient of variation values ≥ 0.25) in 16 of 26 measured root and shoot traits. A strong correlation among most of the selected traits was identified. Principal component analysis indicated four principal components (eigenvalues >1) captured 79.5% of the total variation. Root traits, including total root length, root length at various depths, root diameter and root length ratio (top 20 cm vs. lower section), could be considered in the barley breeding programs. Consistent ranking of the selected eight genotypes based on root biomass and root length in both the semi-hydroponic system and the columns with two different soils confirmed root trait performance in different growth environments as well as the reliability of the phenotyping method. This study identified phenotypic variability in root morphological traits in barley genotypes in the early growth stage. The genotypic variability in root traits represents a basis for mapping quantitative trait loci (QTLs) and molecular markers, particularly focused on breeding lines with optimal root properties for the efficient acquisition of soil resources and adaptation to drought and other abiotic stresses.
1. Introduction
Barley (Hordeum vulgare L.), as the fourth largest broad-acre cereal crop by production, serves as an important feed and food crop [1]. Barley is one of the main cereal crops grown in Mediterranean areas [2], and it is the second-largest cereal crop in Australia; the area of cultivation in southern Australia is around 4.0 million hectares [3]. Barley is considered one of the most highly adapted cereals for its tolerance to cold, drought, alkalinity and salinity [4]. However, barley production is severely hampered in many areas of the world by the abiotic stresses [5], particularly acidity.
Among the cereals, barley is a good genetic model from the Triticeae tribe for genetic and physiological research regarding environmental adaptation. Its inbreeding nature and diploidy make genetic studies relatively more straightforward than in wheat. It is a model species for genetic and physiological research regarding environmental adaption [6]. The wide range of genetic stocks and the extended collinearity with other members of the tribe are additional advantages of a model species [7].
Crop root system architecture (RSA) plays a vital role in the acquisition of water and nutrients, and its effect on growth and yield has been widely reported [8,9,10,11]. For instance, rooting depth is among the most important traits required to sustain plant function under drought stress. A deep and extensive root system architecture would permit plants to extract soil nutrients and water from a large soil volume. The effect of root architecture on yield and other agronomic properties, particularly under biotic or abiotic stresses, has been well-documented in major crops [12,13,14]. However, there is a lack of knowledge on the root system architecture in barley, its role related to the capture of water and nutrients and its adaptation to water-limited, Mediterranean-type environments [15].
Genotypes may differ in their responses to specific environments (genotype-environment interaction, i.e., G × E), which is termed as “phenotypic plasticity”. Phenotypic plasticity involves the alteration of RSA, plant physiology and gene expression, allowing plants to optimize RSA for water and nutrient uptake and to adapt to edaphic stress. Given genetic variability and phenotypic plasticity for root morphological and physiological responses and the plant foraging strategies, there is an opportunity to increase crop productivity by selecting for root traits that are beneficial for nutrient and water uptake in Mediterranean-type environments [11,14,16]. Characterizing the variability in root architecture in a core collection of barley genotypes would provide the basis for breeding new cultivars with suitable root traits for improved adaptation to specific environments.
The objective of this study was to characterize root trait variability among a panel of barley genotypes (advanced breeding lines and key parental germplasm) using a semi-hydroponic system (Exp. 1) recently established and used for other crops [17,18,19], with validation in two types of soil for selected genotypes with contrasting root systems (Exp. 2). This study will provide knowledge on the phenotypic variability in root architectural traits in barley for mapping of relevant quantitative trait loci (QTLs) and molecular markers.
2. Materials and Methods
2.1. Root Phenotyping Experiment (Exp. 1: Semi-Hydroponic System)
Plant Material and Root Phenotyping System
The first experiment for characterizing root trait variability among a set of 189 genotypes of barley (Hordeum vulgare L.) (Exp. 1) was conducted in the established semi-hydroponic system [17] during March–April, 2016. The genotypes were originated from Australia, Europe and Ethiopia and were mainly semi-advanced and parental materials (126) from the InterGrain Pty Ltd. (Bibra Lake, Western Australia) breeding program between 2011 and 2015 that breeds barley cultivars adapted to diverse environments throughout Australia (Table S1). The remaining genotypes were advanced breeding lines from the former Department of Agriculture and Food program in Queensland (Australia) and commercial varieties from the rest of Australia, as well as varieties and advanced breeding lines from Europe, making the panel quite diverse regarding genetic origin.
A randomized complete block design was used in Exp. 1 and conducted in the semi-hydroponic system [17]. Briefly, each bin system consisted of a 240-L plastic wheelie bin (top 75 × 58 cm, height 108 cm) and 20 growth units made of a 5 mm thick acrylic panel (260 × 480 mm) wrapped in a black calico cloth (Figure 1). Each bin contained 40 L of nutrient solution containing (in µM) N (1000), P (40), K (1220), S (1802), Ca (600), Mg (200), Cu (0.2), Zn (0.75), Mn (0.75), B (5), Co (0.2), Na (0.06), Mo (0.03) and Fe (20). An automatic pumping system was used in each bin to provide nutrient solution to the top of pouches containing plants. The pH of the nutrient solution of each bin was adjusted and refreshed weekly. A total of 18 bin systems (240 L each) were used with six bins in each of three replicates. Each bin system accommodated 32 plants, with two plants per growth pouch (Figure 1).

Figure 1.
Layout of barley plants grown in the semi-hydroponic phenotyping system (a), and plants of two barley genotypes with contrasting root systems grown for 30 days after transplanting (b) in a temperature-controlled glasshouse. Bar = 10 cm.
2.2. Plant Growth Environments and Assessments
The experiment was carried out during early summer (May to June, 2016) in a temperature-controlled glasshouse at The University of Western Australia, Perth (31°58′ S, 115°49′ E). The average daily temperature was 24/16 °C (day/night). Barley seeds were germinated in seedling trays filled with washed river sand. Emerged plants were carefully pulled out, washed and then transplanted into the growth pouches. Plants were harvested 30 days after transplanting. Tiller number and shoot height of each plant were determined at harvest; then, shoots were dried in an air-forced oven at 70 °C for 120 h. Root subsamples were collected at harvest by separating the root system into the 0–20 cm, 20–40 cm and below 40 cm sections starting from the shoot-root junction for determining root morphological traits. Root subsamples were placed in plastic bags and stored at 4 °C until scanned at 400 dpi (Epson Perfection V800, Long Beach, CA, USA); afterwards, root section samples from the same plant were combined as one sample and dried as described above for shoots.
Root growth rate for each plant was calculated based on root depth increments between sequential photographs during the experiment. Total root length, root surface area, root volume, average root diameter and diameter class length (DCL, root length in a specific diameter class) were generated by analyzing root images in WinRHIZO Pro (v2009, Regent Instrument, Quebec, QC, Canada). Eleven diameter classes were established (in mm): <0.06, 0.06–0.065, 0.065–0.070, 0.070–0.075, 0.075–0.085, 0.085–0.10, 0.10–0.15, 0.15–0.25, 0.25–0.40, 0.40–0.65 and >0.65.
The root trait data in the upper 0–20 cm section (referred to hereafter as the “top” section) were compared with those for the entire root system. The following traits were considered based on the observed and/or computed data (see Table 1 for a full list of traits):

Table 1.
Description of 23 root- and three shoot-related traits in 189 barley genotypes in the early growth stage assessed in the semi-hydroponic phenotyping system 30 days after transplanting in a temperature-controlled glasshouse.
- Root mass ratio (root dry mass divided by total dry mass)
- Root-to-shoot mass ratio (root dry matter weight divided by shoot dry matter weight)
- Specific root length (SRL) = root length over root dry matter weight (cm mg−1)
- Root tissue density = root dry matter weight divided by root volume (mg cm−3)
- Root growth rate = length of the longest root divided by growth time (cm d−1)
- Relative Diameter Class Length (rDCL) = DCL divided by total root length.
2.3. Validation Experiment (Exp. 2: Soil Columns)
Selected Barley Genotypes
Eight barley genotypes with contrasting root morphological traits were selected from Exp. 1 to validate plant growth and root performance in two types of soil in Exp. 2. The eight genotypes with key root characters observed in Exp. 1 are listed below (genotype rankings with respect to the relevant traits were based on the trait values from the highest to the lowest among the 189 genotypes):
- #112: large root system (ranked 2nd in root biomass and 9th in root length) with high root density at the 20–40 cm depth.
- #190: large root system (ranked 4th in root biomass and 8th in root length) with high root density at the 20–40 cm depth.
- #108: large root system (ranked 6th in root biomass and 14th in root length).
- #128: average root biomass and root length.
- #21: average root biomass and root length with less roots in top 20 cm section than in the 20–40 cm section.
- #49: small root biomass and short root length.
- #48: small root system (ranked 188th in root biomass and 186th in root length).
- #5: small root system (ranked 189th in root biomass and 187th in root length).
A randomized complete block design consisting of eight selected genotypes and two soils with four replications per treatment was used in the column experiment. Cylindrical PVC columns (Bunnings, Willetton, Australia) (10 cm diameter, 60 cm deep) were filled with the same volume of air-dried sandy loam soil and sandy soil weighing 8.00 kg and 9.00 kg, respectively. The sandy soil was collected from a paddock with no cropping and no fertilization in the past 20 years at the UWA’s Shenton Park Field Station, Perth. The sandy loam soil was provided by a local gardening supplier (Amazon Soils Supplies, Perth, WA, Australia). Soils were analyzed for basic properties (CSBP Analytical Laboratories, Bibra Lake, WA, Australia). The sandy soil had pH 6.6 (H2O) and 5.9 (CaCl2), conductivity 0.012 dS m−1, and was low in nutrients (in g kg−1): organic carbon 0.01, nitrate nitrogen <1, ammonium nitrogen 1, K 27 (Colwell), S 0.6 and P 15 (Colwell). The sandy loam soil had pH 7.5 (H2O), 6.8 (CaCl2), conductivity 0.499 dS m−1, and contained (in g kg−1) organic carbon 8.5, nitrate nitrogen 77, ammonium nitrogen 21, K 155 (Colwell), S 183 and P 64 (Colwell).
Six seeds of each barley genotype were sown per PVC column, and were thinned to three seedlings per column after emergence. A diluted nutrient solution identical to that used in the semi-hydroponic system, but with double the N and P concentrations, was applied to plants twice per week. The plants were harvested after 5 weeks, and root trait parameters were measured as described for Exp. 1.
2.4. Statistical Analysis of Root Trait Data
General linear model (GLM) multivariate analysis was used to analyze root trait data for the genotype main effect after identifying non-significant differences among bins [10] by using IBM SPSS Statistics (Version 19, IBM Corp., Armonk, NY, USA). Pearson correlation coefficients (r) were calculated to determine the general relationships between pairs of root traits; correlations were considered statistically significant if P ≤ 0.05 (*) or P ≤ 0.01 (**). Variability in root traits among different genotypes was determined by principal component analysis (PCA).
3. Results
3.1. Variation in Root and Shoot Traits (Exp. 1)
A total of 23 root traits and three shoot traits were characterized in the phenotyping experiment (Table 1). Values of coefficient of variation (CV) ranged from 0.10 (root diameter at the 20–40 cm depth RD_20) to 0.36 (total root volume, RV). Thirteen root traits and three shoot traits had CV values ≥0.25 (Table 2). Among the three shoot traits, large variation among genotypes was observed in shoot biomass (CV = 0.25), shoot height (CV = 0.25) and tiller number (CV = 0.34). At harvest, shoot biomass ranged from 1.4 to 5.3 g/plant and shoot height from 24.4 to 44.5 cm/plant.

Table 2.
Descriptive statistics of 23 root traits and three shoot traits in 189 barley genotypes grown in the semi-hydroponic phenotyping system for 30 days after transplanting in a temperature-controlled glasshouse.
3.2. Root Length in Various Diameter Classes (Exp. 1)
Root morphological traits, including total root length, root surface area and root volume displayed large variation among genotypes, with CV greater than 0.25 for each trait (p < 0.01; Table 2). In addition, large CV values were identified for average root diameter (RD) among the genotypes (CV = 0.26, p < 0.001; Table 2). On average among the genotypes, about 31% of relative diameter class length (rDCL) was in the diameter class greater than 0.15 mm (RDCL_thick), including 2.2% of roots being thicker than 0.65 mm (mostly proximal, top root parts near shoot). The secondary roots were thin (diameter less than 0.15 mm), accounting for 69% of the total root length, including 32% of total root length in the diameter class between 0.10 and 0.15 mm (Figure 2), 18% in the class 0.06–0.065 mm and 19% in the diameter class 0.070–0.075 mm.
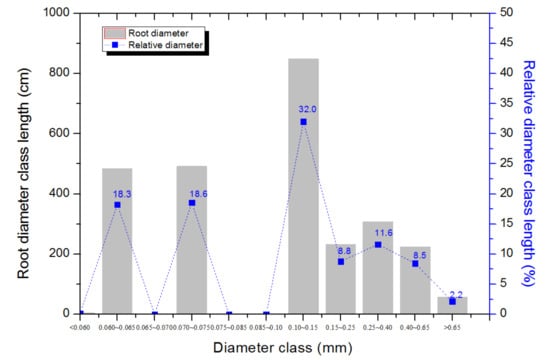
Figure 2.
Root diameter class length (cm) (bars) and relative diameter class length (rDCL, %) (dotted lines) of 189 barley genotypes grown in the semi-hydroponic phenotyping system for 30 days after transplanting in a temperature-controlled glasshouse. Diameter class (mm) including <0.060, 0.060~0.065, 0.065~0.070, 0.070~0.075, 0.075~0.085, 0.085~0.10, 0.10~0.15, 0.15~0.25, 0.25~0.40, 0.40~0.65, >0.65.
3.3. Correlation among Root Traits of Barley (Exp. 1)
Pearson correlation matrix was used to identify correlation among different parameters. A subset of 13 root traits and three shoot parameters were selected with relatively large CV values (CV ≥ 0.25; Table 2). There was significant correlation among most individual traits (Table 3). Root biomass was highly correlated (p < 0.01) with each of 14 traits, except for root length ratio (RLR). Root biomass was strongly correlated with shoot biomass (r = 0.741, p < 0.001, Figure 3a), shoot height, root length in different soil layers and tiller number (Table 3). As expected, total root surface area (RA) and total root volume (RV) showed similar correlations as root biomass: however, root diameter (RD) had no significant correlation with RA, RV or root length.

Table 3.
Pearson’s correlation matric for 13 root traits and 3 shoot traits in 189 barley genotypes grown in the semi-hydroponic phenotyping system for 30 days after transplanting in a temperature-controlled glasshouse.
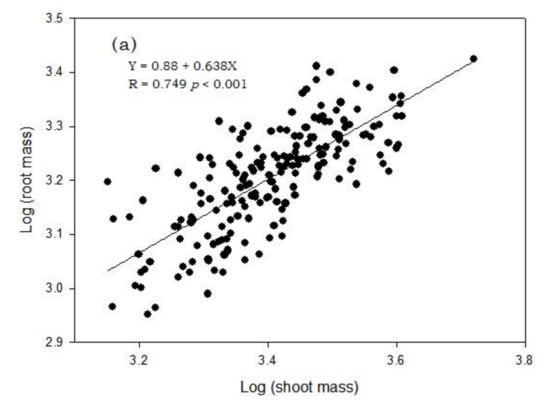
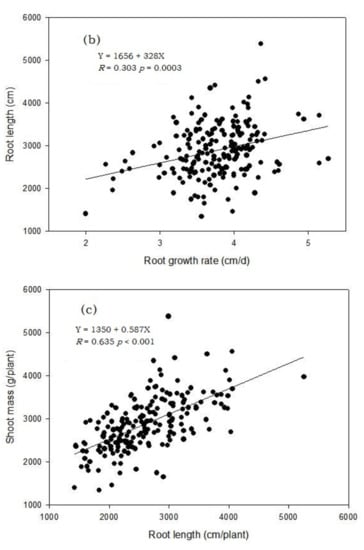
Figure 3.
Correlation between shoot dry mass and root dry mass (both log-transformed) (a), root length and root growth rate (b) and root length and shoot dry mass (c) of 189 barley genotypes grown in the semi-hydroponic phenotyping system for 30 days after transplanting in a temperature-controlled glasshouse (n = 3).
There were strong correlations between root length and other root-related parameters, including growth rate of the longest root (Figure 3b) and shoot mass (Figure 3c).
The hierarchical cluster analysis using the average linkage method between groups separated the same subset of 16 traits into two major clades. Clade I included SB and RL, and Clade II were further divided into two groups (Figure 4). The dendrogram indicated the relationship among the selected traits.
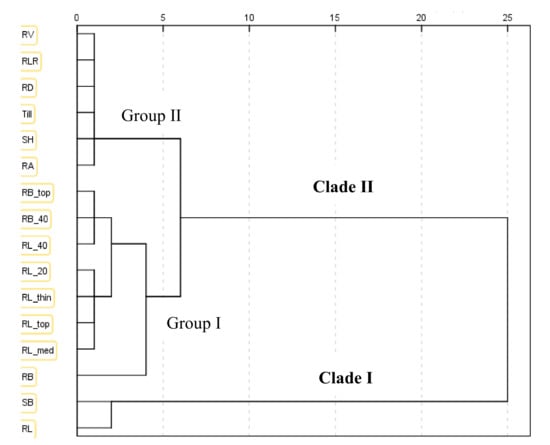
Figure 4.
Dendrogram showing clustering patterns of 16 phenotypic traits (13 root traits and three shoot traits) of 189 barley genotypes grown in the semi-hydroponic phenotyping system for 30 days after transplanting in a temperature-controlled glasshouse. The dendrogram was constructed based on the first principal component (see Table 4). The hierarchical cluster analysis was carried out using the average linkage method between groups.
3.4. Principal Component and Hierarchical Cluster Analyses of Barley Root Traits (Exp. 1)
Sixteen root and shoot traits (CV ≥ 0.25) were selected in the principal component analysis. Four principal components (PCs) were separated with eigenvalues >1, representing 79.5% of the total variation across 189 barley genotypes (Table 4), with the three components (PC1-3) explaining a total of 71.7% of the variance (Figure S1). The first component captured 43.5% of the variability and comprised RL, RB, RV, RA, SB, tiller number and SH, which all related to the overall size or vigor of shoot and root growth. The PC2 was related to root system architecture, represented 14.8% of the total variation and included root diameter (RD) and root biomass below 40 cm (RB_40). The PC3 represented root length ratio (RLR), a component of root architecture, and accounted for 13.4% of the total variation.

Table 4.
Variable loading scores of 16 selected root and shoot traits (all CV ≥ 0.25, see Table 2) and the proportion of variation explained by each principal component. The highest value for each trait among the four components appeared in bold.
3.5. Identification of Phenotypic Diversity (Exp. 1)
An agglomerative hierarchical clustering (AHC) similarity dendrogram (established with the Pearson correlation coefficients of root trait data) indicated a large diversity in root architecture traits among the core collection (Figure S2). Three general clades were clearly distinguished using rescaled distance of 15, and they were further divided into 20 subgroups (G1–G20) at rescaled distance of 5. Clade I comprised genotype #180. Clade II contained #74, and the largest Clade III included 187 genotypes from 18 sub-groups (G3–G20).
3.6. Barley Root and Shoot Trait Consistency in the Two Experiments (Exp. 1 and Exp. 2)
Root biomass, root length, root diameter, shoot biomass and shoot height of eight selected barley genotypes were used to measure the trait consistency in the semi-hydroponic system and two soils (Table 5 and Table 6). All traits measured (except root diameter) had the highest values in sandy loam and lowest values in sandy soil, showing the root plasticity when plants were grown in three different media.

Table 5.
Mean values of root dry mass, shoot dry mass and ratio of root-to-shoot dry mass of eight selected barley genotypes grown in the semi-hydroponic system and two soils in a temperature-controlled glasshouse.

Table 6.
Mean values of total root length, average root diameter and shoot height of eight selected barley genotypes grown in the semi-hydroponic system and two soils in a temperature-controlled glasshouse.
For barley plants grown in the semi-hydroponic system, root biomass and total root length of eight selected barley genotype showed good agreement with the value in the two soils. For instance, the large-root genotypes selected from the semi-hydroponic system (such as #108, #112 and #190) had significantly greater root dry matter and total root length in both soils than the small-root genotypes #5 and #49.
Strong correlations (p < 0.01) of root biomass or root length were observed between the semi-hydroponic system and each of the two soil media (Figure S3), whereas shoot biomass in two soil media and shoot height in sandy soil showed no significant correlation with the same traits in the semi-hydroponic system. Root biomass, root length and shoot biomass of eight selected barley genotypes were highly correlated in the two soils, but there was no significant correlation between the soils regarding shoot height (Figure S4).
Biomass allocation to roots vs. shoots varied among the genotypes and the growth media (Table 5). Comparison of ratio of root-to-shoot dry mass (R/S) across eight genotypes showed that plants grown in the semi-hydroponic system and in the sandy loam (some but not all genotypes in the sandy soil) had relatively higher biomass allocation to roots, as indicated by greater root/shoot ratios ranging from 0.54 to 0.86 in the semi-hydroponic system and 0.53 to 1.07 in the sandy loam. The average R/S ratios were 0.71, 0.66 and 0.75 in the semi-hydroponics, sandy soil and sandy loam, respectively (Table 5). Regarding root dry mass, genotypes #112, #108, #190 and #21 maintained a relatively constant position in the genotype ranking in both the semi-hydroponics and the two soils, whereas #21, #48 and #49 showed growth-medium-dependent plasticity in terms of root dry mass.
Root length ratio (root length of 0–20 cm/below 20 cm) varied among the genotypes and among the growth media (Figure 5). The root length ratio ranged from 0.50 to 0.94 in the sandy loam and from 0.50 to 0.91 in the semi-hydroponic system, whereas it ranged from 1.29 to 2.69 in the sandy soil, indicating genotypes grown in the sandy soil produced relatively shallower root systems with less root length down to the deep profile. The root length ratio of different genotypes showed a similar trend in the three growth media, with genotype #5 having the highest root length ratio in the sandy soil, which was significantly higher than #128 in the sandy loam and sandy soil.
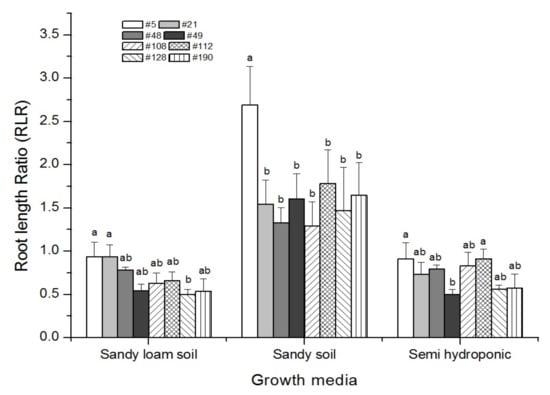
Figure 5.
Root length ratio (root length in the top 20 cm section over the length of the remaining roots) of eight barley genotypes grown in sandy loam soil, sandy soil and semi-hydroponically. These eight genotypes were selected out of 189 barley genotypes grown in the semi-hydroponic system for 30 days after transplanting. Bars with the same significance letter in the given growth medium are not significantly different according to the Tukey test (p ≤ 0.05). Data are means (n = 3) + standard error of means.
4. Discussions
The fibrous root system of barley is broadly divided into seminal roots (developing from the primordia in the seed embryo) and nodal or secondary roots (emerging from the lower nodal parts of the culm throughout tillering) [20]. Phenotyping for different root traits with large variability is crucial for screening and breeding new barley cultivars through marker-assisted selection programs [21].
Accurate quantification of traits observed in plants grown under conditions of environmental stress is crucial for identifying loci of interest in the genome [22]. For barley, many mapping populations have been developed. Moreover, a large number of QTLs controlling complex traits including yield components, agronomic, morphological and physiological traits, disease resistance, tolerance to abiotic stress and malting quality have been detected and validated [23]. However, studies on QTLs associated with drought-related root morphological and physiological traits are still scarce in barley [24,25]. Thus, the established semi-hydroponic phenotyping platform [17] was used to assess the root and shoot trait variability in the panel of barley genotypes, followed by screening selected contrasting genotypes in two soils. The vigorous growth of barley genotypes in our semi-hydroponic system in the 30-d cultivation period indicated an appropriate experimental environment for growth of barley.
The 26 measured traits in this study were associated with root growth (such as total root length, root biomass, root diameter), root distribution (such as root length ratio, maximum root length) and shoot growth (such as shoot mass, shoot height and tiller number). Root size (including total root length and total root dry mass) is considered to be strongly associated with plant foraging for soil water and nutrients [26]. For barley, genotypes with contrasting root size had different grain yield [27]. Root traits such as total root length and total root biomass in the seedling stage were associated with drought tolerance under field conditions in maize, wheat and barley [28]. In the present study, the root mass showed a strong positive correlation with the shoot mass. The barley genotypes with the largest root systems had more than four times the total root length (TRL) and three times the root biomass (RB) of those with the smallest root system in the semi-hydroponic platform.
In the present study, the average root diameter varied up to nearly three–fold among the 189 genotypes in semi-hydroponics but showed little variation among genotypes grown in sandy soil and sandy loam soil; however, there were significant differences among different genotypes in semi-hydroponics and sandy loam soil. It was shown that vigorous growth of root (with increased thickness and root length) was associated with enhanced drought tolerance and increased grain yield under field conditions [13,29]; in addition, increased mechanical impedance also increased root diameter, cortex thickness and the diameter of the xylem vessels in pea, maize and cotton [30]. In the present study, barley root diameter was larger (and total root length shorter) in sandy soil than sandy loam soil, indicating greater mechanical impedance and restricted root growth in the former.
Root distribution was generally as shallow as possible while still fulfilling the transpiration requirement because shallow roots have competitive benefits over deep roots [31]. In the sandy soil, compaction hampered barley root elongation, and root proliferation at shallow depth is a crop strategy to capture most of the water and nutrients in the topsoil layer [32]. Our results confirmed such conservative root growth of barley in soil, with the distribution of almost all roots in 0–20 cm in sandy soil, resulting in higher root length ratios in the three growth media. These findings are in agreement with a field study [33].
Root distribution in the sandy loam showed a consistent trend among different genotypes, with similar values of root length ratio to those in semi-hydroponics at 0–40 cm depth. This indicates that barley growth in semi-hydroponics was representative of the root distribution in soil (at least sandy loam soil).
Some root traits such as total root biomass and length, and also root growth at various depths, can potentially serve as parameters for future germplasm screening. In our study, principal component analysis (PCs) revealed that total root length, root biomass, root diameter (RD), root biomass below 40 cm and root length ratio (RLR) explained 72.1% of total variation. The soil column experiment revealed consistent ranking of the selected eight genotypes in root biomass and root length in both experiments. Therefore, root traits, including total root length, root length at various depths, root diameter and root length ratio (RLR), may be considered in barley breeding programs for target environments.
Theoretically, a deep root system with large root biomass would be optimal for cereal crops grown across the southwest and northern grain-growing region of Australia, and other regions where in-season rainfall is limited and terminal drought stress is common [34]. A deep root system is thought to be conducive to the greater acquisition of resources (water and nutrients) under drought for a number of crops, including barley [16]. In barley, although the value of a narrow and deep RSA for water capture and yield improvement has yet to be explored [20], the genotypes with greater root biomass and root length accompanied by lower ratio of root biomass in the top layer compared to the total root length could be considered for breeding cultivars with increased resistance to drought stress.
Different genotypes showed wide variation in the root distribution in the top layer (0–20 cm), with the ratio of top-layer root biomass to the total root biomass ranging from 0.27 to 0.61 in semi-hydroponics. For example, the ratio of topsoil root biomass to the total root biomass for genotype #5 was 0.51 (ranked the 12th highest among 189 genotypes and with 896 mg of total root dry weight/plant), whereas it was 0.36 for genotype #128 (with 1787 mg of total root dry weight/plant). The root length ratio in two soil media of eight selected genotypes also confirmed that genotype #128 might have deeper root distribution and an advantage in accessing water nutrients from deep parts of the soil profile compared to genotype #5 [35].
Devising high-throughput screening techniques for accurate and efficient phenotyping is vital for determining root-related traits in a diverse germplasm pool [36,37]. However, accurate phenotyping of plant root systems is challenging. Roots may respond to different soil environments in specific ways, with phenotypic plasticity an important component of foraging for soil resources and responding to soil constraints [38]. Phenotyping systems therefore need to be capable of producing reliable rankings of root system phenotypes, and the choice of growth media needs to be carefully considered. A novel semi-hydroponic phenotyping system has recently been developed to characterize genetic variation in root traits in the world collection of lupin accessions [10], and there was a relatively consistent ranking of genotypes between the two independent semi-hydroponic growth systems [17], and between phenotyping experiments in semi-hydroponics and different soil media in the glasshouse in narrow-leafed lupin [10,39], wheat [40,41] and soybean (Yinglong Chen, unpublished). In the present study, the eight selected barley genotypes showed a consistent ranking in root biomass, root length, shoot biomass and shoot height in three different growth media (i.e., semi-hydroponics and two different soils). The eight genotypes showed consistency in their biomass allocation to roots vs. shoots, in particular under the growth conditions of semi-hydroponics and sandy loam. These results indicated the reliability of the semi-hydroponic system to mimic relevant conditions in the early growth stage [17]. There was, however, a poor correlation in shoot biomass between the semi-hydroponic system and each of the two soil media, and between the two soil media, highlighting the challenges involved in ranking genotypes in different media.
5. Conclusions
This phenotyping study demonstrated large variation in root architectural traits across a diverse panel of barley germplasm. The consistent ranking of selected genotypes based on major root traits in the semi-hydroponic phenotyping system and the two different soils confirmed the capacity of the semi-hydroponic phenotyping system to reliably characterize root morphological traits in the early growth stage. The present study and follow-up research under controlled and field environments have potential applications in the breeding selection of suitable root traits for efficient water and nutrient capture in the targeted edaphic environments.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11081583/s1, Table S1: List of 189 genotypes of barley (Hordeum vulgare L.), and their origins, used in this study, Figure S1: Principal component analysis showing the variation of 16 traits in barley germplasm grown in the semi-hydroponic phenotyping system, The position of each trait in 2-D plots is shown for principal component PC2 vs. PC1 presenting 58.3% (A), and PC3 vs. PC1 presenting 56.9% of the total variation (B), Figure S2: Dendrogram of Agglomerative Hierarchical Clustering (AHC) using the Pearson correlation coefficients (>0.25) selected from 26 root and shoot traits tested, The 189 genotypes were assigned into one of three general groups (Clade I to Clade III) at rescaled distance of 15, Figure S3: Correlations of root biomass (A), shoot biomass (B), shoot height (C) and root length (D) of barley genotypes grown in the semi-hydroponic system vs. the two soils (sandy soil and sandy loam). Figure S4: Correlations of root biomass (A), shoot biomass (B), shoot height (C) and root length (D) of barley genotypes grown in sandy soil vs. sandy loam.
Author Contributions
Y.C. and Z.R. conceived, designed and supervised the study. J.W. carried out experimental work. Y.Z. (Yongen Zhang), Y.F. and P.D. assisted in glasshouse work and root scanning. Y.Z. (Yongchun Zhang) and Y.A. helped in data interpretation. J.W. and Y.C. wrote the manuscript. J.W., Y.C., A.D., D.M. and Z.R. revised the manuscript. All authors read and approved the final manuscript.
Funding
Australian Research Council (DP160104434) provided funds for the research experiment. Earmarked Fund for China Agriculture Research System (CARS-10-B-9) and Jiangsu Agricultural Independent Innovation Fund (CX (17)-1001) provided finances to Jidong Wang for data analysis and writing the manuscript.
Data Availability Statement
Relevant data generated or analyzed during this study are included in this article and its supplementary information files. Other data are available upon request to the corresponding author.
Acknowledgments
Jidong Wang, Yongen Zhang and Yupeng Feng were supported by China Scholar Council for their study visits to The University of Western Australia. InterGrain Pty Ltd. Western Australia provided seed materials for this study. We thank Robert Creasy and Bill Piasini for technical support in the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AHC: Agglomerative hierarchical clustering; AWCC: Australia Winter Cereals Collection; CSIRO: Australian Commonwealth Scientific and Industrial Research; CV: Coefficients of variation; DAT: Days after transplanting; GLM: General Linear Model; P: Probability; PCA: Principal component analysis.
References
- Bartlett, J.G.; Alves, S.C.; Smedley, M.; Snape, J.W.; Harwood, W.A. High-throughput Agrobacterium-mediated barley transformation. Plant Methods 2008, 4, 22. [Google Scholar] [CrossRef]
- Araya, A.; Keesstra, S.D.; Stroosnijder, L. Simulating yield response to water of teff (Eragrostis tef) with Fao’s aquacrop model. Field Crop Res. 2010, 116, 196–204. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Hunt, J.; Angessa, T.T.; Meinke, H.; Li, C.; Tian, X.; Zhou, M. Identifying optimal sowing and flowering periods for barley in Australia: A modelling approach. Agric. For. Meteorol. 2020, 282-283, 107871. [Google Scholar] [CrossRef]
- Schulte, D.; Close, T.J.; Graner, A.; Langridge, P.; Matsumoto, T.; Tuehlbauer, G.; Sato, K.; Schulman, A.H.; Waugh, R.; Wise, R.P.; et al. The international barley sequencing consortium—at the threshold of efficient access to the barley genome. Plant Physiology 2009, 149, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Samac, D.A.; Tesfaye, M. Plant improvement for tolerance to aluminum in acid soils—A review. Plant Cell Tissue Organ Cult. 2003, 75, 189–207. [Google Scholar] [CrossRef]
- Mayer, K.F.; Waugh, R.; Brown, J.W.; Schulman, A.; Langridge, P.; Platzer, M.; Fincher, G.B.; Muehlbauer, G.J.; Sato, K.; Close, T.J.; et al. A physical, genetic and functional sequence assembly of the barley genome. Nature 2012, 491, 711–716. [Google Scholar] [PubMed]
- Hayes, P.M.; Castro, A.; Marquez-Cedillo, L.; Corey, A.; Henson, C.; Jones, B.L.; Kling, J.; Mather, D.; Matus, I.; Rossi, C.; et al. Genetic diversity for quantitative inherited agronomic and malting quality traits. In Diversity in Barley; Von-Bothmer, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Dunbabin, V.M.; Postma, J.; Schnepf, A.; Pagès, L.; Javaux, M.; Wu, L.; Leitner, D.; Chen, Y.; Rengel, Z.; Diggle, A.J. Model-ling root–soil interactions using three–dimensional models of root growth, architecture and function. Plant Soil 2013, 372, 93–124. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.; Rengel, Z. Assessing variability in root traits of wild Lupinus an-gustifolius germplasm: Basis for modelling root system structure. Plant Soil 2012, 354, 141–155. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. Lond. 2012, 367, 1598–1604. [Google Scholar] [CrossRef]
- Tuberosa, R.; Sanguineti, M.C.; Landi, P.; Giuliani, M.M.; Salvi, S.; Conti, S. Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol. Biol. 2002, 48, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- De Dorlodot, S.; Forster, B.; Pagès, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef]
- Naz, A.A.; Arifuzzaman, M.; Muzammil, S.; Pillen, K.; Léon, J. Wild barley introgression lines revealed novel QTL alleles for root and related shoot traits in the cultivated barley (Hordeum vulgare L.). BMC Genet. 2014, 15, 1–12. [Google Scholar] [CrossRef]
- Lynch, J.P. Roots of the Second Green Revolution. Aust. J. Bot. 2007, 55, 493–512. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.; Rengel, Z. Development of a novel semi-hydroponic phenotyping system for studying root architecture. Funct. Plant Biol. 2011, 38, 355–363. [Google Scholar] [CrossRef]
- Chen, Y.; Ghanem, M.E.; Siddique, K.H. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2016, 68, 1987–1999. [Google Scholar] [CrossRef]
- Qiao, S.; Fang, Y.; Wu, A.; Xu, B.; Zhang, S.; Deng, X.; Djalovic, I.; Siddique, K.; Chen, Y. Dissecting root trait variability in maize genotypes using the semi-hydroponic phenotyping platform. Plant Soil 2018, 439, 75–90. [Google Scholar] [CrossRef]
- Robinson, H.; Kelly, A.; Fox, G.; Franckowiak, J.; Borrell, A.; Hickey, L. Root architectural traits and yield: Exploring the rela-tionship in barley breeding trials. Euphytica 2018, 214, 1–16. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Mikołajczak, K.; Ogrodowicz, P.; Ćwiek-Kupczyńska, H.; Weigelt-Fischer, K.; Mothukuri, S.R.; Junker, A.; Altmann, T.; Krystkowiak, K.; Adamski, T.; Surma, M.; et al. Image Phenotyping of Spring Barley (Hordeum vulgare L.) RIL Population Under Drought: Selection of Traits and Biological Interpretation. Front. Plant Sci. 2020, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Mora, F.; Castillo, D.; Lado, B.; Matus, I.; Poland, J.; Belzile, F.; Von Zitzewitz, J.; Del Pozo, A. Genome-wide association mapping of agronomic traits and carbon isotope discrimination in a worldwide germplasm collection of spring wheat using SNP markers. Mol. Breed. 2015, 35, 69. [Google Scholar] [CrossRef]
- Mir, R.R.; Zaman-Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645. [Google Scholar] [CrossRef]
- Mansour, E.; Casas, A.M.; Gracia, M.P.; Molina-Cano, J.L.; Moralejo, M.; Cattivelli, L.; Thomas, W.T.B.; Igartua, E. Quantitative trait loci for agronomic traits in an elite barley population for Mediterranean conditions. Mol. Breed. 2013, 33, 249–265. [Google Scholar] [CrossRef]
- Wijesinghe, D.K.; John, E.A.; Beurskens, S.; Hutchings, M.J. Root system size and precision in nutrient foraging: Responses to spatial pattern of nutrient supply in six herbaceous species. J Ecol. 2001, 89, 972–983. [Google Scholar] [CrossRef]
- Svačina, P.; Středa, T.; Chloupek, O. Uncommon selection by root system size increases barley yield. Agron. Sustain. Dev. 2013, 34, 545–551. [Google Scholar] [CrossRef]
- He, Y.B.; Lin, L.R.; Chen, J.Z. Maize root morphology responses to soil penetration resistance related to tillage and drought in a clayey soil. J. Agric. Sci. 2017, 155, 1137–1149. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Redillas, M.C.F.R.; Jang, G.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.-H.; Reuzeau, C.; Kim, J.-K. OsNAC5overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 2012, 11, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, J.; Horn, R.; Pietrusiewicz, J.; Siczek, A. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil Tillage Res. 2012, 121, 74–81. [Google Scholar] [CrossRef]
- Schenk, H.J. Root competition: Beyond resource depletion. J. Ecol. 2006, 94, 725–739. [Google Scholar] [CrossRef]
- Carvalho, P.; Azam-Ali, S.; Foulkes, M.J. Quantifying relationships between rooting traits and water uptake under drought in Mediterranean barley and durum wheat. J. Integr. Plant. Biol. 2013, 56, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.H.; Sepaskhah, A.R.; Zarei, M. Specific root length, soil water status, and grain yields of irrigated and rainfed winter barley in the raised bed and flat planting systems. Agric. Water Manag. 2018, 210, 304–315. [Google Scholar] [CrossRef]
- Chenu, K.; Cooper, M.; Hammer, G.; Mathews, K.; Dreccer, M.F.; Chapman, S. Environment characterization as an aid to wheat improvement: Interpreting genotype–environment interactions by modelling water-deficit patterns in North-Eastern Australia. J. Exp. Bot. 2011, 62, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z. Breeding crops for adaptation to environments with low nutrient availability. In Abiotic stresses: Plant Resistance Through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; The Haworth: New York, NY, USA, 2005. [Google Scholar]
- Chen, Y.; Shan, F.; Nelson, M.N.; Siddique, K.; Rengel, Z. Root trait diversity, molecular marker diversity, and trait-marker associations in a core collection of Lupinus angustifolius. J. Exp. Bot. 2016, 67, 3683–3697. [Google Scholar] [CrossRef][Green Version]
- Kembel, S.W.; De Kroon, H.; Cahill, J.F.; Mommer, L. Improving the scale and precision of hypotheses to explain root foraging ability. Ann Bot. 2008, 101, 1295–1301. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Postma, J.; Diggle, A.J.; Palta, J.; Lynch, J.P.; Siddique, K.; Rengel, Z. Phenotypic variability and modelling of root structure of wild Lupinus angustifolius genotypes. Plant Soil 2011, 348, 345–364. [Google Scholar] [CrossRef]
- Chen, Y.; Palta, J.; Prasad, P.V.V.; Siddique, K. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plan. Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Figueroa-Bustos, V.; Palta, J.A.; Chen, Y.; Siddique, K.H.M. Characterization of root and shoot traits in wheat cultivars with putative differences in root system size. Agronomy 2018, 8, 109. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).