Efficacy of Fungicides against Fusarium Head Blight Depends on the Timing Relative to Infection Rather than on Wheat Growth Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Disease Assessment and Mycotoxin Contamination
2.3. Data Analysis
3. Results
3.1. Timing of Fungicides at Different Wheat Growth Stages (EXP 1)
3.2. Timing of Fungicides Relative to Timing of F. graminearum Inoculation (EXP2)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keller, M.D.; Bergstrom, G.C.; Shields, E.J. The aerobiology of Fusarium graminearum. Aerobiologia (Bologna). 2014, 30, 123–136. [Google Scholar] [CrossRef]
- Savary, S.; Djurle, A.; Yuen, J.; Ficke, A.; Rossi, V.; Esker, P.D.; Fernandes, J.M.C.; Del Ponte, E.M.; Kumar, J.; Madden, L.V.; et al. A White Paper on Global Wheat Health Based on Scenario Development and Analysis. Phytopathology 2017, 107, 1109–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X. Effects of environmental conditions on the development of Fusarium ear blight. Eur. J. Plant Pathol. 2003, 109, 683–689. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Spolti, P.; Ward, T.J.; Gomes, L.B.; Nicolli, C.P.; Kuhnem, P.R.; Silva, C.N.; Tessmann, D.J. Regional and field-specific factors affect the composition of Fusarium head blight pathogens in subtropical no-till wheat agroecosystem of Brazil. Phytopathology 2015, 105, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Nazari, L.; Pattori, E.; Somma, S.; Manstretta, V.; Waalwijk, C.; Moretti, A.; Meca, G.; Rossi, V. Infection incidence, kernel colonisation, and mycotoxin accumulation in durum wheat inoculated with Fusarium sporotrichioides, F. langsethiae or F. poae at different growth stages. Eur. J. Plant Pathol. 2019, 153, 715–729. [Google Scholar] [CrossRef]
- Xu, X.M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Willocquet, L.; Meza, W.R.; Dumont, B.; Klocke, B.; Feike, T.; Kersebaum, K.C.; Meriggi, P.; Rossi, V.; Ficke, A.; Djurle, A.; et al. An outlook on wheat health in Europe from a network of field experiments. Crop Prot. 2021, 139, 105335. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food : Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Bolanos-Carriel, C.; Wegulo, S.N.; Baenziger, P.S.; Funnell-Harris, D.; Hallen-Adams, H.E.; Eskridge, K.M. Effects of fungicide chemical class, fungicide application timing, and environment on Fusarium head blight in winter wheat. Eur. J. Plant Pathol. 2020, 158, 667–679. [Google Scholar] [CrossRef]
- Manstretta, V.; Gourdain, E.; Rossi, V. Deposition patterns of Fusarium graminearum ascospores and conidia within a wheat canopy. Eur. J. Plant Pathol. 2015, 143, 873–880. [Google Scholar] [CrossRef]
- Manstretta, V.; Rossi, V. Effects of weather variables on ascospore discharge from Fusarium graminearum perithecia. PLoS ONE 2015, 10, e0138860. [Google Scholar] [CrossRef] [Green Version]
- Manstretta, V.; Morcia, C.; Terzi, V.; Rossi, V. Germination of Fusarium graminearum ascospores and wheat infection are affected by dry periods and by temperatura and humidity during dry periods. Phytopathology 2016, 106, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Kriss, A.B.; Paul, P.A.; Madden, L.V. Relationship between yearly fluctuations in Fusarium head blight intensity and environmental variables: A window-pane analysis. Phytopathology 2010, 100, 784–797. [Google Scholar] [CrossRef]
- Rossi, V.; Ravanetti, A.; Pattori, E.; Giosuè, S. Influence of temperature and humidity on the infection of wheat spikes by some fungi causing fusarium head blight. J. Plant Pathol. 2001, 83, 189–198. [Google Scholar]
- Del Ponte, E.M.; Fernandes, J.M.C.; Bergstrom, G.C. Influence of growth stage on fusarium head blight and deoxynivalenol production in wheat. J. Phytopathol. 2007, 155, 577–581. [Google Scholar] [CrossRef] [Green Version]
- McCallum, B.D.; Tekauz, A. Influence of inoculation method and growth stage on fusarium head blight in barley. Can. J. Plant Pathol. 2002, 24, 77–80. [Google Scholar] [CrossRef]
- Paul, P.A.; Salgado, J.D.; Bergstrom, G.; Bradley, C.A.; Byamukama, E.; Byrne, A.M.; Chapara, V.; Cummings, J.A.; Chilvers, M.I.; Dill-Macky, R.; et al. Integrated effects of genetic resistance and prothioconazole + tebuconazole application timing on Fusarium head blight in wheat. Plant Dis. 2019, 103, 223–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L. V Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.A.; McMullen, M.P.; Hershman, D.E.; Madden, L.V. Meta-analysis of the effects of triazole-based fungicides on wheat yield and test weight as influenced by Fusarium head blight intensity. Phytopathology 2010, 100, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Haidukowski, M.; Visconti, A.; Perrone, G.; Vanadia, S.; Pancaldi, D.; Covarelli, L.; Balestrazzi, R.; Pascale, M. Effect of prothioconazole-based fungicides on Fusarium head blight, grain yield and deoxynivalenol accumulation in wheat under field conditions. Phytopathol. Mediterr. 2012, 51, 236–246. [Google Scholar]
- D’Angelo, D.L.D.; Bradley, C.A.; Ames, K.A.; Willyerd, K.T.; Madden, L.; Paul, P. Efficacy of fungicide applications during and after anthesis against Fusarium head blight and deoxynivalenol in soft red winter wheat. Plant Dis 2014, 98, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001. [Google Scholar]
- Rossi, V.; Manstretta, V.; Meriggi, P.; Silvestri, M. Le malattie del grano nell’Italia settentrionale. Terra E Vita 2014, 6, 6–9. [Google Scholar]
- Somma, S.; Alvarez, C.; Ricci, V.; Ferracane, L.; Ritieni, A.; Logrieco, A.; Moretti, A. Trichothecene and beauvericin mycotoxin production and genetic variability in Fusarium poae isolated from wheat kernels from northern Italy. Food Addit. Contam. 2010, 27, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FRAC. FRAC Code List 2020: Fungicides Sorted by Mode of Action. Available online: http://www.phi-base.org/images/fracCodeList.pdf (accessed on 31 July 2021).
- Oliver, R.E.; Cai, X.; Friesen, T.L.; Halley, S.; Stack, R.W.; Xu, S.S. Evaluation of Fusarium head blight resistance in tetraploid wheat (Triticum turgidum L.). Crop. Sci. 2008, 48, 213–222. [Google Scholar] [CrossRef]
- R CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Madden, L.V.; Turechek, W.W.; Nita, M. Evaluation of generalized linear mixed models for analyzing disease incidence. Plant Dis. 2002, 86, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douma, J.C.; Weedon, J.T. Analysing continuous proportions in ecology and evolution: A practical introduction to beta and Dirichlet regression. Methods Ecol. Evol. 2019, 10, 1412–1430. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef] [Green Version]
- Crawley, M. The R Book, 2 nd ed.; Wiley: Chichester, UK, 2013. [Google Scholar]
- Quinn, G.; Keough, M. Experimental Design and Data Analysis for Biologists; MIT Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Hartig, F. DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. 2021. Available online: http://florianhartig.github.io/DHARMa/ (accessed on 31 July 2021).
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. A quantitative review of tebuconazole effect on Fusarium head blight and deoxynivalenol content in wheat. Phytopathology 2007, 97, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Machado, F.J.; Santana, F.M.; Lau, D.; Del Ponte, E.M. Quantitative review of the effects of triazole and benzimidazole fungicides on Fusarium head blight and wheat yield in Brazil. Plant Dis. 2017, 101, 1633–1641. [Google Scholar] [CrossRef] [Green Version]
- Rossi, V.; Sperandio, G.; Caffi, T.; Simonetto, A.; Gianni, G. Critical success factors for the adoption of decision tools in IPM. Agronomy 2019, 9, 710. [Google Scholar] [CrossRef] [Green Version]
- Rossi, V.; Caffi, T.; Salinari, F. Helping farmers face the increasing complexity of decision-making for crop protection. Phytopathol. Mediterr. 2012, 51, 457–479. [Google Scholar]
- Mueller, D.; Kiersten, A.; Dufault, N.; Bradley, C.A.; Chilvers, M.I. Part I. Introduction. In Fungicides for Field Crops; APS Press: St Paul, MN, USA,, 2017; pp. 1–13. [Google Scholar]
- Mueller, D.; Kiersten, A.; Dufault, N.; Bradley, C.; Chilvers, M. Part IV. Using fungicides to manage diseases on field crops. In Fungicides for Field Crops; APS Press: St Paul, MN, USA, 2017; pp. 43–101. [Google Scholar]
- Musa, T.; Hecker, A.; Vogelgsang, S.; Forrer, H.R. Forecasting of Fusarium head blight and deoxynivalenol content in winter wheat with FusaProg. EPPO Bull. 2007, 37, 283–289. [Google Scholar] [CrossRef]
- Bondalapati, K.D.; Dakota, S.; Stein, J.M.; Science, P.; Neate, S.M.; Pathology, P.; Dakota, N.; Halley, S.H. Development of weather-based predictive models for Fusarium head blight and deoxynivalenol accumulation for spring malting barley. Plant Disease 2012, 96, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klem, K.; Váňová, M.; Hajšlová, J.; Lancová, K.; Sehnalová, M. A neural network model for prediction of deoxynivalenol content in wheat grain based on weather data and preceding crop. Plant, Soil Environ. 2007, 53, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Gourdain, E.; Piraux, F.; Barrier-Guillot, B. A model combining agronomic and weather factors to predict occurrence of deoxynivalenol in durum wheat kernels. World Mycotoxin J. 2011, 4, 129–139. [Google Scholar] [CrossRef]
- Rossi, V.; Giosuè, S.; Pattori, E.; Spanna, F.; Vecchio, A. Del A model estimating the risk of Fusarium head blight on wheat *. EPPO Bull. 2003, 421–425. [Google Scholar] [CrossRef]
- Rossi, V.; Giosuè, S.; Terzi, V.; Scudellari, D. A decision support system for Fusarium head blight on small grain cereals. EPPO Bull. 2007, 37, 359–367. [Google Scholar] [CrossRef]
- Rossi, V.; Racca, P.; Giosuè, S.; Pancaldi, D.; Alberti, I. A simulation model for the development of brown rust epidemics in winter wheat. Eur. J. Plant Pathol. 1997, 103, 453–465. [Google Scholar] [CrossRef]
- Rossi, V.; Manstretta, V.; Ruggeri, M. A multicomponent decision support system to manage Fusarium head blight and mycotoxins in durum wheat. World Mycotoxin J. 2015, 8, 629–640. [Google Scholar] [CrossRef]
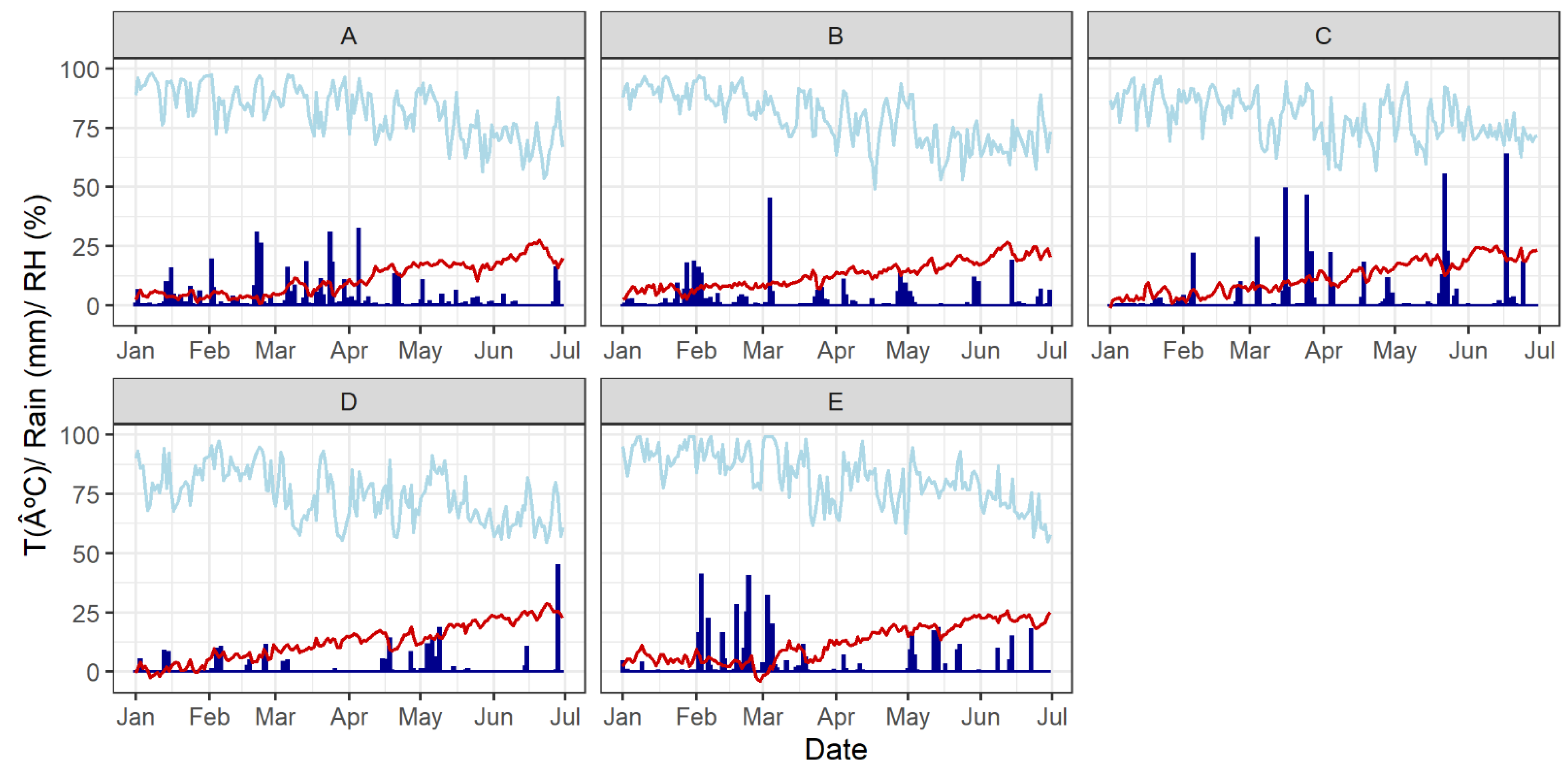
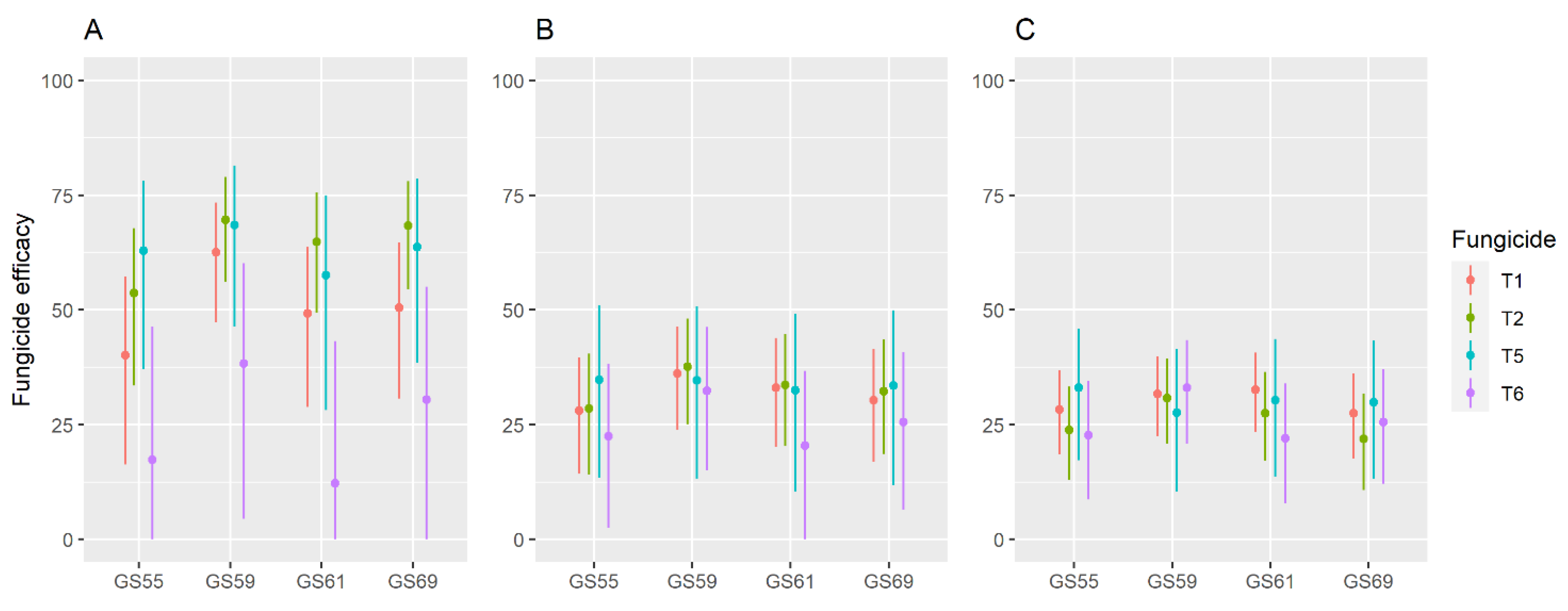

| Experiment 1 | Year | Cultivar | Previous Crop | N. of Subplots 2 | Fungicides 3 |
|---|---|---|---|---|---|
| EXP1 | 2013 | Normanno | Corn | 29 | ARE (T1) / AMIS (T2)/ CAR (T1) / CUST (T2) / ENOV + TEB (T6)/ ORI (T1)/ PROS (T1) |
| 2014 | Normanno | Corn | 29 | AMIS (T2)/ CAR (T1)/ CUST (T2)/ ENOV + TEB (T6)/ FOLI (T1)/ ORI (T1)/ PROS (T1) | |
| 2015 | Normanno | Corn | 17 | MIR + CARN (T2)/ FOLP + ORI (T5)/ ORI (T1)/ PROS (T1) | |
| EXP2 | 2017 | Obelix | Sugarbeet | 36 | CAR (T1)/ ENOV (T4)/ MYST (T1)/ PROL (T1)/ SAK (T1)/ SPOR (T1) |
| 2018 | Obelix | Peas | 36 | BIN (T1)/ CAR (T1)/ MYST (T1)/ PROL (T1)/ SEG (T3)/ SPOR (T1) |
| Code in Table 1 | Commercial Name 1 | Active Ingredients (Concentration %) | Dose 2 | MOA 3 | Producer |
|---|---|---|---|---|---|
| AMIS | Amistar Plus | azoxystrobin (6.8) + tebuconazole (11.7) | 2 l/ha | QoI(11) + DMI(3) | Syngenta |
| ARE | Ares 25 WG | tebuconazole (25.0) | 1 kg/ha | DMI (3) | Nufarm |
| BIN | Binal Pro | tetraconazole (3.9) + prochloraz (21.9) | 1.96 l/ha | DMI (3) | Gowan |
| CAR | Caramba | metconazole (8.6) | 1 l/ha | DMI (3) | BASF |
| CARN | Carnival | prochloraz (35.6) | 1.1 l/ha | DMI (3) | Adama |
| CUST | Custodia | azoxystrobin (11.0) + tebuconazole (18.4) | 1.25 l/ha | QoI(11) + DMI(3) | Adama |
| ENOV | Enovit Metil | thiophanate-methyl (41.7) | 1.25 l/ha | MBC (1) | Sipcam |
| FOLI | Folicur WG | tebuconazole (25.0) | 1 kg/ha | DMI (3) | Bayer |
| FOLP | Folpan 80 WG | folpet (80.0) | 0.9 kg/ha | MS (M04) | Adama |
| ORI | Orius P | tebuconazole (12.3) + prochloraz (24.6) | 1.7 l/ha | DMI (3) | Adama |
| MIR | Mirador SC | azoxystrobin (23.2) | 1 l/ha | QoI (11) | Adama |
| MYST | Mystic 430 SC | tebuconazole (40.2) | 0.58 l/ha | DMI (3) | Nufarm |
| PROL | Proline | prothioconazole (25.0) | 0.8 l/ha | DMI (3) | Bayer |
| PROS | Prosaro | tebuconazole (12.7) + prothioconazole (12.7) | 1 l/ha | DMI (3) | Bayer |
| SAK | Sakura | bromuconazole (15.9) + tebuconazole (10.2) | 1.2 l/ha | DMI (3) | Nufarm |
| SEG | Seguris Era | isopyrazam (11.2) + prothioconazole (13.5) | 1 l/ha | SDHI (7) + DMI(3) | Syngenta |
| SPOR | Sportak 45 EW | prochloraz (39.8) | 1 l/ha | DMI (3) | BASF |
| TEB | Tebusip 46 | tebuconazole (4.5) | 46.5 l/ha | DMI (3) | Sipcam |
| EXP 1 | Variable | Model 2 | Factors 3 | AIC 4 | Deviance | Chisq | P (>Chisq) |
|---|---|---|---|---|---|---|---|
| EXP1 | FHB incidence | 1.1 | (1|YEAR) | 24456 | 24452 | - | - |
| 1.2 | FUNG + (1|YEAR) | 24100 | 24088 | 364.6 | <0.001 | ||
| 1.3 | FUNG + TIME + (1| YEAR) | 24047 | 24027 | 60.7 | <0.001 | ||
| 1.4 | FUNG×TIME + (1|YEAR) | 23991 | 23939 | 88.1 | <0.001 | ||
| FHB severity | 1.5 | (1|YEAR) | −64025 | −64031 | - | - | |
| 1.6 | FUNG + (1|YEAR) | −64411 | −64425 | 393.5 | <0.001 | ||
| 1.7 | FUNG + TIME + (1| YEAR) | −64456 | −64478 | 52.7 | <0.001 | ||
| 1.8 | FUNG×TIME + (1|YEAR) | −64479 | −64533 | 54.8 | <0.001 | ||
| DON | 1.9 | (1|YEAR) | −10765 | −10771 | - | - | |
| 1.10 | FUNG + (1|YEAR) | −11326 | −11340 | 569.1 | <0.001 | ||
| 1.11 | FUNG + TIME + (1| YEAR) | −11404 | −11426 | 86.0 | <0.001 | ||
| 1.12 | FUNG×TIME + (1|YEAR) | −11455 | −11509 | 83.0 | <0.001 | ||
| EXP2 | FHB incidence | 2.1 | (1|YEAR) | 10796 | 10792 | - | - |
| 2.2 | FUNG + (1|YEAR) | 10040 | 10030 | 761.3 | <0.001 | ||
| 2.3 | FUNG + TIME + (1| YEAR) | 9582 | 9560 | 470.3 | <0.001 | ||
| 2.4 | FUNG×TIME + (1|YEAR) | 9359 | 9301 | 258.5 | <0.001 | ||
| FHB severity | 2.5 | (1|YEAR) | −55317 | −55323 | - | - | |
| 2.6 | FUNG + (1|YEAR) | −55886 | −55898 | 574.6 | <0.001 | ||
| 2.7 | FUNG + TIME + (1| YEAR) | −56070 | −56094 | 196.6 | <0.001 | ||
| 2.8 | FUNG×TIME + (1|YEAR) | −56287 | −56347 | 252.8 | <0.001 | ||
| DON | 2.9 | (1|YEAR) | −18734 | −18740 | - | - | |
| 2.10 | FUNG + (1|YEAR) | −19790 | −19802 | 1061.7 | <0.001 | ||
| 2.11 | FUNG + TIME + (1| YEAR) | −20127 | −20151 | 349.5 | <0.001 | ||
| 2.12 | FUNG×TIME + (1|YEAR) | −20622 | −20682 | 530.8 | <0.001 |
| Growth Stage 1 | Fungicide Group 2 | FHB Incidence 3 | FHB Severity | DON | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L 4 | se (L) 5 | P(Z) 6 | L | se (L) | P(Z) | L | se (L) | P(Z) | ||
| GS55 | T1 | −0.514 | 0.171 | 0.003 | −0.330 | 0.089 | <0.001 | −0.332 | 0.065 | <0.001 |
| T2 | −0.769 | 0.185 | <0.001 | −0.335 | 0.093 | <0.001 | −0.272 | 0.068 | <0.001 | |
| T5 | −0.990 | 0.269 | <0.001 | −0.428 | 0.144 | 0.003 | −0.402 | 0.109 | <0.001 | |
| T6 | −0.190 | 0.221 | 0.389 | −0.254 | 0.117 | 0.030 | −0.257 | 0.085 | <0.001 | |
| GS59 | T1 | −0.981 | 0.174 | <0.001 | −0.448 | 0.090 | <0.001 | −0.382 | 0.065 | <0.001 |
| T2 | −1.190 | 0.187 | <0.001 | −0.473 | 0.093 | <0.001 | −0.368 | 0.068 | <0.001 | |
| T5 | −1.155 | 0.271 | <0.001 | −0.426 | 0.144 | 0.003 | −0.323 | 0.109 | 0.003 | |
| T6 | −0.483 | 0.223 | 0.030 | −0.392 | 0.117 | <0.001 | −0.402 | 0.085 | <0.001 | |
| GS61 | T1 | −0.678 | 0.172 | <0.001 | −0.402 | 0.089 | <0.001 | −0.394 | 0.065 | <0.001 |
| T2 | −1.043 | 0.185 | <0.001 | −0.410 | 0.093 | <0.001 | −0.321 | 0.068 | <0.001 | |
| T5 | −0.857 | 0.269 | <0.001 | −0.393 | 0.144 | 0.006 | −0.361 | 0.109 | 0.001 | |
| T6 | −0.130 | 0.222 | 0.558 | −0.229 | 0.117 | 0.050 | −0.249 | 0.085 | 0.003 | |
| GS69 | T1 | −0.704 | 0.172 | <0.001 | −0.361 | 0.089 | <0.001 | −0.321 | 0.065 | <0.001 |
| T2 | −1.150 | 0.186 | <0.001 | −0.389 | 0.093 | <0.001 | −0.247 | 0.068 | <0.001 | |
| T5 | −1.014 | 0.270 | <0.001 | −0.409 | 0.144 | 0.004 | −0.356 | 0.109 | 0.001 | |
| T6 | −0.363 | 0.222 | 0.102 | −0.295 | 0.117 | 0.011 | −0.296 | 0.085 | 0.001 | |
| Fungicide Timing 1 | Fungicide Group 2 | FHB Incidence 3 | FHB Severity | DON | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L4 | se (L) 5 | P(Z) 6 | L | se (L) | P(Z) | L | se (L) | P(Z) | ||
| -10DI | T1 | −1.364 | 0.271 | <0.001 | −0.730 | 0.105 | <0.001 | −1.376 | 0.126 | <0.001 |
| T3 | −2.554 | 0.494 | <0.001 | −1.377 | 0.154 | <0.001 | −2.286 | 0.192 | <0.001 | |
| T4 | −0.081 | 0.376 | 0.830 | −0.049 | 0.161 | 0.761 | 0.025 | 0.197 | 0.898 | |
| -7DI | T1 | −2.075 | 0.273 | <0.001 | −0.691 | 0.106 | <0.001 | −1.133 | 0.129 | <0.001 |
| T3 | −3.350 | 0.495 | <0.001 | −1.414 | 0.155 | <0.001 | −2.196 | 0.192 | <0.001 | |
| T4 | −0.630 | 0.393 | 0.109 | 0.009 | 0.161 | 0.953 | 0.137 | 0.197 | 0.488 | |
| -4DI | T1 | −2.332 | 0.274 | <0.001 | −0.911 | 0.105 | <0.001 | −1.597 | 0.126 | <0.001 |
| T3 | −4.185 | 0.516 | <0.001 | −1.739 | 0.155 | <0.001 | −2.756 | 0.189 | <0.001 | |
| T4 | −0.953 | 0.419 | 0.023 | −0.216 | 0.161 | 0.179 | −0.409 | 0.194 | 0.035 | |
| -1DI | T1 | −2.513 | 0.276 | <0.001 | −0.902 | 0.106 | <0.001 | −1.539 | 0.127 | <0.001 |
| T3 | −4.302 | 0.524 | <0.001 | −1.721 | 0.155 | <0.001 | −2.675 | 0.189 | <0.001 | |
| T4 | −0.483 | 0.397 | 0.224 | −0.094 | 0.161 | 0.558 | −0.084 | 0.196 | 0.667 | |
| 3DI | T1 | −1.044 | 0.271 | <0.001 | −0.660 | 0.105 | <0.001 | −1.305 | 0.126 | <0.001 |
| T3 | −1.827 | 0.499 | <0.001 | −1.102 | 0.153 | <0.001 | −1.925 | 0.193 | <0.001 | |
| T4 | −0.663 | 0.406 | 0.103 | −0.192 | 0.161 | 0.232 | −0.397 | 0.194 | 0.041 | |
| 5DI | T1 | −0.871 | 0.270 | 0.001 | −0.342 | 0.106 | 0.001 | −0.598 | 0.128 | <0.001 |
| T3 | −0.868 | 0.544 | 0.111 | −0.519 | 0.152 | 0.001 | −0.829 | 0.193 | <0.001 | |
| T4 | −0.564 | 0.390 | 0.148 | 0.013 | 0.161 | 0.935 | 0.090 | 0.197 | 0.648 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Domínguez, E.; Meriggi, P.; Ruggeri, M.; Rossi, V. Efficacy of Fungicides against Fusarium Head Blight Depends on the Timing Relative to Infection Rather than on Wheat Growth Stage. Agronomy 2021, 11, 1549. https://doi.org/10.3390/agronomy11081549
González-Domínguez E, Meriggi P, Ruggeri M, Rossi V. Efficacy of Fungicides against Fusarium Head Blight Depends on the Timing Relative to Infection Rather than on Wheat Growth Stage. Agronomy. 2021; 11(8):1549. https://doi.org/10.3390/agronomy11081549
Chicago/Turabian StyleGonzález-Domínguez, Elisa, Pierluigi Meriggi, Matteo Ruggeri, and Vittorio Rossi. 2021. "Efficacy of Fungicides against Fusarium Head Blight Depends on the Timing Relative to Infection Rather than on Wheat Growth Stage" Agronomy 11, no. 8: 1549. https://doi.org/10.3390/agronomy11081549
APA StyleGonzález-Domínguez, E., Meriggi, P., Ruggeri, M., & Rossi, V. (2021). Efficacy of Fungicides against Fusarium Head Blight Depends on the Timing Relative to Infection Rather than on Wheat Growth Stage. Agronomy, 11(8), 1549. https://doi.org/10.3390/agronomy11081549







