Optimization of the Microwave-Assisted Extraction of Simple Phenolic Compounds from Grape Skins and Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Products and Reagents
2.2. Plant Material
2.3. Extraction Process
2.4. Separation and Quantification of Phenolic Compounds
2.5. Experimental Design
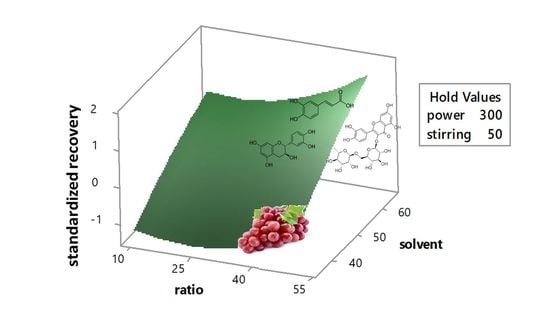
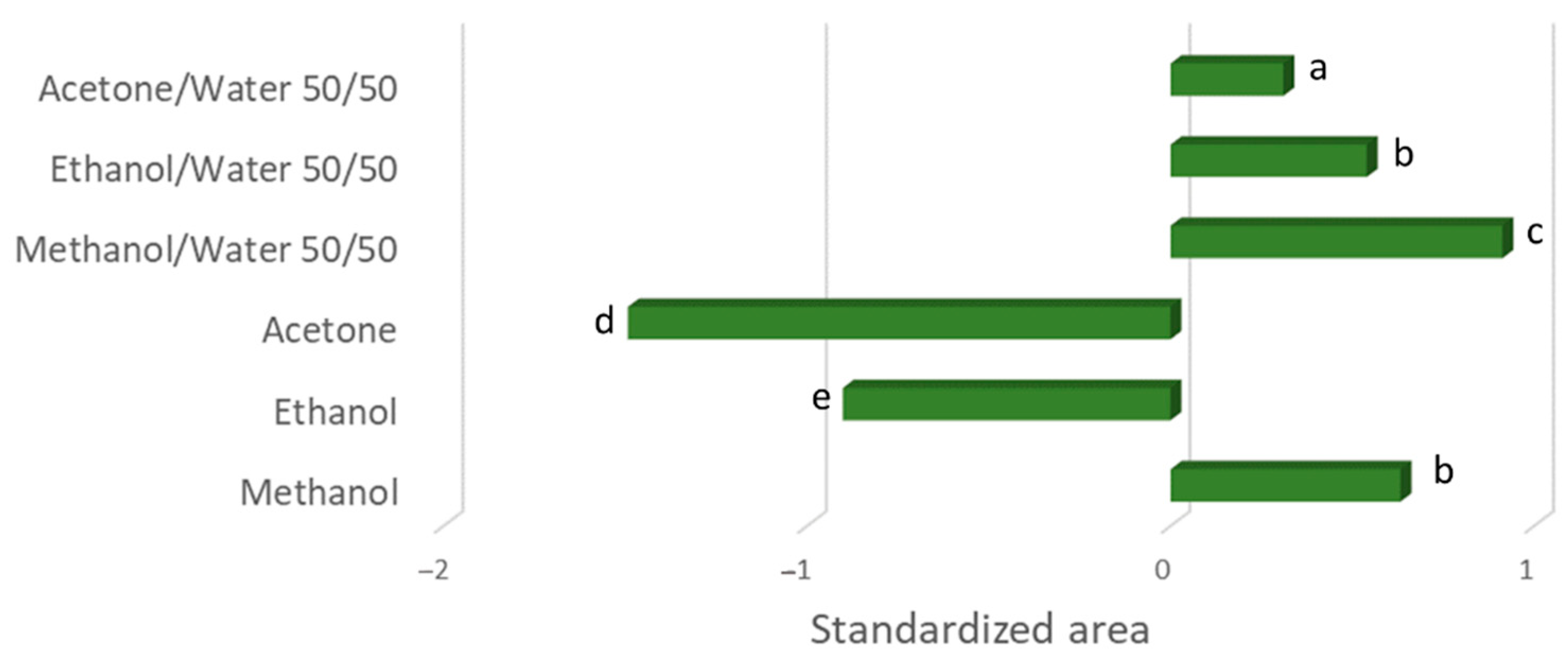
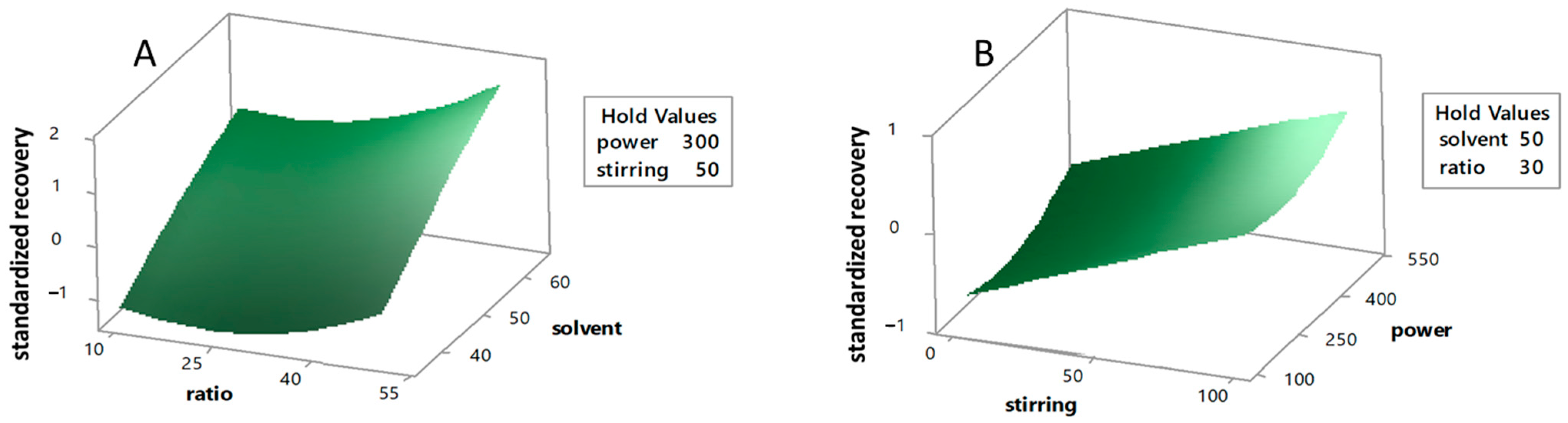
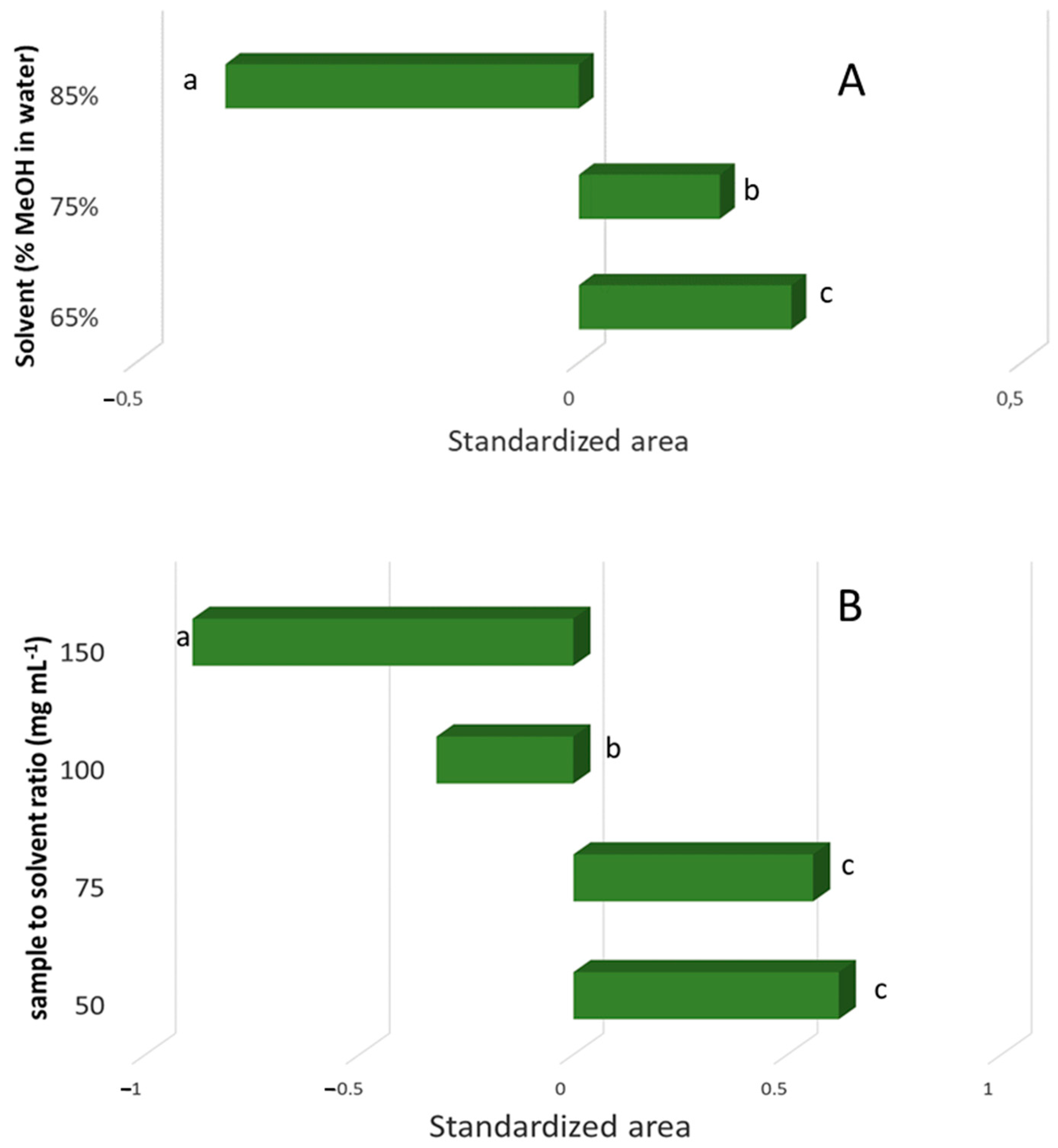
3. Results and Discussion
3.1. Preliminary Test to Evaluate the Best Solvent
3.2. Analysis of Response Surface
3.3. Effects by the Significant Extraction Variables on the Recovery
3.4. Extraction Time
3.5. Repeatability and Intermediate Precision
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unterkofler, J.; Muhlack, R.A.; Jeffery, D.W. Processes and purposes of extraction of grape components during winemaking: Current state and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 4737–4755. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.R.; Schieber, A.; Carle, R. Characterization and recovery of phenolic compounds from grape pomace—A review. J. Appl. Bot. Food Qual. 2005, 79, 189–196. [Google Scholar]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Escott, C.; Suárez Lepe, J.A. Evolution of the Phenolic Fraction and Aromatic Profile of Red Wines Aged in Oak Barrels. ACS Omega 2020, 5, 7235–7243. [Google Scholar] [CrossRef] [Green Version]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than resveratrol: New insights into stilbene-based compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Trans-Resveratrol: A Review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R. Wine Science; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780123736468. [Google Scholar]
- Pedroza, M.A.; Salinas, M.R.; Alonso, G.L.; Zalacain, A. Oenological Applications of Winemaking By-Products; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128098714. [Google Scholar]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic aspects of waste recovery in the wine industry. A multidisciplinary approach. Sci. Total Environ. 2021, 759. [Google Scholar] [CrossRef] [PubMed]
- Xavier Machado, T.D.O.; Portugal, I.B.M.; Padilha, C.V.D.S.; Ferreira Padilha, F.; dos Santos Lima, M. New trends in the use of enzymes for the recovery of polyphenols in grape byproducts. J. Food Biochem. 2021, 45. [Google Scholar] [CrossRef] [PubMed]
- Allcca-Alca, E.E.; León-Calvo, N.C.; Luque-Vilca, O.M.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Hot pressurized liquid extraction of polyphenols from the skin and seeds of vitis vinifera L. Cv. negra criolla pomace a peruvian native pisco industry waste. Agronomy 2021, 11, 866. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Amendola, D.; Maggi, L.; Zalacain, A.; De Faveri, D.M.; Spigno, G. Microwave-Assisted Extraction of Phenolic Compounds from Dried Waste Grape Skins. Int. J. Food Eng. 2015, 11, 359–370. [Google Scholar] [CrossRef]
- Hong, N.; Yaylayan, V.A.; Vijaya Raghavan, G.S.; Paré, J.R.J.; Bélanger, J.M.R. Microwave-assisted Extraction of Phenolic Compounds from Grape Seed. Nat. Prod. Lett. 2001, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.Z.; Fu, X.Q.; Deng, L.; Li, Z.L.; Dang, Y.Y. Phenolic extraction from grape (Vitis vinifera) seed via enzyme and microwave co-assisted salting-out extraction. Food Biosci. 2021, 40, 100919. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of Microwave-Assisted Extraction of Phenolic Antioxidants from Grape Seeds (Vitis vinifera). Food Bioprocess Technol. 2013, 6, 441–455. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Raks, V.; Al-Suod, H.; Buszewski, B. Isolation, Separation, and Preconcentration of Biologically Active Compounds from Plant Matrices by Extraction Techniques. Chromatographia 2018, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Sammani, M.S.; Clavijo, S.; Cerdà, V. Recent, advanced sample pretreatments and analytical methods for flavonoids determination in different samples. TrAC Trends Anal. Chem. 2021, 138. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Ayuso, J.; Barbero, G.F.; Álvarez, J.Á. Extraction of anthocyanins and total phenolic compounds from açai (Euterpe oleracea Mart.) using an experimental design methodology. part 3: Microwave-assisted extraction. Agronomy 2020, 10, 179. [Google Scholar] [CrossRef] [Green Version]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M.; Barroso, C.G. Multiresponse optimization of a UPLC method for the simultaneous determination of tryptophan and 15 tryptophan-derived compounds using a Box-Behnken design with a desirability function. Food Chem. 2017, 225, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Duros, E.; Palma, M.; Barroso, C.G. Optimization of the ultrasound-assisted extraction of melatonin from red rice (Oryza sativa) grains through a response surface methodology. Appl. Acoust. 2016, 103, 129–135. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Piovesana, S.; Ventura, S. Chromatographic methods coupled to mass spectrometry detection for the determination of phenolic acids in plants and fruits. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 353–370. [Google Scholar] [CrossRef]
- Liazid, A.; Barbero, G.F.; Azaroual, L.; Palma, M.; Barroso, C.G. Stability of anthocyanins from red grape skins under pressurized liquid extraction and ultrasound-assisted extraction conditions. Molecules 2014, 19, 21034–21043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liazid, A.; Schwarz, M.; Varela, R.M.; Palma, M.; Guillén, D.A.; Brigui, J.; Macías, F.A.; Barroso, C.G. Evaluation of various extraction techniques for obtaining bioactive extracts from pine seeds. Food Bioprod. Process. 2010, 88, 247–252. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of isoflavones from soybeans. Anal. Chim. Acta 2004, 522, 169–177. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Ayuso, J.; Álvarez, J.Á.; Barbero, G.F. Extraction of anthocyanins and total phenolic compounds from açai (Euterpe oleracea Mart.) using an experimental design methodology. part 2: Ultrasound-assisted extraction. Agronomy 2020, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Marín, R.; Fernandes, S.C.M.; Andrés, M.A.; Labidi, J. Microwave-assisted extraction of curcuma longa l. Oil: Optimization, chemical structure and composition, antioxidant activity and comparison with conventional soxhlet extraction. Molecules 2021, 26, 1516. [Google Scholar] [CrossRef] [PubMed]
- Chmelová, D.; Škulcová, D.; Legerská, B.; Horník, M.; Ondrejovič, M. Ultrasonic-assisted extraction of polyphenols and antioxidants from Picea abies bark. J. Biotechnol. 2020, 314–315, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box-Behnken experimental design and kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]

| Experiment | Methanol in Water (%) | Ratio (mg mL−1) | Power (W) | Stirring (%) | Standardized Average Area |
|---|---|---|---|---|---|
| 1 | 65 | 30 | 500 | 50 | 1.489 |
| 2 | 65 | 30 | 100 | 50 | 0.758 |
| 3 | 35 | 30 | 300 | 100 | −0.797 |
| 4 | 50 | 30 | 100 | 100 | 0.680 |
| 5 | 50 | 50 | 300 | 0 | 0.676 |
| 6 | 50 | 10 | 500 | 50 | 0.379 |
| 7 | 35 | 30 | 100 | 50 | −0.766 |
| 8 | 50 | 30 | 500 | 100 | 0.895 |
| 9 | 35 | 30 | 300 | 0 | −1.287 |
| 10 | 50 | 50 | 500 | 50 | 1.082 |
| 11 | 50 | 30 | 300 | 50 | 0.613 |
| 12 | 65 | 30 | 300 | 0 | −0.046 |
| 13 | 50 | 30 | 500 | 0 | −1.059 |
| 14 | 35 | 30 | 500 | 50 | −1.514 |
| 15 | 50 | 10 | 100 | 50 | −0.118 |
| 16 | 50 | 30 | 100 | 0 | −1.325 |
| 17 | 50 | 30 | 300 | 50 | −0.752 |
| 18 | 65 | 50 | 300 | 50 | 1.537 |
| 19 | 35 | 50 | 300 | 50 | −0.8667 |
| 20 | 35 | 10 | 300 | 50 | −1.462 |
| 21 | 50 | 30 | 300 | 50 | −0.608 |
| 22 | 65 | 10 | 300 | 50 | 0.382 |
| 23 | 50 | 10 | 300 | 100 | −0.005 |
| 24 | 50 | 50 | 100 | 50 | 0.578 |
| 25 | 50 | 50 | 300 | 100 | 1.049 |
| 26 | 50 | 10 | 300 | 0 | −0.425 |
| 27 | 65 | 30 | 300 | 100 | 0.811 |
| Term | Coefficient | p-Value |
|---|---|---|
| solvent | 0.969 | 0.001 |
| ratio | 0.442 | 0.016 |
| power | 0.122 | 0.453 |
| stirring | 0.508 | 0.007 |
| solvent*solvent | −0.123 | 0.613 |
| ratio*ratio | 0.448 | 0.082 |
| power*power | 0.232 | 0.345 |
| stirring*stirring | −0.007 | 0.978 |
| solvent*ratio | 0.140 | 0.617 |
| solvent*power | 0.370 | 0.202 |
| solvent*stirring | 0.092 | 0.743 |
| ratio*power | 0.002 | 0.994 |
| ratio*stirring | −0.012 | 0.967 |
| power*stirring | −0.013 | 0.964 |
| Levels (mg kg−1) | Repeatability | Intermediate Precision | |
|---|---|---|---|
| (+)-Catechin | 453.2 | 3.5 | 2.8 |
| (−)-Epicatechin | 306.3 | 4.7 | 3.6 |
| Caftaric acid 1 | 22.37 | 3.0 | 2.8 |
| Dihydrokaempferol-glycoside 2 | 11.13 | 4.8 | 3.4 |
| Quercetin-3-glucuronide 2 | 18.28 | 6.6 | 6.5 |
| Quercetin-3- glucoside 2 | 20.09 | 3.6 | 6.6 |
| Kaempferol-3 -Glucoside 2 | 11.10 | 4.6 | 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azaroual, L.; Liazid, A.; Mansouri, F.E.; Brigui, J.; Ruíz-Rodriguez, A.; Barbero, G.F.; Palma, M. Optimization of the Microwave-Assisted Extraction of Simple Phenolic Compounds from Grape Skins and Seeds. Agronomy 2021, 11, 1527. https://doi.org/10.3390/agronomy11081527
Azaroual L, Liazid A, Mansouri FE, Brigui J, Ruíz-Rodriguez A, Barbero GF, Palma M. Optimization of the Microwave-Assisted Extraction of Simple Phenolic Compounds from Grape Skins and Seeds. Agronomy. 2021; 11(8):1527. https://doi.org/10.3390/agronomy11081527
Chicago/Turabian StyleAzaroual, Latifa, Ali Liazid, Fouad El Mansouri, Jamal Brigui, Ana Ruíz-Rodriguez, Gerardo F. Barbero, and Miguel Palma. 2021. "Optimization of the Microwave-Assisted Extraction of Simple Phenolic Compounds from Grape Skins and Seeds" Agronomy 11, no. 8: 1527. https://doi.org/10.3390/agronomy11081527
APA StyleAzaroual, L., Liazid, A., Mansouri, F. E., Brigui, J., Ruíz-Rodriguez, A., Barbero, G. F., & Palma, M. (2021). Optimization of the Microwave-Assisted Extraction of Simple Phenolic Compounds from Grape Skins and Seeds. Agronomy, 11(8), 1527. https://doi.org/10.3390/agronomy11081527