Producing Superphosphate with Sewage Sludge Ash: Assessment of Phosphorus Availability and Potential Toxic Element Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Superphosphate Preparation and Analysis
2.2. Greenhouse Experiment
2.3. Plant P Content and P Use Efficiency
2.4. Soil Sampling and Analysis
2.5. Statistical Analysis
3. Results
3.1. Phosphorus and PTE Content in Fertilizers
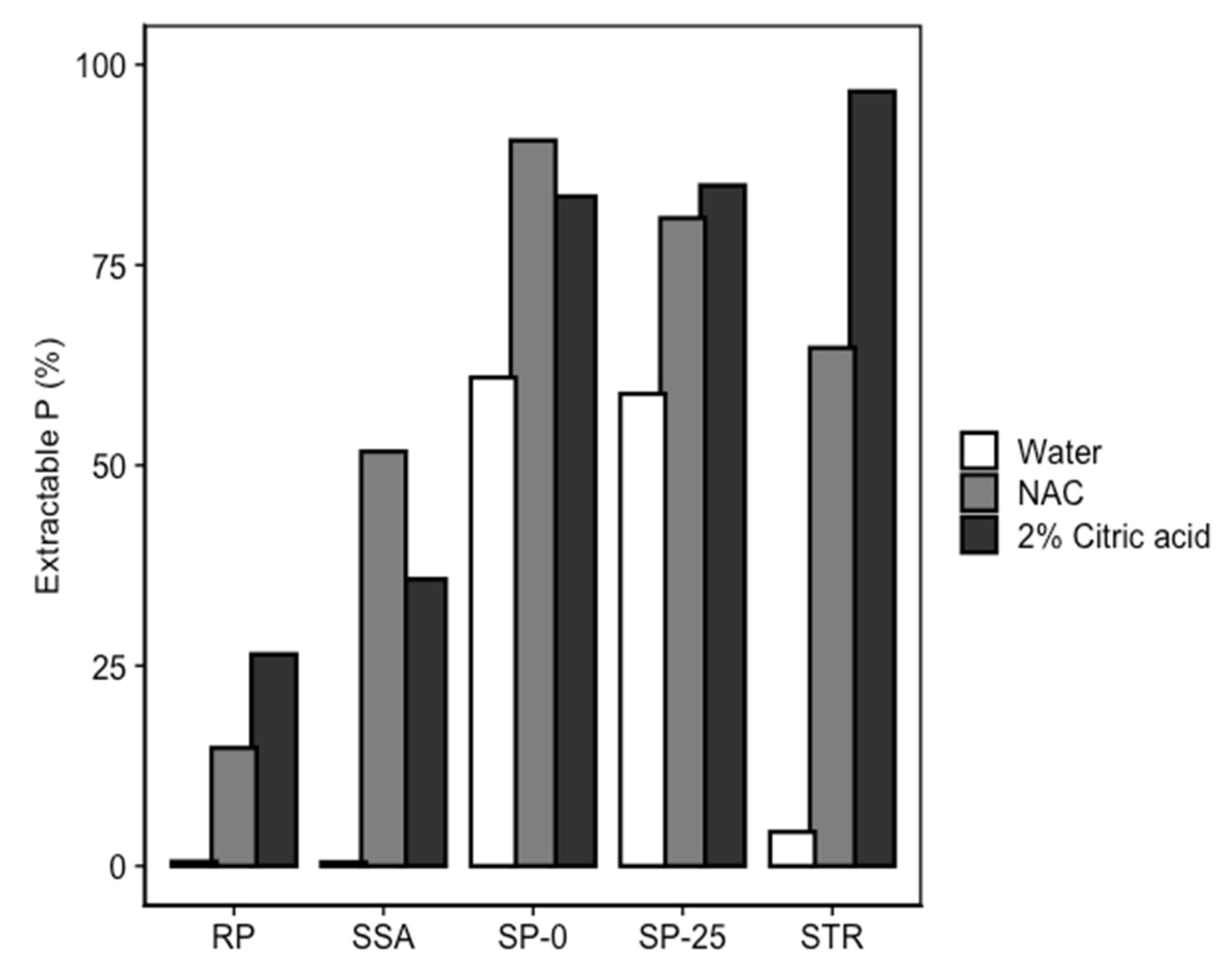
3.2. Bioavailability of P and PTEs in Fertilized Soil
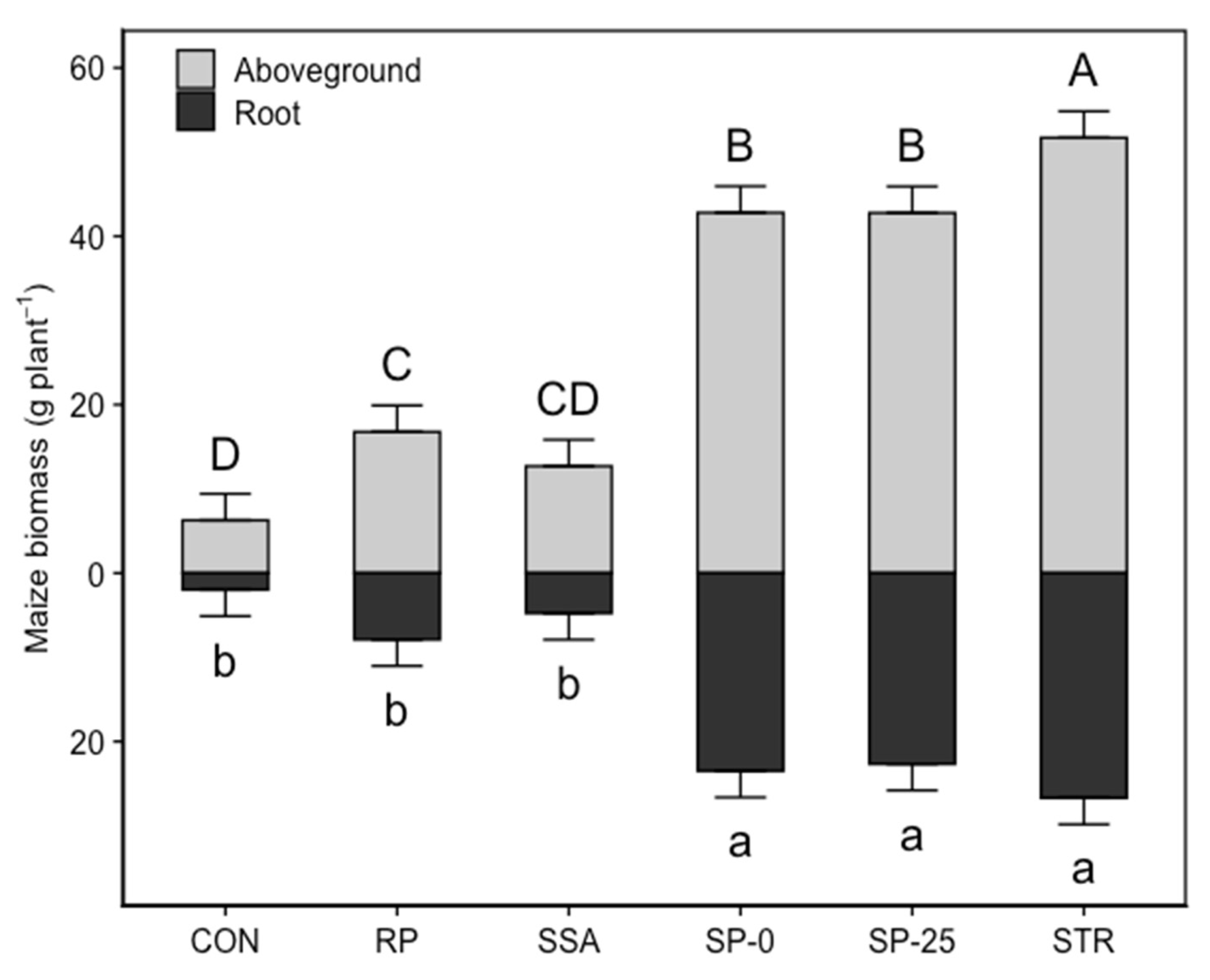
3.3. Maize Growth and Aboveground Biomass Nutrient (P, Mg, Fe) Content
3.4. Aboveground Biomass PTE Content under Different Fertilizer Treatments
4. Discussion
4.1. Limitation of SSA as a Secondary Raw Material in P Fertilizer Production
4.2. P Availability of Produced Superphosphates
4.3. PTE Content in Aboveground Biomass
4.4. PTE Accumulation in Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Study on EU’s List of Critical Raw Materials, Factsheets on Critical Raw Materials; European Commission: Luxembourg, 2020. [Google Scholar]
- German Environmental Agency. Environmetal Criticality of Raw Materials; German Environmental Agency: Dessau-Roßlau, Germany, 2019.
- Kratz, S.; Schick, J.; Schnug, E. Trace Elements in Rock Phosphates and P Containing Mineral and Organo-Mineral Fertilizers Sold in Germany. Sci. Total Environ. 2016, 542, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus Flows and Balances of the European Union Member States. Sci. Total Environ. 2016, 542, 1078–1093. [Google Scholar] [CrossRef]
- Eurostat. Sewage Sludge Production and Disposal from Urban Wastewater (in Dry Substance (d.s)); European Commission: Luxembourg, 2021. [Google Scholar]
- Krüger, O.; Grabner, A.; Adam, C. Complete Survey of German Sewage Sludge Ash. Environ. Sci. Technol. 2014, 48, 11811–11818. [Google Scholar] [CrossRef]
- Donatello, S. Recycling and Recovery Routes for Incinerated Sewage Sludge Ash (ISSA): A Review. Waste Manag. 2013, 33, 2328–2340. [Google Scholar] [CrossRef]
- Donatello, S.; Tong, D.; Cheeseman, C.R. Production of Technical Grade Phosphoric Acid from Incinerator Sewage Sludge Ash (ISSA). Waste Manag. 2010, 30, 1634–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage Sludge Ash—A Promising Secondary Phosphorus Source for Fertilizer Production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef]
- Hartmann, T.E.; Möller, K.; Meyer, C.; Müller, T. Partial Replacement of Rock Phosphate by Sewage Sludge Ash for the Production of Superphosphate Fertilizers. J. Plant Nutr. Soil Sci. 2020, 183, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Leikam, D.F.; Achorn, F.P. Phosphate Fertilizers: Production, Characteristics, and Technologies. In Agronomy Monographs; Thomas Sims, J., Sharpley, A.N., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2015; Volume 46, pp. 23–50. ISBN 978-0-89118-269-6. [Google Scholar]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A Review and Meta-analysis of the Agricultural Potential of Struvite as a Phosphorus Fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- KOPF SynGas GmbH & Co. KG. Klärschlammverwertung: Nachhaltige Phosphornutzung Aus Vergasungsaschen; KOPF SynGas GmbH & Co. KG: Sulz am Neckar, Germany, 2018. [Google Scholar]
- Aufl. VDLUFA Verlag. VDLUFA Methode 2.1.3: Mikrowellenbeheitzer Druckaufschluss. In Handbuch Der Landwirtschaftlichen Versuchs-Und Untersuchungsmethodik (VDLUFA Methodenbuch); Band VII Umweltanalytik; Aufl. VDLUFA Verlag: Darmstadt, Germany, 2011; Volume 4. [Google Scholar]
- Aufl. VDLUFA Verlag. VDLUFA Methode 4.1.4: Bestimmung des wasser-und neutralammoniumcitratlöslichen Phosphats nach FRESENIUS-NEUBAUER, Extraktion. In Handbuch Der Landwirtschaftlichen Versuchs-Und Untersuchungsmethodik (VDLUFA Methodenbuch); Band II Die Untersuchung von Düngemitteln; Aufl. VDLUFA Verlag: Darmstadt, Germany, 1995; Volume 4. [Google Scholar]
- Aufl. VDLUFA Verlag. VDLUFA Methode 4.1.3: Bestimmung des citronensäurelöslichen Phosphats, Extraktion. In Handbuch Der Landwirtschaftlichen Versuchs- Und Untersuchungsmethodik (VDLUFA Methodenbuch); Band II Die Untersuchung von Düngemitteln; Aufl. VDLUFA Verlag: Darmstadt, Germany, 1995; Volume 4. [Google Scholar]
- Meyer, C.; Preyl, V.; Steinmetz, H.; Maier, W.; Mohn, R.-E.; Schönberger, H. The Stuttgart Process (Germany). In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 283–295. ISBN 978-981-10-8031-9. [Google Scholar]
- Aufl. VDLUFA Verlag. VDLUFA Methode 6.2.1.1: Bestimmung von Phosphor und Kalium im Calcium-Acetat-Lactat-Auszug. In Handbuch Der Landwirtschaftlichen Versuchs-Und Untersuchungsmethodik (VDLUFA Methodenbuch); Band I Die Untersuchung von Böden; Aufl. VDLUFA Verlag: Darmstadt, Germany, 2012; Volume 4. [Google Scholar]
- Aufl. VDLUFA Verlag. VDLUFA Methode 6.4.1: Bestimmung von Magnesium, Natrium und den Spurennährstoffen Kupfer, Mangan, Zink und Bor im Calciumchlorid/DTPA-Auszug. In Handbuch Der Landwirtschaftlichen Versuchs-Und Untersuchungsmethodik (VDLUFA Methodenbuch); Band I Die Untersuchung von Böden; Aufl. VDLUFA Verlag: Darmstadt, Germany, 2002; Volume 4. [Google Scholar]
- Aufl. VDLUFA Verlag. VDLUFA Methode 7.6.1: Bestimmung von Spurennährstoffen und von umweltrelevanten Schwermetallen im EDTA-Auszug. In Handbuch Der Landwirtschaftlichen Versuchs-Und Untersuchungsmethodik (VDLUFA Methodenbuch); Band I Die Untersuchung von Böden; Aufl. VDLUFA Verlag: Darmstadt, Germany, 1997; Volume 4. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Düngemittelverordnung. Verordnung Über Das Inverkehrbringen von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten Und Pflanzenhilfsmitteln (Düngemittelverordnung—DüMV); Bundesrepublik Deutschland: Berlin, Germany, 2012. [Google Scholar]
- European Council. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; European Council: Brussels, Belgium, 2019; p. 114. [Google Scholar]
- Wiesler, F.; Appel, T.; Dittert, K.; Ebertseder, T.; Müller, T.; Nätscher, L.; Olfs, H.-W.; Rex, M.; Schweitzer, K.; Steffens, D.; et al. Phosphordüngung Nach Bodenuntersuchung Und Pflanzenbedarf; VDLUFA: Speyer, Germany, 2018. [Google Scholar]
- European Council. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; European Council: Brussels, Belgium, 2002. [Google Scholar]
- Langeveld, K. Phosphorus Recovery into Fertilizers and Industrial Products by ICL in Europe. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 235–252. ISBN 978-981-10-8031-9. [Google Scholar]
- Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E. Extracting Phosphorous from Incinerated Sewage Sludge Ash Rich in Iron or Aluminum. Chemosphere 2013, 91, 963–969. [Google Scholar] [CrossRef]
- Krüger, O.; Adam, C. Recovery Potential of German Sewage Sludge Ash. Waste Manag. 2015, 45, 400–406. [Google Scholar] [CrossRef]
- Lemming, C.; Bruun, S.; Jensen, L.S.; Magid, J. Plant Availability of Phosphorus from Dewatered Sewage Sludge, Untreated Incineration Ashes, and Other Products Recovered from a Wastewater Treatment System. J. Plant Nutr. Soil Sci. 2017, 180, 779–787. [Google Scholar] [CrossRef]
- Severin, M.; Breuer, J.; Rex, M.; Stemann, J.; Ch, A.; Van den Weghe, H.; Kücke, M. Phosphate Fertilizer Value of Heat Treated Sewage Sludge Ash. Plant Soil Environ. 2014, 60, 555–561. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Li, H.; Cheng, F. Evaluating Heavy Metal Accumulation and Potential Risks in Soil-Plant Systems Applied with Magnesium Slag-Based Fertilizer. Chemosphere 2018, 197, 382–388. [Google Scholar] [CrossRef]
- Vatehová, Z.; Malovíková, A.; Kollárová, K.; Kučerová, D.; Lišková, D. Impact of Cadmium Stress on Two Maize Hybrids. Plant Physiol. Biochem. 2016, 108, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hatch, D.J.; Jones, L.H.P.; Burau, R.G. The Effect of PH on the Uptake of Cadmium by Four Plant Species Grown in Flowing Solution Culture. Plant Soil 1988, 105, 121–126. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Role of Potentially Toxic Elements in Soils. In Soil Components and Human Health; Springer: Dordrecht, The Netherlands, 2018; pp. 375–450. ISBN 978-94-024-1221-5. [Google Scholar]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Kochian, L.V. Transport Interactions between Cadmium and Zinc in Roots of Bread and Durum Wheat Seedlings. Physiol. Plant. 2002, 116, 73–78. [Google Scholar] [CrossRef]
- Wiesler, F. Nutrition and Quality. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 271–282. ISBN 978-0-12-384905-2. [Google Scholar]
- Li, J.; Tsang, D.C.W.; Wang, Q.; Fang, L.; Xue, Q.; Poon, C.S. Fate of Metals before and after Chemical Extraction of Incinerated Sewage Sludge Ash. Chemosphere 2017, 186, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Q.; Fang, L.; Poon, C.S. Characteristics and Metal Leachability of Incinerated Sewage Sludge Ash and Air Pollution Control Residues from Hong Kong Evaluated by Different Methods. Waste Manag. 2017, 64, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. EU Landfill Waste Acceptance Criteria and EU Hazardous Waste Directive Compliance Testing of Incinerated Sewage Sludge Ash. Waste Manag. 2010, 30, 63–71. [Google Scholar] [CrossRef]
- Armienta, M.A.; Beltrán, M.; Martínez, S.; Labastida, I. Heavy Metal Assimilation in Maize (Zea mays L.) Plants Growing near Mine Tailings. Environ. Geochem. Health 2020, 42, 2361–2375. [Google Scholar] [CrossRef]
- Möller, K.; Oberson, A.; Bünemann, E.K.; Cooper, J.; Friedel, J.K.; Glæsner, N.; Hörtenhuber, S.; Løes, A.-K.; Mäder, P.; Meyer, G.; et al. Chapter Four—Improved Phosphorus Recycling in Organic Farming: Navigating between Constraints. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 147, pp. 159–237. [Google Scholar]
- Weissengruber, L.; Möller, K.; Puschenreiter, M.; Friedel, J.K. Long-Term Soil Accumulation of Potentially Toxic Elements and Selected Organic Pollutants through Application of Recycled Phosphorus Fertilizers for Organic Farming Conditions. Nutr. Cycl. Agroecosyst. 2018, 110, 427–449. [Google Scholar] [CrossRef] [Green Version]
- Groenenberg, J.E.; Römkens, P.F.A.M.; Comans, R.N.J.; Luster, J.; Pampura, T.; Shotbolt, L.; Tipping, E.; de Vries, W. Transfer Functions for Solid-Solution Partitioning of Cadmium, Copper, Nickel, Lead and Zinc in Soils: Derivation of Relationships for Free Metal Ion Activities and Validation with Independent Data. Eur. J. Soil Sci. 2010, 61, 58–73. [Google Scholar] [CrossRef] [Green Version]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. ISBN 978-0-12-384905-2. [Google Scholar]
- Zhang, W.; Chen, X.-X.; Liu, Y.-M.; Liu, D.-Y.; Chen, X.-P.; Zou, C.-Q. Zinc Uptake by Roots and Accumulation in Maize Plants as Affected by Phosphorus Application and Arbuscular Mycorrhizal Colonization. Plant Soil 2017, 413, 59–71. [Google Scholar] [CrossRef]
- Adam, C.; Peplinski, B.; Michaelis, M.; Kley, G.; Simon, F.-G. Thermochemical Treatment of Sewage Sludge Ashes for Phosphorus Recovery. Waste Manag. 2009, 29, 1122–1128. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Abbreviation | Process | P | N | Mg | Fe/P |
|---|---|---|---|---|---|---|
| mg g−1 | Molar Ratio | |||||
| Sewage sludge ash | SSA | Gasification | 89 | <1 | 10 | 0.53 |
| Rock phosphate | RP | Mining | 118 | <1 | 40 | 0.07 |
| SP solely from RP | SP-0 | RP digested with 95% H2SO4 | 73 | <1 | 10 | 0.12 |
| SP from 75% RP + 25% SSA | SP-25 | 75% RP and 25% SSA digested with 95% H2SO4 | 61 | <1 | 9 | 0.34 |
| Struvite | STR | Enforced P dissolution | 120 | 52 | 100 | 0.04 |
| No P fertilizer addition | CON | - | - | - | - | - |
| Fertilizer | Cd | Cr | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|
| (mg kg−1 P2O5) | (mg kg−1) | (mg kg−1) | |||||
| SSA | 3 d | 0.6 d | 123 a | 641 a | 98.8 a | 129 a | 3189 a |
| RP | 7.4 c | 2.0 a | 45.4 c | 11.6 c | 25.5 b | 20.0 c | 97.3 c |
| SP-0 | 7.8 b | 1.3 b | 75.9 b | 9.2 c | 20.7 b | 11.4 d | 75.9 cd |
| SP-25 | 8.6 a | 1.2 c | 53.7 c | 104 b | 31.5 b | 29.1 b | 642 b |
| STR | <0.06 1 | <0.06 1 | 8.4 d | 1.4 c | 5.0 b | <0.06 1 | 10.7 d |
| Analysis of Variance | *** | *** | *** | *** | ** | *** | *** |
| Maximum concentrations | |||||||
| EU regulation | 60.0 2 | 3.0 3 | 600 | 100 | 120 | 1500 | |
| German Fertilizer Ordinance | 50.0 2 | 1.5 3 | - | 80 | 150 | - | |
| Fertilizer | Cd | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|
| (mg kg−1 P) | ||||||
| SSA | 7.2 | 1380 | 7207 | 1110 | 1450 | 35,832 |
| RP | 16.6 | 382 | 97 | 214 | 168 | 818 |
| SP-0 | 18.1 | 1040 | 126 | 284 | 157 | 1039 |
| SP-25 | 19.3 | 881 | 1700 | 516 | 477 | 10,527 |
| STR | - | 70 | 11 | 42 | - | 89 |
| Treatment | CAT-Extraction | EDTA-Extraction |
|---|---|---|
| Mg | Fe | |
| (mg kg−1) | ||
| CON | 139.3 | 137 bc |
| SSA | 145.6 | 220 a |
| RP | 160.5 | 139 bc |
| SP-0 | 143.3 | 161 bc |
| SP-25 | 153.3 | 166 b |
| STR | 172.4 | 136 c |
| Analysis of Variance | ns | *** |
| P Concentration (mg kg−1) | P Content (mg P plant−1) | |
|---|---|---|
| CON | 0.94 ab | 5.87 c |
| SSA | 0.82 b | 10.17 c |
| RP | 0.78 b | 13.06 c |
| SP-0 | 0.98 ab | 41.87 b |
| SP-25 | 0.99 ab | 42.44 b |
| STR | 1.1 a | 57.31 a |
| Treatment | Mg | Fe | Cu | Zn | Cd | Cr | Ni | Pb |
|---|---|---|---|---|---|---|---|---|
| Concentration (mg kg−1) | ||||||||
| CON | 2674 a | 53.61 a | 3.84 a | 14.44 a | 0.10 | 0.22 | 0.50 a | 0.32 |
| SSA | 2055 b | 45.09 b | 3.14 b | 11.83 ab | 0.08 | 0.17 | 0.30 b | 0.23 |
| RP | 1999 bc | 44.97 b | 2.67 bc | 10.55 bc | 0.07 | 0.28 | 0.24 b | 0.14 |
| SP-0 | 1712 bc | 26.44 c | 2.06 d | 6.87 cd | <0.05 | 0.11 | 0.18 b | 0.34 |
| SP-25 | 1681 c | 27.06 c | 2.08 d | 9.36 bcd | <0.05 | 0.17 | 0.25 b | 0.22 |
| STR | 1987 bc | 27.79 c | 2.24 cd | 7.17 cd | <0.05 | 0.28 | 0.19 b | 0.16 |
| Directive 2002/32/EC | - | - | - | - | 0.44 | - | - | 26.4 |
| ANOVA | *** | *** | *** | *** | ns | ns | *** | ns |
| Content (mg plant−1) | ||||||||
| CON | 16.78 d | 0.34 d | 0.02 d | 0.09 b | <0.01 | <0.01 | <0.01 | <0.01 |
| SSA | 26.10 cd | 0.57 c | 0.04 cd | 0.15 b | <0.01 | <0.01 | <0.01 | <0.01 |
| RP | 33.53 c | 0.75 c | 0.04 c | 0.18 b | <0.01 | <0.01 | <0.01 | <0.01 |
| SP-0 | 73.27 b | 1.13 b | 0.08 b | 0.29 a | <0.01 | <0.01 | 0.01 | 0.01 |
| SP-25 | 71.90 b | 1.16 b | 0.08 b | 0.40 a | <0.01 | <0.01 | 0.01 | 0.01 |
| STR | 102.73 a | 1.44 a | 0.11 a | 0.37 a | <0.01 | 0.01 | 0.01 | 0.01 |
| ANOVA | *** | *** | *** | *** | - | - | - | - |
| RP | SSA | SP-0 | SP-25 | STR | |
|---|---|---|---|---|---|
| PUE (%) | 0.63 ± 0.11 | 0.38 ± 0.16 | 3.57 ± 0.50 | 3.47 ± 0.73 | 5.26 ± 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Klein, J.; Hartmann, T.E.; Nkebiwe, P.M.; Yang, H.; Zhang, W.; Chen, X.; Müller, T. Producing Superphosphate with Sewage Sludge Ash: Assessment of Phosphorus Availability and Potential Toxic Element Contamination. Agronomy 2021, 11, 1506. https://doi.org/10.3390/agronomy11081506
You Y, Klein J, Hartmann TE, Nkebiwe PM, Yang H, Zhang W, Chen X, Müller T. Producing Superphosphate with Sewage Sludge Ash: Assessment of Phosphorus Availability and Potential Toxic Element Contamination. Agronomy. 2021; 11(8):1506. https://doi.org/10.3390/agronomy11081506
Chicago/Turabian StyleYou, Yawen, Jakob Klein, Tobias Edward Hartmann, Peteh Mehdi Nkebiwe, Huaiyu Yang, Wei Zhang, Xinping Chen, and Torsten Müller. 2021. "Producing Superphosphate with Sewage Sludge Ash: Assessment of Phosphorus Availability and Potential Toxic Element Contamination" Agronomy 11, no. 8: 1506. https://doi.org/10.3390/agronomy11081506
APA StyleYou, Y., Klein, J., Hartmann, T. E., Nkebiwe, P. M., Yang, H., Zhang, W., Chen, X., & Müller, T. (2021). Producing Superphosphate with Sewage Sludge Ash: Assessment of Phosphorus Availability and Potential Toxic Element Contamination. Agronomy, 11(8), 1506. https://doi.org/10.3390/agronomy11081506






