Softening of Processed Plant Virus Infected Cucumis sativus L. Fruits
Abstract
1. Introduction
2. Materials and Methods
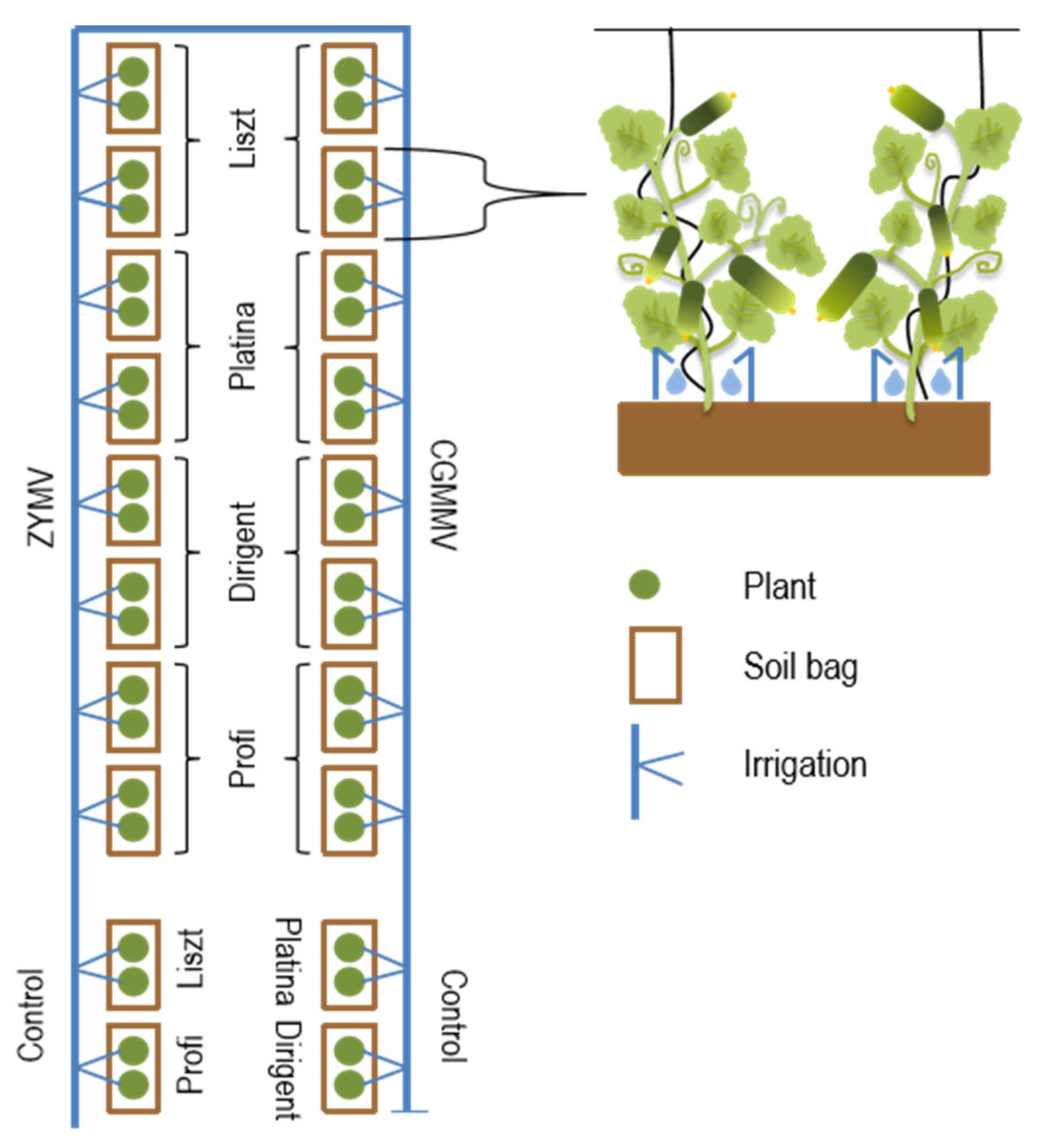
2.1. Virus Inoculation and Cultivation of Plants
2.2. Diagnosis of Virus Infection
2.3. Harvesting and Processing of Cucumber Fruits
2.4. Instrumental Measurement of Texture of Pasteurized and Fresh Cucumbers
2.5. Light Microscopy
2.5.1. Sample Preparation and Sectioning
2.5.2. Staining and Image Recording
3. Results
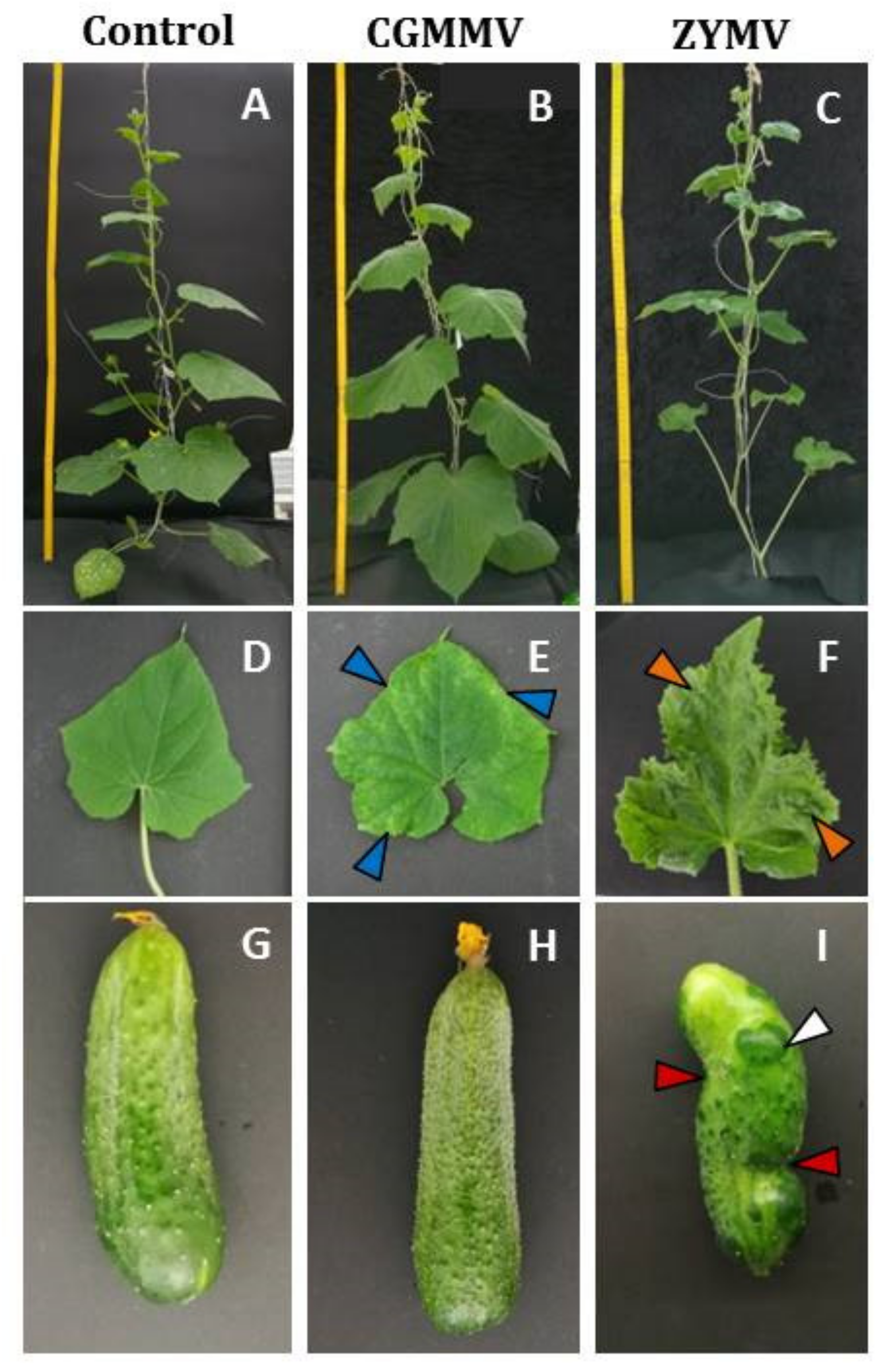
3.1. Virus Induced Phenotypic Symptoms on Plants and Fruits
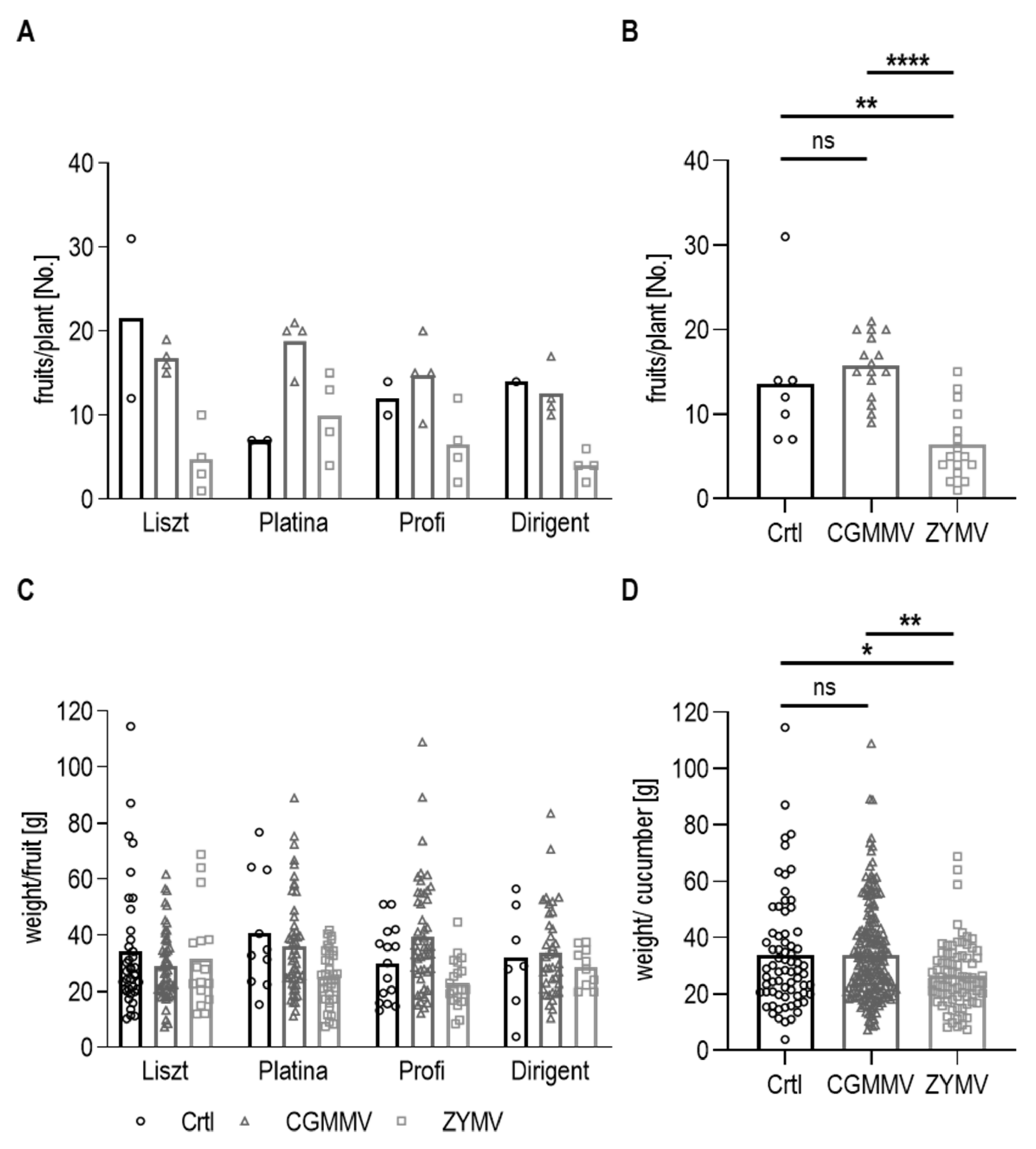
3.2. Yield and Influence of Plant Viruses
3.3. Virus Detection by Serological Assay
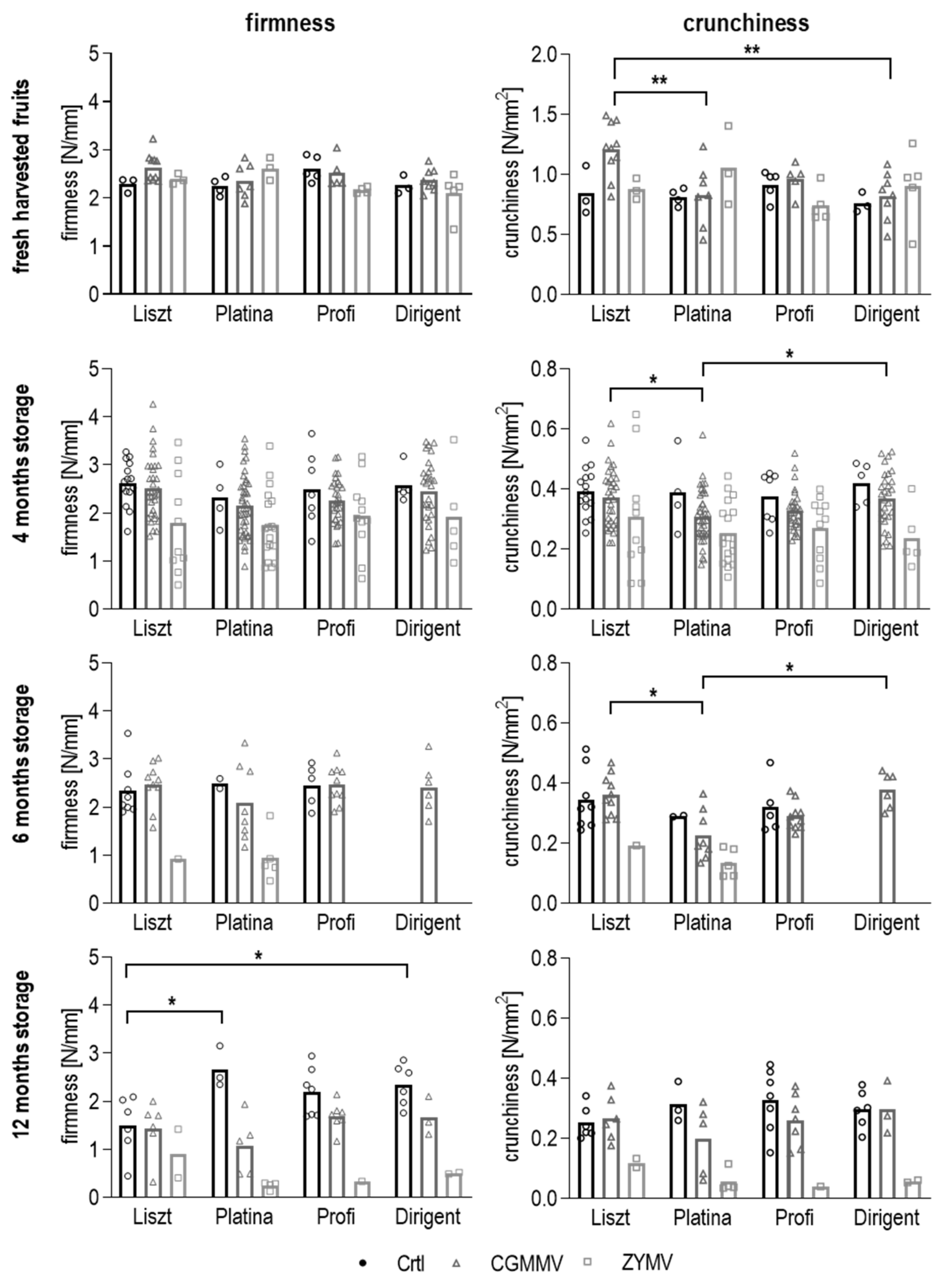
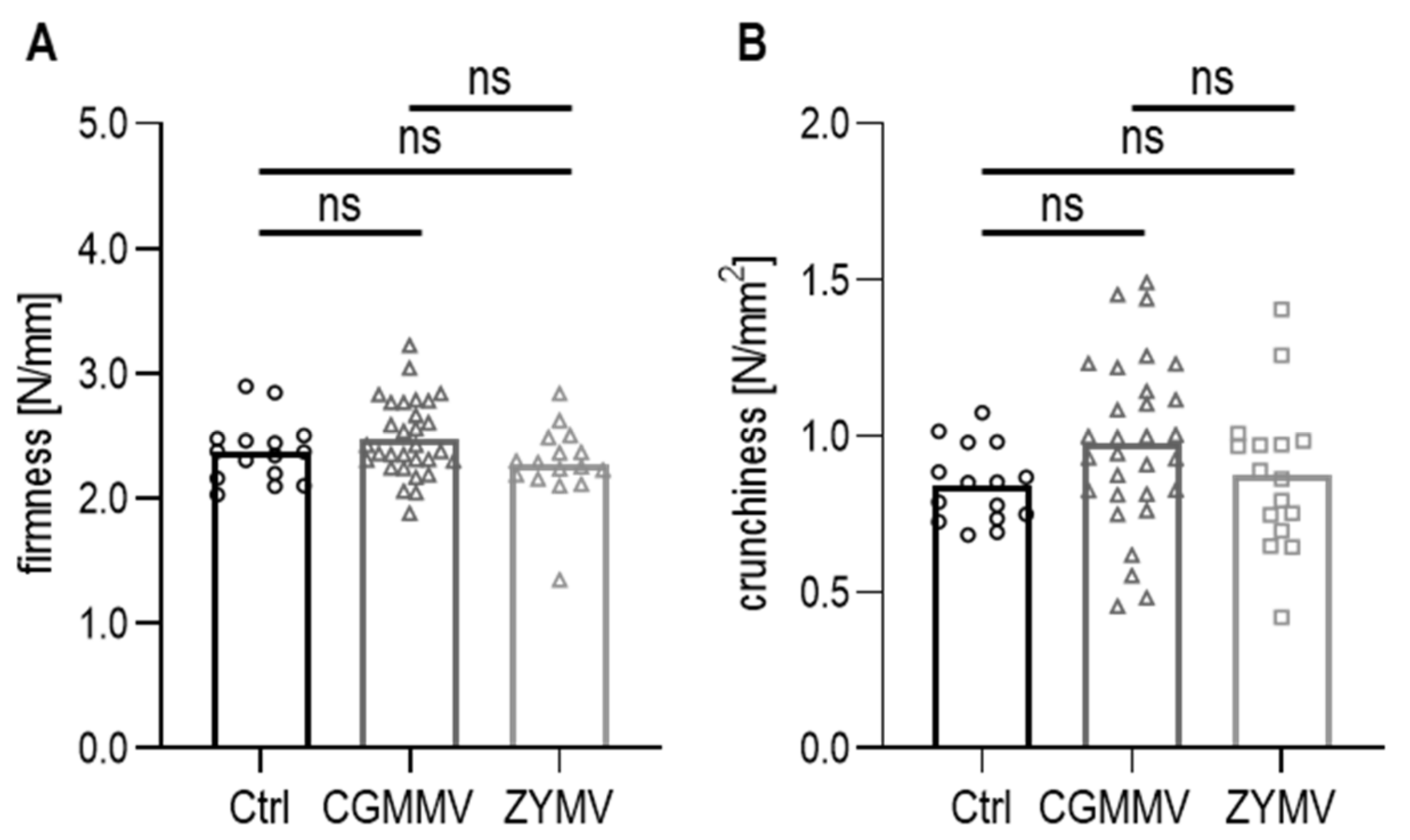
3.4. Measurement of Firmness and Crunchiness
3.4.1. Texture of Fresh Cucumber Fruits
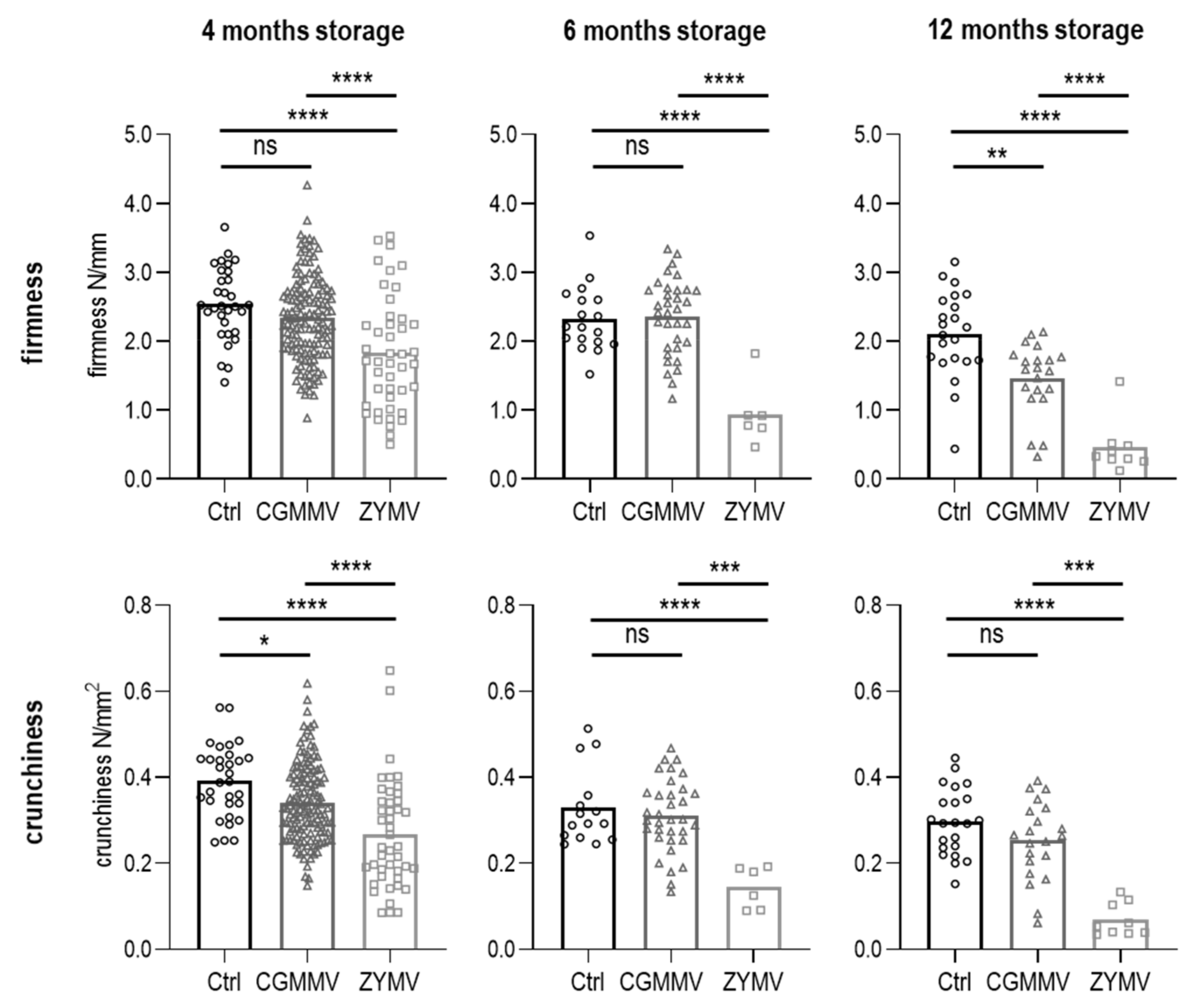
3.4.2. Texture of Pasteurized Pickles
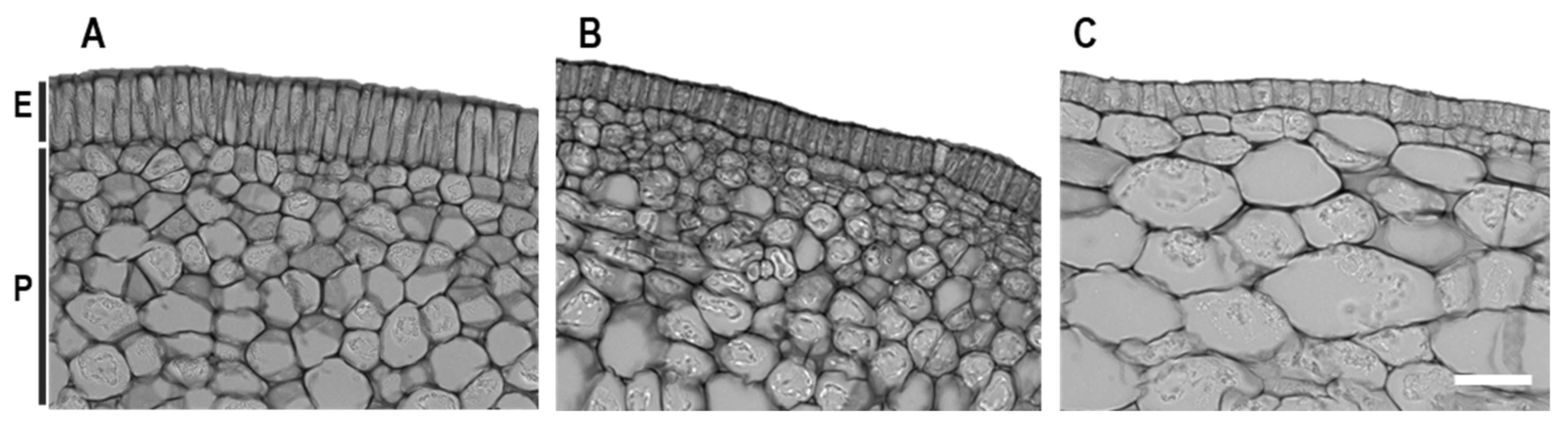
3.5. Histological Alterations
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pal, S.; Sharma, H.R.; Thakur, A.K.; Dogra, R.K. Morpho-agronomic characterization of cucumber (Cucumis sativus L.) germplasm through principal component analysis. J. Pharmacogn. Phytochem. 2018, 7, 2573–2577. [Google Scholar]
- Sebastian, P.; Schaefer, H.; Telford, I.R.H.; Renner, S.S. Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc. Natl. Acad. Sci. USA 2010, 107, 14269–14273. [Google Scholar] [CrossRef] [PubMed]
- Breene, W.M.; Chou, H.; Davis, D.W. Texture profile analysis of cucumbers. J. Food Sci. 1972, 37, 113–117. [Google Scholar] [CrossRef]
- Sahoo, M.; Prakash, J. Formulation and standardization of dill based gherkin pickles: A study on physico-chemical and sensory attributes. Indian J. Nutr. Diet. 2017, 54. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Marangon, M.; Magli, M.; Cianciabella, M.; Predieri, S.; Curioni, A.; Vincenzi, S. Sensory characterization of cucumbers pickled with verjuice as novel acidifying agent. Food Chem. 2019, 286, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Phaff, H.J. Softening of cucumbers during curing. J. Agric. Food Chem. 1957, 5, 60–64. [Google Scholar] [CrossRef]
- Reeve, R.M. Relationships of histological structure to texture of fresh and processed fruits and vegetables. J. Texture Stud. 1970, 1, 247–284. [Google Scholar] [CrossRef]
- Szczesniak, A.S.; Ilker, R. The meaning of textural characteristics—Juiciness in plant foodstuffs. J. Texture Stud. 1988, 19, 61–78. [Google Scholar] [CrossRef]
- Sajnín, C.; Gamba, G.; Gerschenson, L.N.; Rojas, A.M. Textural, histological and biochemical changes in cucumber (Cucumis sativus L) due to immersion and variations in turgor pressure. J. Sci. Food Agric. 2003, 83, 731–740. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Chang, Y.; Xu, X.; Nie, S. Influences of high temperature stress on membrane permeability and activity of cell defence enzymes in leaves of cucumber seedlings. J. Changjiang Veg. 2007, 9, 59–61. [Google Scholar]
- Alsadon, A.; Al-Helal, I.; Ibrahim, A.; Abdel-Ghany, A.; Al-Zaharani, S.; Ashour, T. The effects of plastic greenhouse covering on cucumber (Cucumis sativus L.) growth. Ecol. Eng. 2016, 87, 305–312. [Google Scholar] [CrossRef]
- Bell, T.A.; Etchells, J.L. Influence of salt (NaCI) on pectinolytic softening of cucumbers. J. Food Sci. 1961, 26, 84–90. [Google Scholar] [CrossRef]
- Mcfeeters, R.F.; Fleming, H.P. pH effect on calcium inhibition of softening of cucumber mesocarp tissue. J. Food Sci. 1991, 56, 730–732. [Google Scholar] [CrossRef]
- Bell, T.A.; Turney, L.J.; Etchells, J.L. Influence of different organic acids on the firmness of fresh-pack pickles. J. Food Sci. 1972, 37, 446–449. [Google Scholar] [CrossRef]
- Mcfeeters, R.F.; Fleming, H.P. Effect of calcium-ions on the thermodynamics of cucumber tissue softening. J. Food Sci. 1990, 55, 446–449. [Google Scholar] [CrossRef]
- Sistrunk, W.A.; Kozup, J. Influence of processing methodology on quality of cucumber pickles. J. Food Sci. 1982, 47, 949–953. [Google Scholar] [CrossRef]
- Thompson, R.L.; Fleming, H.P.; Monroe, R.J. Effects of storage-conditions on firmness of brined cucumbers. J. Food Sci. 1979, 44, 843–846. [Google Scholar] [CrossRef]
- Etchells, J.L.; Bell, T.A.; Monroe, R.J.; Masley, P.M.; Demain, A.L. Populations and softening enzyme activity of filamentous fungi on flower, ovaries, and fruit of pickling cucumbers. Appl. Microbiol. 1958, 6, 427–440. [Google Scholar] [CrossRef]
- Raymond, F.L.; Etchells, J.L.; Bell, T.A.; Masley, P.M. Filamentous fungi from blossoms, ovaries, and fruit of pickling cucumbers. Mycologia 1959, 51, 492–511. [Google Scholar] [CrossRef]
- Voldřich, M.; Horsáková, I.; Čeřovský, M.; Čížková, H.; Opatová, H. Factors affecting the softening of pickled pasteurised cucumbers. Czech J. Food Sci. 2009, 27, 314–318. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in plant-virus interaction. Front. Microbiol. 2016, 7, 1565. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Ainsworh, G.C. Mosaic disease of cucumber. Ann. Appl. Biol. 1935, 22, 55–67. [Google Scholar] [CrossRef]
- Lisa, V.; Boccardo, G.; Dagostino, G.; Dellavalle, G.; Daquilio, M. Characterization of a potyvirus that causes zucchini yellow mosaic. Phytopathology 1981, 71, 667–672. [Google Scholar] [CrossRef]
- Lovisolo, O. Virus and viroid diseases of cucurbits. Acta Hortic. 1980. [Google Scholar] [CrossRef]
- Desbiez, C.; Lecoq, H. Zucchini yellow mosaic virus. Plant Pathol. 1997, 46. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.A.C. Cucumber green mottle mosaic virus: Rapidly increasing global distribution, etiology, epidemiology, and management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef]
- Kersten, A.-K.; Scharf, S.; Bandte, M.; Meurer, P.; Lentzsch, P.; Büttner, C. Influence of plant viruses on the texture of fresh and processed fruits of Cucumis sativus. In Proceedings of the 4th International Symposium on Horticulture in Europe, Stuttgart, Germany (Online), 9–11 March 2021. [Google Scholar]
- Thompson, R.L.; Fleming, H.P.; Hamann, D.D.; Monroe, R.J. Methode for determination of firmness in cucumber slices. J. Texture Stud. 1982, 13, 311–324. [Google Scholar] [CrossRef]
- Fowke, L.C.; Rennie, P.J. Botanical microtechnique for plant cultures. In Plant Cell, Tissue and Organ Culture; Springer: Berlin/Heidelberg, Germany, 1995; pp. 217–228. [Google Scholar]
- Soukup, A.; Tylová, E. Essential methods of plant sample preparation for light microscopy. In Plant Cell Morphogenesis; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1992, pp. 1–23. [Google Scholar]
- Gamborg, O.; Phillips, G.C. Plant Cell, Tissue and Organ Culture: Fundamental Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zubkova, K.; Stoianova, O. Study of quality of snack gherkin tinned food. EUREKA Life Sci. 2020, 1, 25–31. [Google Scholar] [CrossRef]
- Thöne, M. (Obst- und Gemüseverarbeitung Spreewaldkonserve Golßen GmbH, Golßen, Brandenburg, Germany). Personal communication, 2019.
- Zechmann, B.; Muller, M.; Zellnig, G. Cytological modifications in zucchini yellow mosaic virus (ZYMV)-infected Styrian pumpkin plants. Arch. Virol. 2003, 148, 1119–1133. [Google Scholar] [CrossRef]
- Suojala-Ahlfors, T.J.H. Fruit firmness of pickling cucumber cultivars. HortTechnology 2005, 15, 777–781. [Google Scholar] [CrossRef]
- Shimomura, K.; Horie, H.; Sugiyama, M.; Kawazu, Y.; Yoshioka, Y.J.S.H. Quantitative evaluation of cucumber fruit texture and shape traits reveals extensive diversity and differentiation. Sci. Hort. 2016, 199, 133–141. [Google Scholar] [CrossRef]
- Malmstrom, C.M.; Melcher, U.; Bosque-Perez, N.A. The expanding field of plant virus ecology: Historical foundations, knowledge gaps, and research directions. Virus Res. 2011, 159, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.M.; Cilia, M. A molecular tug-of-war: Global plant proteome changes during viral infection. Curr. Plant Biol. 2016, 5, 13–24. [Google Scholar] [CrossRef]
- Carmo, L.S.; Resende, R.O.; Silva, L.P.; Ribeiro, S.G.; Mehta, A. Identification of host proteins modulated by the virulence factor AC2 of Tomato chlorotic mottle virus in Nicotiana benthamiana. Proteomics 2013, 13, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Sheng, J.; Hind, G.; Handa, A.K.; Citovsky, V. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J. 2000, 19, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Brizard, J.P.; Carapito, C.; Delalande, F.; van Dorsselaer, A.; Brugidou, C. Proteome analysis of plant-virus interactome: Comprehensive data for virus multiplication inside their hosts. Mol. Cell. Proteom. 2006, 5, 2279–2297. [Google Scholar] [CrossRef]
- Kofalvi, S.A.; Gao, J.-G.; Nassuth, A. Biochemical investigation into the wall collapse of wheat leaf cells caused by wheat streak mosaic virus infection. Physiol. Mol. Plant Pathol. 1995, 47, 379–389. [Google Scholar] [CrossRef]
- Zellnig, G.; Pockl, M.H.; Mostl, S.; Zechmann, B. Two and three dimensional characterization of Zucchini Yellow Mosaic Virus induced structural alterations in Cucurbita pepo L. plants. J. Struct. Biol. 2014, 186, 245–252. [Google Scholar] [CrossRef][Green Version]
- Sochor, J.; Babula, P.; Adam, V.; Krska, B.; Kizek, R. Sharka: The past, the present and the future. Viruses 2012, 4, 2853–2901. [Google Scholar] [CrossRef]
- El-Deeb, S.H.; IsHak, J. Histological and cytological changes due to the infection with Sweetpotato feathery mottle virus (SPFMV). J. Plant Dis. Prot. 2004, 111, 247–256. [Google Scholar]
- Sajjaanantakul, T.; van Buren, J.P.; Downing, D.L. Effect of methyl ester content on heat degradation of chelator-soluble carrot pectin. Food Sci. 1989, 54, 1272–1277. [Google Scholar] [CrossRef]
- Sams, C.E. Preharvest factors affecting postharvest texture. Postharvest Biol. Technol. 1999, 15, 249–254. [Google Scholar] [CrossRef]
- Lin, W.C.; Jolliffe, P.A. Light intensity and spectral quality affect fruit growth and shelf life of greenhouse-grown long English cucumber. J. Am. Soc. Hortic. Sci. 1996, 121, 1168–1173. [Google Scholar] [CrossRef]
- Toivonen, P.M.; Hodges, D.M. Abiotic stress in harvested fruits and vegetables. In Abiotic Stress in Plants-Mechanisms and Adaptations; InTech: Rijeka, Croatia, 2011; pp. 39–58. [Google Scholar]
- Kubo, Y.; Xue, Y.; Nakatsuka, A.; Mathooko, F.M.; Inaba, A.; Nakamura, R. Expression of a water stress-induced polygalacturonase gene in harvested cucumber fruit. J. Jpn. Soc. Hortic. Sci. 2000, 69, 273–279. [Google Scholar] [CrossRef]
- Shibairo, S.I.; Upadhyaya, M.K.; Toivonen, P.M. Changes in water potential, osmotic potential, and tissue electrolyte leakage during mass loss in carrots stored under different conditions. Sci. Hortic. 2002, 95, 13–21. [Google Scholar] [CrossRef]
- Morawicki, R.O.; Schmalko, M.E. Prediction of out-of-container pasteurization of pickled cucumbers using the finite-difference method. J. Food Eng. 2011, 107, 289–295. [Google Scholar] [CrossRef]
- Al-Shahwan, I.M.; Abdalla, O.A.; Al-Saleh, M.A. Response of greenhouse-grown cucumber cultivars to an isolate of zucchini yellow mosaic virus (ZYMV). APS 1995, 79. [Google Scholar] [CrossRef]
- Fletcher, J.T.; George, A.J.; Green, D.E. Cucumber green mottle mosaic virus, its effect on yield and its control in the Lea Valley, England. Plant Pathol. 1969, 18, 16–22. [Google Scholar] [CrossRef]
- Prendeville, H.R.; Tenhumberg, B.; Pilson, D. Effects of virus on plant fecundity and population dynamics. New Phytol. 2014, 202, 1346–1356. [Google Scholar] [CrossRef]
- Fraile, A.; García-Arenal, F. Environment and evolution modulate plant virus pathogenesis. Curr. Opin. Virol. 2016, 17, 50–56. [Google Scholar] [CrossRef] [PubMed]
| Virusinfection | Cultivar | ||||
|---|---|---|---|---|---|
| Liszt | Platina | Profi | Dirigent | ||
| Harvested cucumbers | CGMMV | 67 | 75 | 59 | 50 |
| ZYMV | 19 | 40 | 26 | 16 | |
| control | 43 | 14 | 24 | 14 | |
| Processed cucumbers | CGMMV | 50 | 53 | 47 | 38 |
| ZYMV | 13 | 27 | 12 | 7 | |
| control | 29 | 9 | 19 | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kersten, A.-K.; Scharf, S.; Bandte, M.; Martin, P.; Meurer, P.; Lentzsch, P.; Büttner, C. Softening of Processed Plant Virus Infected Cucumis sativus L. Fruits. Agronomy 2021, 11, 1451. https://doi.org/10.3390/agronomy11081451
Kersten A-K, Scharf S, Bandte M, Martin P, Meurer P, Lentzsch P, Büttner C. Softening of Processed Plant Virus Infected Cucumis sativus L. Fruits. Agronomy. 2021; 11(8):1451. https://doi.org/10.3390/agronomy11081451
Chicago/Turabian StyleKersten, Anne-Katrin, Sabrina Scharf, Martina Bandte, Peer Martin, Peter Meurer, Peter Lentzsch, and Carmen Büttner. 2021. "Softening of Processed Plant Virus Infected Cucumis sativus L. Fruits" Agronomy 11, no. 8: 1451. https://doi.org/10.3390/agronomy11081451
APA StyleKersten, A.-K., Scharf, S., Bandte, M., Martin, P., Meurer, P., Lentzsch, P., & Büttner, C. (2021). Softening of Processed Plant Virus Infected Cucumis sativus L. Fruits. Agronomy, 11(8), 1451. https://doi.org/10.3390/agronomy11081451






