Protective Effects of Fruit Wines against Hydrogen Peroxide—Induced Oxidative Stress in Rat Synaptosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Production
2.2. Chemicals and Reagents
2.3. Lyophilization of Fruit Wines
2.4. Preparation of Crude Synaptosomal Fraction
2.5. Synaptosomal Treatment with Fruit Wines
2.6. Determination of Antioxidant Enzyme Activities
2.7. Lipid Peroxidation Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Various Types of Fruit Wines on the Activities of Antioxidant Enzymes and Lipid Peroxidation in H2O2-Treated Synaptosomes
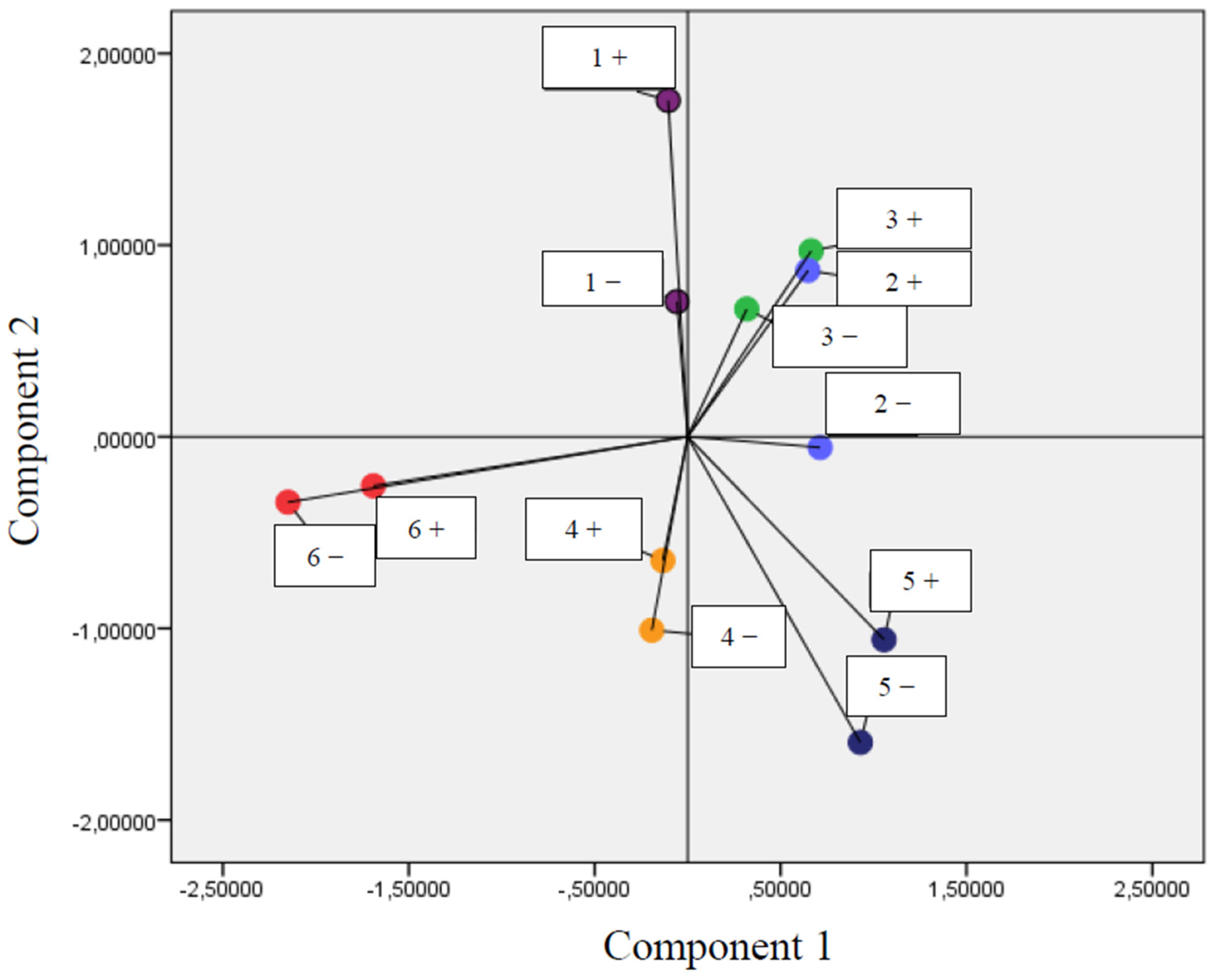
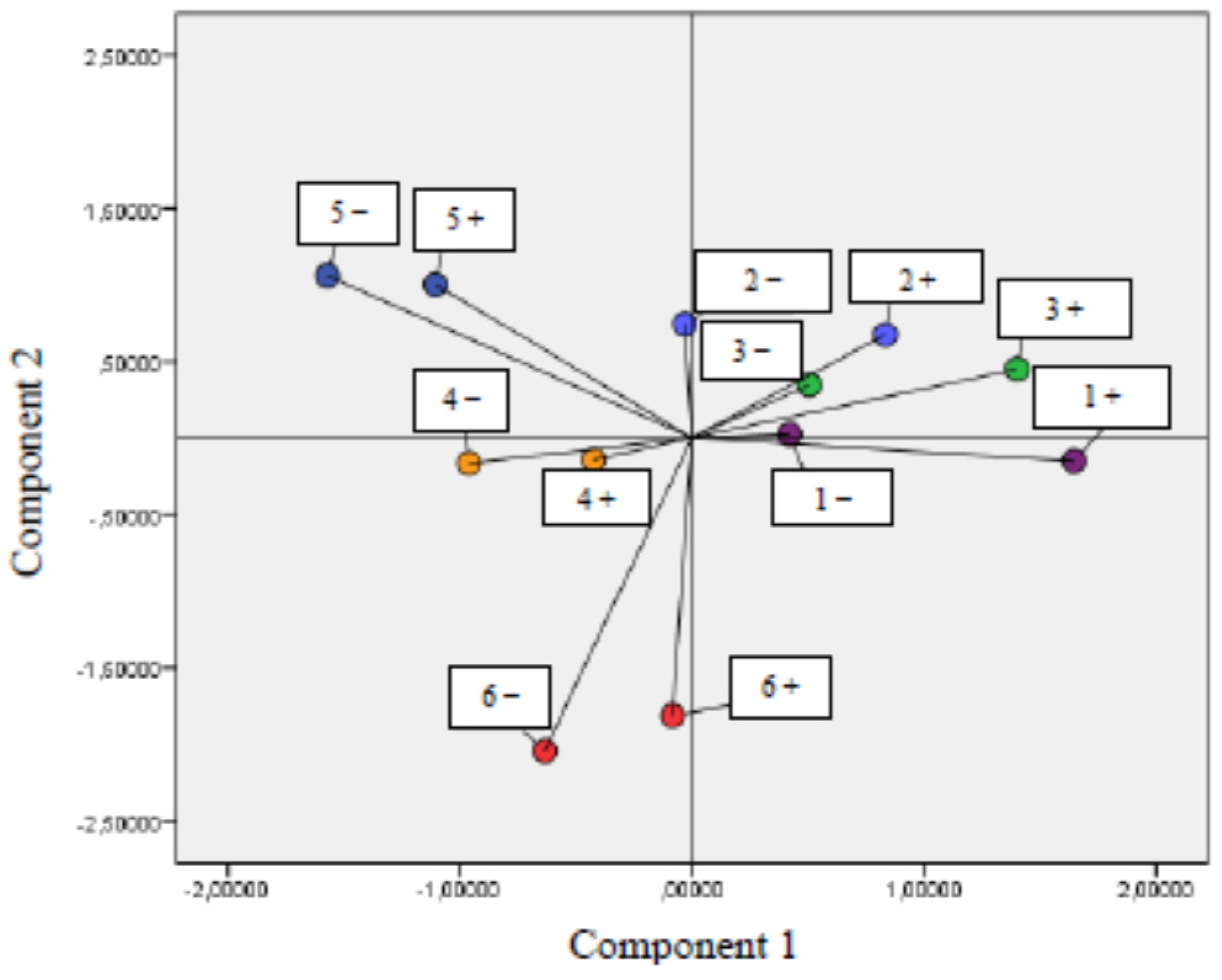
3.2. Correlation between Parameters of Oxidative Stress and Antioxidant Properties—PCA Analysis
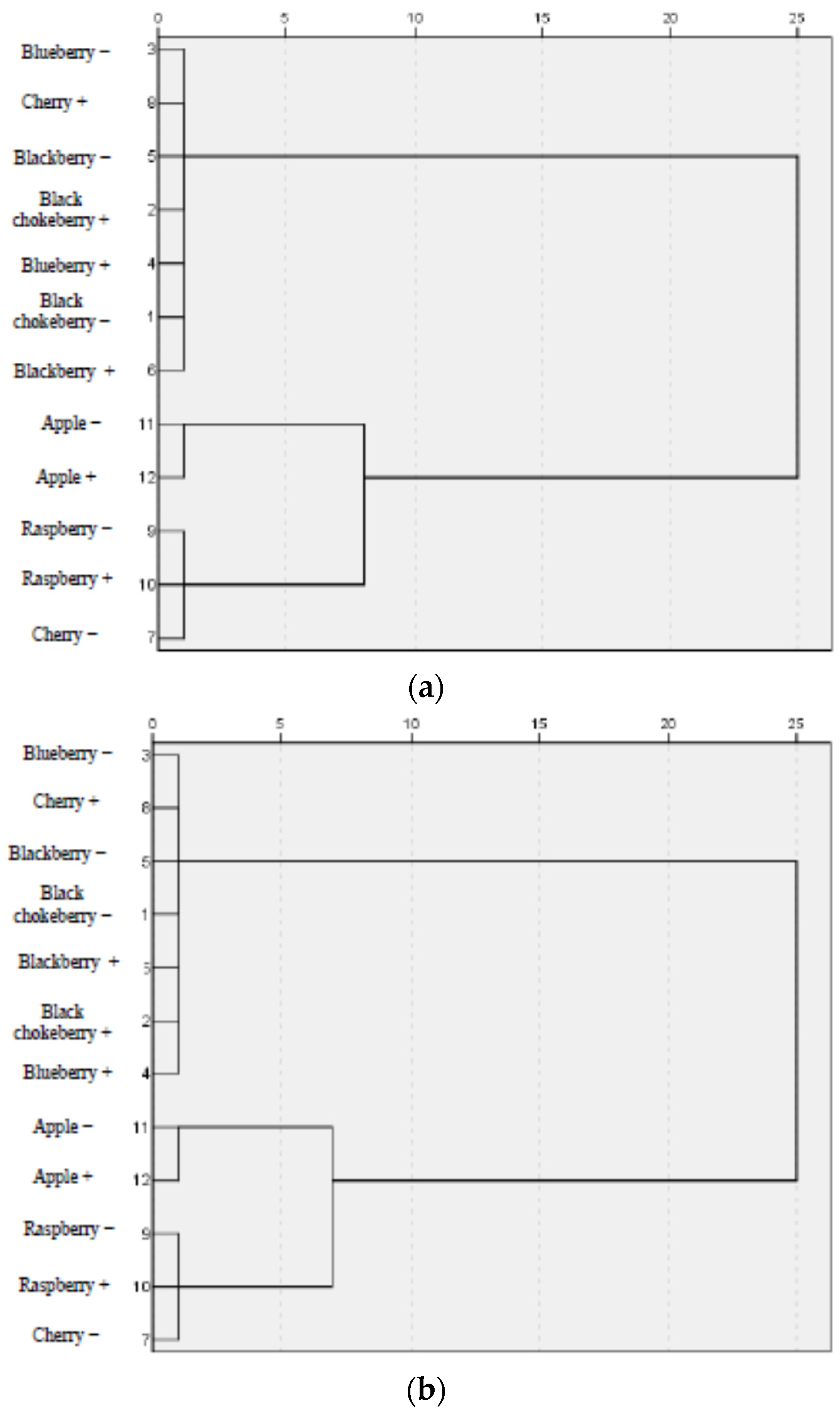
3.3. Correlation between Parameters of Oxidative Stress and Antioxidant Properties—HCA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. How to characterize a biological antioxidant. Free Radic. Res. Commun. 1990, 9, 1–32. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2017, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. The evolution of phenolic compounds in Vitis vinifera L. red berries during ripening: Analysis and role on wine sensory—A review. Agronomy 2021, 11, 999. [Google Scholar] [CrossRef]
- Jabs, T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999, 57, 231–245. [Google Scholar] [CrossRef]
- Kelsey, N.; Hulick, W.; Winter, A.; Ross, E.; Linseman, D. Neuroprotective effects of anthocyanins on apoptosis induced by mitochondrial oxidative stress. Nutr. Neurosci. 2011, 14, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and In Vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Junli, Y.; Yantao, H.; Chunbo, W.; Wengong, Y. Cytoprotective effect of polypeptide from Chlamys farreri on neuroblastoma (SH-SY5Y) cells following H2O2 exposure involves scavenging ROS and inhibition JNK phosphorylation. J. Neurochem. 2009, 111, 441–451. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Ghinea, I.O.; Ionica Mihaila, M.D.; Blaga Costea, G.-V.; Avramescu, S.M.; Cudalbeanu, M.; Isticioaia, S.-F.; Dinica, R.M.; Furdui, B. HPLC-DAD polyphenolic profiling and antioxidant activities of Sorghum bicolor during germination. Agronomy 2021, 11, 417. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- de la Portilla, N.; Vaca, R.; Mora-Herrera, M.E.; Salinas, L.; del Aguila, P.; Yañez-Ocampo, G.; Lugo, J. Soil amendment with biosolids and inorganic fertilizers: Effects on biochemical properties and oxidative stress in basil (Ocimum basilicum L.). Agronomy 2020, 10, 1117. [Google Scholar] [CrossRef]
- Elavarasan, J.; Velusamy, P.; Ganesan, T.; Ramakrishnan, S.K.; Rajasekaran, D.; Periandavan, K. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J. Pharm. Pharmacol. 2012, 64, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Doronicheva, N.; Yasui, H.; Sakurai, H. Chemical structure-dependent differential effects of flavonoids on the catalase activity as avaluated by a chemiluminescent method. Biol. Pharm. Bull. 2007, 30, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Srikanta, A.H.; Kumar, A.; Sukhdeo, S.V.; Peddha, M.S.; Govindaswamy, V. The antioxidant effect of mulberry and jamun fruit wines by ameliorating oxidative stress in streptozotocin-induced diabetic Wistar rats. Food Funct. 2016, 7, 4422–4431. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In Vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: Its relationship to phenolic profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antúneza, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: A randomised cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 46–53. [Google Scholar] [CrossRef]
- Pourabdolhossein, F.; Ghasemi, A.; Shahroukhi, A.; Sherafat, M.A.; Khoshbaten, A.; Asgari, A. In vitro assessment of paraoxon effects on GABA uptake in rat hippocampal synaptosomes. Toxicol. Vitro 2009, 23, 868–873. [Google Scholar] [CrossRef]
- Čolović, M.B.; Vasić, V.M.; Avramović, N.S.; Gajić, M.M.; Djurić, D.M.; Krstić, D.Z. In vitro evaluation of neurotoxicity potential and oxidative stress responses of diazinon and its degradation products in rat brain synaptosomes. Toxicol. Lett. 2015, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Čakar, U.; Petrović, A.; Janković, M.; Pejin, B.; Vajs, V.; Čakar, M.; Djordjević, B. Differentiation of wines made from berry and drupe fruits according to their phenolic profiles. Eur. J. Hortic. Sci. 2018, 83, 49–61. [Google Scholar] [CrossRef]
- Cohen, R.S.; Blomberg, F.; Berzins, K.; Siekevitz, P. The structure of postsynaptic densities isolated from dog cerebral cortex: I. overall morphology and protein composition. J. Cell Biol. 1977, 74, 181–203. [Google Scholar] [CrossRef] [Green Version]
- Towle, A.C.; Sze, P.Y. Steroid binding to synaptic plasma membrane: Differential binding of glucocorticoids and gonadal steroids. J. Steroid Biochem. 1983, 18, 135–143. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Xu, H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism. In A Manual of Biochemical Methods, 3rd ed.; Beutler, E., Ed.; Grune and Startton: New York, NY, USA, 1984; pp. 77–136. [Google Scholar]
- Wendel, A. Glutathione peroxidase. In Enzymatic Basis of Detoxication; Jakoby, W.B., Ed.; Academic Press: New York, NY, USA, 1980; pp. 333–353. [Google Scholar]
- Aruoma, O.I.; Halliwell, B.; Laughton, M.J.; Quinlan, G.J.; Gutteridge, J.M.C. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochem. J. 1989, 258, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Cho, B.O.; Ryu, H.W.; Jin, C.H.; Choi, D.S.; Kang, S.Y.; Kim, D.S.; Byun, M.-W.; Jeong, I.Y. Blackberry extract attenuates oxidative stress through up-regulation of Nrf2-dependent antioxidant enzymes in carbon tetrachloride-treated rats. J. Agric. Food Chem. 2011, 59, 11442–11448. [Google Scholar] [CrossRef]
- Noguer, M.A.; Cerezo, A.B.; Donoso Navarro, E.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012, 65, 609–614. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the control of gastric inflammation: In Vitro and In Vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [Green Version]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J. Nutr. Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Kardum, N.; Takić, M.; Šavikin, K.; Zec, M.; Zdunić, G.; Spasić, S.; Konić-Ristić, A. Effects of polyphenol-rich chokeberry juice on cellular antioxidant enzymes and membrane lipid status in healthy women. J. Funct. Foods 2014, 9, 89–97. [Google Scholar] [CrossRef]
- Miller, M.G.; Shukitt-Hale, B. Berry fruit enhances beneficial signaling in the brain. J. Agric. Food Chem. 2012, 60, 5709–5715. [Google Scholar] [CrossRef]
- de Figueiredo, E.A.; Alves, N.F.B.; de Monteiro, M.M.O.; de Cavalcanti, C.O.; da Silva, T.M.S.; da Silva, T.M.G.; de Braga, V.A.; Oliveira, E. Antioxidant and antihypertensive effects of a chemically defined fraction of syrah red wine on spontaneously hypertensive rats. Nutrients 2017, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Tenore, G.C.; Manfra, M.; Stiuso, P.; Coppola, L.; Russo, M.; Ritieni, A.; Campiglia, P. Polyphenolic pattern and In Vitro cardioprotective properties of typical red wines from vineyards cultivated in Scafati (Salerno, Italy). Food Chem. 2013, 140, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čakar, U.; Grozdanić, N.; Pejin, B.; Vasić, V.; Čakar, M.; Petrović, A.; Djordjević, B. Impact of vinification procedure on fruit wine inhibitory activity against α-glucosidase. Food Biosci. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Szwajgier, D.; Halinowski, T.; Helman, E.; Tylus, K.; Tymcio, A. Influence of different heat treatments on the content of phenolic acids and their derivatives in selected fruits. Fruits 2014, 69, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Klarić, D.A.; Klarić, I.; Mornar, A. Polyphenol content and antioxidant activity of commercial blackberry wines from Croatia: Application of multivariate analysis for geographic origin differentiation. J. Food Nutr. Res. 2011, 50, 199–209. [Google Scholar]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic Acid Profiles in Some Small Berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of Free and Total Phenolic Acids in Plant-Derived Foods by HPLC with Diode-Array Detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of Foods Containing Proanthocyanidins and Their Structural Characterization Using LC-MS/MS and Thiolytic Degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Anti-atherosclerotic effects of fruit bioactive compounds: A review of current scientific evidence. Can. J. Plant Sci. 2012, 92, 407–419. [Google Scholar] [CrossRef]
- Czyzowska, A.; Pogorzelski, E. Changes to polyphenols in the process of production of must and wines from blackcurrants and cherries. Part I. Total polyphenols and phenolic acids. Eur. Food Res. Technol. 2002, 214, 148–154. [Google Scholar] [CrossRef]
- Xiao, Z.; Fang, L.; Niu, Y.; Yu, H. Effect of cultivar and variety on phenolic compounds and antioxidant activity of cherry wine. Food Chem. 2015, 186, 69–73. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Su, M.-S.; Chien, P.-J. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) fluid products as affected by fermentation. Food Chem. 2007, 104, 182–187. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. Winemaking Biochemistry and Microbiology: Current Knowledge and Future Trends. Crit. Rev. Food Sci. Nutr. 2005, 45, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Eder, R.; Wendelin, S.; Vrhovsek, U. Influence of viticultural and enological factors on the concentration of resveratrols in grapes and wine. In Proceedings of the 25th Congres Mondial de la Vigne et du Vin, Paris, France, 19–23 June 2000; pp. 79–86. [Google Scholar]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
| Type of Fruit | Type of Microvinification | Lievito Secco Yeast | ICV D254 Yeast |
|---|---|---|---|
| U/mg Protein | U/mg Protein | ||
| Black chokeberry | −sugar −enzyme | 5.81 ± 0.50 | 5.95 ± 0.55 |
| Black chokeberry | +sugar +enzyme | 6.26 ± 0.62 a,* | 6.22 ± 0.52 b,* |
| Blueberry | −sugar −enzyme | 6.08 ± 0.45 | 5.86 ± 0.40 |
| Blueberry | +sugar +enzyme | 6.35 ± 0.50 a,* | 6.22 ± 0.56 b,* |
| Blackberry | −sugar −enzyme | 6.17 ± 0.47 | 6.26 ± 0.51 |
| Blackberry | +sugar +enzyme | 6.53 ± 0.62 a,* | 6.81 ± 0.65 b,* |
| Cherry | −sugar −enzyme −pit | 5.45 ± 0.48 | 5.59 ± 0.55 |
| Cherry | +sugar +enzyme +pit | 5.63 ± 0.52 a,* | 5.50 ± 0.58 b,* |
| Raspberry | −sugar −enzyme | 5.23 ± 0.49 | 5.01 ± 0.38 |
| Raspberry | +sugar +enzyme | 5.09 ± 0.41 a,* | 5.32 ± 0.40 b,* |
| Apple | −sugar −enzyme | 4.67 ± 0.38 | 4.58 ± 0.42 |
| Apple | +sugar +enzyme | 4.79 ± 0.40 a,* | 4.73 ± 0.45 b,* |
| Type of Fruit | Type of Microvinification | Lievito Secco Yeast | ICV D254 Yeast |
|---|---|---|---|
| U/mg Protein | U/mg Protein | ||
| Black chokeberry | −sugar −enzyme | 0.041 ± 0.005 | 0.042 ± 0.004 |
| Black chokeberry | +sugar +enzyme | 0.049 ± 0.005 a,* | 0.048 ± 0.004 b,* |
| Blueberry | −sugar −enzyme | 0.047 ± 0.005 | 0.043 ± 0.004 |
| Blueberry | +sugar +enzyme | 0.058 ± 0.004 a,* | 0.054 ± 0.005 b,* |
| Blackberry | −sugar −enzyme | 0.042 ± 0.004 | 0.043 ± 0.004 |
| Blackberry | +sugar +enzyme | 0.051 ± 0.005 a,* | 0.050 ± 0.004 b,* |
| Cherry | −sugar −enzyme −pit | 0.034 ± 0.003 | 0.035 ± 0.004 |
| Cherry | +sugar +enzyme +pit | 0.039 ± 0.003 a,* | 0.037 ± 0.004 b,* |
| Raspberry | −sugar −enzyme | 0.035 ± 0.003 | 0.036 ± 0.003 |
| Raspberry | +sugar +enzyme | 0.039 ± 0.004 a,* | 0.040 ± 0.003 b,* |
| Apple | −sugar −enzyme | 0.036 ± 0.003 | 0.034 ± 0.003 |
| Apple | +sugar +enzyme | 0.038 ± 0.003 a,* | 0.039 ± 0.004 b,* |
| Type of Fruit | Type of Microvinification | Lievito Secco Yeast | ICV D254 Yeast |
|---|---|---|---|
| U/mg Protein | U/mg Protein | ||
| Black chokeberry | −sugar −enzyme | 0.0147 ± 0.002 | 0.0141 ± 0.001 |
| Black chokeberry | +sugar +enzyme | 0.0163 ± 0.002 a,* | 0.0159 ± 0.002 b,* |
| Blueberry | −sugar −enzyme | 0.0153 ± 0.002 | 0.0148 ± 0.002 |
| Blueberry | +sugar +enzyme | 0.017 ± 0.002 a,* | 0.0166 ± 0.002 b,* |
| Blackberry | −sugar −enzyme | 0.0158 ± 0.002 | 0.0138 ± 0.001 |
| Blackberry | +sugar +enzyme | 0.0142 ± 0.002 a,* | 0.0155 ± 0.002 b,* |
| Cherry | −sugar −enzyme −pit | 0.00972 ± 0.001 | 0.0099 ± 0.001 |
| Cherry | +sugar +enzyme +pit | 0.0105 ± 0.001 a,* | 0.0110 ± 0.001 b,* |
| Raspberry | −sugar −enzyme | 0.0128 ± 0.001 | 0.0131 ± 0.001 |
| Raspberry | +sugar +enzyme | 0.0137 ± 0.002 a,* | 0.0141 ± 0.002 b,* |
| Apple | −sugar −enzyme | 0.0117 ± 0.001 | 0.0112 ± 0.001 |
| Apple | +sugar +enzyme | 0.0121 ± 0.001 a,* | 0.0125 ± 0.001 b,* |
| Type of Fruit | Type of Microvinification | Lievito Secco Yeast | ICV D254 Yeast |
|---|---|---|---|
| nmol/mg Protein | nmol/mg Protein | ||
| Black chokeberry | −sugar −enzyme | 1.65 ± 0.15 | 1.74 ± 0.14 |
| Black chokeberry | +sugar +enzyme | 1.42 ± 0.11 a,* | 1.50 ± 0.12 b,* |
| Blueberry | −sugar −enzyme | 2.69 ± 0.22 | 2.57 ± 0.15 |
| Blueberry | +sugar +enzyme | 2.25 ± 0.18 a,* | 2.32 ± 0.17 b,* |
| Blackberry | −sugar −enzyme | 2.15 ± 0.16 | 2.31 ± 0.18 |
| Blackberry | +sugar +enzyme | 1.63 ± 0.16 a,* | 1.85 ± 0.15 b,* |
| Cherry | −sugar −enzyme −pit | 1.91 ± 0.15 | 1.79 ± 0.12 |
| Cherry | +sugar +enzyme +pit | 1.72 ± 0.14 a,* | 1.85 ± 0.13 b,* |
| Raspberry | −sugar −enzyme | 2.91 ± 0.25 | 2.62 ± 0.14 |
| Raspberry | +sugar +enzyme | 2.52 ± 0.13 a,* | 2.43 ± 0.15 b,* |
| Apple | −sugar −enzyme | 3.49 ± 0.22 | 3.37 ± 0.25 |
| Apple | +sugar +enzyme | 3.62 ± 0.20 a,* | 3.52 ± 0.23 b,* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čakar, U.; Čolović, M.; Milenković, D.; Medić, B.; Krstić, D.; Petrović, A.; Đorđević, B. Protective Effects of Fruit Wines against Hydrogen Peroxide—Induced Oxidative Stress in Rat Synaptosomes. Agronomy 2021, 11, 1414. https://doi.org/10.3390/agronomy11071414
Čakar U, Čolović M, Milenković D, Medić B, Krstić D, Petrović A, Đorđević B. Protective Effects of Fruit Wines against Hydrogen Peroxide—Induced Oxidative Stress in Rat Synaptosomes. Agronomy. 2021; 11(7):1414. https://doi.org/10.3390/agronomy11071414
Chicago/Turabian StyleČakar, Uroš, Mirjana Čolović, Danijela Milenković, Branislava Medić, Danijela Krstić, Aleksandar Petrović, and Brižita Đorđević. 2021. "Protective Effects of Fruit Wines against Hydrogen Peroxide—Induced Oxidative Stress in Rat Synaptosomes" Agronomy 11, no. 7: 1414. https://doi.org/10.3390/agronomy11071414
APA StyleČakar, U., Čolović, M., Milenković, D., Medić, B., Krstić, D., Petrović, A., & Đorđević, B. (2021). Protective Effects of Fruit Wines against Hydrogen Peroxide—Induced Oxidative Stress in Rat Synaptosomes. Agronomy, 11(7), 1414. https://doi.org/10.3390/agronomy11071414








