Enhancing the Quality of Two Species of Baby Leaves Sprayed with Moringa Leaf Extract as Biostimulant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants Materials
2.2. Preparation and Analysis of Moringa Leaf Extract
2.3. Chlorophyll A Fluorescence
2.4. Measurement of Growth Parameters
2.5. Chlorophyll and Carotenoid Pigments
2.6. 2.2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical-Scavenging Activity and Total Phenolic Contents
2.7. Total Sugars
2.8. Ascorbic Acid Analysis and Nitrate Concentration
2.9. Determination of Mineral Elements
2.10. Statistical Analysis
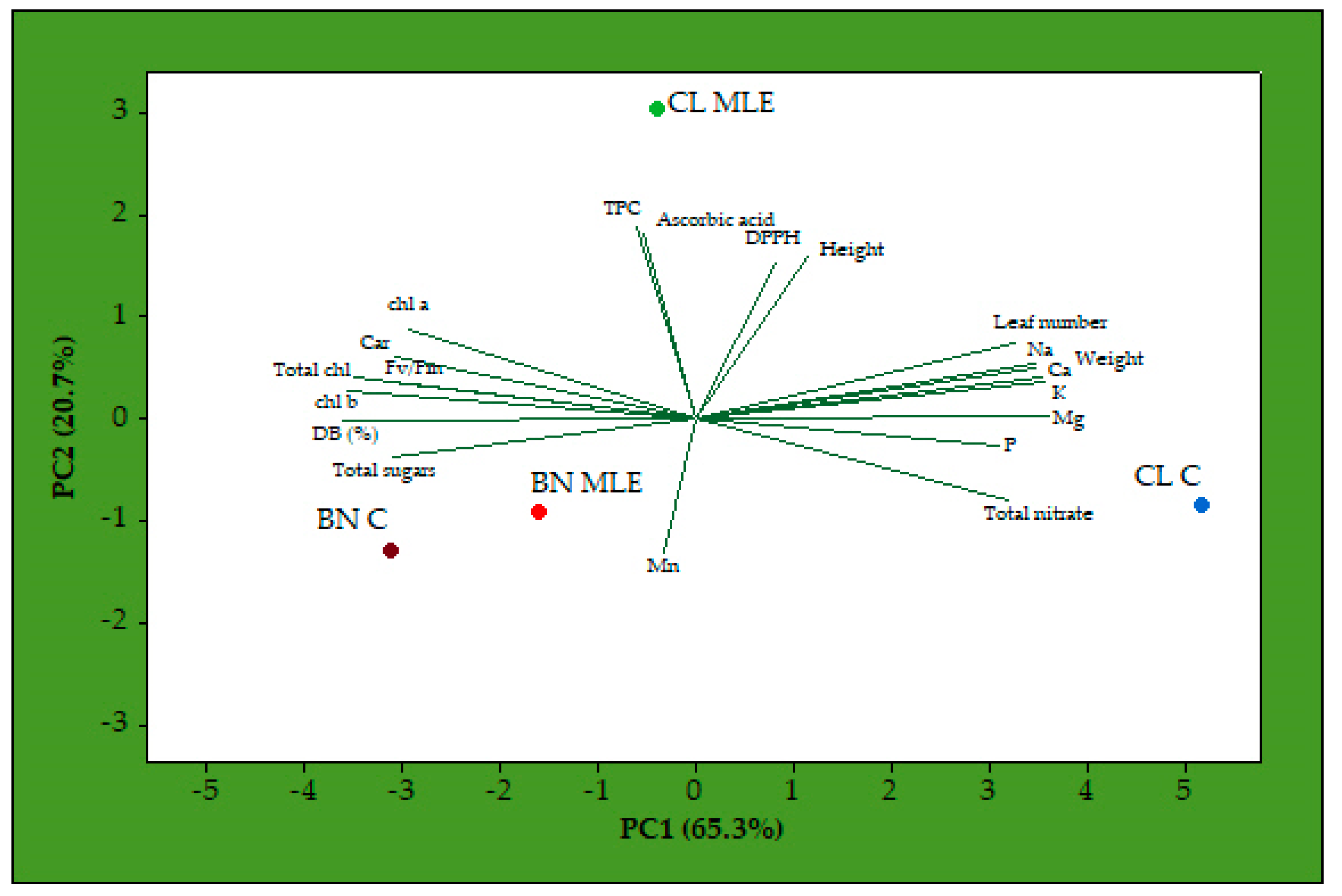
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kader, A.A. Effects on Nutritional Quality. In Modified and Controlled Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities; Yahia, E.M., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 111–118. [Google Scholar]
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric characteristics, polyphenols and ascorbic acid variation in Brassica oleracea L. Novel Foods: Sprouts, Microgreens and Baby Leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of pre-harvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Ryder, E.J. The new salad crop revolution. In Proceedings of the Fifth National Symposium Trends in New Crops and New Uses, Atlanta, GA, USA, 10–13 November 2001; pp. 408–412. [Google Scholar]
- Subhasree, B.; Baskar, R.; Keerthana, R.L.; Susan, R.L.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, microgreens and “baby leaf” vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. [Google Scholar]
- Ali, A.M. Effect of food processing methods on the bioactive compound of cauliflower. Egypt. J. Agric. Res. 2015, 93, 117–131. [Google Scholar]
- Wu, X.; Huang, H.; Childs, H.; Wu, Y.; Yu, L.; Pehrsson, P.R. Glucosinolates in Brassica Vegetables: Characterization and Factors That Influence Distribution, Content, and Intake. Annu. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef]
- Lester, G.E.; Makus, D.J.; Hodges, D.M. Relationship between fresh-packaged spinach leaves exposed to continuous light or dark and bioactive contents: Effects of cultivar, leaf size, and storage duration. J. Agric. Food Chem. 2010, 58, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Regulated water deficits improve phytochemical concentration in lettuce. J. Am. Soc. Hortic. Sci. 2010, 135, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Kunicki, E.; Grabowska, A.; Sekara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Amanda, A.; Valagussa, M.; Piaggesi, A.; Ferrante, A. Effect of biostimulants on quality of baby leaf lettuce grown under plastic tunnel. Acta Hortic. 2009, 807, 407–412. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S. Review: Long-term research activity on the biostimulant properties of natural origin compounds. Acta Hortic. 2013, 1009, 81–187. [Google Scholar] [CrossRef]
- Ferrini, F.; Nicese, F.P. Response of English oak (Quercus robur L.) trees to biostimulants application in the urban environment. J. Arboric. 2002, 28, 70–75. [Google Scholar]
- Howladar, S.M. A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef]
- Rady, M.M.; Bhavya Varma, C.; Howladar, S.M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F.; Abdalla, A.M.; Ahmed Yasmin, H.M. Integrated application of salicylic acid and Moringa oleifera leaf extract alleviates the salt-induced adverse effects in common bean plants. Int. J. Agric. Technol. 2015, 11, 1595–1614. Available online: https://www.thaiscience.info/journals/Article/IJAT/10976758.pdf (accessed on 21 May 2021).
- Semida, W.M.; Rady, M.M. Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Hortic. Sci. Biotechnol. 2014, 89, 338–344. [Google Scholar] [CrossRef]
- Manzoor, M.; Anwar, F.; Iqbal, T.; Bhanger, M.I. Physico-chemical characterization of Moringa concanensis seeds and seed oil. J. Am. Oil Chem. Soc. 2007, 84, 413–419. [Google Scholar] [CrossRef]
- Vlahov, G.; Chepkwony, P.K.; Ndalut, P.K. 13C NMR Characterization of triacylglycerols of Moringa oleifera Seed Oil: An “Oleic-Vaccenic Acid” Oil. J. Agric. Food Chem. 2002, 50, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drum- stick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Foidl, N.; Makkar, H.P.S.; Becker, K. The potential of Moringa oleifera for agricultural and industrial uses. In The Miracle Tree: The Multipurpose Attributes of Moringa; Fuglie, J., Ed.; CTA Publications: Wageningen, The Netherlands, 2001; pp. 45–76. [Google Scholar]
- Yasmeen, A.; Basra, S.M.A.; Farooq, M.; ur Rehman, H.; Hussain, N. Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul. 2013, 69, 225–233. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Hamad, G.M.; Mohamed, S.E.; Amar, M.H.; Al-Hindi, R.R. Biochemical and functional properties of Moringa oleifera leaves and their potential as a functional food. Glob. Adv. Res. J. Agric. Sci. 2015, 4, 188–199. [Google Scholar]
- Fuglie, L.J. The Miracle Tree: Moringa oleifera: Natural Nutrition for the Tropics; CWS: Dakar, Senegal, 2000; p. 172. [Google Scholar]
- Iqbal, J.; Irshad, J.; Bashir, S.; Khan, S.; Yousaf, M.; Shah, A.N. Comparative study of water extracts of Moringa leaves and roots to improve the growth and yield of sunflower. S. Afr. J. Bot. 2020, 129, 221–224. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Pervez, K.; Ullah, F.; Mehmood, S.; Khattak, A. Effect of Moringa oleifera Lam. leaf aqueous extract on growth attributes and cell wall bound phenolics accumulation in maize (Zea mays L.) under drought stress. Kuwait J. Sci. 2017, 44, 110–118. Available online: https://journalskuwait.org/kjs/index.php/KJS/article/view/2015/218 (accessed on 26 May 2021).
- Sakr, M.T.; Ibrahim, H.M.; ElAwady, A.E.; AboELMakarm, A.A. Growth, yield and biochemical constituents as well as post-harvest quality of water-stressed broccoli (Brassica oleracea L. var. italica) as affected by certain biomodulators. Sci. Hortic. 2021, 275, 109605. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Cocetta, G.; Rossoni, M.; Gardana, C.; Mignani, I.; Ferrante, A.; Spinardi, A. Methyl jasmonate affects phenolic metabolism and gene expression in blueberry (Vaccinium corymbosum). Physiol. Plant. 2015, 153, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Janghel, E.K.; Gupta, V.K.; Rai, M.K.; Rai, J.K. Micro determination of ascorbic acid using methyl viologen. Talanta 2007, 72, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.A.; Haroon, M.; Sehrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by titration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Bulgari, R.; Trivellini, A.; Ferrante, A. Effects of two doses of organic extract-based biostimulant on greenhouse lettuce grown under increasing NaCl concentrations. Front. Plant Sci. 2019, 9, 1870. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Ferrante, A. Borage extracts affect wild rocket quality and influence nitrate and carbon metabolism. Physiol. Mol. Biol. Plants 2020, 1–12. [Google Scholar] [CrossRef]
- Yasmeen, A.; Nouman, W.; Basra, S.M.A.; Wahid, A.; Hussain, N.; Afzal, I. Morphological and physiological response of tomato (Solanum lycopersicum L.) to natural and synthetic cytokinin sources: A comparative study. Acta Physiol. Plant. 2014, 36, 3147–3155. [Google Scholar] [CrossRef]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Culver, M.; Tagwira, F.A.; Chiteka, Z. Effect of moringa extract on growth and yield of tomato. Greener J. Agric. Sci. 2012, 2, 207–211. Available online: https://miracletrees.org/moringa-doc/moringa-increase-plant-growth.pdf (accessed on 26 May 2021).
- Makkar, H.P.S.; Becker, K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J. Agric. Sci. 1997, 128, 311–322. [Google Scholar] [CrossRef]
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933. [Google Scholar] [CrossRef] [Green Version]
- Merwad, A.R.M. Using Moringa oleifera extract as biostimulant enhancing the growth, yield and nutrients accumulation of pea plants. J. Plant Nutr. 2018, 41, 425–431. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Yameogo, C.W.; Bengaly, M.D.; Savadogo, A.; Nikiema, P.A.; Traore, S.A. Determination of chemical composition and nutritional values of Moringa oleifera leaves. Pak. J. Nutr. 2011, 10, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Afzal, I.; Hussain, B.; Basra, S.M.A.; Rehman, H. Priming with moringa leaf extract reduces imbibitional chilling injury in spring maize. Seed Sci. Technol. 2012, 40, 271–276. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.S.A.; Abdel-Kader, A.A.S. Response of Fennel (Foeniculum vulgare Mill) plants to foliar application of moringa leaf extract and benzyladenine (BA). S. Afr. J. Bot. 2020, 129, 113–122. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Stress physiology. In Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002; pp. 591–623. [Google Scholar]
- Alkuwayti, M.A.; El-Sherif, F.; Yap, Y.K.; Khattab, S. Foliar application of Moringa oleifera leaves extract altered stress-responsive gene expression and enhanced bioactive compounds composition in Ocimum basilicum. S. Afr. J. Bot. 2020, 129, 291–298. [Google Scholar] [CrossRef]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The effect of plant-derived biostimulants on white head cabbage seedlings grown under controlled conditions. Sustainability 2019, 11, 5317. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Aslam, M.; Sultana, B.; Anwar, F.; Munir, H. Foliar spray of selected plant growth regulators affected the biochemical and antioxidant attributes of spinach in a field experiment. Turk. J. Agric. For. 2016, 40, 136–145. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomas-Barberan, F.A.; Garcia-Viguera, C. Potential bioactive compounds in health promotion from broccoli cultivars grown in Spain. J. Sci. Food Agric. 2002, 82, 1293–1297. [Google Scholar] [CrossRef]
- Podsędek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Int. J. Food Sci. Technol. 2006, 41, 49–58. [Google Scholar] [CrossRef]
- Dudaš, S.; Šola, I.; Sladonja, B.; Erhatić, R.; Ban, D.; Poljuha, D. The effect of biostimulant and fertilizer on “low input” lettuce production. Acta Bot. Croat. 2016, 75, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Q.; Lee, K.S. Effect of mixed amino acids on crop growth. In Agricultural Science; Aflakpui, G., Ed.; InTech Europe Publisher: Rijeka, Croatia, 2012; pp. 119–158. [Google Scholar]
- Vernieri, P.; Borghesi, E.; Ferrante, A.; Magnani, G. Application of biostimulants in floating system for improving rocket quality. J. Food Agric. Environ. 2005, 3, 86. [Google Scholar]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef] [Green Version]
| Component | Value |
|---|---|
| Phosphorus (P) | 4.9 g kg−1 DW |
| Potassium (K) | 16.0 g kg−1 DW |
| Calcium (Ca) | 16.2 g kg−1 DW |
| Iron (Fe) | 0.2 g kg−1 DW |
| Magnesium (Mg) | 3.0 g kg−1 DW |
| DPPH 1 | 130 mg TE g−1 DW |
| Total polyphenols | 22.6 mg GAE g−1 DW |
| Chlorophyll a | 1.2 µg mg−1 FW |
| Chlorophyll b | 1.4 µg mg−1 FW |
| Carotenoids | 0.10 µg mg−1 FW |
| Nitrate concentration | 515.5 mg kg−1 FW |
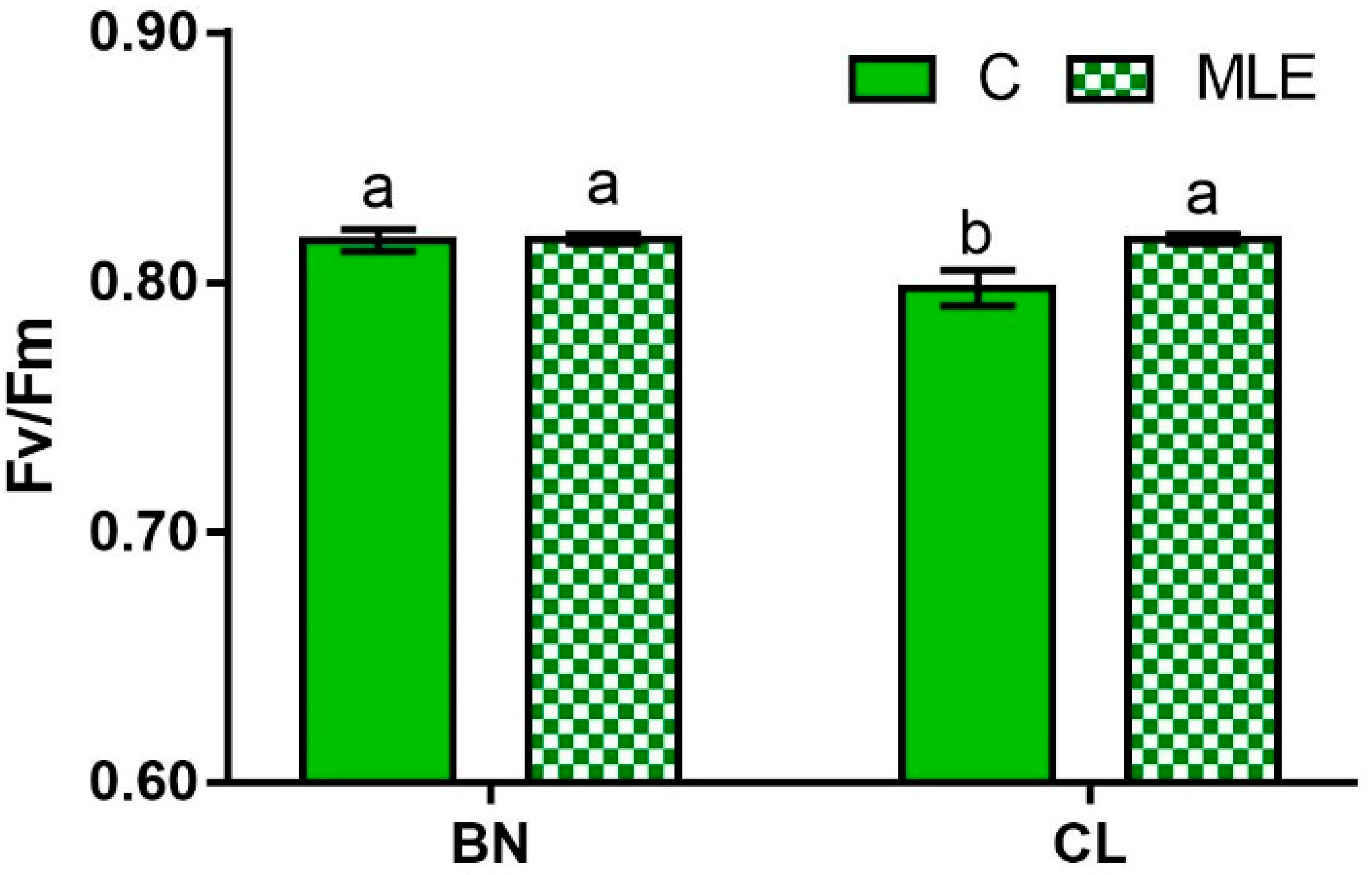
| Genotype (G) | Treatment (T) | F0 | Fm | Fv/Fm |
|---|---|---|---|---|
| BN | C | 433.5 ± 6.4 | 2379.8 ± 53.2 | 0.82 ± 0.0 a |
| MLE | 426.0 ± 20.5 | 2201.6 ± 28.9 | 0.82 ± 0.0 a | |
| CL | C | 449.5 ± 15.6 | 2232.8 ± 24.0 | 0.80 ± 0.0 b |
| MLE | 449.0 ± 14.1 | 2364.5 ± 51.9 | 0.81 ± 0.0 a | |
| BN | 429.8 ± 9.2 | 2290.7 ± 37.8 | 0.82 ± 0.0 | |
| CL | 449.3 ± 9.9 | 2298.5 ± 33.9 | 0.80 ± 0.0 | |
| C | 441.5 ± 8.1 | 2306.1 ± 36.6 | 0.80 ± 0.0 | |
| MLE | 437.5 ± 11.4 | 2283.1 ± 34.9 | 0.81 ± 0.1 | |
| Significance | ||||
| G | ns | ns | ns | |
| T | ns | ns | ns | |
| G × T | ns | ns | * |
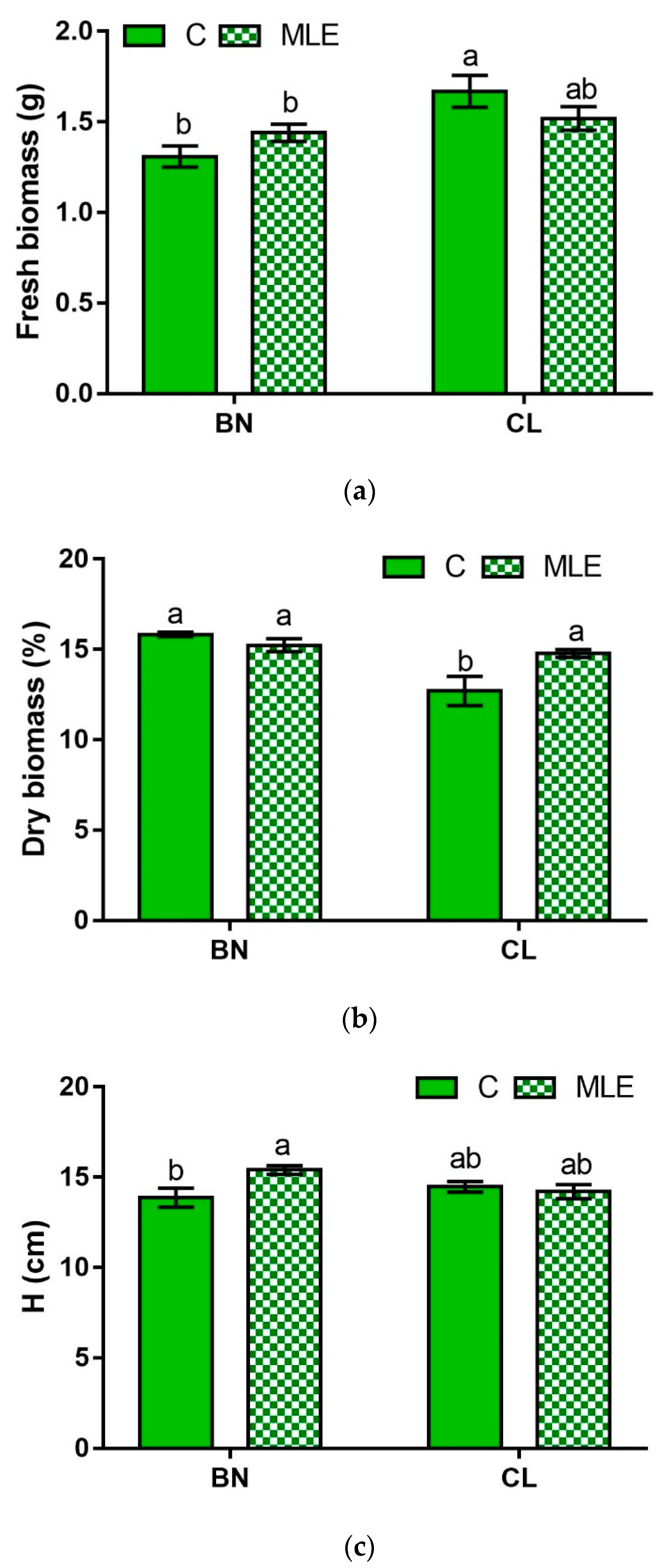
| Genotype | Treatment | Fresh Biomass (g plant−1) | Dry Biomass (%) | Height (cm) | Leaf Number (n) |
|---|---|---|---|---|---|
| BN | C | 1.31 ± 0.06 b | 15.83 ± 0.13 a | 13.87 ± 0.53 b | 3.27 ± 0.12 |
| MLE | 1.44 ± 0.05 b | 15.23 ± 0.36 a | 15.40 ± 0.24 a | 3.47 ± 0.13 | |
| CL | C | 1.67 ± 0.09 a | 12.70 ± 0.80 b | 14.47 ± 0.29 ab | 3.73 ± 0.15 |
| MLE | 1.52 ± 0.07 ab | 14.77 ± 0.22 a | 14.20 ± 0.39 ab | 3.60 ± 0.13 | |
| BN | 1.37 ± 0.04 b | 15.53 ± 0.22 a | 14.63 ± 0.32 | 3.37 ± 0.09 b | |
| CL | 1.59 ± 0.06 a | 13.73 ± 0.60 b | 14.33 ± 0.10 | 3.67 ± 0.10 a | |
| C | 1.49 ± 0.06 | 14.27 ± 0.80 | 14.20 ± 0.81 | 3.50 ± 0.10 | |
| MLE | 1.48 ± 0.04 | 15.00 ± 0.21 | 14.80 ± 0.25 | 3.53 ± 0.09 | |
| Significance | |||||
| G | ** | ** | ns | * | |
| T | ns | ns | ns | ns | |
| G × T | * | * | * | ns |
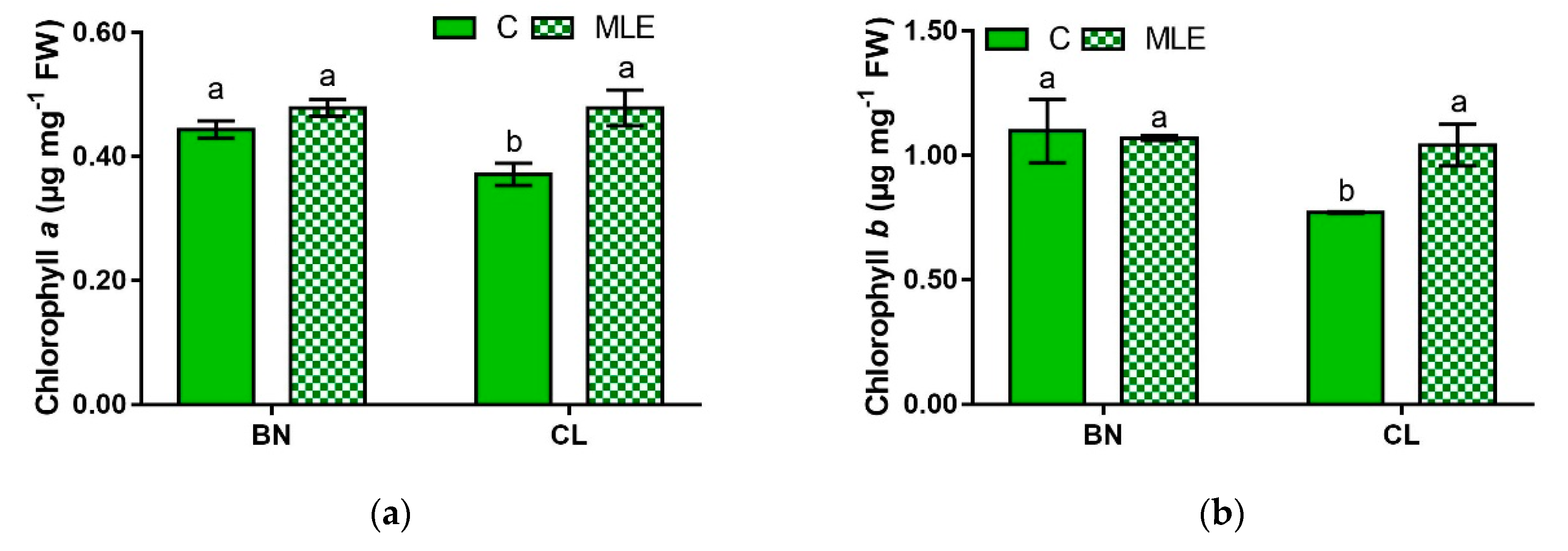
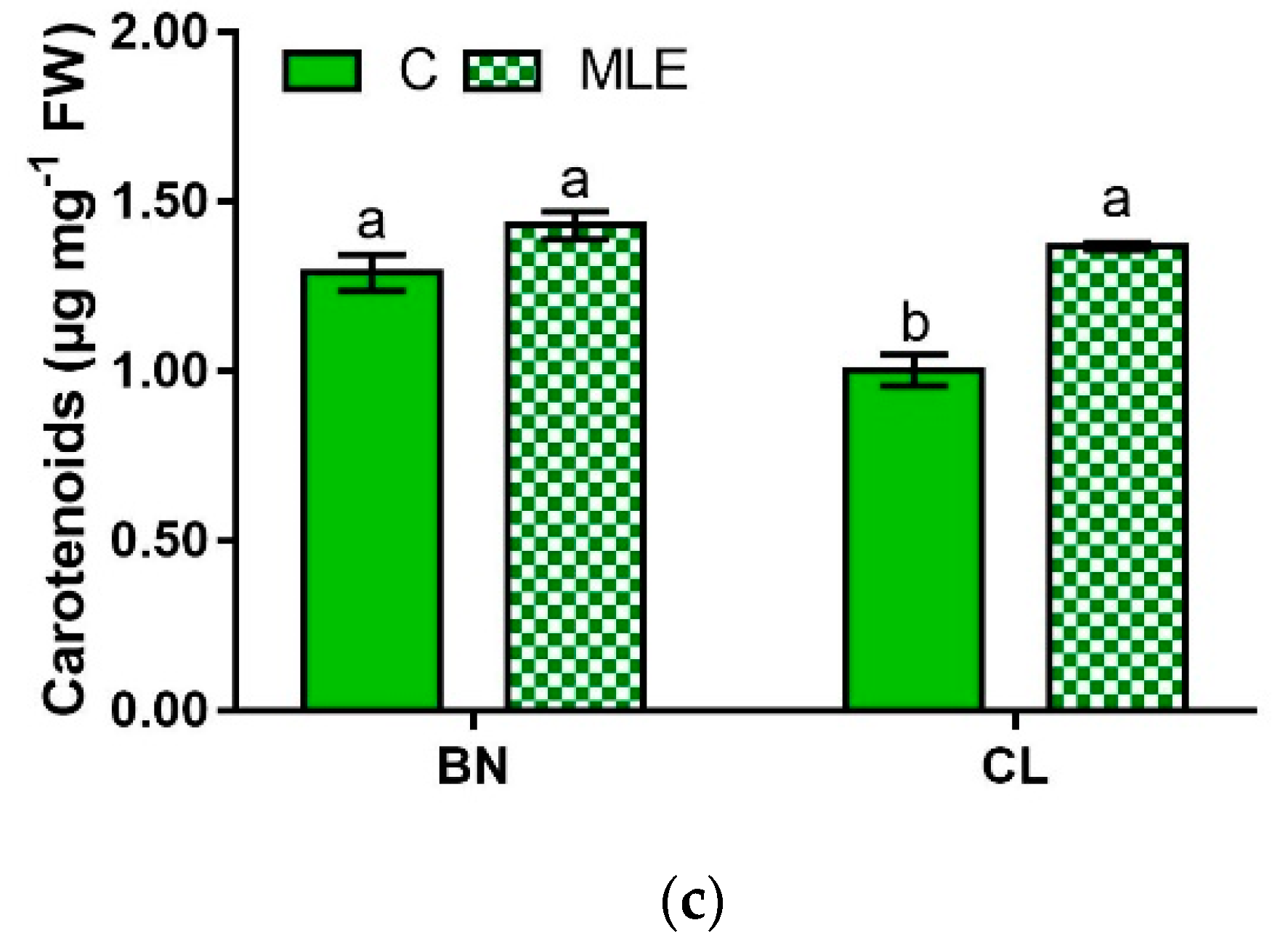
| Genotypes | Treatment | Chl a (µg mg−1 FW) | Chl b (µg mg−1 FW) | Carotenoids (µg mg−1 FW) |
|---|---|---|---|---|
| BN | C | 0.44 ± 0.01 a | 1.10 ± 0.13 a | 1.29 ± 0.05 a |
| MLE | 0.48 ± 0.01 a | 1.07 ± 0.00 a | 1.43 ± 0.04 a | |
| CL | C | 0.37 ± 0.02 b | 0.77 ± 0.00 b | 1.00 ± 0.05 b |
| MLE | 0.48 ± 0.02 a | 1.04 ± 0.08 a | 1.37 ± 0.01 a | |
| BN | 0.46 ± 0.01 | 0.91 ± 0.07 b | 1.36 ± 0.04 a | |
| CL | 0.42 ± 0.03 | 1.08 ± 0.06 a | 1.19 ± 0.08 b | |
| C | 0.41 ± 0.02 b | 0.93 ± 0.09 | 1.15 ± 0.07 b | |
| ME | 0.48 ± 0.01 a | 1.06 ± 0.04 | 1.40 ± 0.02 a | |
| Significance | ||||
| G | ns | * | ** | |
| T | ** | ns | *** | |
| G × T | * | * | ** |
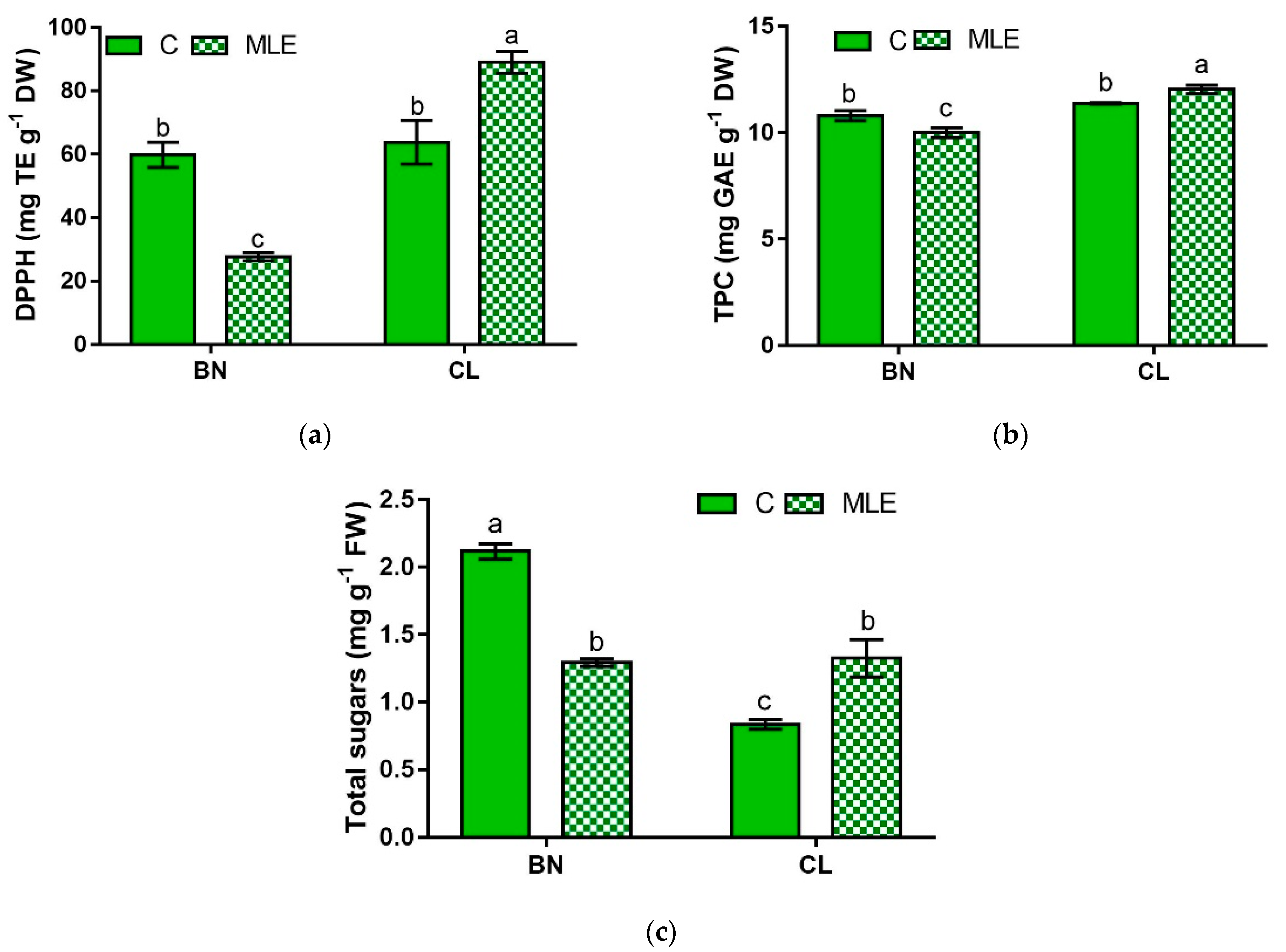
| Genotype | Treatment | DPPH (mg TE g−1 DW) | TPC (mg GAE g−1 DW) | Total Sugars (mg g−1 FW) |
|---|---|---|---|---|
| BN | C | 59.8 ± 3.9 b | 10.8 ± 0.2 b | 2.10 ± 0.06 a |
| MLE | 27.7 ± 1.3 c | 10.0 ± 0.4 c | 1.29 ± 0.03 b | |
| CL | C | 63.8 ± 6.9 b | 11.4 ± 0.0 b | 0.8 ± 0.04 c |
| MLE | 89.0 ± 3.4 a | 12.0 ± 0.2 a | 1.3 ± 0.14 b | |
| BN | 43.7 ± 17.9 b | 10.4 ± 0.2 b | 1.08 ± 0.13 b | |
| CL | 76.4 ± 31.2 a | 11.7 ± 0.2 a | 1.70 ± 0.19 a | |
| C | 61.8 ± 3.7 | 11.1 ± 0.2 | 1.48 ± 0.29 | |
| LME | 58.4 ± 13.8 | 11.0 ± 0.5 | 1.31 ± 0.06 | |
| Significance | ||||
| G | *** | *** | *** | |
| T | ns | ns | ns | |
| G × T | *** | * | *** |
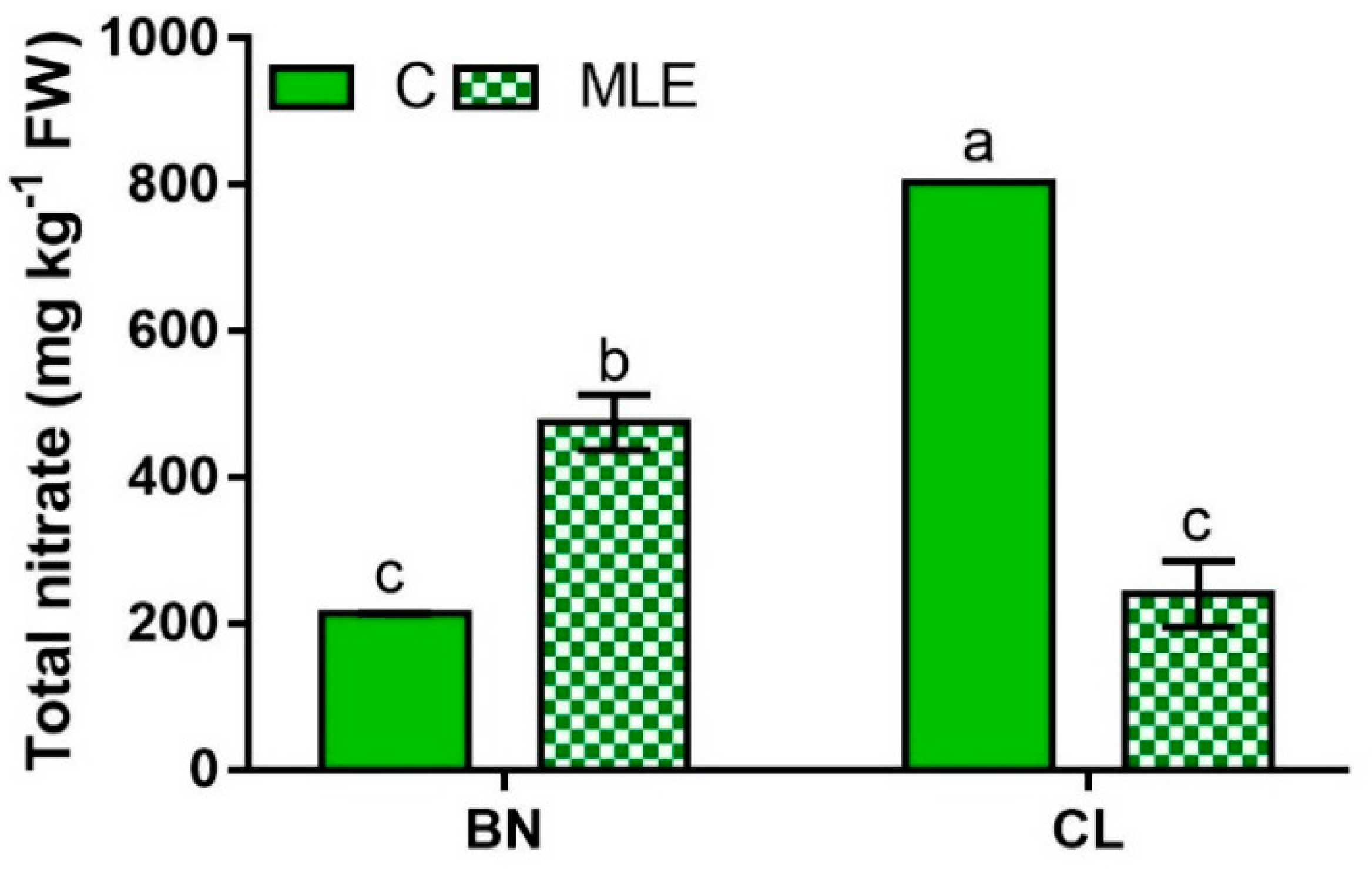
| Genotype | Treatment | Asc (mg 100 g−1 FW) | Nitrate (mg kg−1 FW) |
|---|---|---|---|
| BN | C | 129.0 ± 16.2 | 213.1 ± 1.3 c |
| MLE | 149.2 ± 2.0 | 475.0 ± 38.3 b | |
| CL | C | 132.8 ± 1.6 | 803.0 ± 0.1 a |
| MLE | 167.6 ± 0.4 | 240.1 ± 45.0 c | |
| BN | 139.1 ± 8.6 | 344.1 ± 61.0 b | |
| CL | 150.2 ± 7.8 | 521.6 ± 127.5 a | |
| C | 130.9 ± 7.4 b | 508.1 ± 131.9 a | |
| LME | 158.4 ± 4.2 a | 357.6 ± 58.8 b | |
| Significance | |||
| G | ns | *** | |
| T | * | *** | |
| G × T | ns | *** |
| Na (g kg−1 DW) | Mg (g kg−1 DW) | K (g kg−1 DW) | Ca (g kg−1 DW) | Mn (g kg−1 DW) | Fe (mg kg−1 DW) | Ni (mg kg−1 DW) | Cu (mg kg−1 DW) | Zn (mg kg−1 DW) | P (g kg−1 DW) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BN | C | 1.0 ± 0.0 c | 2.5 ± 0.0 c | 8.4 ± 0.1 d | 14.8 ± 0.2 d | 51.2 ± 0.7 bc | 137.3 ± 8.7 | 2.2 ± 0.6 | 8.0 ± 0.8 | 24.1 ± 1.4 | 7.8 ± 0.1 b |
| MLE | 1.1 ± 0.0 d | 2.6 ± 0.1 c | 9.1 ± 0.1 c | 16.1 ± 0.4 c | 58.0 ± 0.7 a | 139.8 ± 10.8 | 1.6 ± 0.1 | 7.7 ± 1.1 | 27.9 ± 2.0 | 8.1 ± 0.2 ab | |
| CL | C | 1.8 ± 0.0 a | 3.4 ± 0.0 a | 11.2 ± 0.2 a | 20.2 ± 0.2 a | 52.3 ± 0.9 b | 150.1 ± 8.1 | 1.7 ± 0.4 | 9.0 ± 1.5 | 29.0 ± 0.5 | 8.3 ± 0.1 a |
| MLE | 1.4 ± 0.0 b | 2.8 ± 0.0 b | 9.9 ± 0.1 b | 17.5 ± 0.4 b | 48.2 ± 1.0 c | 148.2 ± 10.0 | 1.1 ± 0.3 | 8.7 ± 0.5 | 23.0 ± 1.0 | 8.0 ± 0.1 ab | |
| Genotypes (G) | BN | 1.1 ± 0.0 b | 2.5 ± 0.0 b | 8.8 ± 0.2 b | 15.4 ± 0.4 b | 54.6 ± 1.7 a | 138.5 ± 6.2 | 1.9 ± 0.3 | 7.9 ± 0.6 | 26.0 ± 1.4 | 7.9 ± 0.1 |
| CL | 1.6 ± 0.1 a | 3.1 ± 0.1 a | 10.5 ± 0.3 a | 18.9 ± 0.6 a | 50.2 ± 1.1 b | 149.2 ± 5.8 | 1.4 ± 0.2 | 8.9 ± 0.7 | 26.0 ± 1.4 | 8.1 ± 0.1 | |
| Treatment (T) | C | 1.4 ± 0.2 a | 3.0 ± 0.2 a | 9.8 ± 0.6 a | 17.5 ± 1.2 a | 51.7 ± 0.6 | 143.7 ± 6.0 | 1.9 ± 0.3 | 8.5 ± 0.8 | 26.5 ± 1.3 | 8.0 ± 0.1 |
| MLE | 1.3 ± 0.1 b | 2.7 ± 0.1 b | 9.5 ± 0.2 b | 16.8 ± 0.4 b | 53.1 ± 0.4 | 144.0 ± 6.9 | 1.4 ± 0.2 | 8.2 ± 0.6 | 25.5 ± 1.5 | 8.0 ± 0.1 | |
| Significance | |||||||||||
| G | *** | *** | *** | *** | ** | ns | ns | ns | ns | ns | |
| T | *** | *** | * | * | ns | ns | ns | ns | ns | ns | |
| G × T | *** | *** | *** | *** | ** | ns | ns | ns | ns | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, S.; Ferrante, A.; Branca, F.; Romano, D. Enhancing the Quality of Two Species of Baby Leaves Sprayed with Moringa Leaf Extract as Biostimulant. Agronomy 2021, 11, 1399. https://doi.org/10.3390/agronomy11071399
Toscano S, Ferrante A, Branca F, Romano D. Enhancing the Quality of Two Species of Baby Leaves Sprayed with Moringa Leaf Extract as Biostimulant. Agronomy. 2021; 11(7):1399. https://doi.org/10.3390/agronomy11071399
Chicago/Turabian StyleToscano, Stefania, Antonio Ferrante, Ferdinando Branca, and Daniela Romano. 2021. "Enhancing the Quality of Two Species of Baby Leaves Sprayed with Moringa Leaf Extract as Biostimulant" Agronomy 11, no. 7: 1399. https://doi.org/10.3390/agronomy11071399
APA StyleToscano, S., Ferrante, A., Branca, F., & Romano, D. (2021). Enhancing the Quality of Two Species of Baby Leaves Sprayed with Moringa Leaf Extract as Biostimulant. Agronomy, 11(7), 1399. https://doi.org/10.3390/agronomy11071399









