Monitoring of Long-Lasting Effects of Fumigation with Dimethyl Disulfide (DMDS) on Root-Gall Index, Root-Knots, Other Nematode Populations, and Crop Yield over Three Protected Cucumber Crops in Bulgaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Root-Gall Index and Root Mapping
2.4. Yield
2.5. Soil Sampling and Processing and Nematode Identification
2.6. Statistical Analysis
3. Results
3.1. Root-Knot Nematode Identification
3.2. Root-Gall Index and Root Mapping
3.3. Yield
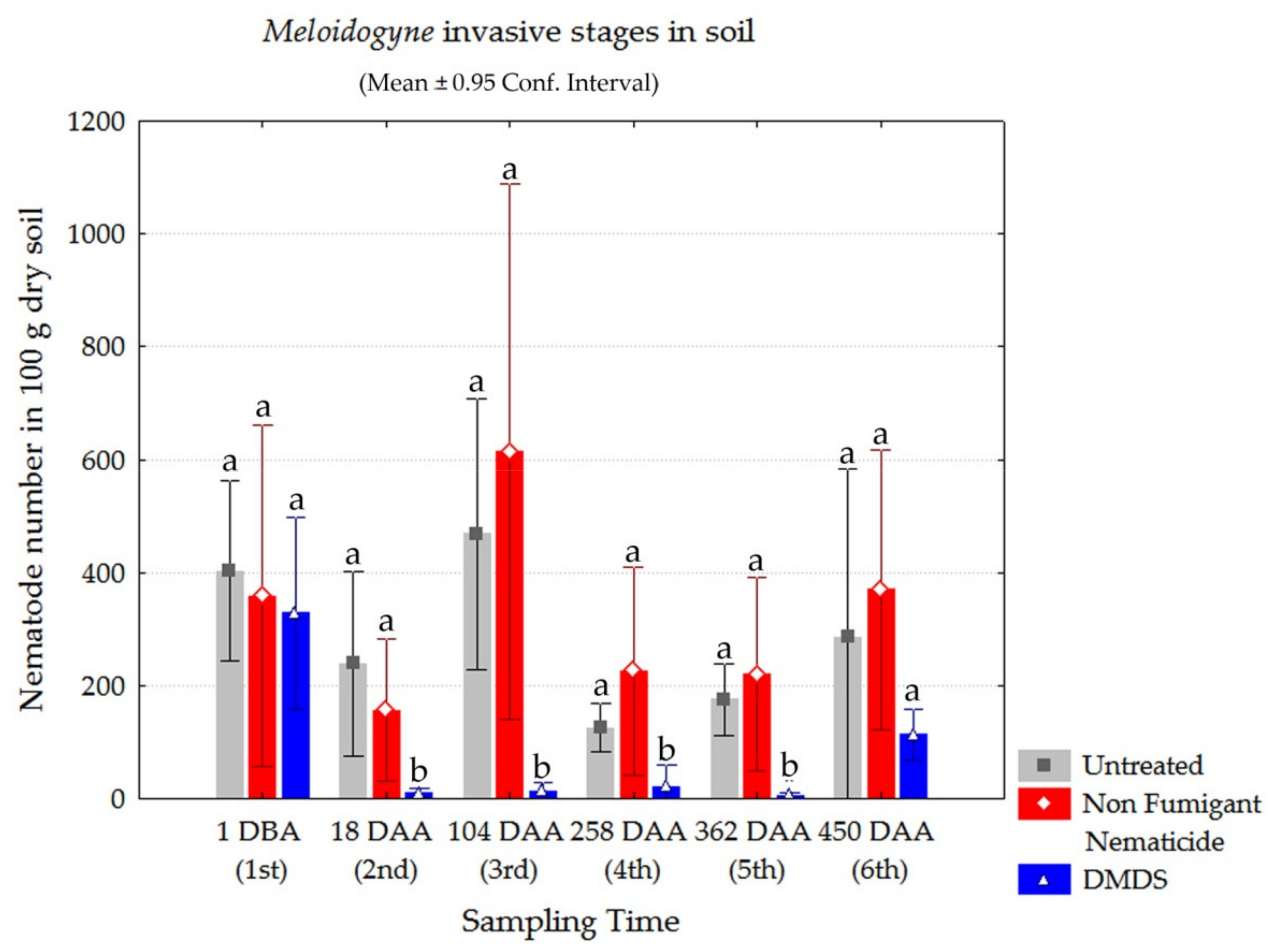
3.4. Root-Knot Nematode Invasive Stages in Soil
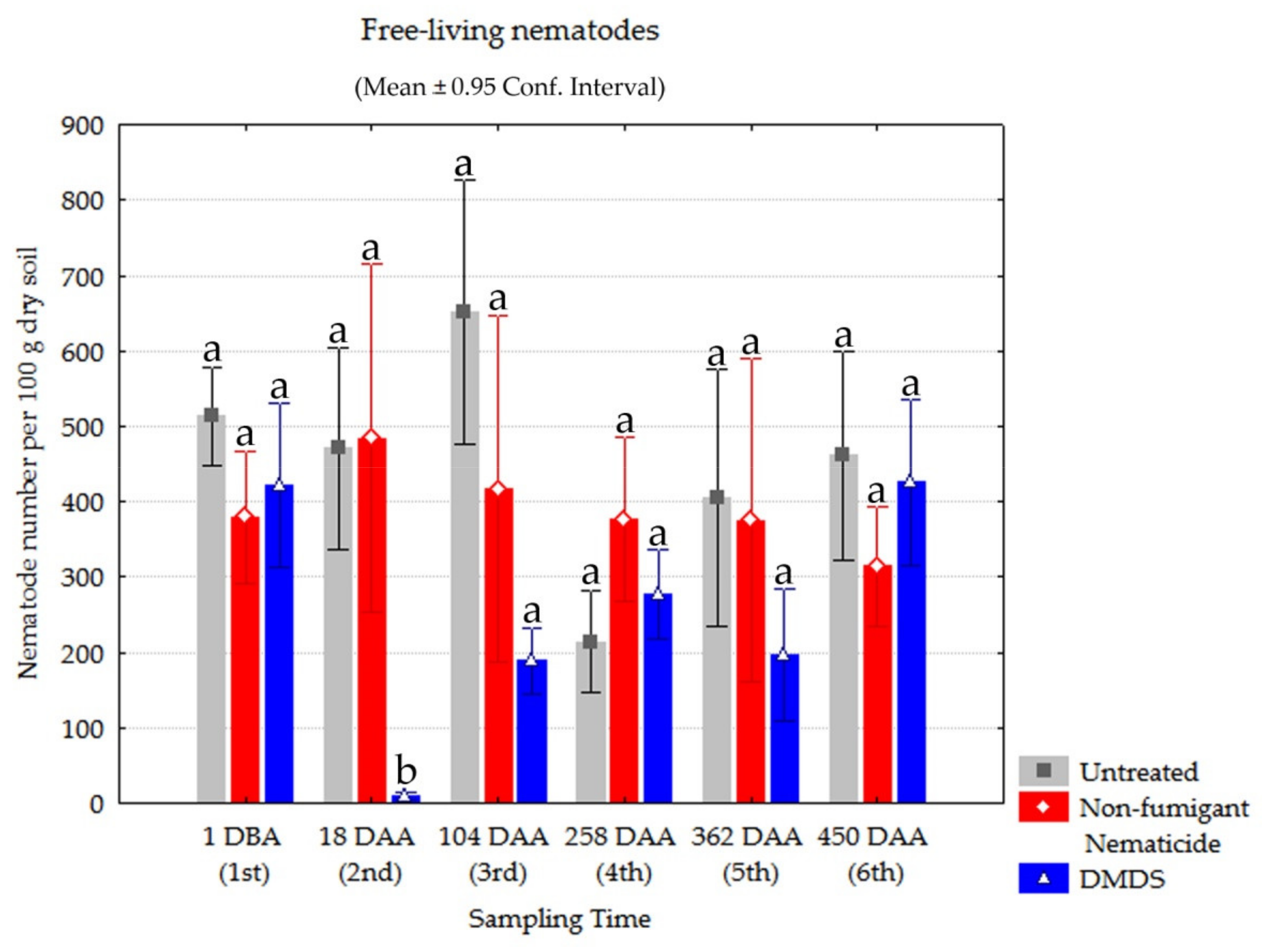
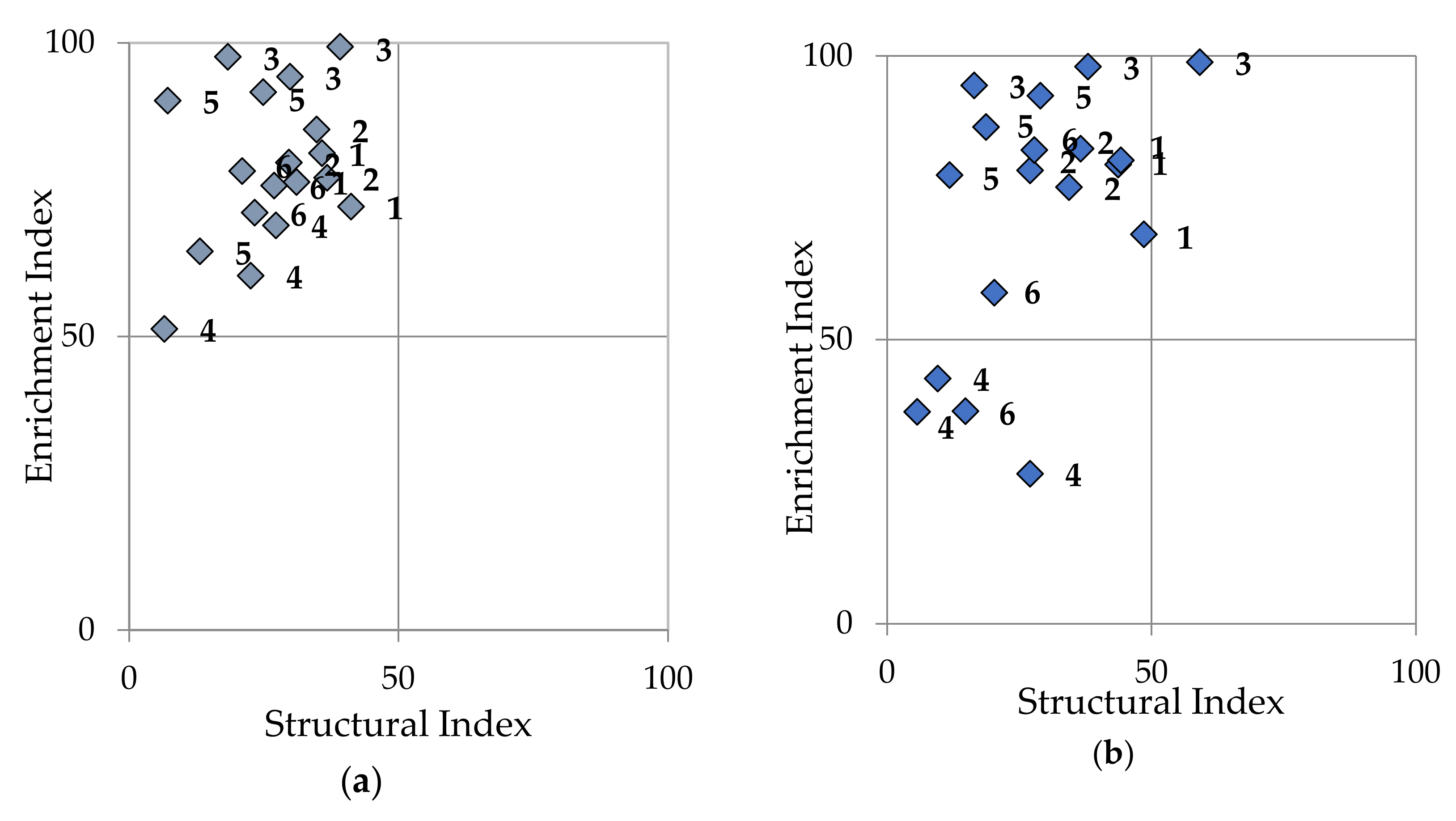
3.5. Monitoring Other Nematode Populations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
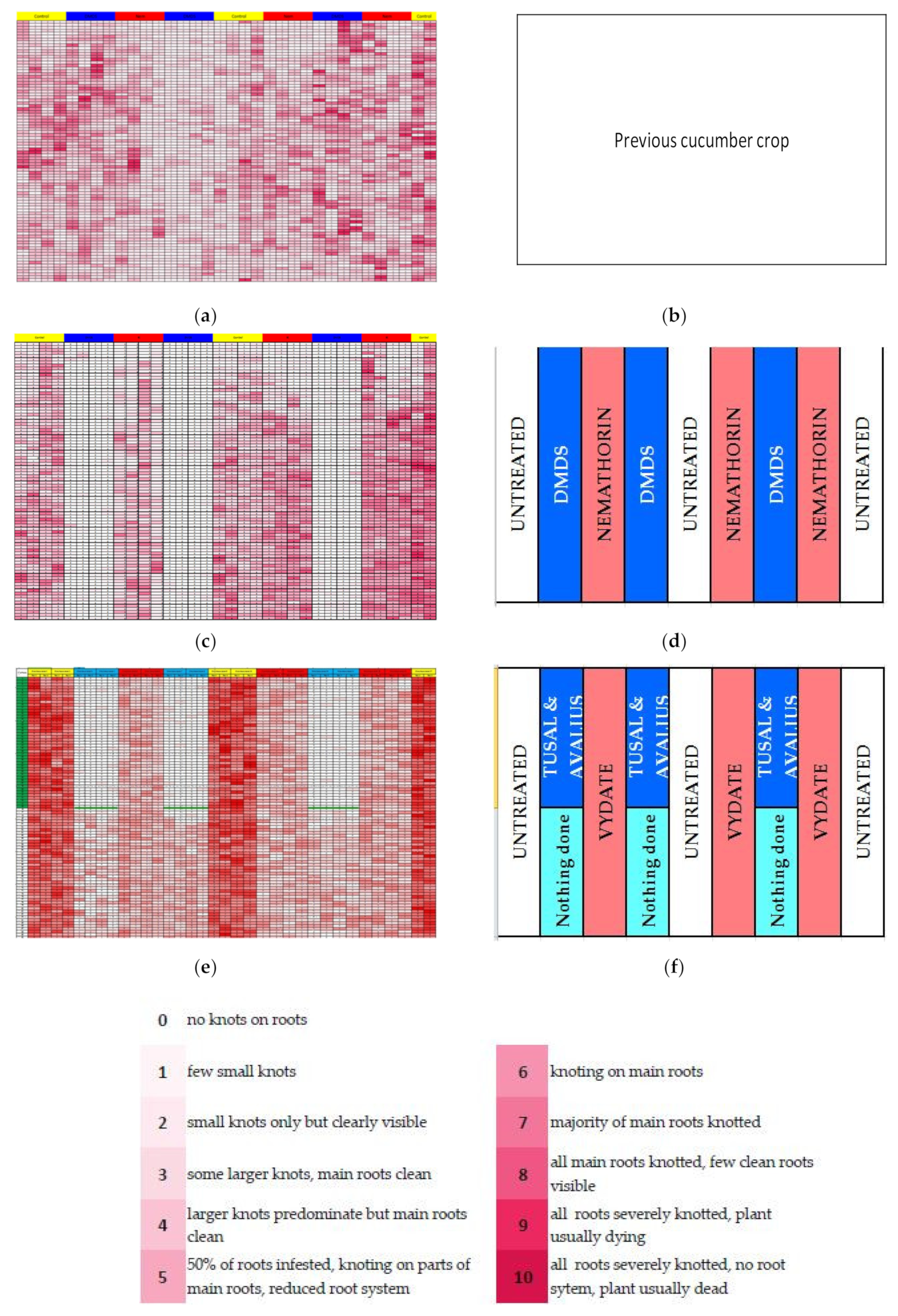
Appendix B
| Guild | Genus | Sampling Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DBA | 18 DAA | 104 DAA | 258 DAA | 362 DAA | 450 DAA | ||||||||
| Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | ||
| Untreated | |||||||||||||
| Pp-a-3 | Meloidogyne | 401.8 | 100% | 246.0 | 100% | 454.1 | 100% | 94.3 | 100% | 93.9 | 100% | 268.8 | 100% |
| Pp-d-2 | Paratylenchus | 4.7 | 33% | 3.7 | 58% | 1.2 | 42% | 2.4 | 42% | ||||
| Pp-d-3 | Criconemella | 4.7 | 17% | 1.2 | 25% | 0.9 | 8% | 2.4 | 17% | ||||
| Pp-d-3 | Tylenchorhynchus | 0.2 | 8% | ||||||||||
| Pp-e-2 | Psilenchus | 4.7 | 33% | 5.5 | 75% | 2.3 | 42% | ||||||
| Total | 415.9 | 256.7 | 454.1 | 94.3 | 96.1 | 275.9 | |||||||
| Non-fumigant Nematicide | |||||||||||||
| Pp-a-3 | Meloidogyne | 358.8 | 100% | 153.4 | 100% | 640.7 | 100% | 367.0 | 100% | 138.1 | 92% | 295.0 | 100% |
| Pp-d-2 | Paratylenchus | 6.6 | 42% | 1.8 | 42% | 0.9 | 25% | 3.1 | 67% | ||||
| Pp-d-3 | Criconemella | 6.6 | 33% | 2.7 | 33% | 10.5 | 8% | 2.1 | 17% | 3.4 | 8% | 6.1 | 25% |
| Pp-d-3 | Tylenchorhynchus | 0.5 | 8% | ||||||||||
| Pp-e-2 | Psilenchus | 1.9 | 17% | 3.0 | 33% | 0.2 | 8% | 0.8 | 17% | ||||
| Total | 373.9 | 161.3 | 651.2 | 369.1 | 142.6 | 305.0 | |||||||
| DMDS | |||||||||||||
| Pp-a-3 | Meloidogyne | 328.2 | 100% | 10.5 | 75% | 14.0 | 50% | 43.8 | 58% | 6.0 | 50% | 113.1 | 92% |
| Pp-d-2 | Paratylenchus | 8.5 | 25% | 0.7 | 17% | 3.6 | 67% | ||||||
| Pp-d-3 | Criconemella | 0.9 | 8% | 0.2 | 8% | ||||||||
| Pp-d-3 | Tylenchorhynchus | 0.9 | 8% | 4.5 | 58% | 0.2 | 8% | 0.3 | 8% | ||||
| Pp-e-2 | Psilenchus | 1.9 | 17% | 0.2 | 8% | ||||||||
| Total | 340.4 | 10.5 | 14.0 | 48.3 | 6.9 | 117.3 | |||||||
| Guild | Genus | Sampling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DBA | 18 DAA | 104 DAA | 258 DAA | 362 DAA | 450 DAA | ||||||||
| Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | ||
| Untreated | |||||||||||||
| Ba 1 | Cruznema | 69.4 | 83% | 36.3 | 100% | 435.4 | 67% | 10.2 | 67% | 13.2 | 75% | 104.8 | 92% |
| Ba 1 | Cuticularia | 0.5 | 17% | 3.6 | 33% | ||||||||
| Ba 1 | Diplagaster | ||||||||||||
| Ba 1 | Diploscapter | 68.9 | 83% | 39.0 | 92% | 48.3 | 92% | 54.7 | 92% | ||||
| Ba 1 | Mesorhabditis | 51.2 | 67% | 18.7 | 75% | 0.7 | 25% | 0.5 | 8% | 33.1 | 67% | ||
| Ba 1 | Panagrolaimus | 6.6 | 33% | 16.9 | 67% | 117.7 | 25% | 43.6 | 92% | 1.9 | 17% | 6.3 | 42% |
| Ba 1 | Rhabditidae | 96.5 | 100% | 187.8 | 100% | ||||||||
| Ba 2 | Acrobeles | 16.1 | 75% | 12.4 | 83% | 5.7 | 58% | 4.1 | 58% | 10.9 | 83% | ||
| Ba 2 | Acrobeloides | 44.7 | 83% | 16.1 | 100% | 6.1 | 83% | 1.7 | 42% | 32.4 | 83% | ||
| Ba 2 | Cephalobus | 6.6 | 42% | 5.1 | 58% | 1.0 | 8% | 4.1 | 25% | 10.9 | 67% | ||
| Ba 2 | Chiloplacus | 1.9 | 25% | 3.9 | 42% | 16.8 | 100% | 5.9 | 42% | 3.8 | 25% | ||
| Ba 2 | Heterocephalobus | 7.5 | 50% | 8.3 | 83% | 13.6 | 75% | 10.4 | 75% | ||||
| Ba 2 | Plectus | 3.8 | 33% | 8.2 | 58% | 1.1 | 17% | 2.7 | 50% | ||||
| Ba 2 | Ypsilonellus | 0.9 | 8% | 3.3 | 42% | 0.4 | 8% | ||||||
| Ba 2 | Zeldia | 156.5 | 100% | 93.6 | 100% | 49.8 | 92% | 118.0 | 100% | 76.1 | 100% | 171.1 | 100% |
| Ba 3 | Acromadora | 0.2 | 8% | 0.5 | 17% | ||||||||
| Ba 3 | Prismatolaimus | 8.5 | 50% | 5.8 | 67% | 3.1 | 1.2 | 42% | 2.3 | 58% | 5.2 | 75% | |
| Ba 3 | Teratocephalus | 0.2 | 8% | 1.1 | 8% | ||||||||
| Ba 4 | Alaimus | ||||||||||||
| Total | 442.7 | 364.7 | 607.3 | 203.8 | 364.5 | 446.8 | |||||||
| Non-fumigant Nematicide | |||||||||||||
| Ba 1 | Cruznema | 78.8 | 100% | 36.9 | 100% | 16.1 | 67% | 46.6 | 92% | 84.4 | 100% | ||
| Ba 1 | Cuticularia | 0.3 | 17% | 4.7 | 50% | ||||||||
| Ba 1 | Diplagaster | 0.2 | 8% | 0.9 | 17% | ||||||||
| Ba 1 | Diploscapter | 36.9 | 83% | 24.1 | 100% | 18.8 | 50% | 11.0 | 83% | ||||
| Ba 1 | Mesorhabditis | 16.0 | 42% | 16.1 | 75% | 1.5 | 42% | 6.0 | 58% | 6.1 | 42% | ||
| Ba 1 | Panagrolaimus | 13.1 | 25% | 13.3 | 50% | 545.5 | 100% | 25.2 | 100% | 1.3 | 25% | 1.4 | 17% |
| Ba 1 | Rhabditidae | 123.9 | 100% | 160.9 | 100% | ||||||||
| Ba 2 | Acrobeles | 14.2 | 58% | 12.2 | 75% | 4.2 | 75% | 9.8 | 83% | 9.4 | 67% | ||
| Ba 2 | Acrobeloides | 16.1 | 75% | 9.5 | 67% | 3.5 | 67% | 6.7 | 67% | 18.5 | 92% | ||
| Ba 2 | Cephalobus | 5.7 | 50% | 3.9 | 67% | 0.7 | 25% | 13.0 | 92% | ||||
| Ba 2 | Chiloplacus | 7.7 | 67% | 32.5 | 100% | 2.8 | 67% | 0.4 | 8% | ||||
| Ba 2 | Heterocephalobus | 2.8 | 25% | 5.0 | 67% | 0.2 | 8% | 14.4 | 92% | 4.4 | 83% | ||
| Ba 2 | Plectus | 7.6 | 42% | 19.6 | 83% | 0.2 | 8% | 5.5 | 67% | ||||
| Ba 2 | Ypsilonellus | 3.4 | 50% | ||||||||||
| Ba 2 | Zeldia | 111.0 | 100% | 89.8 | 100% | 78.7 | 83% | 273.5 | 100% | 65.9 | 100% | 130.5 | 100% |
| Ba 3 | Acromadora | 0.2 | |||||||||||
| Ba 3 | Prismatolaimus | 4.7 | 42% | 4.1 | 58% | 1.0 | 8% | 0.5 | 17% | 1.9 | 67% | ||
| Ba 3 | Teratocephalus | 0.9 | 8% | 0.1 | 8% | ||||||||
| Ba 4 | Alaimus | 0.2 | |||||||||||
| Total | 306.9 | 369.7 | 626.1 | 357.4 | 340.4 | 286.6 | |||||||
| DMDS | |||||||||||||
| Ba 1 | Cruznema | 58.9 | 92% | 3.6 | 58% | 3.8 | 42% | 45.2 | 100% | ||||
| Ba 1 | Cuticularia | 3.9 | 50% | 0.7 | 8% | ||||||||
| Ba 1 | Diplagaster | ||||||||||||
| Ba 1 | Diploscapter | 28.2 | 83% | 0.5 | 17% | 39.6 | 83% | 22.1 | 92% | ||||
| Ba 1 | Mesorhabditis | 36.5 | 50% | 2.9 | 58% | 0.2 | 8% | 39.6 | 50% | ||||
| Ba 1 | Panagrolaimus | 0.9 | 8% | 0.7 | 25% | 64.6 | 100% | 127.0 | 100% | 1.8 | 17% | ||
| Ba 1 | Rhabditidae | 13.4 | 50% | 48.9 | 100% | ||||||||
| Ba 2 | Acrobeles | 29.1 | 33% | 5.7 | 67% | 6.8 | 50% | 26.4 | 58% | ||||
| Ba 2 | Acrobeloides | 26.4 | 67% | 0.5 | 17% | 62.7 | 100% | 8.8 | 83% | 16.0 | 100% | ||
| Ba 2 | Cephalobus | 4.7 | 25% | 0.9 | 17% | 5.7 | 8% | 4.7 | 50% | ||||
| Ba 2 | Chiloplacus | 1.9 | 17% | 6.0 | 92% | 3.3 | 50% | 5.4 | 42% | ||||
| Ba 2 | Heterocephalobus | 2.8 | 25% | 0.7 | 8% | 0.5 | 17% | 2.1 | 50% | ||||
| Ba 2 | Plectus | 0.9 | 8% | 0.2 | 8% | 2.5 | 50% | 0.8 | 25% | ||||
| Ba 2 | Ypsilonellus | 1.9 | 8% | ||||||||||
| Ba 2 | Zeldia | 178.9 | 100% | 6.2 | 75% | 115.0 | 100% | 54.2 | 100% | 70.5 | 100% | 237.3 | 100% |
| Ba 3 | Acromadora | ||||||||||||
| Ba 3 | Prismatolaimus | 13.2 | 75% | 0.5 | 8% | 0.6 | 0% | 0.7 | 25% | 3.0 | 67% | ||
| Ba 3 | Teratocephalus | 0.5 | 25% | ||||||||||
| Ba 4 | Alaimus | ||||||||||||
| Total | 384.2 | 7.8 | 193.5 | 269.0 | 192.0 | 404.2 | |||||||
| Guild | Genus | Sampling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DBA | 18 DAA | 104 DAA | 258 DAA | 362 DAA | 450 DAA | ||||||||
| Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | ||
| Untreated | |||||||||||||
| Fu 2 | Aphelenchoides | 9.5 | 33% | 2.8 | 67% | 0.9 | 25% | 5.7 | 33% | ||||
| Fu 2 | Aphelenchus | 7.5 | 42% | 23.0 | 67% | 4.1 | 25% | 4.0 | 67% | 12.9 | 100% | 3.8 | 42% |
| Fu 2 | Ditylenchus | 1.8 | 33% | ||||||||||
| Fu 2 | Filenchus | 1.1 | 8% | ||||||||||
| Fu 2 | Lelenchus | 0.8 | 25% | ||||||||||
| Fu 2 | Paraphelenchus | 11.3 | 58% | 6.2 | 75% | 10.7 | 67% | ||||||
| Total | 28.3 | 33.8 | 5.3 | 5.0 | 13.7 | 20.2 | |||||||
| Non-fumigant Nematicide | |||||||||||||
| Fu 2 | Aphelenchoides | 8.4 | 17% | 5.2 | 42% | 1.6 | 25% | 2.8 | 17% | ||||
| Fu 2 | Aphelenchus | 14.1 | 75% | 28.5 | 100% | 1.9 | 8% | 6.0 | 75% | 24.1 | 67% | 5.2 | 75% |
| Fu 2 | Ditylenchus | 1.0 | 8% | 0.5 | 17% | 0.2 | 8% | ||||||
| Fu 2 | Filenchus | 1.9 | 8% | 1.9 | 17% | 0.1 | 8% | ||||||
| Fu 2 | Lelenchus | ||||||||||||
| Fu 2 | Paraphelenchus | 6.1 | 25% | ||||||||||
| Total | 25.4 | 40.3 | 3.9 | 7.7 | 24.1 | 8.2 | |||||||
| DMDS | |||||||||||||
| Fu 2 | Aphelenchoides | 0.7 | 17% | ||||||||||
| Fu 2 | Aphelenchus | 3.8 | 25% | 1.4 | 8% | 0.9 | 42% | 0.7 | 17% | 0.6 | 17% | ||
| Fu 2 | Ditylenchus | ||||||||||||
| Fu 2 | Filenchus | 0.2 | 8% | 0.1 | 8% | 0.3 | 8% | ||||||
| Fu 2 | Lelenchus | 0.7 | 17% | ||||||||||
| Fu 2 | Paraphelenchus | 0.9 | 8% | 0.6 | 17% | ||||||||
| Total | 4.7 | 0.0 | 2.4 | 1.7 | 0.9 | 1.2 | |||||||
| Guild | Genus | Sampling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DBA | 18 DAA | 104 DAA | 258 DAA | 362 DAA | 450 DAA | ||||||||
| Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | ||
| Untreated | |||||||||||||
| Om4 | Ecumenicus | 2.8 | 25% | 0.9 | 33% | 0.2 | 8% | 1.2 | 25% | ||||
| Om4 | Eudorylaimus | 0.5 | 17% | ||||||||||
| Om4 | Mesodorylaimus | 1.9 | 17% | 0.9 | 33% | 2.8 | 42% | 0.8 | 17% | ||||
| Om4 | Thonus | 7.6 | 58% | 4.1 | 83% | 0.7 | 17% | 4.0 | 58% | ||||
| Om5 | Aporcelaimus | 0.9 | 8% | 0.2 | 8% | 0.5 | 8% | ||||||
| Om5 | Metaporcelaimus | 2.9 | 25% | 1.8 | 42% | 2.6 | 42% | ||||||
| Om5 | Aporcelaimellus | 3.8 | 33% | 2.1 | 58% | 4.1 | 58% | 0.4 | 17% | 3.0 | 42% | ||
| Om5 | Aporcella | 2.4 | 8% | 0.2 | 8% | ||||||||
| Total | 19.9 | 10.5 | 2.4 | 5.0 | 3.4 | 12.2 | |||||||
| Non-fumigant Nematicide | |||||||||||||
| Om4 | Ecumenicus | 4.7 | 33% | 1.4 | 33% | 10.8 | 17% | 0.4 | 17% | 1.2 | 42% | ||
| Om4 | Eudorylaimus | 0.5 | 17% | 0.1 | 8% | ||||||||
| Om4 | Mesodorylaimus | 2.9 | 25% | 2.8 | 42% | 0.8 | 25% | 5.3 | 50% | 2.4 | 33% | ||
| Om4 | Thonus | 8.5 | 50% | 3.7 | 67% | 1.9 | 17% | 1.7 | 42% | 1.8 | 58% | ||
| Om5 | Aporcelaimus | 0.2 | 8% | 2.4 | 25% | ||||||||
| Om5 | Metaporcelaimus | ||||||||||||
| Om5 | Aporcelaimellus | 7.6 | 50% | 2.2 | 42% | 1.1 | 42% | 2.2 | 50% | ||||
| Om5 | Aporcella | 3.7 | 17% | 1.1 | 33% | 0.2 | 8% | 0.3 | 8% | ||||
| Total | 27.4 | 11.9 | 4.3 | 14.6 | 5.9 | 8.0 | |||||||
| DMDS | |||||||||||||
| Om4 | Ecumenicus | 2.8 | 25% | 0.5 | 17% | 0.7 | 25% | 0.7 | 25% | ||||
| Om4 | Eudorylaimus | 0.9 | 8% | 0.2 | 17% | 0.2 | 8% | ||||||
| Om4 | Mesodorylaimus | 1.9 | 8% | 0.5 | 17% | 0.1 | 8% | 0.2 | 8% | ||||
| Om4 | Thonus | 4.7 | 42% | 0.8 | 17% | 0.2 | 8% | 4.0 | 42% | ||||
| Om5 | Aporcelaimus | 0.9 | 8% | ||||||||||
| Om5 | Metaporcelaimus | 6.6 | 42% | 0.7 | 8% | 3.0 | 58% | ||||||
| Om5 | Aporcelaimellus | 11.3 | 50% | 1.0 | 42% | 0.5 | 17% | 5.8 | 75% | ||||
| Om5 | Aporcella | 5.6 | 42% | 0.5 | 8% | 1.6 | 42% | ||||||
| Total | 34.8 | 0.0 | 1.0 | 2.7 | 2.3 | 15.4 | |||||||
| Guild | Genus | Sampling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DBA | 18 DAA | 104 DAA | 258 DAA | 362 DAA | 450 DAA | ||||||||
| Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | Mean | Occurence | ||
| Untreated | |||||||||||||
| Ca-2 | Seinura | 1.9 | 8% | 0.7 | 8% | 0.8 | 17% | ||||||
| Ca-3 | Tobrilus | 0.9 | 8% | 0.2 | 8% | 0.2 | 8% | ||||||
| Ca-4 | Clarcus | ||||||||||||
| Ca-4 | Mononchus | 1.9 | 17% | 0.7 | 17% | 1.3 | 17% | ||||||
| Ca-4 | Mylonchulus | 3.8 | 25% | 3.0 | 58% | 0.2 | 8% | 2.5 | 33% | ||||
| Ca-5 | Carcharolaimus | ||||||||||||
| Ca-5 | Sectonema | ||||||||||||
| Total | 8.5 | 4.6 | 0.0 | 0.0 | 0.3 | 4.6 | |||||||
| Non-fumigant Nematicide | |||||||||||||
| Ca-2 | Seinura | 0.4 | 8% | 1.5 | 8% | ||||||||
| Ca-3 | Tobrilus | 1.0 | 8% | 0.7 | 17% | 0.2 | 8% | ||||||
| Ca-4 | Clarcus | 0.2 | 8% | ||||||||||
| Ca-4 | Mononchus | 0.9 | 8% | 0.4 | 8% | 0.2 | 8% | ||||||
| Ca-4 | Mylonchulus | 1.9 | 17% | 0.7 | 25% | 0.9 | 33% | 0.4 | 17% | ||||
| Ca-5 | Carcharolaimus | 0.7 | 17% | ||||||||||
| Ca-5 | Sectonema | 0.9 | 8% | 0.4 | 8% | 0.2 | 8% | ||||||
| Total | 4.7 | 3.0 | 0.0 | 0.0 | 2.4 | 1.7 | |||||||
| DMDS | |||||||||||||
| Ca-2 | Seinura | ||||||||||||
| Ca-3 | Tobrilus | ||||||||||||
| Ca-4 | Clarcus | 0.2 | 8% | ||||||||||
| Ca-4 | Mononchus | 0.2 | 8% | ||||||||||
| Ca-4 | Mylonchulus | 1.0 | 8% | 0.2 | 8% | ||||||||
| Ca-5 | Carcharolaimus | ||||||||||||
| Ca-5 | Sectonema | ||||||||||||
| Total | 1.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.2 | |||||||
References
- Agrostatistica. Available online: https://www.mzh.government.bg/bg/statistika-i-analizi/izsledvane-rastenievadstvo/danni/ (accessed on 11 June 2021).
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial Formulates of Trichoderma Induce Systemic Plant Resistance to Meloidogyne incognita in Tomato and the Effect Is Additive to That of the Mi-1.2 Resistance Gene. Front. Microbiol. 2020, 10, 3042. [Google Scholar] [CrossRef] [PubMed]
- Wesemael, W.; Viaene, N.; Moens, M. Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology 2011, 13, 3–16. [Google Scholar] [CrossRef]
- Samaliev, H. Root-knot nematodes (Genus Meloidogyne Goeldi) on Vegetable Crops Grown in Greenhouses in Bulgaria. Rast. Nauk. (Plant Sci.) 2009, 46, 351–360. [Google Scholar]
- Greco, N.; Aranda, J.M.; López, S.M.; Maccarini, C.; de Tommaso, N.; Myrta, A. Sustainability of European vegetable and strawberry production in relation to fumigation practices in the EU. Acta Hortic. 2020, 1270, 203–210. [Google Scholar] [CrossRef]
- Fritsch, J.; Fargier-Puech, P.; Ramponi-Bur, C.; Du Fretay, G.; Fouillet, T.; Charles, P.; Descamps, S.; Myrta, A. French Experiences with Dimethyl Disulfide (DMDS) as a Nematicide in Vegetable Crops. Acta Hortic. 2014, 1044, 427–433. [Google Scholar] [CrossRef]
- Leocata, S.; Pirruccio, G.; Myrta, A.; Medico, E.; Greco, N. Dimethyl Disulfide (DMDS): A New Soil Fumigant to Control Root-Knot Nematodes, Meloidogyne spp., in Protected Crops in Sicily, Italy. Acta Hortic. 2014, 1044, 415–420. [Google Scholar] [CrossRef]
- Sasanelli, N.; Santori, A.; Dongiovanni, C.; Myrta, A. Control of the Root-Knot Nematode Meloidogyne incognita by Dimethyl Disulfide (DMDS) Applied in Drip Irrigation on Melon and Tomato in Apulia and Basilicata (Italy). Acta Hortic. 2014, 1044, 401–404. [Google Scholar] [CrossRef]
- Zanon, M.J.; Gutierrez, L.A.; Myrta, A. Spanish experiences with dimethyl disulfide (DMDS) on the control of root-knot nematodes Meloidogyne spp. in fruiting vegetables in protected crops. VIII International symposium on chemical and non-chemical soil and substrate desinfestation. Acta Hortic. 2014, 1044, 421–425. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; de Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T. Maturity index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil 1999, 212, 13–22. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Bridge, J.; Page, S.L.J. Estimation of root-knot nematode infestation levels on roots using a rating chart. Trop. Pest Manag. 1980, 26, 296–298. [Google Scholar] [CrossRef]
- Leocata, S. Valutazione dell’indice galligeno sulla coltura precedente come metodo affidabile per un corretto posizionamento di prove sperimentali sui nematodi del genere Meloidogyne. Atti. Giornate Fitopatol. 2020, 1, 321–333. [Google Scholar]
- Coolen, W.A. Methods for the extraction of Meloidogyne spp. and other nematodes from roots and soil. In Root-Knot Nematodes (Meloidogyne spp.); Lamberty, F., Taylor, C.E., Eds.; Academic Press: London, UK, 1979; pp. 317–329. [Google Scholar]
- Van Bezooijen, J. Methods and Techniques for Nematology; Manual of WUR, The Netherlands. 2006, p. 112. Available online: http://www.nematologia.com.br/files/tematicos/5.pdf (accessed on 4 May 2018).
- Adam, M.A.M.; Phillips, M.S.; Blok, V.C. Molecular diagnostic key for identification of single juveniles of seven common and economically-important species of root-knot nematode (Meloidogyne spp.). Plant Pathol. 2007, 56, 190–197. [Google Scholar] [CrossRef]
- Bongers, T.; de Goede, R.G.N.; Korthals, G.W.; Yeates, G.W. Proposed changes of c-p classification for nematodes. Russ. J. Nematol. 1995, 3, 61–62. [Google Scholar]
- Toth, E.; Bogdányi, F.T.; Petrikovszki, R.; Gódor, A.; Zalai, M.; Bálint, B.; Sunder, P.; Myrta, A. Control of the Root-Knot Nematode Meloidogyne incognita and Weeds in Protected Cucumber with Dimethyl Disulfide (DMDS) over Two Crop Cycles: The First Results in Hungary. Acta Phytopathol. Entomol. Hung. 2019, 54, 267–278. [Google Scholar] [CrossRef]
- Gerik, J.S. Evaluation of soil fumigants applied by drip irrigation for Liatris production. Plant. Dis. 2005, 89, 883–887. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garibaldi, A.; Baudino, M.; Minuto, A.; Gullino, M.L. Effectiveness of fumigants and grafting against tomato brown root rot caused by Colletotrichum coccodes. Phytoparasitica 2008, 36, 483–488. [Google Scholar] [CrossRef]
- Gilardi, G.; Gullino, M.L.; Garibaldi, A.; Gaudino, M. Effectiveness of fumigants alone and in combination with grafting to control Verticillium wilt and root-knot nematodes in eggplant and tomato brown root rot caused by Colletotricum coccodes. Acta Hortic. 2010, 883, 21–25. [Google Scholar] [CrossRef]
- Abou Zeid, N.M.; Noher, A.M. Efficacy of DMDS as methyl bromide alternative in controlling soil borne diseases, root-knot nematode and weeds on pepper, cucumber and tomato in Egypt. Acta Hortic. 2014, 1044, 411–414. [Google Scholar] [CrossRef]
- Li, Y.; Mao, L.; Yan, D.; Ma, T.; Shen, J.; Guo, M.; Wang, Q.; Ouyang, C.; Cao, A. Quantification of Fusarium oxysporum in fumigated soils by a newly developed real-time PCR assay to assess the efficacy of fumigants for Fusarium wilt disease in strawberry plants. Pest Manag. Sci. 2014, 70, 1669–1675. [Google Scholar] [CrossRef]
- Mao, L.; Yan, D.; Wang, Q.; Li, Y.; Ouyang, C.; Liu, P.; Cao, A. Evaluation of the combination of dimethyl disulfide and dazomet as an efficient methyl bromide alternative for cucumber production in China. J. Agric. Food Chem. 2014, 62, 4864–4869. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.; Domínguez, P.; Medina, J.J.; Miranda, L.; Soria, C.; Romero, F.; López Aranda, J.M.; Daugovish, O.; Mertely, J.; De los Santos, B. Assessment of chemical and biosolarization treatments for the control of Macrophomina phaseolina in strawberries. Sci. Hortic. 2015, 192, 361–368. [Google Scholar] [CrossRef]
- Gomez-Tenorio, M.A.; Zanon, M.J.; de Cara, M.; Lupion, B.; Tello, J.C. Efficacy of dimethyl disulfide (DMDS) against Meloidogyne sp. and three formae speciales of Fusarium oxysporum under controlled conditions. Crop Prot. 2015, 78, 263–269. [Google Scholar] [CrossRef]
- Gilardi, G.; Gullino, M.L.; Garibaldi, A. Soil disinfestation with dimethyl disulfide for management of Fusarium wilt on lettuce in Italy. J. Plant Dis. Prot. 2017, 124, 361–370. [Google Scholar] [CrossRef]
- Pecchia, S.; Franceschini, A.; Santori, A.; Vannacci, G.; Myrta, A. Efficacy of dimethyl disulfide (DMDS) for the control of chrysanthemum Verticillium wilt in Italy. Crop Prot. 2017, 93, 28–32. [Google Scholar] [CrossRef]
- Pitrel, B.; Chianella, M.; Saathof, W.; van de Griend, P.; Santori, A.; Sunder, P.; Myrta, A. Dimethyl Disulfide, successful to control nutsedge (Cyperus spp.) in Europe. Acta Hortic. 2018, 1270, 225–230. [Google Scholar] [CrossRef]
- Sanchez-Moreno, S.; Jimenez, L.; Alonso-Prados, J.L.; Garcia-Baudin, J.M. Nematodes as indicators of fumigant effects on soil food webs in strawberry crops in southern Spain. Ecol. Indic. 2010, 10, 148–156. [Google Scholar] [CrossRef]
- Timper, P.; Davis, R.; Jagdale, G.; Herbert, J. Resiliency of a nematode community and suppressive service to tillage and nematicide application. Appl. Soil Ecol. 2012, 59, 48–59. [Google Scholar] [CrossRef]
- Watson, T.T.; Desaeger, J.A. Evaluation of non-fumigant chemical and biological nematicides for strawberry production in Florida. Crop. Prot. 2019, 117, 100–107. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Mauldin, M.D.; Habteweld, A.; Carter, E.T. Nematicide efficacy at managing Meloidogyne arenaria and non-target effects on free-living nematodes in peanut production. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hua, J.; Jiang, Y.; Li, Q.; Wen, D. Nematode communities in greenhouse soil of different ages from Shenyang suburb. Helminthologia 2006, 43, 51–55. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Y.; Liang, W.; Lou, Y.; Zhang, E.; Liang, C. Long-term effect of fertility management on the soil nematode community in vegetable production under greenhouse conditions. Appl. Soil Ecol. 2010, 46, 111–118. [Google Scholar] [CrossRef]
- Ruan, W.B.; Ren, T.; Chen, Q.; Zhu, X.; Wang, J.G. Effects of conventional and reduced N inputs on nematode communities and plant yield under intensive vegetable production. Appl. Soil Ecol. 2013, 66, 48–55. [Google Scholar] [CrossRef]

| 1st Cucumber Crop (July–October 2018) | 2nd Cucumber Crop (April–June 2019) | 3rd Cucumber Crop (July–October 2019) |
|---|---|---|
| DMDS | No treatment | No treatment |
| Trichoderma spp. + garlic extract | No treatment | |
| Fosthiazate | Oxamyl | No treatment |
| Untreated control | Untreated control | No treatment |
| 1st Crop | 2nd Crop | 3rd Crop | |
|---|---|---|---|
| Planting date | 30 June 2018 | 12 April 2019 | 10 June 2019 |
| Replicates | 6 | 6 | 6 |
| Plot size (m2) | 168 | 168/84 * | 168 |
| Previous crop | cucumber | lettuce | cucumber |
| Cucumber cultivar | Pert | Delano | Delano |
| Nr. plants/ha | 18,000 | 18,000 | 18,000 |
| Last harvest date | 22 September 2018 | 14 May 2019 | 14 October 2019 |
| Treatment (Nr. Application) | Root Damage | Total Yield/Ha | ||
|---|---|---|---|---|
| Rgi (70 Dat) | % Reduction | M Tons | % Increase | |
| Untreated control | 5.0 a | - | 19.4 c | - |
| Fosthiazate 10G 40 kg/ha (1) | 2.4 ab | 52 | 26.7 b | 37.6 |
| DMDS 94.1% 400 L/ha (1) | 0.2 b | 96 | 32.1 a | 65.5 |
| Treatments First Crop (Nr. Application) | Treatments Second Crop (Nr. Application) | Root Damage | Total Yield/Ha | ||
|---|---|---|---|---|---|
| RGI (80DAT) | % Reduction | M Tons | % Increase | ||
| Untreated control | Untreated control | 6.5 a | - | 10.3 b | - |
| Fosthiazate 10G 40 kg/ha (1) | Oxamyl 10 L/ha (1) | 1.9 b | 70.8 | 11.7 b | 13.6 |
| DMDS 94.1% 400 L/ha (1) | Trichoderma (3) + Garlic extract (3) 0.5 kg/ha + 4 L/ha | 0.0 c | 100 | 16.7 a | 62.1 |
| No treatment done | 1.3 b | 80.0 | 15.5 a | 50.5 | |
| Treatments First Crop (Nr. Application) | Treatments Second Crop (Nr. Application) | Treatments Third Crop | Root Damage | |
|---|---|---|---|---|
| RGI (96 DAT) | % Reduction | |||
| Untreated control | Untreated control | No treatment | 6.4 a | - |
| Fosthiazate 10 G 40 kg/ha (1) | Oxamyl 10 L/ha (1) | No treatment | 5.2 b | 18.8 |
| DMDS 94.1% 400 L/ha (1) | Trichoderma (3) + Garlic extract (3) 0.5 kg/ha + 4 L/ha | No treatment | 3.7 c | 42.2 |
| No treatment done | No treatment | 5.1 b | 20.3 | |
| Treatment | 1 DBA | 18 DAA | 104 DAA | 252 DAA | 362 DAA | 450 DAA |
|---|---|---|---|---|---|---|
| Taxonomical richness | ||||||
| Untreated | 31 a | 37 a | 9 bc | 16 ac | 25 a | 31 a |
| Non-fumigant nematicide | 28 a | 37 a | 10 ac | 21 a | 26 a | 29 a |
| DMDS | 30 a | 5 b | 11 ac | 23 a | 26 a | 28 a |
| H’—Shannon–Wiener diversity index | ||||||
| Untreated | 2.0 a | 2.3 a | 1.2 b | 1.6 a | 1.9 a | 2.1 a |
| Non-fumigant nematicide | 1.8 a | 2.4 a | 1.0 b | 1.3 ab | 2.0 a | 1.7 a |
| DMDS | 1.9 a | 1.0 b | 1.2 b | 1.8 a | 1.9 a | 1.9 a |
| Eχ—Evenness | ||||||
| Untreated | 0.6 a | 0.6 a | 0.5 a | 0.6 a | 0.6 a | 0.6 a |
| Non-fumigant nematicide | 0.5 a | 0.7 a | 0.4 a | 0.4 a | 0.6 a | 0.5 a |
| DMDS | 0.6 a | 0.6 a | 0.5 a | 0.6 a | 0.6 a | 0.6 a |
| MI—Maturity index | ||||||
| Untreated | 1.8 ac | 1.6 a | 1.2 b | 1.8 ac | 1.5 a | 1.7 ac |
| Non-fumigant nematicide | 1.8 ac | 1.6 a | 1.1 b | 2.0 c | 1.5 a | 1.8 ac |
| DMDS | 1.9 c | 1.9 ac | 1.6 a | 1.6 a | 1.6 a | 1.9 ac |
| PPI—Plant-parasitic index | ||||||
| Untreated | 3.0 a | 2.9 a | 3.0 a | 3.0 a | 3.0 a | 3.0 a |
| Non-fumigant nematicide | 3.0 a | 2.9 a | 3.0 a | 3.0 a | 3.0 a | 3.0 a |
| DMDS | 2.9 a | 3.0 a | 3.0 a | 3.0 a | 2.9 a | 2.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, Z.; Lazarova, T.; Mitev, A.; Myrta, A. Monitoring of Long-Lasting Effects of Fumigation with Dimethyl Disulfide (DMDS) on Root-Gall Index, Root-Knots, Other Nematode Populations, and Crop Yield over Three Protected Cucumber Crops in Bulgaria. Agronomy 2021, 11, 1206. https://doi.org/10.3390/agronomy11061206
Ilieva Z, Lazarova T, Mitev A, Myrta A. Monitoring of Long-Lasting Effects of Fumigation with Dimethyl Disulfide (DMDS) on Root-Gall Index, Root-Knots, Other Nematode Populations, and Crop Yield over Three Protected Cucumber Crops in Bulgaria. Agronomy. 2021; 11(6):1206. https://doi.org/10.3390/agronomy11061206
Chicago/Turabian StyleIlieva, Zhenya, Tanya Lazarova, Aleksander Mitev, and Arben Myrta. 2021. "Monitoring of Long-Lasting Effects of Fumigation with Dimethyl Disulfide (DMDS) on Root-Gall Index, Root-Knots, Other Nematode Populations, and Crop Yield over Three Protected Cucumber Crops in Bulgaria" Agronomy 11, no. 6: 1206. https://doi.org/10.3390/agronomy11061206
APA StyleIlieva, Z., Lazarova, T., Mitev, A., & Myrta, A. (2021). Monitoring of Long-Lasting Effects of Fumigation with Dimethyl Disulfide (DMDS) on Root-Gall Index, Root-Knots, Other Nematode Populations, and Crop Yield over Three Protected Cucumber Crops in Bulgaria. Agronomy, 11(6), 1206. https://doi.org/10.3390/agronomy11061206






