Rice Paddy Soil Seedbanks Composition in a Mediterranean Wetland and the Influence of Winter Flooding
Abstract
1. Introduction
2. Materials and Methods
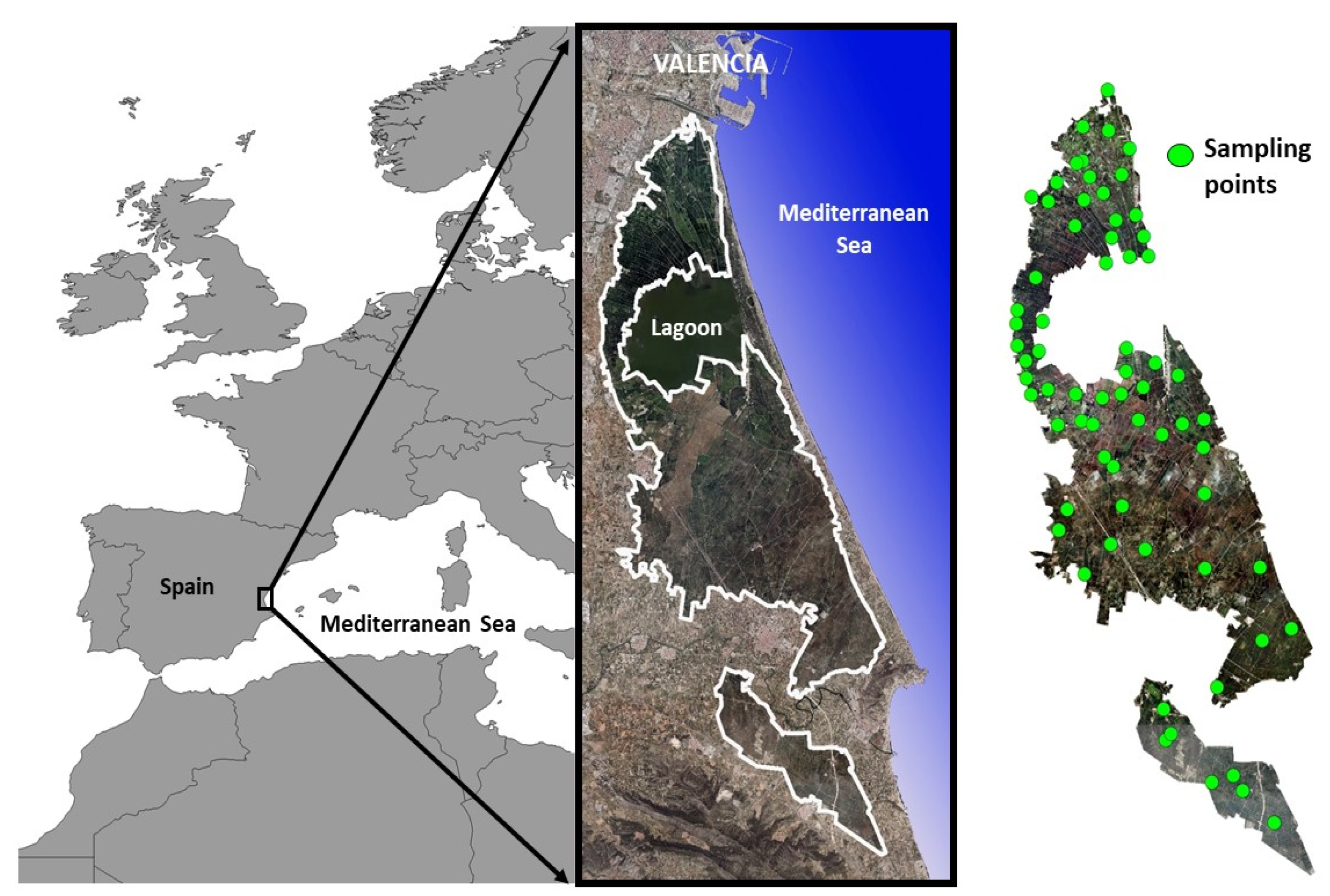
2.1. Study Area
2.2. Sampling Process
2.3. Seedbank Determination
2.3.1. Tray Method (TM)
2.3.2. Extraction Method (EM)
2.4. Calculation of the Frequency, Abundance, and Specific Contribution of Each Species
2.5. Winter Flooding and Geostatistical Methods
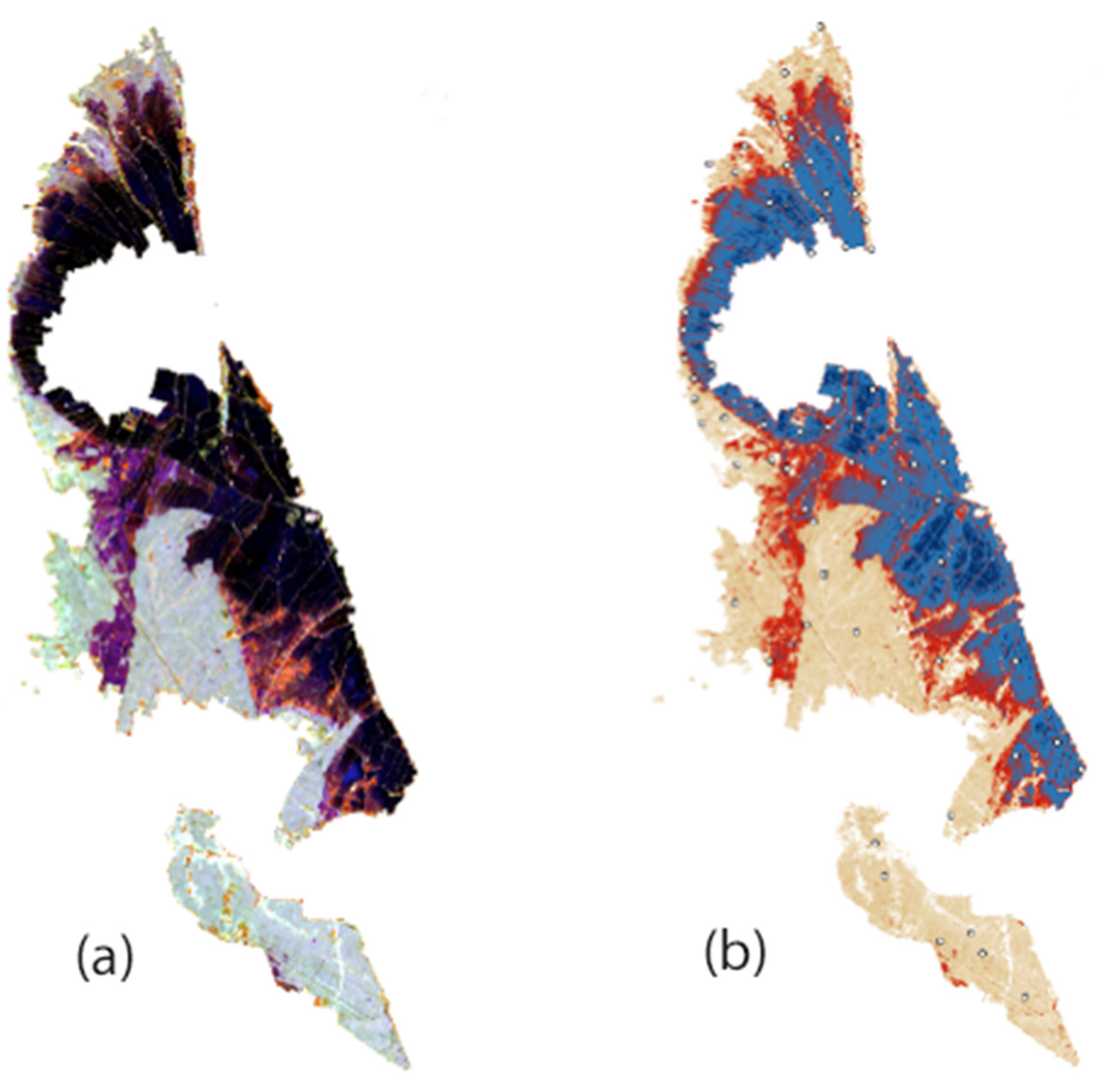
- Phase 1 Delimitation of paddy field areas: With multispectral image No. 1, the rice-growing area was delimited by combining bands B8A, B11, and B02 (Figure 1). At this time, rice plants in Albufera de Valencia were between the final tillering phase and the beginning of stem elongation (BBCH 29-32);
- Phase 2 Characterisation of the winter flood area: The multispectral image No. 2 of January 2019 was used, corresponding to the moment when the paddy fields reached the highest levels of winter flooding. For this, layers B8A, B11, and B04 were combined and a false colour image was obtained, which was cut with a layer of polygons corresponding to the paddy fields obtained from Phase 1 (Figure 2a). Four levels of WF were defined (Table 2) from the graphic of the pixel values of the cropped image bands (Figure 3). These levels were represented with new colours to improve visualisation (Figure 2b);
- Phase 3 Assignation of flood level in sample plots: For each of the sixty-nine sampling points, the winter flood level was obtained by intersecting the sampling point layers with the flood level layer obtained in Phase 2 with GIS;
- Phase 4 Validation: The four winter flood levels were validated according to the assignation obtained in Phase 3 with real field data from the georeferenced plots in the area and whose winter flood level was previously known.
2.6. Models
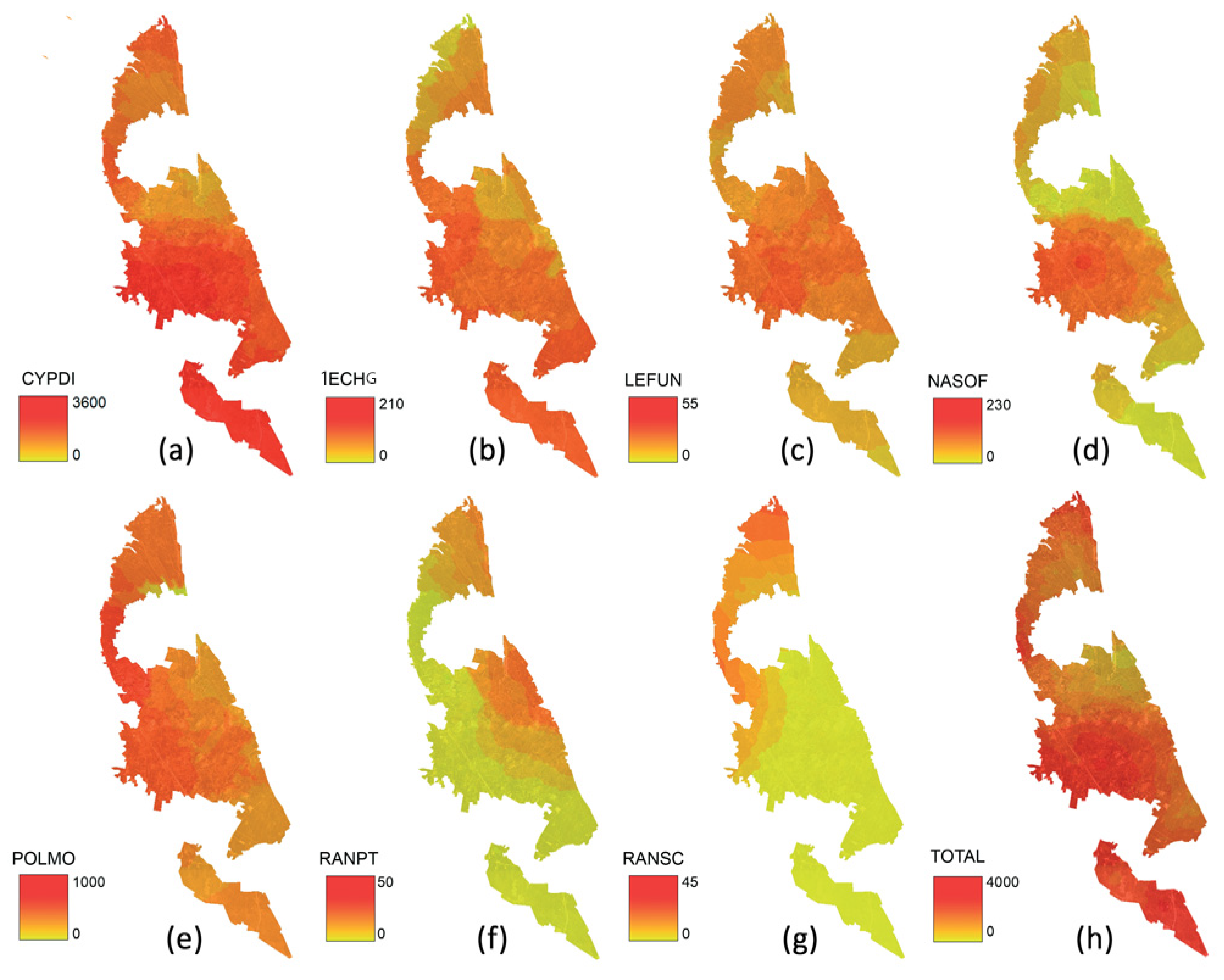
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawson, H.M. The use of weed seedbank in selection of herbicide recommendations. Weed Res. 1988, 28, 486–487. [Google Scholar]
- Forcella, F. Prediction of weed seedling densities from buried seed reserves. Weed Res. 1992, 32, 29–38. [Google Scholar] [CrossRef]
- Cardina, J.; Sparrow, D.H. A comparison of methods to predict weed seedling populations from the soil seedbank. Weed Sci. 1996, 44, 46–51. [Google Scholar] [CrossRef]
- Akter, F.; Begum, M.; Salam, M.A. Floristic diversity of the soil weed seedbank in boro rice fields: In situ and ex situ evaluation. J. Bangladesh Agric. Univ. 2018, 16, 396–402. [Google Scholar] [CrossRef]
- Barralis, G.; Chadoeuf, R. Etude de la dynamique d’une communaute adventice: I-Evolution de la flore adventice au cours du cycle vegetatif d’une culture. Weed Res. 1980, 20, 231–237. [Google Scholar] [CrossRef]
- Menalled, F.D.; Gross, K.L.; Hammond, M. Weed aboveground and seedbank community reponses to agricultural management systems. Ecol. Appl. 2001, 11, 1586–1601. [Google Scholar] [CrossRef]
- He, M.; Lv, L.; Li, H.; Meng, W.; Zhao, N. Analysis on soil seed bank characteristics ans its relation with soil properties after substrate addition. PLoS ONE 2016, 11, e0147439. [Google Scholar] [CrossRef]
- Altop, E.K.; Mennan, H.; Philippo, C.J.; Zandstra, B.H. Effect of the burial depth and environmental factors on the seasonal germination of bearded sprangletop (Leptochloa fusca [L.] ssp. fascicularis [Lam.] N. Snow). Weed Biol. Manag. 2015, 15, 147–158. [Google Scholar] [CrossRef]
- McIntyre, S.; Mitchell, D.; Ladiges, P. Germination and Seedling Emergence in Diplachne fusca: A Semi-Aquatic Weed of Rice Fields. J. Appl. Ecol. 1989, 26, 551–562. [Google Scholar] [CrossRef]
- Osca, J.M. Expansion of Leptochloa fusca ssp. uninervia and Leptochloa fusca ssp. fascicularis in rice fields in Valencia, eastern Spain. Weed Res. 2013, 53, 479–488. [Google Scholar] [CrossRef]
- Driver, K.E.; Al-Khatib, K.; Godar, A. Bearded sprangletop (Diplachne fusca ssp. fascicularis) flooding tolerance in California rice. Weed Technol. 2020, 34, 193–196. [Google Scholar] [CrossRef]
- Luo, Y.; Fu, H.; Xiong, F.; Xiang, M.Z.; Wang, F.; Bugingo, Y.C.; Khan, S.; Cui, Y. Effects of water-saving irrigation on weed infestation and diversity in paddy fields in East China. Paddy Water Environ. 2017, 15, 593–604. [Google Scholar] [CrossRef]
- Perellonet, A.A.; Cavallo, F.; Simpson, D.; Gauthier-Clerc, M.; Guillemain, M. Seed density and Waterfowl Use of Rice Fields in Camargue, France. J. Wildl. Manag. 2017, 81, 96–117. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Burns, E.G.; Edaie, J.M.; Horwath, W.R.; van Kessel, C. Effects of foraing waterfowl in winter flooded rice fields an weed stress and residue decomposition. Agric. Ecosyst. Environ. 2002, 95, 289–296. [Google Scholar] [CrossRef]
- Stafford, J.D.; Kaminski, R.M.; Reinecke, K.J. Avian foods, foraging and habitat conservation in World rice fields. Waterbirds 2010, 33, 133–150. [Google Scholar] [CrossRef]
- Greer, D.D.; Dugger, B.D.; Reinecke, K.J.; Petrie, M.J. Depletion of rice as food of waterfowl wintering in the Mississipi Alluvial Valley. J. Wildl. Manag. 2009, 73, 1125–1133. [Google Scholar] [CrossRef]
- Kaneko, K.; Nakamura, T. Effects of the inhibition of weed communities by winter-flooding. Agric. Sci. 2011, 2, 383–391. [Google Scholar] [CrossRef]
- Barret, S.C.H.; Seaman, D.E. The weed flora of Califormanian rice fields. Aquat. Bot. 1980, 9, 351–376. [Google Scholar] [CrossRef]
- Peterson, J.E.; Baldwin, A.H. Seedling emergence from seed banks of tidal freshwater wetlands: Response to inundation and sedimentation. Aquat. Bot. 2004, 78, 243–254. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed seed movement and dispersal strategies in the agricultural environment. Weed Biol. Manag. 1980, 7, 141–157. [Google Scholar] [CrossRef]
- Hayashi, H.; Shimatani, Y.; Shigematsu, K. A study of seed dispersal by flood flow in an artificially restored floodplain. Landsc. Ecol. Eng. 2012, 8, 129–143. [Google Scholar] [CrossRef]
- Salazar, L.C. Malezas Asociadas a Los Cultivos de Panamá; Universidad de Panamá: Panamá City, Panamá, 2012. [Google Scholar]
- Ghazali, M.F.; Wikantika, K.; Harto, A.B. Generating soil salinity, soil moisture, soil pH from satellite imagery and its analysis. Inf. Process. Agric. 2019, 7, 294–306. [Google Scholar] [CrossRef]
- Yue, J.; Tian, J.; Tian, Q.; Xu, K.; Xu, N. Development of soil moisture indices from differences in water absorption between shortwave-infrared bands ISPRS. J. Photogramm. Remote Sens. 2019, 154, 216–230. [Google Scholar] [CrossRef]
- Ambrosone, M.; Matese, A.; Di Gennaro, S.F. Retrieving soil moisture in rainfed and irrigated fields using Sentinel-2 observations and a modified OPTRAM approach. Int. J. Appl. Earth Obs. Geoinf. 2020, 89, 102113. [Google Scholar] [CrossRef]
- Alvarez-Mendoza, C.I.; Teodoro, A.; Ramirez-Cando, L. Improving NDVI by removing cirrus clouds with optical remote sensing data from Landsat-8—A case study in Quito, Ecuador. Remote Sens. Appl. Soc. Environ. 2019, 13, 257–274. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, G. Estimating green biomass ratio with remote sensing in arid grasslands. Ecol. Indic. 2019, 98, 568–574. [Google Scholar] [CrossRef]
- López-Granados, F.; Jurado-Expósito, M.; Peña-Barragán, J.M. Using remote sensing for identification of late-season grass weed patches in wheat. Weed Sci. 2006, 54, 346–353. [Google Scholar] [CrossRef]
- Gerhards, R. Spatial and Temporal Dynamics of Weed Populations. In Precision Crop Protection—The Challenge and Use of Heterogeneity; Oerke, E.C., Gerhards, R., Menz, G., Sikora, R., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Adamchuk, V.I.; Ferguson, R.B.; Hergert, G.W. Soil Heterogeneity and Crop Growth. In Precision Crop Protection—The Challenge and Use of Heterogeneity; Oerke, E.C., Gerhards, R., Menz, G., Sikora, R., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Heijting, S.; Van der Werf, W.; Stein, A.; Kropff, M.J. Are patches stable in location? Application of an explicitly two-dimensional methodology. Weed Res. 2007, 47, 381–395. [Google Scholar] [CrossRef]
- Moreno-Ramón, H.; Marqués-Mateu, A.; Ibáñez-Asensio, S.; Gisbert, J.M. Wetland soils under rice management and seawater intrusion: Characterization and classification. Span. J. Soil Sci. 2015, 5, 111–129. [Google Scholar] [CrossRef]
- Reinhardt, T.; Leon, R. Extractable and germinable seedbank methods provide different quantifications of weed communities. Weed Sci. 2018, 66, 715–720. [Google Scholar] [CrossRef]
- Farina, R.; Marchetti, A.; Francaviglia, R.; Napoli, R.; Di Bene, C. Modeling regional soil C stocks and CO2 emissions under Mediterranean cropping systems and soil types. Agric. Ecosyst. Environ. 2017, 238, 128–141. [Google Scholar] [CrossRef]
- Samsonova, V.P.; Blagoveshchenskii, Y.N.; Meshalkina, Y.L. Use of empirical Bayesian kriging for revealing heterogeneities in the distribution of organic carbon on agricultural lands. Eurasian Soil Sci. 2017, 50, 305–311. [Google Scholar] [CrossRef]
- Oliveira, U.; Soares-Filho, B.S.; Santos, A.J. Modelling highly biodiverse areas in Brazil. Sci. Rep. 2019, 9, 6355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cai, Y.; Yang, W.; Yi, Y.; Yang, Z.; Fu, Q. Multiple spatio-temporal patterns of vegetation coverage and its relationship with climatic factors in a large dam-reservoir-river system. Ecol. Eng. 2019, 138, 188–199. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Wang, X.; Guo, K. Driving factors of recent vegetation changes in Hexi Region, northwest China based on a new classification framework. Remote Sens. 2020, 12, 1758. [Google Scholar] [CrossRef]
- Begum, M.; Juraimi, A.; Azmi, M.; Omar, S.R.S.; Rajan, A. Soil seedbank of the Muda rice granary in northwest Peninsular Malaysia invaded by the weed Fimbristylis miliacea (L.) Vahl. Plant Prot. Q. 2008, 23, 157–161. [Google Scholar]

| No. | Date | Function |
|---|---|---|
| 1 | 15 July 2018 | Describe the perimeter of paddy fields |
| 2 | 1 January 2019 | Calculate and classify winter flooding areas |
| 3 | 27 March 2019 | Soil samples |
| Pixel Value | Flooding Level | Description |
|---|---|---|
| 0–150 | 1 | Flooding |
| 150–170 | 2 | Flooding |
| 170–1200 | 3 | Puddles or Saturated |
| 1200–1900 | 4 | Dry |
| >1900 | - | No crop |
| Family | Species | Germinable Seedbank (No) 1 | |
|---|---|---|---|
| TM | EM | ||
| Cyperaceae | Cyperus difformis L. | 11,057 a | 34,760 b |
| Poaceae | Polypogon monspeliensis (L.) Desf. | 1281 a | 1264 a |
| Araceae | Lemna sp. | 227 a | 520 b |
| Brassicaceae | Nasturtium officinale R.Br. | 237 a | 454 b |
| Poaceae | Echinochloa sp. | 461 b | 204 a |
| Poaceae | Leptochloa sp. | 247 a | 225 a |
| Ranunculaceae | Ranunculus sceleratus L. | 175 a | 160 a |
| Ranunculaceae | Ranunculus peltatus Schrank | 66 a | 106 b |
| Compositae | Sonchus oleraceus L. | 37 a | 47 a |
| Pontederiaceae | Heteranthera reniformis Ruiz & Pav. | 66 a | 2 b |
| Elatinaceae | Bergia capensis L. | 55 a | 3 b |
| Amaranthaceae | Amaranthus sp. | 6 nd | 4 nd |
| Poaceae | Oryza sativa L. | 19 nd | 0 nd |
| Solanaceae | Solanum nigrum L. | 1 nd | 2 nd |
| Compositae | Senecio vulgaris L. | 3 nd | 1 nd |
| Malvaceae | Malva parviflora L. | 0 nd | 3 nd |
| Portulaceae | Portulaca oleracea L. | 0 nd | 2 nd |
| Geraniaceae | Erodium malacoides (L.) L’Hér | 1 nd | 0 nd |
| Other/non identified | 607 nd | 771 nd | |
| Total | 14546 a | 38528 b | |
| Weed Species | TM | EM | ||||||
|---|---|---|---|---|---|---|---|---|
| FA | F (%) | AM | Cs | FA | F (%) | AM | Cs | |
| Cyperus difformis L. | 61 | 88.41 | 45.32 ± 68.91 | 75.98 | 67 | 97.10 | 129.70 ± 228.07 | 90.16 |
| Polypogon monspeliensis (L.) Desf. | 32 | 46.38 | 10.01 ± 33.43 | 8.88 | 32 | 46.38 | 9.88 ± 19.55 | 3.28 |
| Echinochloa sp. | 35 | 50.72 | 3.29 ± 6.6 | 3.17 | 30 | 43.48 | 1.70 ± 3.67 | 0.53 |
| Leptochloa sp. | 28 | 40.58 | 2.21 ± 3.66 | 1.70 | 29 | 42.03 | 1.94 ± 3.00 | 0.58 |
| Nasturtium officinale R.Br. | 19 | 27.54 | 3.12 ± 4.54 | 1.63 | 24 | 34.78 | 4.73 ± 8.89 | 1.18 |
| Ranunculus sceleratus L. | 22 | 31.88 | 1.99 ± 2.29 | 1.20 | 18 | 26.09 | 2.22 ± 2.48 | 0.41 |
| Ranunculus peltatus Schrank | 18 | 26.09 | 0.92 ± 1.25 | 0.45 | 19 | 27.53 | 1.39 ± 2.19 | 0.25 |
| Sonchus oleraceus L. | 19 | 27.54 | 0.49 ± 0.87 | 0.25 | 19 | 27.54 | 0.62 ± 1.22 | 0.12 |
| Lemna sp. | 8 | 11.59 | 7.09 ± 7.24 | 1.56 | 17 | 24.64 | 7.65 ± 8.32 | 1.35 |
| Amaranthus sp. | 5 | 7.25 | 0.3 ± 0.47 | 0.04 | 13 | 18.84 | 0.5 ± 0.93 | 0.07 |
| Oryza sativa L. | 8 | 11.59 | 0.59 ± 1.07 | 0.13 | - | - | - | - |
| Bergia capensis L. | 11 | 15.94 | 1.25 ± 1.43 | 0.38 | 1 | 1.45 | 0.75 ± 0.50 | 0.01 |
| Heteranthera reniformis Ruiz & Pav. | 3 | 4.35 | 5.5 ± 6.2 | 0.45 | 1 | 1.45 | 0.5 ± 0.58 | 0.01 |
| Senecio vulgaris L. | 3 | 4.35 | 0.25 ± 0.45 | 0.02 | 1 | 1.45 | 0.25 ± 0.5 | 0.00 |
| Solanum nigrum L. | 1 | 1.45 | 0.25 ± 0.50 | 0.01 | 2 | 2.90 | 0.25 ± 0.46 | 0.01 |
| Malva parviflora L. | - | - | - | - | 1 | 1.45 | 0.75 ± 0.96 | 0.01 |
| Portulaca oleracea L. | - | - | - | - | 1 | 1.45 | 0.50 ± 0.58 | 0.01 |
| Erodium malacoides (L.) L’Hér | 1 | 1.45 | 0.25 ± 0.50 | 0.01 | - | - | - | - |
| Other/nd | 65 | 94.20 | 2.70 ± 1.54 | 4.17 | 69 | 100 | 2.84 ± 1.61 | 2.00 |
| Variable | A | CYPDI | 1ECHG | POLMO | LEFSS | NASOF | RANSC | RANPT | SONOL | 1LEMG |
|---|---|---|---|---|---|---|---|---|---|---|
| Constant | 1.5202 * | 1.7275 * | −0.1817 | −0.2349 | 0.2432 * | −0.16402 | −0.07917 | 0.25409 * | −0.00644 | 0.253573 |
| Flooding level | 1.0476 * | 0.9334 * | 0.3378* | 0.4187 * | 0.0399 | 0.2660 * | 0.1797 * | −0.0573 * | 0.0498 * | 0.0955 |
| F | 283.63 | 76.43 | 42.78 | 73.92 | 1.70 | 62.88 | 48.31 | 8,72 | 13.61 | 2.37 |
| R2 | 0.34 | 0.22 | 0.14 | 0.12 | 0.00 | 0.10 | 0.08 | 0.02 | 0.02 | 0.00 |
| Standard error | 1.2754 | 1.5480 | 0.7487 | 0.9985 | 0.6263 | 0.6879 | 0.5301 | 0.3976 | 0.2749 | 0.8933 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osca, J.M.; Galán, F.; Moreno-Ramón, H. Rice Paddy Soil Seedbanks Composition in a Mediterranean Wetland and the Influence of Winter Flooding. Agronomy 2021, 11, 1199. https://doi.org/10.3390/agronomy11061199
Osca JM, Galán F, Moreno-Ramón H. Rice Paddy Soil Seedbanks Composition in a Mediterranean Wetland and the Influence of Winter Flooding. Agronomy. 2021; 11(6):1199. https://doi.org/10.3390/agronomy11061199
Chicago/Turabian StyleOsca, José M., Felip Galán, and Héctor Moreno-Ramón. 2021. "Rice Paddy Soil Seedbanks Composition in a Mediterranean Wetland and the Influence of Winter Flooding" Agronomy 11, no. 6: 1199. https://doi.org/10.3390/agronomy11061199
APA StyleOsca, J. M., Galán, F., & Moreno-Ramón, H. (2021). Rice Paddy Soil Seedbanks Composition in a Mediterranean Wetland and the Influence of Winter Flooding. Agronomy, 11(6), 1199. https://doi.org/10.3390/agronomy11061199






