Abstract
The effects of growth stimulants consist of activating plant physiological processes, which positively affects vegetative and generative growth, increasing the yield and its quality. The aim of the experiment was to study the effect of microbial and amino acid products on the quality of grassland silage; their application in different combinations was an experimental factor. According to the results, the foliar application of the amino acid and microbial products had a statistically significant effect on the chemical composition of silage. In comparison to control, silage produced from plants treated with those growth stimulants contained significantly more total protein in dry matter, while crude fiber content changed only slightly. In addition, due to higher lactic acid content, the quality of silage treated with the stimulants was higher than the quality of control plants. To sum up, the foliar treatment of plants with microorganisms in combination with amino acids resulted in a significant increase in the content of desired components, with a higher nutritional value of the silage.
1. Introduction
Plants are exposed to stress that limits the yield and its quality. According to its origin, external stress can be divided into biotic, e.g., pathogens, insects, and abiotic, e.g., drought, extreme temperatures, salinity, etc. Using various techniques and production systems, growing methods are aimed at minimizing the impact of stress on crops [1].
Currently, supporting physiological processes, biostimulants are of increasing interest on both domestic and international markets [2]. According to Brown and Saa [3], du Jardin [4] and De Pascale et al. [5], biostimulants improve the overall condition of plants by stimulating growth and/or reducing the adverse effects of stress factors such as salinity, drought, temperature fluctuations or pathogens. They promote plant productivity by affecting their communication pathways, thereby reducing negative responses to stress [3,6]. In recent years, scientists in Poland and abroad have defined this previously unregulated group of products as any substance or microorganism delivered to plants primarily in order to increase nutrient uptake efficiency but also to increase tolerance to abiotic stress and/or plant quality characteristics [4]. Yakhin et al. [7] define plant biostimulants as products of biological origin that affect plant performance by improving the properties of the nutrient complex, without providing the essential elements that plants need and without acting as growth regulators or plant protection products. Gawrońska and Przybysz [8] classify biostimulants as synthetic compounds (amino acids, giberelin acid, cytokinins and phenolic compounds, the latter being growth regulators) and substances of natural origin (plant extracts, products based on algae, fulvic and humic acids). Many authors such as Chojnacka [9], Lovatt et al. [10] and Yakhin et al. [7] refine the overall characteristics adopted by du Jardin [4] excluding fertilizers and pesticides. They also describe them as low-dose, environmentally friendly agents.
Based on the current knowledge, new solutions aiming at improving the yield and quality of silage have been developed, among others, through the use of products containing amino acids and effective microorganisms. Such products do not adversely affect the state of the natural environment and can also be used in organic farming. Amino acids present in some fertilizers form complexes with metal ions [11], which allows for the quick transport of nutrients in the plant. Unlike technical salts or synthetic chelates, amino acid molecules are electrically inactive so they can be transferred to a plant cell through ectodesmata to plasmalemma [12]. Finally, amino acids also affect transport of minerals from plasmalemma into the cytoplasm. Therefore, the time of absorbing such complexes of amino acids and nutrients is very short, ranging from 2 to 4 h. In conclusion, thanks to their full compatibility with the metabolism of treated plants, amino acids ensure fast and easy transport of nutrients to the places with the greatest deficit of a given ingredient [13]. According to some studies, the foliar use of amino acids eliminates the effects of biotic and abiotic stress, increasing the yield and improving its quality as a result [14]. In turn, the soil application of amino acids is aimed at improving soil structure and the activity of microorganisms, which facilitates the uptake of nutrients by plants and lengthens the process of their photosynthesis [15].
Plants synthesize amino acids from basic chemical elements taken up from the soil complex and water. However, this process requires time and a lot of energy. The use of fertilizers with the addition of amino acids reduces the amount of energy needed for nitrogen assimilation, which is important in stress conditions and critical stages of plant development [16]. In adverse conditions, the plant focuses on confronting the stresses, not on its growth. Thus, a consequence of stress is the limited use of biological yield potential and slower growth and development [17]. However, the supply of amino acids to plants will effectively defend them against stress and the yield is higher.
Amino acids applied to plants are obtained through enzymatic hydrolysis. The content of free amino acids with low molecular weight is particularly important because they can be taken up by plants quickly. Free amino acids should be in the left-handed form since only this determines the activity of metabolic processes in the plant. They act as an organic carrier—a chelator, which allows for fast and extremely efficient delivery of nutrients to plants. Amino acids form very small, electrically inactive molecules with nutrients, which accelerates the absorption and transport of the latter inside the plant [18].
Microorganisms used in crop production in the form of microbial fertilizers improve plant growth and development conditions. They positively affect the rebalancing of soil microflora disturbed by environmental pressures. This promotes the creation of desirable probiotic consortia in the environment, which results in the proper growth and development of plants and limits the harmful effects of pathogenic microbes, effectively isolating them [19,20,21]. Microorganisms strengthen the natural resistance of plants to fungal diseases and pests, accelerate the decomposition of organic matter and the formation of the humus layer, regulate the ratio of air to water, eliminate putrefactive processes and reduce pathogens and pests. They also make hard-to-absorb macro and micronutrients available to plants, contribute to a significant increase in microflora conducive to biological soil activity, increase the amount of microorganisms of the rhizosphere and optimize the ratio of carbon (C) to nitrogen (N). Both amino acids and microorganisms are biostimulants with a growth-promoting effect that may, or may not, be accompanied by an enhancement of the defensive system of plants against pests and pathogens [1]. In Poland, for several years, research has been conducted on the effects of amino acids and effective microorganisms on crops. Unfortunately, there have been no reports so far on the results of the use of these products on grassland. The nutritional quality of grassland silage depends mainly on nutrient content in the plants and varies across species and varieties and in particular is affected by the treatment and the growing stage at which grass is harvested.
Therefore, the aim of the paper is to investigate the effect of microbial and amino acid products on the quality of silage from meadow plants.
2. Materials and Methods
2.1. Study Site and Soil Analysis
The survey was carried out on a farm located in Ratajów in the area of Krakow, southern Poland (50°13′ N, 20°05′ E). With a randomized block design, the field experiment was established in four replications on permanent grassland; the area of an experimental plot was 10 m2. The experiment was set up on degraded dark earth soil developed from loess, with moderate content of available forms of phosphorus [5.5 mg (100 g)−1], potassium [13.4 mg (100 g)−1] and magnesium [8.1 mg (100 g)−1].
Prior to the establishment of the experiment, the dominant grass species in the meadow were as follows (Table 1): perennial ryegrass (Loliumperenne), meadow fescue (Festuca pratensis), timothy (Phleum pratense) and bluegrass (Poa pratensis). Of Fabaceae plants, only red clover (Trifoliumpratense) was found with a share of 7%. Other dicotyledonous plants of eight species represented 13% of meadow vegetation.

Table 1.
Floristic composition of the grassland before the establishment of the experiment.
2.2. Weather Conditions
Annual rainfall during the study period (2019) was 735 mm, with 534 mm for the growing period from April to September (Figure 1). The average annual temperature was 7.9 °C, and the average temperature from April to September was 14.6 °C.
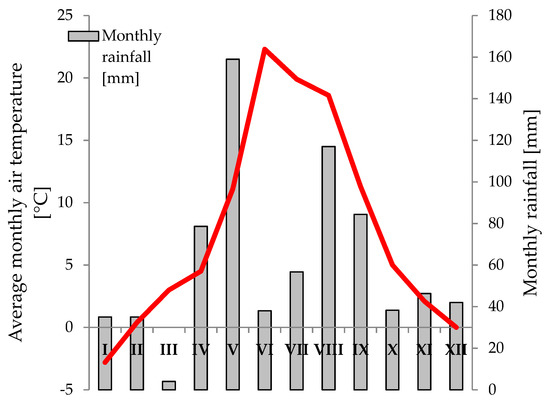
Figure 1.
Rainfall and average air temperature at the Plant Breeding Station in Polanowice in 2019.
2.3. Materials and Experimental Designs
The experiment included four combinations: (1) control (with no treatment); (2) plants sprayed with ProBios® at a dose of 20.0 dm3 ha−1; (3) plants sprayed with 2 dm3 ha−1 of AGRO-SORB® Folium; (4) plants sprayed with both ProBios® 20.0 dm3 ha−1 and AGRO-SORB® Folium 2 dm3 ha−1. Plants were treated before each growth cycle with the first foliar spray within five days after the growing period started, while the second and third was applied 5–6 days after the first and second harvests.
Adequate amounts of microbial and amino acid products were dissolved in water to produce the spraying liquid applied at 300 dm3 ha−1. Containing amino acids, AGRO-SORB® Folium is produced by Biopharmacotech Limited Partnership with its registered office in Częstochowa (Poland). It is a growth stimulant with 18 biologically active free amino acids (L-alpha) obtained by enzymatic hydrolysis. In its composition it contains a high amount of the following biologically active free amino acids of (at least 9.3% as mass percentage, or 100 g per 1000 mL: aspargic acid 0.450%, serine 0.321%, glutamate acid 1.814%, glycine 2.743%, histidine 0.208%, arginine 0.131%, threonine 0.323%, alanine 0.524%, proline 0.347%, cysteine 0.435%, tyrosine 0.174%, waline 0.551%, methionine 0.349%, lysine 0.661%, isoleucine 0.308%, leucine 0.180%, phenylalanine 0.218% and tryptophan 0.05%.
In turn, ProBios®, a microbiological product with pH of 3.4–3.6, contains lactic acid bacteria, phototropic bacteria, yeast, fermenting fungi, actinomycetes, ethanol, natural acetic acid (3.5%), sugar cane molasses (3.5%), plant extracts (about 1%), dechlorinate water (92%), the cultures of Bifidobacterium lactis, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus salivarius, Lactococcus lactis, Streptococcus thermophilus (non-pathogenic strain) and herbs: Pimpinella anisum, Glycyrrhiza glabra, Foeniculum vulgare, Ocimumbasilicum, Matricariarecutita, Anthriscus cerefolium, Anethumgraveolens, Sambucus nigra, Trigonellafoenum-graecum, Zingiberofficinale, Angelica archangelica, Juniperuscommunis, Urticadioica, Origanum vulgare, Petroselinum crispum, Mentha piperata, Rosmarinus officinalis, Salvia officinalis and Thymus vulgaris. It is produced by Eco-Natural (11-600 Węgorzewo, Przemysłowa 7 L, Poland).
During the experiment, mineral fertilizers were also used at the following doses: 80 kg Nha−1 before the first growth cycle and 60 kg N ha−1 before the second and third in the form of 34% N ammonium nitrate. Phosphorus was used once in the spring in the amount of 34.9 kg P2O5ha−1 in the form of enriched superphosphate with 17.4% P. Potassium was applied at 49.8 kg K2O ha−1 as 49.8% K potassium salt before the first and third cycle.
Plants were harvested at a height of 6–7 cm, the first time when the dominant grass species were in the boot stage. After the second and third growth cycle, they were mown seven weeks after the previous harvest. Then, the green matter was dried for one day. In the next stage, the partially dried material was cut into 2-centimeter-long shreds using a chaff cutter. The shredded and semi-dry matter was compacted in 5 L plastic containers and then tightly closed. The density of the plant material was 200 kg DM in 1 m3. Containers with silage had been stored for four months in rooms with a temperature of about 15 °C. After this period, the containers were opened and silage samples collected for chemical analysis. The above procedure was replicated three times. The chemical composition of the silage was carried out with the Weenden method [22], and pH was measured with a ph-meter N 517. Lactic acid content was determined by a Varian 3400 CX Gas Chromatograph with a flame ionization detector (FID), J&W Scientific DB-FFAP column with a length of 30 m and a diameter of 0.53 mm, argon carrier gas, dispenser temperature of 200 °C, detector temperature of 240 °C and column temperature of 60–210 °C. The acetic acid content was determined by a INGOS liquid chromatograph LCP 5020, with a steel column 8 × 250 mm filled with OSTION LG-KS 0800 H+ (Tessek), mobile phase: 5 mM H2SO4.
2.4. Statistical Analysis
A two-factor analysis of variance was carried out to determine the main effects of treatment combinations and cut as well as treatment combinations × cut interaction on the variability of 11 studied silage traits. Mean values and standard deviations were calculated for all traits. The least significant differences (LSDs) tests were used to determine differences across concentration for all traits. The relationships between the observed traits were estimated using Pearson’s linear correlation coefficients, for each treatment combination and for control independently. The elementary comparisons between means across cuts of particular traits were tested using the two-sample t-test, on the 0.05, 0.01 and 0.001 levels, for control and the effects of amino acids, microorganisms and amino acids with microorganisms, independently. To account for multiple comparison, the Bonferroni correction was used. All the analyses were conducted using the GenStat v. 18 statistical software package.
3. Results
The basic chemical composition of the silage is presented in Table 2 and Table 3. The content of most nutrients in the silage produced from plants treated with the stimulants was significantly different (p < 0.05) from control (Table 2 and Table 3). Total protein content varied from 97.3 to 133.0 g kg−1 DM, depending on the treatment and the harvest, being the lowest for control plants (Table 2). The foliar application of amino acids and microbial products increased this content for all harvests, and it was on average 5% higher than for control plants, although the differences were not statistically significant (Table 2). Compared to control, in biomass treated with microbial and amino acid products applied together, there was a statistically significant 23% increase (p ≤ 0.05) in ether extract content in dry matter (Table 2). It ranged between 22.9 and 32.6 g kg−1 DM, depending on the treatment combination and on the harvest. The mean for silage from control was 24.8 g kg−1 DM, with 24.9 g kg−1 DM for plots with amino acid application, 25.5 g kg−1 DM for plots with the application of the microbial product and 30.7 g kg−1 DM for combined application.

Table 2.
The effect of effective microorganisms and amino acids on the chemical composition of grassland silage.

Table 3.
Chemical compositions of the silage.
In the silage from plants treated with stimulants applied separately, a small decrease in crude fiber content was recorded only in the third harvest, while their combined application did not affect it (Table 2). Different treatment combinations did not significantly affect (p > 0.05) neutral detergent fiber (NDF) content either (Table 2). In contrast, statistically significant differences in acidic detergent fiber (ADF) content (p < 0.05) between silage from different plots were recorded.
A higher concentration of water-soluble carbohydrates was observed in silage from plots where the microbial product was applied (Table 2), but the increase was not statistically significant (p > 0.05). However, significant differences (p < 0.05) in pH between silage from plots with treatment combinations were recorded. This value ranged from 5.81 to 6.64 and was the lowest in control silage (Table 3).
Lactic acid content considerably varied and was dependent on the treatment and the date of harvesting (Table 3). It increased in silage from plants treated with the microbial product and on plots where the latter and amino acids were applied together. The amount of acetic acid was the smallest in silage made from control plants. On the other hand, its largest amount was recorded in silage from plants treated with a combined application of microbial and amino acid products (Table 3). Silage made from plants treated with microbial and amino acid product contained the smallest amount of propionic acid (Table 3).
In control plants and those treated with amino acids (Figure 2) and microorganisms, crude protein content was correlated with the content of ether extract, crude fiber, NDF, ADF and with pH (Figure 3). Additionally, crude fiber content was significantly correlated with the amounts of NDF and ADF in silage from control and plots treated with amino acids (Figure 2) and with microorganisms (Figure 3). Silage pH was significantly correlated with ADF and water-soluble carbohydrates in silage from control and plots treated with amino acids (Figure 2) and with microorganisms (Figure 3).
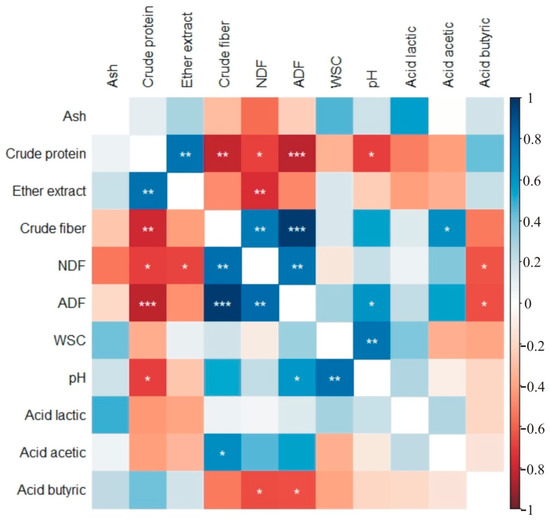
Figure 2.
Heatmaps of correlation coefficients for traits of control silage (above diagonal) and of silage treated with amino acids (below diagonal), rcr = 0.576. (NDF—neutral detergent fiber, ADF—acidic detergent fiber, WSC—water-soluble carbohydrates), * p < 0.05, ** p < 0.01, *** p < 0.001.
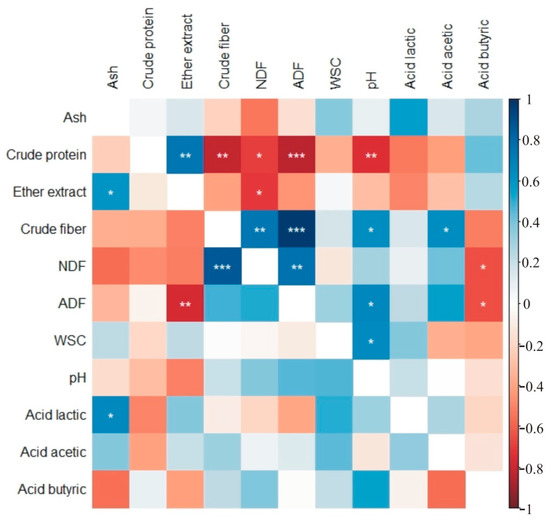
Figure 3.
Heatmaps of correlation coefficients for traits of silage treated with microorganisms (above diagonal) and treated with the combined use of microorganisms and amino acids (below diagonal), rcr = 0.576. (NDF—neutral detergent fiber, ADF—acidic detergent fiber, WSC—water-soluble carbohydrates), * p < 0.05, ** p < 0.01, *** p < 0.001.
The statistically significant differences between cuts I and III for crude fiber, ADF and pH were observed for control plants and those treated with amino acids and microorganisms (Table 4). Means for cut I were larger than for cut III. For crude protein, significant contrasts between means for I and III cuts were observed for all four groups of plants: control, treated with amino acids and microorganisms and treated with both amino acids with microorganisms (Table 4). Comparisons between cuts II and III for ash were observed for control and amino acids (Table 4). The largest statistically significant differences were observed for lactic acid (Table 4).

Table 4.
Statistical significance of differences between the average values of each pairs of cuts for control, amino acids, microorganisms and amino acids with microorganisms, independently.
4. Discussion
The stimulants used in the experiment significantly increased the concentration of (p < 0.05) basic nutrients. Products containing amino acids are mainly used as a stimulant in the production of cereals, rapeseed, corn, fruit and vegetables [23,24,25]. However, some authors also indicate that such substances have a beneficial effect on the growth of grassland plants [26]. The high efficiency of amino acid fertilizers is due to the fact that they are a source of nitrogen readily available to plant cells, taken up faster than inorganic nitrogen. In addition, amino acids play a key role in plant metabolism, including assimilation of protein, one of the most important factors conducive to the proper formation of cells, resulting in an increase in fresh and dry mater yields. An increase in the plant yield due to the use of amino acids has been demonstrated in many scientific studies [23,27,28,29]. One of those studies found that amino acid treatment significantly increased soybean yields [27]. In another experiment, it increased the height of roselle plants (Hibiscus sabdariffa), stem diameter and the fresh and dry matter yield of leaves [23]. It was also found that amino acids increased the plant height and dry matter yield of potato [28]. Similar effects were noted in the cultivation of herbs treated with amino acids. Tarraf et al. [29] recorded more leaves and more branches, a larger plant height, as well as a higher yield of fresh and dry matter in fenugreek (Trigonellafoenum-graecum L.), and Gamal El-Din and Abd El-Wahed [30] noted similar results in their experiment with chamomile (Matricaria chamomilla). This may be due to the fact that amino acids play an important role in plant metabolism and assimilation of proteins necessary for the formation of new cells, this way increasing the yield of fresh and dry matter [25].
The present studies showed that the treatment of meadow plants with amino acids increased the content of total protein and ether extract in silage. In the silage from the third harvest, a significant decrease in crude fiber content and a higher concentration of water-soluble sugars were recorded. Zewail [31] and Pooryousef and Alizadeh [32] found higher protein content in plants treated with amino acids; the use of Aminol Forte resulted in an increase in the content of crude protein to 22%, compared with 17% in control. Goss [33] points out that when plants are lacking in carbohydrates they can use amino acids as an alternative source of carbon and energy, releasing ammonia and organic acid. As bioactive compounds, amino acids can accelerate oil biosynthesis from sugars in plants. Its exact mechanism is strictly related to plant growth and development [34].
Treating plants with biostimulants containing amino acids strengthens their defense mechanism, prevents the loss of water and positively affects plasmalysis. Amino acid-based products stimulate the process of photosynthesis, and at the same time, they determine the rate and direction of metabolic processes [35]. Additionally, they affect hormonal and enzymatic activity in plants. Ertani et al. [36] point out that at a favorable concentration of NH4+, the activity of glutamine synthetase and glutamate synthase increases, which increases nitrogen concentration in plants. Bettoni et al. [37] found that biostimulants improved absorption and translocation of nitrogen from roots to shoots, increasing leaf protein content as a result [38].
In the literature, there are also reports that along with an increase in plant total protein content, the content of fat falls. However, studying the effect of Fylloton (containing free amino acids and seaweed extracts) on soybean yields, Kocira et al. [39] found that the biostimulant increased fat and protein content in the seeds, compared to control samples. Some researchers have claimed that with lower air temperature and moisture the fat content of soya seeds increases [40]. Ebinezer et al. [41] report that biostimulant application brings about changes in plant transcriptome and proteome. Those changes affect the metabolism and biosynthesis of many essential compounds in plants. In addition, biostimulants increase the activity of proteins, positively affecting fatty acid synthesis and lipid metabolism [41]. Rouphael et al. [42] reported that plants responded to stimulant application with lipid accumulation in different parts. Biostimulants increase the synthesis of such nutrients as proteins and lipids, carbohydrate metabolism and ion transport. According to Kumar et al. [43], they affect gene transcription and, in effect, lipid and nitrogen metabolism, increasing plant yields and nutritional value.
In the present experiment, the chemical composition of the silage indicated that it was of good nutritional value. For its proper digestion in the gastrointestinal tract of bovine animals, the minimum content of total protein in the feed should range from 150 to 170 g kg−1 DM [44]. The total protein content of the silage varied and was slightly below the optimal standard, especially in plants from the control plot where no stimulants were used.
Grassland forage for ruminant animals should contain approximately 200–250 g kg−1 DM crude fiber not exceeding 280 g kg−1 DM [44]. The use of stimulants in the form of amino acids and effective microorganisms positively influenced the ether extract content of the silage, and these values across treatment combinations were within the limits optimal for roughage, ranging from 23 to 33 g kg−1 DM [45].
An important indicator determining the quality of silage and its usefulness in animal nutrition is organic acid content [46]. Good silage should have a pH of approximately 4.2. The lower the pH, i.e., the higher the acidity, the more lactic and acetic acid it contains. On the other hand, pH of 5.0 and above indicates the presence of butyric acid [47]. The content of butyric acid reduces the quality of silage, and the quantity of above 10 g kg−1 DM disqualifies it for feeding. In the present studies, silage pH was slightly above the optimal level.
The yield and quality of silage material from permanent grassland is significantly influenced by environmental factors during plant growth. Intensive cultivation of plants negatively affects nutrient content in soil and leads to its degradation and acidification. One of the methods of improving its properties can be the use of microbiological products. Probiotic bacteria introduced into the soil improve its physical and chemical properties, but this effect largely depends on the dose of the product and its type [48,49]. Kaczmarek et al. [50] reported that the microorganism application increased the number of bacteria, fungi, actinomycetes and copiotrophic microorganisms in the soil. At the same time, these microorganisms inhibited the development of oligotrophic microbes and had a positive effect on soil dehydrogenase activity. However, Kucharski and Jastrzębska [51] point out that the growth and development of fungi and other soil microbes after the application of microorganisms are limited. According to Badura [52], microbial products work better on poor, damaged and degraded soils where microbial balance is shaken.
Additionally, Vázquez-Hernández et al. [1] point out that plants inoculated with plant-growth-promoting rhizobacterias (PGPRs) respond with better morphological and biochemical development and increased tolerance to abiotic stress. The increased growth and stress resistance are effects of various factors such as a higher content of ACC deaminase and plant antioxidative enzymes, a lower content of stress ethylene and heavy metals and more efficient nutrient uptake [1].
According to Ollel and Williams [53], most studies have found that effective microorganisms (EM) positively affect vegetable growth, improving their yield and quality, lowering the incidence of pests and diseases and even reducing the number of weeds. Other researchers, however, did not record any significant impact of EM on plants. The present experiment demonstrated that foliar feeding of meadow plants with effective microorganisms and amino acids resulted in a higher nutrient concentration and is advisable even on soil rich in minerals.
5. Conclusions
In the field experiment, it was found that microbial and amino acid products had a positive effect on the chemical composition of silage made from meadow plants. On the basis of the results, it was concluded that the treatment significantly increased the content of total protein and dry mater compared to control, while crude fiber content changed only slightly. The quality of silage due to lactic acid content was higher in plants treated with the microbial and amino acid products than in control ones. In conclusion, foliar treatment with microorganisms in combination with amino acids resulted in a significant increase, relative to control, in the desired components in the chemical composition of plants. In view of the potential benefits, it would also be advisable to extend and update research on the effects of forage from plants treated with microorganisms and amino acids on the milk yield and health of dairy cows, especially high-productive ones.
Author Contributions
Conceptualization, A.R. and J.B.; methodology, A.R., I.R., J.B. and A.C.; software, J.B. and A.C.; validation, A.R., I.R. and J.B.; formal analysis, J.B. and A.C.; investigation, A.R.; resources, A.R.; data curation, A.R.; writing—original draft preparation, A.R., I.R., J.B. and A.C.; writing—review and editing, A.R., I.R., J.B., A.C., K.W. and H.B.; visualization, J.B. and A.C.; supervision, A.R.; project administration, A.R.; funding acquisition, K.W. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Gugała, M.; Zarzecka, K.; Sikorska, A.; Mystkowska, I.; Dołęga, H. Wpływ herbicydów i biostymulatorów wzrostu na organiczenie zachwaszczenia i plonowanie ziemniaka jadalnego. Fragm. Agron. 2017, 34, 59–66. [Google Scholar]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A new trend towards solving an old problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Gawrońska, H.; Przybysz, A. Biostymulatory: Mechanizmy Działania i Przykłady Zastosowań; Mat. Konferencyjne, Targi sadownictwa i warzywnictwa: Warsaw, Poland, 2011; pp. 5–6. [Google Scholar]
- Chojnacka, K. Innovative bio-products for agriculture. Open Chem. 2015, 13, 932–937. [Google Scholar] [CrossRef]
- Lovatt, C.J. Use of a Natural Metabolite to Increase Crop Production. U.S. Patent 14/503,276, 21 May 2015. [Google Scholar]
- Wang, H.; Wu, L.; Tao, Q. Influence of partial replacement of nitrate by amino acids on nitrate accumulation of pakchoi (Brassica chinensis L.). China Environ. Sci. 2004, 24, 19–23. [Google Scholar]
- Persson, J.; Näshom, T. Regulation of amino acid uptake in conifers by exogenous and endogenous nitrogen. Planta 2002, 215, 639–644. [Google Scholar] [CrossRef]
- Persson, J.; Näshom, T. Regulation of amino acid uptake by carbon and nitrogen in Pinus sylvestris. Planta 2003, 217, 309–315. [Google Scholar] [CrossRef]
- Persson, J.; Hogberg, P.; Ekblad, A.; Hogberg, M.N.; Nordgren, A.; Nasholm, T. Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 2003, 137, 252–257. [Google Scholar] [CrossRef]
- Pessarakli, M. Handbook of Plant and Crop Physiology, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 385–394. [Google Scholar]
- Popova, O.V.; Dietz, K.J.; Golldack, D. Salt–dependent expression of a nitrate transporter and two amino acid transporter genes in Mesembryanthemum crystallinum. Plant Mol. Biol. 2003, 52, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Werdin-Pfisterer, N.R.; Kielland, K.; Boone, R.D. Soil amino acid composition across a boreal forest successional sequence. Soil Biol. Biochem. 2009, 41, 1210–1220. [Google Scholar] [CrossRef]
- Jones, D.L.; Shannon, D.; Junvee-Fortune, T.; Farrarc, J.F. Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol. Biochem. 2005, 37, 179–181. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L. Cell-to-cell communication in bacteria: A chemical discourse. Harvey Lect. 2004, 100, 123–142. [Google Scholar] [PubMed]
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Method 935.14 and 992.24; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Ahmed, Y.M.; Shalaby, E.A.; Shanan, N.T. The use of organic and inorganic cultures in improving vegetative growth, yield characters and antioxidant activity of roselle plants (Hibiscus sabdariffa L.). Afr. J. Biotechnol. 2011, 10, 1988–1996. [Google Scholar] [CrossRef]
- Sadak, M.S.H.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of amino acids on plant yield and physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.M.; Seadh, S.E.; AlTai, D.S.K. Role of humic acid and amino acids in limiting loss of nitrogen fertilizer and increasing productivity of some wheat cultivars grown under newly reclaimed sandy soil. Int. J. Adv. Res. Biol. Sci. 2016, 3, 123–136. [Google Scholar]
- Radkowski, A.; Radkowska, I.; Bocianowski, J. Effect of the fertilization of meadow sward with amino acids obtained from enzymatic hydrolysis on silage quality. J. Elem. 2020, 25, 259–277. [Google Scholar] [CrossRef]
- Saeed, M.R.; Kheir, A.M.; Al-Sayed, A.A. Supperssive effect of some amino acids against Meloidogyne incognita on soybeans. J. Agric. Sci. Mansoura Univ. 2005, 30, 1097–1103. [Google Scholar]
- El-Zohiri, S.S.M.; Asfour, Y.M. Effect of some organic compounds on growth and productivity of some potato cultivars. Ann. Agric. Sci. Moshtohor. J. 2009, 47, 403–415. [Google Scholar]
- Tarraf, S.A.; Talaat, I.M.; El-Sayed, A.E.B.; Balbaa, L.K. Influence of foliar application of algae extract and amino acids mixture on fenugreek plants in sandy and clay soils. Nusant. Biosci. 2015, 7, 33–37. [Google Scholar] [CrossRef]
- Gamal El-Din, K.M.; Abd El-Wahed, M.S.A. Effect of some amino acids on growth and essential oil content of chamomile plant. J. Agri. Biol. 2005, 7, 376–380. [Google Scholar]
- Zewail, R.M.Y. Effect of seaweed extract and amino acids on growth and productivity and some biocostituents of common bean (Phaseolus vulgaris L.) plants. J. Plant Prod. 2014, 5, 1441–1453. [Google Scholar] [CrossRef]
- Pooryousef, M.; Alizadeh, K. Effect of foliar application of free amino acids on alfalfa performance under rainfed conditions. Res. Crops 2014, 15, 254–258. [Google Scholar] [CrossRef]
- Goss, J.A. Amino acid synthesis and metabolism. In Physiology of Plants and Their Cells; Pergamon Press, Inc.: New York, NY, USA, 1973; p. 202. [Google Scholar] [CrossRef]
- Mulabagal, V.; Tsay, H.S. Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int. J. Appl. Sci. Eng. 2004, 2, 29–48. [Google Scholar]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, A.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Bettoni, M.M.; Mogor, Á.F.; Pauletti, V.; Goicoechea, N.; Aranjuelo, I.; Garmendia, I. Nutritional quality and yield of onion as affected by different application methods and doses of humic substances. J. Food Comp. Anal. 2016, 51, 37–44. [Google Scholar] [CrossRef]
- Shahabivand, S.; Padash, A.; Aghaee, A.; Nasiri, Y.; Rezaei, P.F. Plant biostimulants (Funneliformis mosseae and humic substances) rather than chemical fertilizer improved biochemical responses in peppermint. Iran. J. Plant Physiol. 2018, 8, 2333–2344. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wojtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef]
- Hołubowicz-Kliza, G. Soybean Cultivation; IUNG Puławy: Puławy, Poland, 2007. [Google Scholar]
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arrigoni, G.; Masi, A. Quantitative proteomics of maize roots treated with a protein hydrolysate: A comparative study with transcriptomics highlights the molecular mechanisms responsive to Biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic Responses of Maize Shoots and Roots Elicited by Combinatorial Seed Treatments With Microbial and Non-microbial Biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Trivedi, K.; Anand, K.G.V.; Ghosh, A. Science behind biostimulant action of seaweed extract on growth and crop yield: Insights into transcriptional changes in roots of maize treated with Kappaphycus alvarezii seaweed extract under soil moisture stressed conditions. J. Appl. Phycol. 2020, 32, 599–613. [Google Scholar] [CrossRef]
- Brzóska, F. Roughage feeds from grassland and their use in feeding of livestock. Wieś Jutra 2008, 3, 28–33. [Google Scholar]
- Brzóska, F.; Śliwiński, B. Jakość pasz objętościowych w żywieniu przeżuwaczy i metody jej oceny. Cz. II. Metody analizy i oceny wartości pokarmowej pasz objętościowych. Wiad. Zoot. 2011, 4, 57–68. [Google Scholar]
- Podkówka, W.; Potkański, A. Wpływ czynników chemicznych i fizycznych na przydatność pasz do zakiszania. Post. Nauk Rol. 1993, 40, 29–42. [Google Scholar]
- Nowak, J.; Šařec, P. Wybrane czynniki decydujące o jakości kiszonek w belach cylindrycznych. Post. Nauk Rol. 2001, 5, 95–110. [Google Scholar]
- Tokeshi, H.; Aloes, M.C.; Sanches, A.B.; Harada, D.Y. Effective Microorganisms for controlling the phytopathogenic fungus Sclerotinia sclerotiorum in lettuce. In Proceedings of the Conference on Effective Microorganisms for a sustainable agriculture and environment. In Proceedings of the 4th International Conference on Kyusei Nature Farming, Bellingham, WA, USA, 19–21 June 1998; pp. 131–139. [Google Scholar]
- Kaczmarek, Z.; Jakubus, M.; Grzelak, M.; Mrugalska, L. Impact of the addition of various doses of Effective Microorganisms to arable-humus horizons of mineral soils on their physical and water properties. J. Res. Appl. Agric. Eng. 2008, 53, 118–121. [Google Scholar]
- Kaczmarek, Z.; Wolna-Maruwka, A.; Jakubus, M. Zmiany liczebności wybranych grup drobnoustrojów glebowych oraz aktywności enzymatycznej w glebie inokulowanej efektywnymi mikroorganizmami (EM). J. Res. Appl. Agric. Eng. 2008, 53, 122–128. [Google Scholar]
- Kucharski, J.; Jastrzębska, E. Rola mikroorganizmów efektywnych (EM) i glebowych w kształtowaniu właściwości mikrobiologicznych gleby. Zesz. Probl. Post. Nauk Rol. 2005, 507, 315–322. [Google Scholar]
- Badura, L. Czy znamy wszystkie uwarunkowania funkcji mikroorganizmów w ekosystemach lądowych? Kosm. Probl. Nauk Biol. 2004, 53, 373–379. [Google Scholar]
- Ollel, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotech. 2013, 88, 380–386. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).