Effect of Naturally Occurring Compounds on Fumonisin Production and fum Gene Expression in Fusarium verticillioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Tested Compounds
2.3. In Vitro Activity
2.4. Mycelial Growth Estimation and Fumonisin Quantification
2.5. In Vivo Activity in Maize Kernels
2.6. Fum Gene Expression Analysis upon Phenolic Compound Treatment at 1.0 mM
2.7. Statistical Analysis
3. Results
3.1. In Vitro Effect of Phenolic Compounds on FvH Growth and Fumonisin Production
3.2. In Vitro Effect of Phenolic Compounds on FvL Growth and Fumonisin Production
3.3. In Vitro Effects of Reduced ELL and ISO Dosages on FvH and FvL
3.4. In Vivo Effect of Phenolic Compounds on Growth and FB Production by FvH
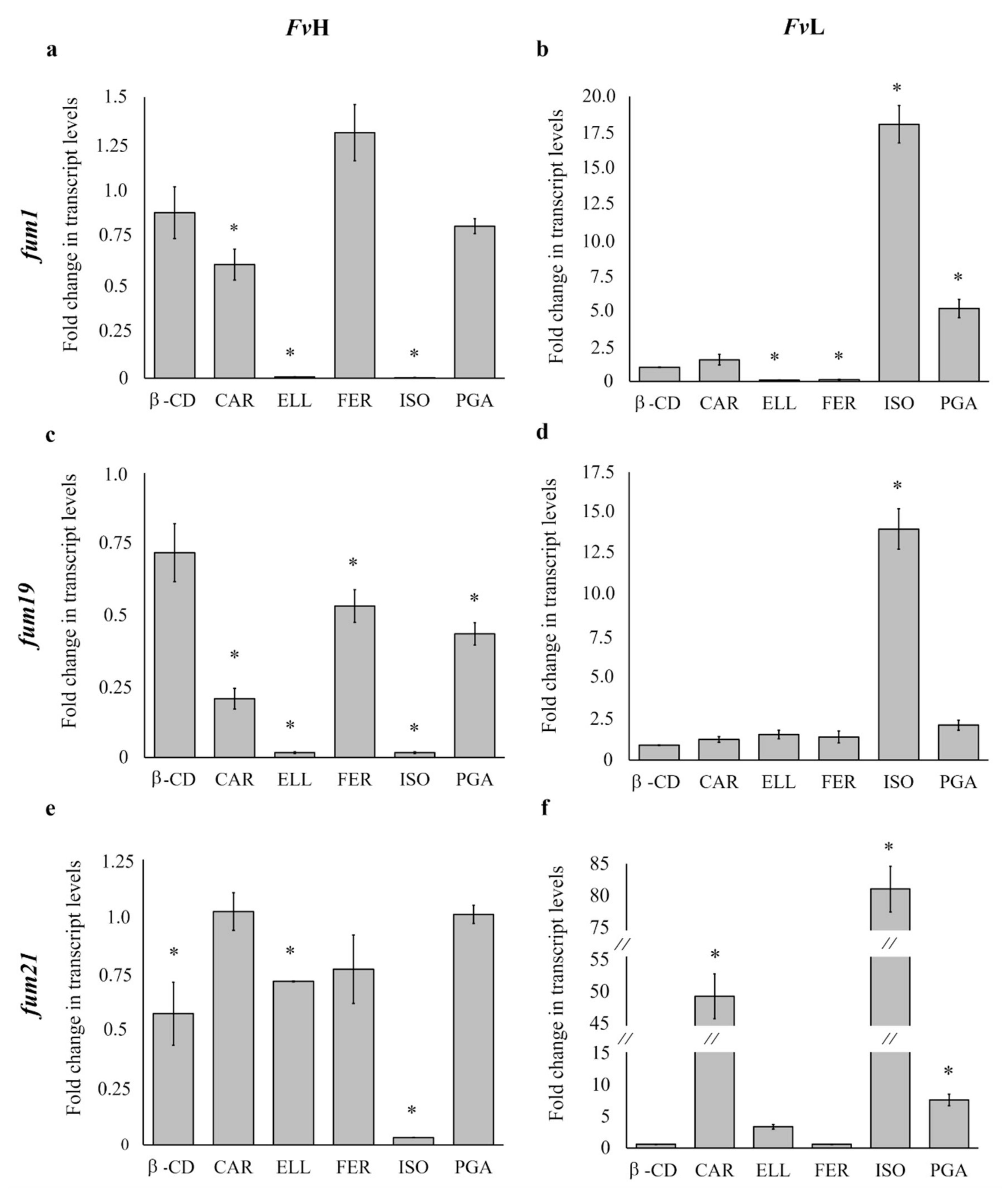
3.5. Effect of Phenolic Compounds on fum1 Expression
3.6. Effect of Phenolic Compounds on fum19 Expression
3.7. Effect of Phenolic Compounds on fum21 Expression
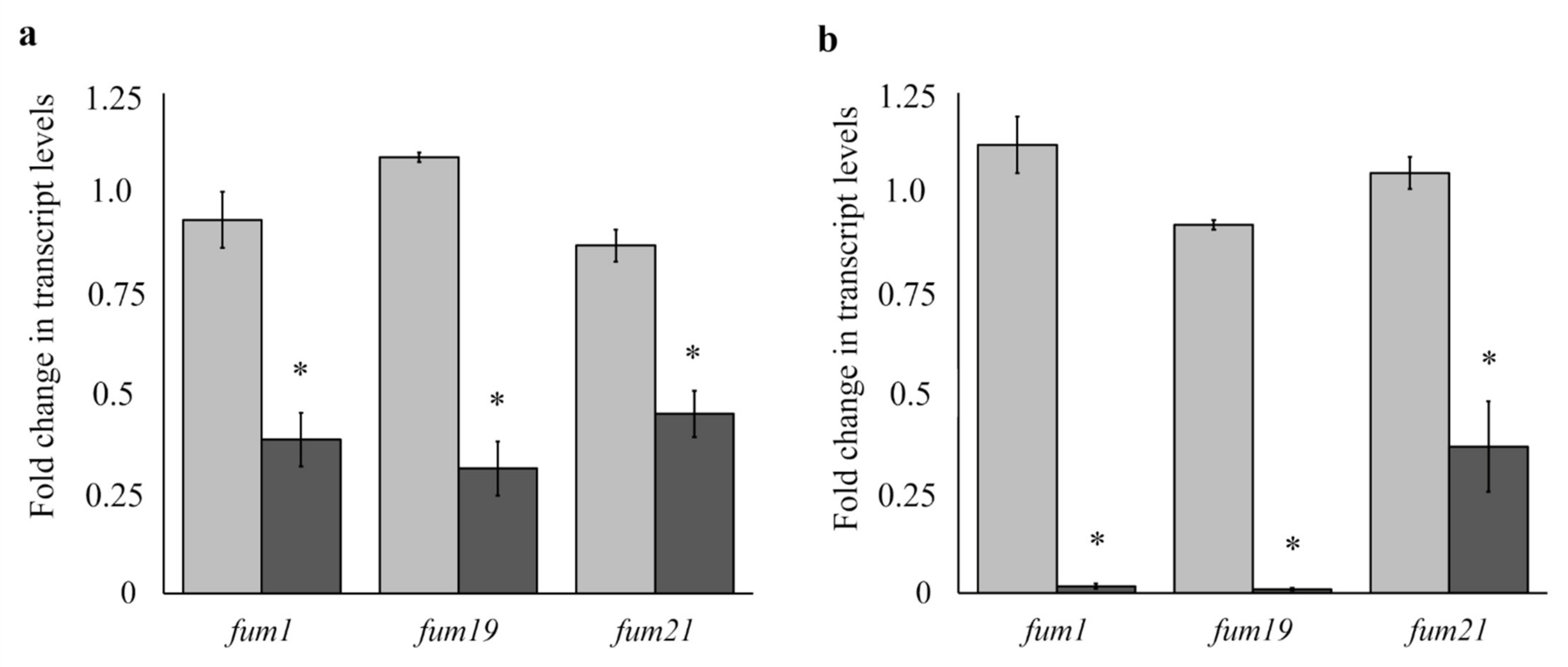
3.8. Effect of Under-Dosed ELL Treatment on Fum Gene Expression
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, G.W. Fumonisins. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1003–1018. [Google Scholar]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, B.J.; Mc Laren, N.W.; Schoeman, A.; Flett, B.C. The effects of cultivar and prophylactic fungicide spray for leaf diseases on colonisation of maize ears by fumonisin producing Fusarium spp. and fumonisin synthesis in South Africa. Crop Prot. 2016, 79, 56–63. [Google Scholar] [CrossRef]
- Becher, R.; Hettwer, U.; Karlovsky, P.; Deising, H.B.; Wirsel, S.G. Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology 2010, 100, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Ramirez, M.L.; Arroyo, M.; Chulze, S.N.; Magan, N. Potential use of antioxidants for control of growth and fumonisin production by Fusarium verticillioides and Fusarium proliferatum on whole maize grain. Int. J. Food Microbiol. 2003, 83, 319–324. [Google Scholar] [CrossRef]
- Farnochi, M.C.; Torres, A.M.; Magan, N.; Chulze, S.N. Effect of antioxidants and competing mycoflora on Fusarium verticillioides and F. proliferatum populations and fumonisin production on maize grain. J. Stored Prod. Res. 2005, 41, 211–219. [Google Scholar] [CrossRef]
- Dambolena, J.S.; López, A.G.; Meriles, J.M.; Rubinstein, H.R.; Zygadlo, J.A. Inhibitory effect of 10 natural phenolic compounds on Fusarium verticillioides. A structure-property-activity relationship study. Food Control 2012, 28, 163–170. [Google Scholar] [CrossRef]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to decipher the chemical defense of cereals against Fusarium graminearum and deoxynivalenol accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants; Lavoisier Publishing: Paris, France, 1995; ISBN 2-7430-0028-7. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; Nigro, F.; De Girolamo, A.; Ippolito, A. Effect of quercetin and umbelliferone on the transcript level of Penicillium expansum genes involved in patulin biosynthesis. Eur. J. Plant Pathol. 2009, 125, 223–233. [Google Scholar] [CrossRef]
- Oufensou, S.; Balmas, V.; Azara, E.; Fabbri, D.; Dettori, M.A.; Schüller, C.; Zehetbauer, F.; Strauss, J.; Delogu, G.; Migheli, Q. Naturally occurring phenols modulate vegetative growth and deoxynivalenol biosynthesis in Fusarium graminearum. ACS Omega 2020, 5, 29407–29415. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Boutigny, A.-L.; Barreau, C.; Richard-Forget, F. Cinnamic-Derived acids significantly affect Fusarium graminearum growth and in vitro synthesis of type B trichothecenes. Phytopathology 2011, 101, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Narasaiah, K.V.; Sashidhar, R.B.; Subramanyam, C. Biochemical analysis of oxidative stress in the production of aflatoxin and its precursor intermediates. Mycopathologia 2006, 162, 179. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Zjalic, S.; Ricelli, A.; Fabbri, A.A.; Fanelli, C. Oxidant/Antioxidant balance in Aspergillus parasiticus affects aflatoxin biosynthesis. Mycotoxin Res. 2006, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0-470-27646-0. [Google Scholar]
- Mulè, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Tuite, J. Plant Pathological Methods: Fungi and Bacteria; Burgess Publishing Co.: Minneapolis, MN, USA, 1969. [Google Scholar]
- Nesci, A.V.; Etcheverry, M.G. Control of Aspergillus growth and aflatoxin production using natural maize phytochemicals under different conditions of water activity. Pest Manag. Sci. Former. Pestic. Sci. 2006, 62, 775–784. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’Keeffe, T.L.; Mahoney, N.E. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia 2007, 164, 241–248. [Google Scholar] [CrossRef]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahanshiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessì, A. Natural and natural-like phenolic inhibitors of type B trichothecene in vitro production by the wheat (Triticum sp.) pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef] [PubMed]
- Žilius, M.; Ramanauskienė, K.; Briedis, V. Release of propolis phenolic acids from semisolid formulations and their penetration into the human skin in vitro. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. Methods to measure the antioxidant activity in plant material. A comparative discussion. Free Radic. Res. 1999, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Braumann, T. Determination of hydrophobic parameters by reversed-phase liquid chromatography: Theory, experimental techniques, and application in studies on quantitative structure-activity relationships. J. Chromatogr. A 1986, 373, 191–225. [Google Scholar] [CrossRef]
- Kfoury, M.; Lounès-Hadj Sahraoui, A.; Bourdon, N.; Laruelle, F.; Fontaine, J.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem. 2016, 196, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Divakar, S.; Maheswaran, M.M. Structural studies on inclusion compounds of β-cyclodextrin with some substituted phenols. J. Incl. Phenom. Mol. Recognit. Chem. 1997, 27, 113–126. [Google Scholar] [CrossRef]
- Blackwell, B.A.; Miller, J.D.; Savard, M.E. Production of carbon 14-labeled fumonisin in liquid culture. J. AOAC Int. 1994, 77, 506–511. [Google Scholar] [CrossRef]
- Balmas, V.; Delogu, G.; Sposito, S.; Rau, D.; Migheli, Q. Use of a complexation of tebuconazole with β-cyclodextrin for controlling foot and crown rot of durum wheat incited by Fusarium culmorum. J. Agric. Food Chem. 2006, 54, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Venturini, G.; Assante, G.; Toffolatti, S.L.; Vercesi, A. Pathogenicity variation in Fusarium verticillioides populations isolated from maize in Northern Italy. Mycoscience 2013, 54, 285–290. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Bogialli, S.; Bortolini, C.; Tapparo, A.; Causin, R. In vitro production of fumonisins by Fusarium verticillioides under oxidative stress induced by H2O2. J. Agric. Food Chem. 2015, 63, 4879–4885. [Google Scholar] [CrossRef]
- Lazzaro, I.; Susca, A.; Mulè, G.; Ritieni, A.; Ferracane, R.; Marocco, A.; Battilani, P. Effects of temperature and water activity on FUM2 and FUM21 gene expression and fumonisin B production in Fusarium verticillioides. Eur. J. Plant Pathol. 2012, 134, 685–695. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Simon, P. Q-Gene: Processing quantitative real-time RT–PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, R.P.; Jacquat, A.G.; Usseglio, V.L.; Achimón, F.; Cuello, A.E.; Zygadlo, J.A.; Dambolena, J.S. Quantitative-Structure-activity relationship study to predict the antifungal activity of essential oils against Fusarium verticillioides. Food Control 2020, 108, 106836. [Google Scholar] [CrossRef]
- Pagnussatt, F.A.; Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Inhibition of Fusarium graminearum growth and mycotoxin production by phenolic extract from Spirulina sp. Pestic. Biochem. Physiol. 2014, 108, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; De Meulenaer, B.; Osei-Nimoh, D.; Lamboni, Y.; Debevere, J.; Devlieghere, F. Can phenolic compounds be used for the protection of corn from fungal invasion and mycotoxin contamination during storage? Food Microbiol. 2007, 24, 465–473. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef]
- Gauthier, L.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Buśko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Trans-Cinnamic and chlorogenic acids affect the secondary metabolic profiles and ergosterol biosynthesis by Fusarium culmorum and F. graminearum sensu stricto. Toxins 2017, 9, 198. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Bernillon, S.; Marchegay, G.; Lornac, A.; Pinson-Gadais, L.; Ponts, N.; Zehraoui, E.; Barreau, C.; Richard-Forget, F. Bioguided isolation, characterization, and biotransformation by Fusarium verticillioides of Maize kernel compounds that inhibit fumonisin production. Mol. Plant Microbe Interact. 2014, 27, 1148–1158. [Google Scholar] [CrossRef]
- Pani, G.; Dessì, A.; Dallocchio, R.; Scherm, B.; Azara, E.; Delogu, G.; Migheli, Q. Natural phenolic inhibitors of trichothecene biosynthesis by the wheat fungal pathogen Fusarium culmorum: A computational insight into the structure-activity relationship. PLoS ONE 2016, 11, e0157316. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H.R. Antifumonisin activity of natural phenolic compounds: A structure-property-activity relationship study. Int. J. Food Microbiol. 2011, 145, 140–146. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce fusarium growth and mycotoxin production in cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [PubMed]
- Beekrum, S.; Govinden, R.; Padayachee, T.; Odhav, B. Naturally occurring phenols: A detoxification strategy for fumonisin B1. Food Addit. Contam. 2003, 20, 490–493. [Google Scholar] [CrossRef]
- Ferruz, E.; Atanasova-Pénichon, V.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Lorán, S.; Ariño, A.; Barreau, C.; Richard-Forget, F. Effects of phenolic acids on the growth and production of T-2 and HT-2 toxins by Fusarium langsethiae and F. sporotrichioides. Molecules 2016, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Ferrochio, L.; Cendoya, E.; Farnochi, M.C.; Massad, W.; Ramirez, M.L. Evaluation of ability of ferulic acid to control growth and fumonisin production of Fusarium verticillioides and Fusarium proliferatum on maize based media. Int. J. Food Microbiol. 2013, 167, 215–220. [Google Scholar] [CrossRef]
- Nesci, A.; Marín, S.; Etcheverry, M.; Sanchis, V. Natural maize phytochemicals for control of maize mycoflora and aflatoxigenic fungi. World Mycotoxin J. 2009, 2, 305–312. [Google Scholar] [CrossRef]
- Dambolena, J.S.; López, A.G.; Cánepa, M.C.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 2008, 51, 37–44. [Google Scholar] [CrossRef]
- Jayashree, T.; Subramanyam, C. Antiaflatoxigenic activity of eugenol is due to inhibition of lipid peroxidation. Lett. Appl. Microbiol. 1999, 28, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Appell, M.; Tu, Y.-S.; Compton, D.L.; Evans, K.O.; Wang, L.C. Quantitative structure-activity relationship study for prediction of antifungal properties of phenolic compounds. Struct. Chem. 2020, 31, 1621–1630. [Google Scholar] [CrossRef]
- Yang, D.; Donovan, S.; Black, B.C.; Cheng, L.; Taylor, A.G. Relationships between compound lipophilicity on seed coat permeability and embryo uptake by soybean and corn. Seed Sci. Res. 2018, 28, 229–235. [Google Scholar] [CrossRef]
- Menniti, A.M.; Gregori, R.; Neri, F. Activity of natural compounds on Fusarium verticillioides and fumonisin production in stored maize kernels. Int. J. Food Microbiol. 2010, 136, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Barz, W.; Hoesel, W. Metabolism and degradation of phenolic compounds in plants. In Biochemistry of Plant Phenolics; Springer: Cham, Switzerland, 1979; pp. 339–369. [Google Scholar]
- Friedman, M.; Jürgens, H.S. Effect of PH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Guillén, F.; Evans, C.S. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 1994, 60, 2811–2817. [Google Scholar] [CrossRef]
- Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Varga, J.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C.; Campbell, B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef]
- Proctor, R.H.; Desjardins, A.E.; Plattner, R.D.; Hohn, T.M. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population A. Fungal Genet. Biol. 1999, 27, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Brown, D.W.; Plattner, R.D.; Desjardins, A.E. Co-Expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet. Biol. 2003, 38, 237–249. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Munkvold, G.P.; Plattner, R.D.; Proctor, R.H. FUM1—A gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliformis in field tests. Mol. Plant Microbe Interact. 2002, 15, 1157–1164. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Mokhtari, A.R. Efficacy of Cuminum cyminum essential oil on FUM1 gene expression of fumonisin-producing Fusarium verticillioides strains. Avicenna J. Phytomed. 2015, 5, 34. [Google Scholar] [PubMed]
- Li, R.; Jiang, Z.-T. Chemical composition of the essential oil of Cuminum cyminum L. from China. Flavour Fragr. J. 2004, 19, 311–313. [Google Scholar] [CrossRef]
- Etzerodt, T.; Maeda, K.; Nakajima, Y.; Laursen, B.; Fomsgaard, I.S.; Kimura, M. 2, 4-Dihydroxy-7-Methoxy-2H-1, 4-Benzoxazin-3 (4H)-One (DIMBOA) inhibits trichothecene production by Fusarium graminearum through suppression of Tri6 expression. Int. J. Food Microbiol. 2015, 214, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jian, Q.; Chen, F.; Wang, Y.; Gong, L.; Duan, X.; Yang, B.; Jiang, Y. Influence of butylated hydroxyanisole on the growth, hyphal morphology, and the biosynthesis of fumonisins in Fusarium proliferatum. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Schmidt-Heydt, M.; Cárdenas-Chávez, D.L.; Parra, R.; Geisen, R.; Magan, N. Integrating Toxin gene expression, growth and fumonisin B1 and B2 production by a strain of Fusarium verticillioides under different environmental factors. J. R. Soc. Interface 2013, 10, 20130320. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium Mycotoxins: Chemistry, Genetics, and Biology; American Phytopathological Society (APS Press): Saint Paul, MN, USA, 2006; ISBN 0-89054-335-6. [Google Scholar]
- Cruz, A.; Marin, P.; Magan, N.; Gonzalez-Jaen, M.T. Combined effects of benomyl and environmental factors on growth and expression of the fumonisin biosynthetic genes FUM1 and FUM19 by Fusarium verticillioides. Int. J. Food Microbiol. 2014, 191, 17–23. [Google Scholar] [CrossRef] [PubMed]
- López-Errasquín, E.; Vázquez, C.; Jiménez, M.; González-Jaén, M.T. Real-Time RT-PCR assay to quantify the expression of FUM1 and FUM19 genes from the fumonisin-producing Fusarium verticillioides. J. Microbiol. Methods 2007, 68, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, K.; Rose, L.J.; Viljoen, A. Fusarium verticillioides FUM1 and FUM19 gene expression in maize kernels during early infection. Physiol. Mol. Plant Pathol. 2019, 108, 101430. [Google Scholar] [CrossRef]
- Sun, L.; Chen, X.; Gao, J.; Zhao, Y.; Liu, L.; Hou, Y.; Wang, L.; Huang, S. Effects of disruption of five FUM genes on fumonisin biosynthesis and pathogenicity in Fusarium proliferatum. Toxins 2019, 11, 327. [Google Scholar] [CrossRef]
- Jurado, M.; Marín, P.; Callejas, C.; Moretti, A.; Vázquez, C.; González-Jaén, M.T. Genetic variability and fumonisin production by Fusarium proliferatum. Food Microbiol. 2010, 27, 50–57. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Rocha, L.; Reis, G.M.; da Silva, V.N.; Braghini, R.; Teixeira, M.M.G.; Corrêa, B. Molecular Characterization and fumonisin production by Fusarium verticillioides isolated from corn grains of different geographic origins in Brazil. Int. J. Food Microbiol. 2011, 145, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Butchko, R.A.E.; Busman, M.; Proctor, R.H. The Fusarium verticillioides FUM gene cluster encodes a Zn(II)2Cys6 protein that affects FUM gene expression and fumonisin production. Eukaryot. Cell 2007, 6, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.-B.; Woloshuk, C.P. Regulation of fumonisin B1 biosynthesis and conidiation in Fusarium verticillioides by a cyclin-like (C-Type) gene, FCC1. Appl. Environ. Microbiol. 2001, 67, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, J.E.; Woloshuk, C.P. Regulation of fumonisin biosynthesis in Fusarium verticillioides by a zinc binuclear cluster-type gene, ZFR1. Appl. Environ. Microbiol. 2004, 70, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, B.H.; Woloshuk, C.P. Fck1, a C-Type cyclin-dependent kinase, interacts with Fcc1 to regulate development and secondary metabolism in Fusarium verticillioides. Fungal Genet. Biol. 2006, 43, 146–154. [Google Scholar] [CrossRef]
- Kim, H.; Woloshuk, C.P. Role of AREA, a regulator of nitrogen metabolism, during colonization of maize kernels and fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 2008, 45, 947–953. [Google Scholar] [CrossRef]
- Myung, K.; Li, S.; Butchko, R.A.; Busman, M.; Proctor, R.H.; Abbas, H.K.; Calvo, A.M. FvVE1 Regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides. J. Agric. Food Chem. 2009, 57, 5089–5094. [Google Scholar] [CrossRef]

| Compound Name | Type | TEAC a | Lipophilicity Log P b |
|---|---|---|---|
| Apocynin (APO) | Acetophenones | 0.41 | 0.83 |
| Caffeic acid (CAF) | Cinnamic Acids | 1.25 | 1.15 |
| Carvacrol (CAR) | Phenylpropanoids | 0.29 | 3.37 |
| Ellagic acid (ELL) | Hydroxylated Biphenyls | 3.05 | 1.05 |
| Ferulic acid (FER) | Cinnamic Acids | 1.45 | 1.42 |
| Isoeugenol (ISO) | Phenylpropanoids | 0.72 | 2.52 |
| Propyl gallate (PGA) | Benzoic Acids | 3.32 | 1.51 |
| Vanillic acid (VAN) | Benzoic Acids | 0.76 | 1.24 |
| FvH | Dry Fungal Biomass (rel. Yield ± SE) | Effect on Biomass † | FBs Content (rel. Yield ± SE) | Effect on FBs † |
| Control | 100 ab | - | 100 ab | |
| β-CD | 99.8 ± 1.2 ab | - | 123.5 ± 3.8 a | - |
| APO | 80.3 ± 1.1 abc | - | 76.6 ± 0.9 bc | - |
| CAF | 94.1 ± 1.2 ab | - | 90.0 ± 5.0 bc | - |
| CAR | 93.6 ± 4.2 abc | - | 68.2 ± 2.2 c | −31.8% |
| ELL | 53.1 ± 3.4 cd | −46.9% | n.a. d | −99.2% |
| FER | 72.2 ± 2.1 c | −27.8% | 136.1 ± 3.9 ab | - |
| ISO | 18.8 ± 0.7 d | −81.2% | n.a. d | −99.2% |
| PGA | 106.8 ± 8.0 a | - | 59.7 ± 4.8 c | −40.3% |
| VAN | 103.4 ± 6.1 a | - | 70.0 ± 0.5 c | −30.0% |
| FvL | Dry Fungal Biomass (rel. Yield ± SE) | Effect on Biomass † | FBs Content (rel. Yield ± SE) | Effect on FBs † |
| Control | 100 a | - | 100.0 a | |
| β-CD | 86.0 ± 3.8 b | −14.0% | 42.1 ± 0.6 bc | −57.9% |
| APO | 63.3 ± 0.9 cd | −36.7% | 13.5 ± 0.8 de | −86.5% |
| CAF | 93.0 ± 1.7 ab | - | 79.4 ± 1.5 ab | - |
| CAR | 82.5 ± 2.2 bc | −17.5% | 41.5 ± 0.1 de | −58.5% |
| ELL | 45.5 ± 4.1 d | −54.5% | n.a e | −97.2% |
| FER | 79.5 ± 3.9 c | −20.5% | 21.9 ± 0.8 de | −78.1% |
| ISO | 61.9 ± 2.1 cd | −38.1% | 49.1 ± 0.4 cd | −50.9% |
| PGA | 115.3 ± 4.8 a | - | 46.2 ± 0.8 cd | −53.8% |
| VAN | 95.5 ± 0.5 ab | - | 78.6 ± 0.8 ab | - |
| FvH | Dry Fungal Biomass (rel. Yield ± SE) | Effect on Biomass † | FBs Content (rel. Yield ± SE) † | Effect on FBs † | |
| Control | 100 ab | - | 100 b | - | |
| ELL | 1.0 mM | 48.4 ± 5.4 c | −51.6% | n.a.d | −99.5% |
| 0.5 mM | 46.4 ± 5.6 c | −53.6% | n.a.d | −99.5% | |
| 0.25 mM | 96.1 ± 3.9 b | - | n.a.d | −99.5% | |
| ISO | 1.0 mM | 19.7 ± 0.9 d | −80.3% | n.a.d | −99.5% |
| 0.5 mM | 39.6 ± 0.8 cd | −60.4% | 55.9 ± 4.9 bc | - | |
| 0.25 mM | 120.9 ± 2.9 a | - | 209.9 ± 15.4 a | +109.9% | |
| FvL | Dry Fungal Biomass (rel. Yield ± SE) | Effect on Biomass † | FBs Content (rel. Yield ± SE)† | Effect on FBs † | |
| Control | 100 b | 100 b | - | ||
| ELL | 1.0 mM | 39.9 + 0.6 d | −60.1% | n.a.d | −97.5% |
| 0.5 mM | 38.7 + 2.3 d | −61.3% | n.a.d | −97.5% | |
| 0.25 mM | 46.2 + 1.2 cd | −53.8% | n.a.d | −97.5% | |
| ISO | 1.0 mM | 62.9 + 7.0 bc | - | 55.5 + 6.2 cd | −44.5% |
| 0.5 mM | 204.9 + 16.6 a | +104.9% | 249.6 + 10.1 a | +149.6% | |
| 0.25 mM | 189.5 + 15.9 a | +89.5% | 177.1 + 12.4 ab | - | |
| 1.0 mM Treatment | 3.0 mM Treatment | |||
|---|---|---|---|---|
| FBs Content (rel. Yield ± SE) † | Effect on FBs † | FBs Content (rel. Yield ± SE) † | Effect on FBs † | |
| Control | 100 c | 100 a | ||
| β-CD | 98.9 ± 8.8 cd | - | 34.3 ± 1.3 bc | −65.7 |
| CAR | 48.6 ± 4.3 d | −51.4 | 3.4 ± 1.5 d | −96.6% |
| ELL | 43.5 ± 2.2 d | −56.5 | 18.8 ± 3.6 cd | −81.2% |
| FER | 365.7 ± 16.1 ab | +265.7 | 72.5 ± 6.9 ab | - |
| ISO | 587.8 ± 3.9 a | +487.8 | 23.1 ± 1.9 bcd | −76.9 |
| PGA | 157.8 ± 17.2 bc | - | 31.4 ± 3.5 bc | −68.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrigo, D.; Bharti, S.; Mondin, M.; Raiola, A. Effect of Naturally Occurring Compounds on Fumonisin Production and fum Gene Expression in Fusarium verticillioides. Agronomy 2021, 11, 1060. https://doi.org/10.3390/agronomy11061060
Ferrigo D, Bharti S, Mondin M, Raiola A. Effect of Naturally Occurring Compounds on Fumonisin Production and fum Gene Expression in Fusarium verticillioides. Agronomy. 2021; 11(6):1060. https://doi.org/10.3390/agronomy11061060
Chicago/Turabian StyleFerrigo, Davide, Sharda Bharti, Massimiliano Mondin, and Alessandro Raiola. 2021. "Effect of Naturally Occurring Compounds on Fumonisin Production and fum Gene Expression in Fusarium verticillioides" Agronomy 11, no. 6: 1060. https://doi.org/10.3390/agronomy11061060
APA StyleFerrigo, D., Bharti, S., Mondin, M., & Raiola, A. (2021). Effect of Naturally Occurring Compounds on Fumonisin Production and fum Gene Expression in Fusarium verticillioides. Agronomy, 11(6), 1060. https://doi.org/10.3390/agronomy11061060






