Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extractions from Leaves
2.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Antioxidant Activity Assay
2.4. 2′-Azinobis (3-Ethylbenzothiazoline 6-Sulfonate) (ABTS) Antioxidant Activity Assay
2.5. Total Flavonoid Content (TFC)
2.6. Total Polyphenol Content (TPC)
2.7. Data Analysis
3. Results
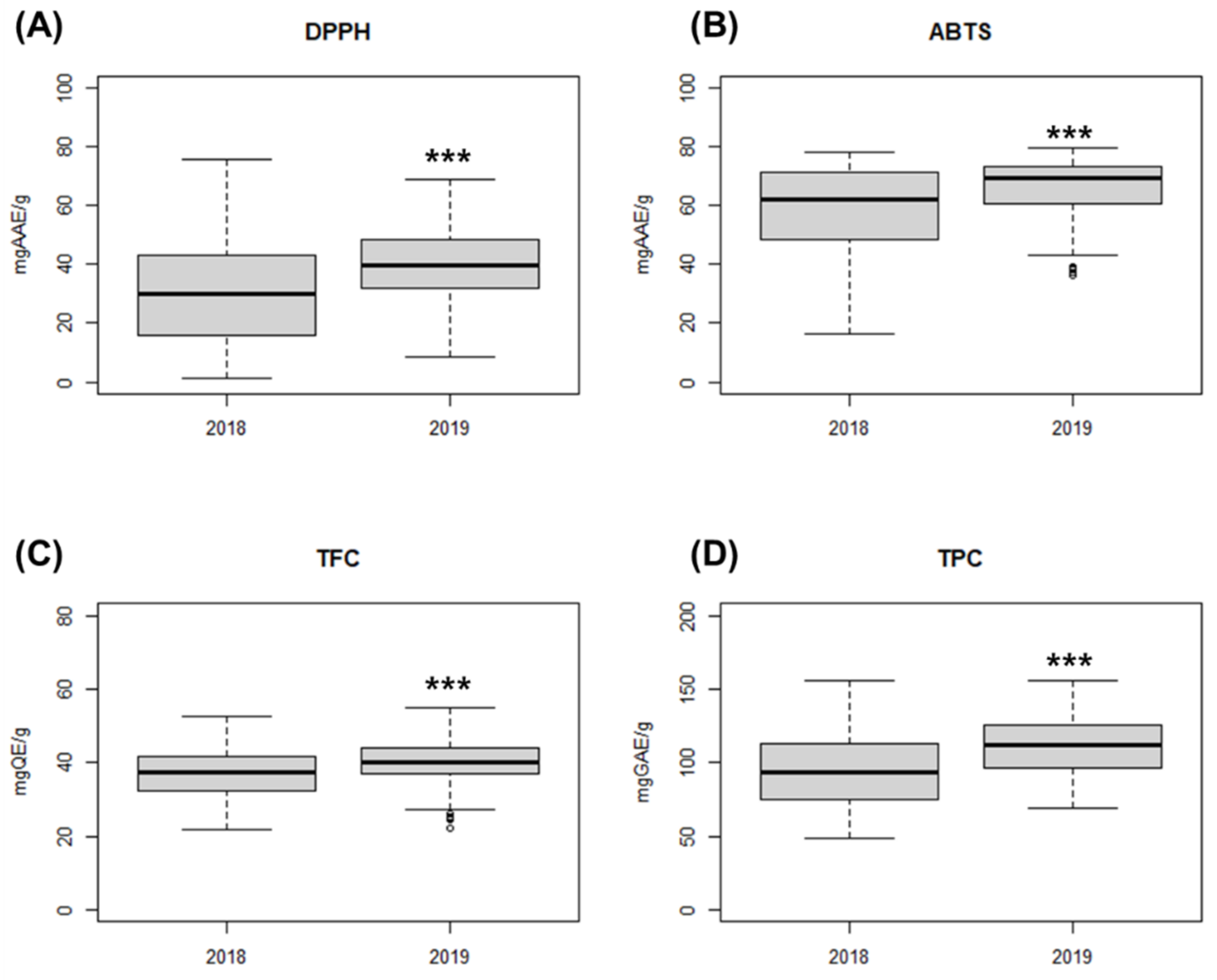
3.1. Antioxidant Activity and Phytochemical Content
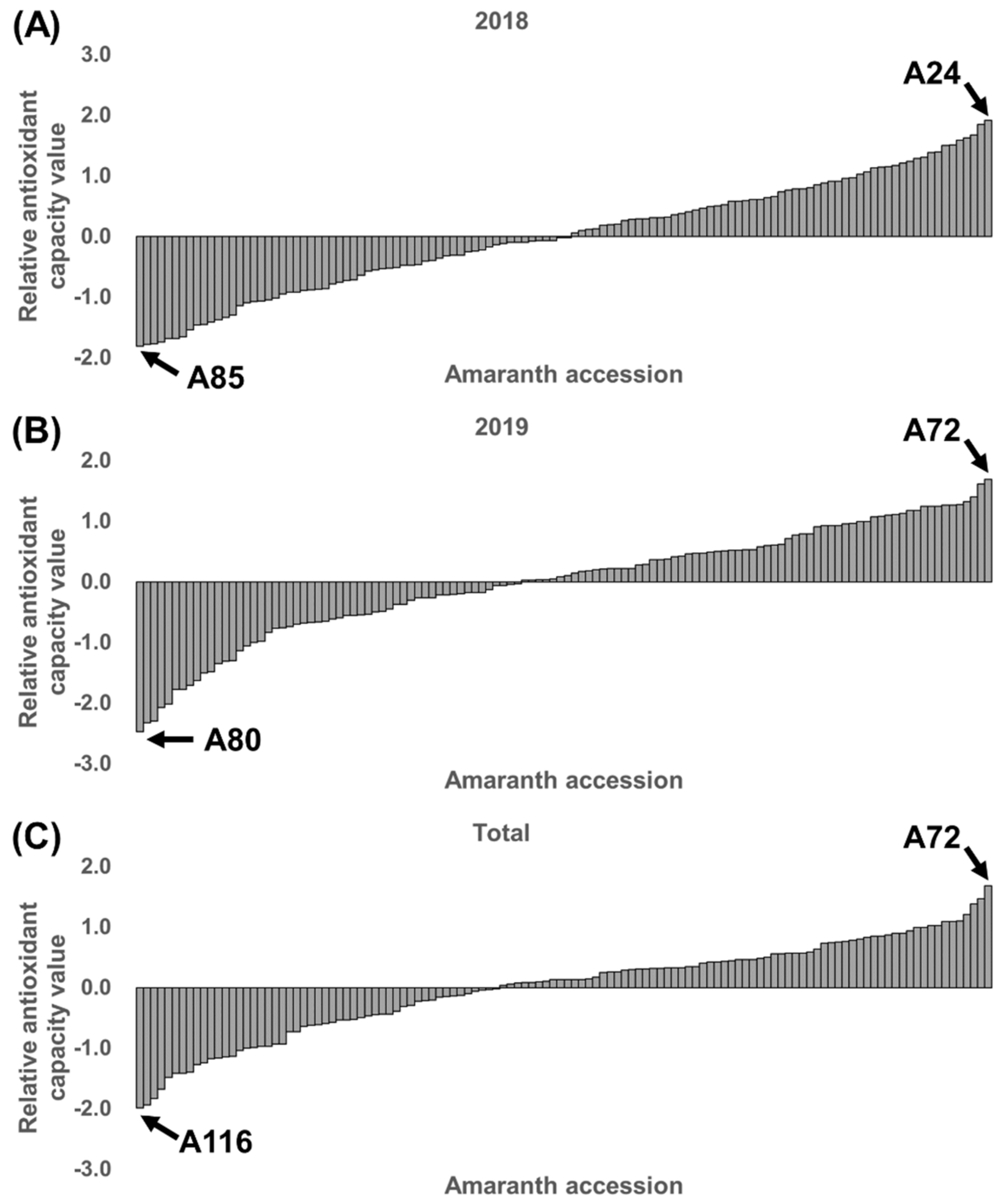
3.2. Relative Antioxidant Capacity Index
3.3. Antioxidant Activities in Amaranthus Species
3.4. Total Flavonoid Content and Total Polyphenol Content in Amaranthus Species
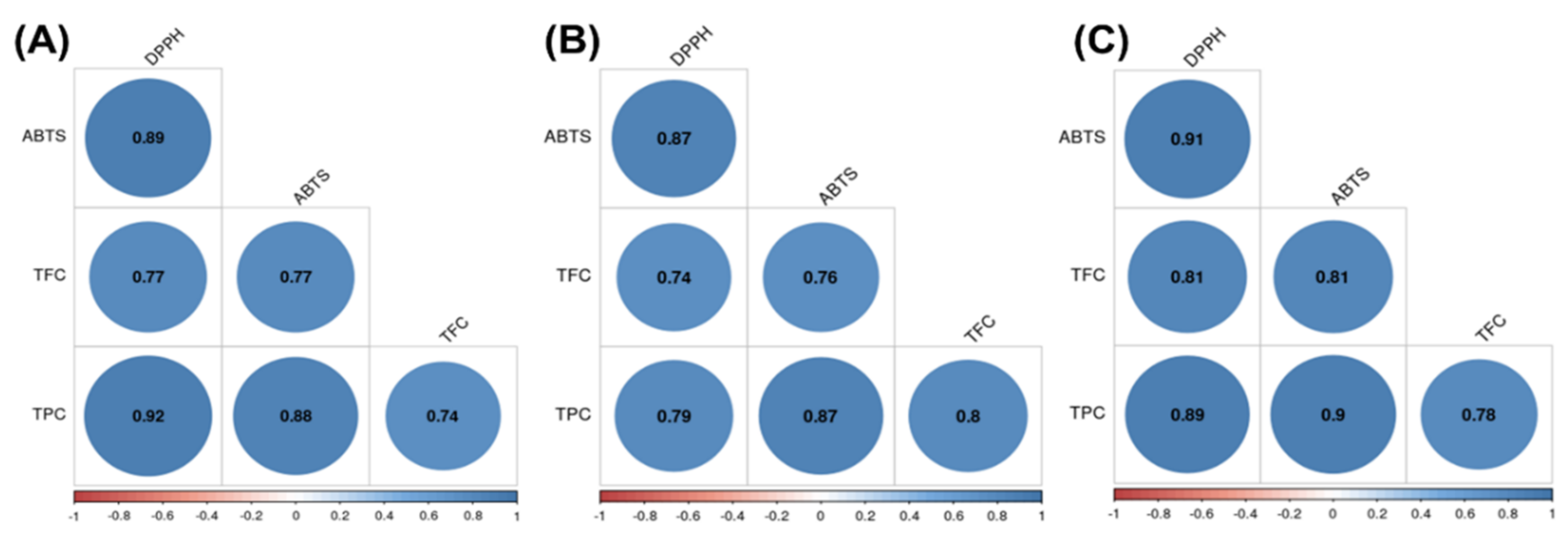
3.5. Correlations among Antioxidant Activities, Total Phenolic Content, and Total Flavonoid Content
3.6. Hierarchical Clustering Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rastogi, A.; Shukla, S. Amaranth: A New Millennium Crop of Nutraceutical Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Amaranths: The Crop of Great Prospect. In Amaranthus: A Promising Crop of Future; Springer: Singapore, 2016; pp. 13–48. [Google Scholar]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Pandey, A.C.; Mishra, B.K. Diversity in phenotypic and nutritional traits in vegetable amaranth (Amaranthus tricolor), a nutritionally underutilised crop. J. Sci. Food Agric. 2010, 90, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mejía, O.A.; Lopez-Malo, A.; Palou, E. Antioxidant capacity of extracts from amaranth Amaranthus hypochondriacus L. seeds or leaves. Ind. Crop. Prod. 2014, 53, 55–59. [Google Scholar] [CrossRef]
- Jin, Y.; Xuan, Y.; Chen, M.; Chen, J.; Jin, Y.; Piao, J.; Tao, J. Antioxidant, antiinfammatory and anticancer activities of Amaranthus viridis L. Extracts. Asian J. Chem. 2013, 25, 8901–8904. [Google Scholar] [CrossRef]
- Bulbul, I.J.; Nahar, L.; Ripa, F.A.; Haque, O. Antibacterial, cytotoxic and antioxidant activity of chloroform, n-hexane and ethyl acetate extract of plant Amaranthus spinosus. Int. J. PharmTech Res. 2011, 33, 1675–1680. [Google Scholar]
- Kumar, B.S.A.; Lakshman, K.; Jayaveera, K.N.; Shekar, D.S.; Kumar, A.A.; Manoj, B. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac. J. Trop. Med. 2010, 3, 702–706. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Hanif, S.; Iftkhar, T. Phytochemical profiling with antioxidant and antimicrobial screening of Amaranthus viridis L. Leaf and Seed Extracts. Open J. Med. Microbiol. 2013, 3, 16–171. [Google Scholar] [CrossRef]
- De Leon, N.; Jannink, J.-L.; Edwards, J.W.; Kaeppler, S.M. Introduction to a Special Issue on Genotype by Environment Interaction. Crop Sci. 2016, 56, 2081–2089. [Google Scholar] [CrossRef]
- Falconer, D.S. Selection for large and small size in mice. J. Genet. 1953, 51, 470–501. [Google Scholar] [CrossRef]
- Lee, K.J.; Baek, D.-Y.; Lee, G.-A.; Cho, G.-T.; So, Y.-S.; Lee, J.-R.; Ma, K.-H.; Chung, J.-W.; Hyun, D.Y. Phytochemicals and Antioxidant Activity of Korean Black Soybean (Glycine max L.) Landraces. Antioxidants 2020, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Bukhori, M.F.M.; Rahmat, M.H.; Rahmat, A. Assessment and comparison of phytochemical constituents and biological activities of bitter bean (Parkia speciosa Hassk.) collected from different locations in Malaysia. Chem. Cent. J. 2018, 12, 12. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Barba de la Rosa, A.P.; Fomsgaard, I.S.; Laursen, B.; Mortensen, A.G.; Olvera-Martínez, L.; Silva-Sánchez, C.; Mendoza-Herrera, A.; González-Castañeda, J.; De León-Rodríguez, A. Amaranth (Amaranthus hypochondriacus) as an alternative crop for sustainable food production: Phenolic acids and flavonoids with potential impact on its nutraceutical quality. J. Cereal Sci. 2009, 49, 117–121. [Google Scholar] [CrossRef]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Gowri, S.; Vasantha, K. Free radical scavenging and antioxidant activity of leaves from agathi (Sesbania grandiflora) (L.) Pers. Am. Eur. J. Sci. Res. 2010, 5, 114–119. [Google Scholar]
- Amarowicz, R.; Pegg, R.B. Legumes as a source of natural antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 865–878. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S.A. An Integrated Approach to Evaluate Food Antioxidant Capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.R.; Russo, D.; Cetera, P.; Faraone, I.; Lo Giudice, V.; Milella, L.; Todaro, L.; Sinisgalli, C.; Fritsch, C.; Dumarçay, S.; et al. Chemical analysis and antioxidant properties of orange-tree (Citrus sinensis L.) biomass extracts obtained via different extraction techniques. Biofuels Bioprod. Biorefin. 2020, 14, 509–520. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Tepe, B. Astragalus gymnolobus, A. leporinus var. hirsutus, and A. onobrychis: Phytochemical analysis and biological activity. Ind. Crop. Prod. 2020, 150, 112366. [Google Scholar] [CrossRef]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant Activity and Phenolic Composition of Amaranth (Amaranthus caudatus) during Plant Growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Ishtiaq, S.; Ahmad, M.; Hanif, U.; Akbar, S.; Kamran, S.H. Phytochemical and in-vitro antioxidant evaluation of different fractions of Amaranthus graecizan subsp. Silvestris Vill. Brenan. Asian Pac. J. Trop. Biomed. 2014, 412, 965–971. [Google Scholar] [CrossRef]
- Barku, V.; Yaw, O.-B.; Owusu-Ansah, E.; Mensah, A. Antioxidant activity and the estimation of total phenolic and flavonoid contents of the root extract of Amaranthus spinosus. Asian J. Plant Sci. Res. 2013, 3, 69–74. [Google Scholar]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Lee, K.J.; Ma, K.-H.; Cho, Y.-H.; Lee, J.-R.; Chung, J.-W.; Lee, G.-A. Phytochemical Distribution and Antioxidant Activities of Korean Adzuki Bean (Vigna angularis) Landraces. J. Crop Sci. Biotechnol. 2017, 20, 205–212. [Google Scholar] [CrossRef]
- Lee, K.J.; Shin, M.-J.; Cho, G.-T.; Lee, G.-A.; Ma, K.-H.; Chung, J.-W.; Lee, J.-R. Evaluation of Phytochemical econtents and antioxidant activity of Korean common bean (Phaseolus vulgaris L.) landraces. Korean Soc. Int. Agric. 2018, 30, 1–13. [Google Scholar] [CrossRef]
- Kim, E.H.; Song, H.K.; Park, Y.J.P.; Lee, J.R.; Kim, M.Y.; Chung, I.-M.C. Determination of Phenolic Compounds in Adzuki bean (Vigna angularis) Germplasm. Korean J. Crop Sci. 2011, 56, 375–384. [Google Scholar] [CrossRef][Green Version]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Lee, J.-R.; Ma, K.-H.; Cho, Y.-H.; Lee, G.-A.; Chung, J.-W. Anthocyanin and Isoflavone Contents in Korean Black Soybean Landraces and their Antioxidant Activities. Plant Breed Biotechnol. 2016, 4, 441–452. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Baraniak, B.; Karaś, M. Antioxidant activity of polyphenols of Adzuki bean (Vigna angularis) germinated in abiotic stress conditions. Acta Sci. Pol. Technol. Aliment. 2015, 14, 55–62. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org (accessed on 14 September 2020).
- Maiyo, Z.C.; Ngure, R.N.; Matasyoh, J.C.; Chepkorir, R. Phytochemical constituents and antimicrobial activity of leaf extract of three Amaranthus plant species. Afr. J. Biotechnol. 2010, 9, 3178–3182. [Google Scholar]
- Pasko, P.; Barton, H.; Fołta, M.; Gwiżdż, J. Evaluation of antioxidant activity of Amaranth Amaranthus cruentus grain and by-products flour, popping, cereal. Roczniki Państwowego Zakładu Higieny 2007, 581, 35–40. [Google Scholar] [PubMed]
- Tatiya, A.U.; Surana, S.J.; Khope, S.D.; Gokhale, S.B.; Sutar, M.P. Phytochemical investigation and immunomodulatory activity of Amaranthus spinosus linn. Indian J. Pharm. Educ. Res. 2007, 444, 337–341. [Google Scholar]
- Sarker, U.; Oba, S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci. Rep. 2019, 9, 20413. [Google Scholar] [CrossRef]
- Pamela, E.A.I.; Olufemi, T.A.; Yemisi, O.O.; Aduloju, O.A.; Usifo, G.A. Phytochemical Content and Antioxidant Activity of Five Grain Amaranth Species. Am. J. Food Sci. Technol. 2017, 5, 249–255. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH. Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Kainama, H.; Fatmawati, S.; Santoso, M.; Papilaya, P.M.; Ersam, T. The Relationship of Free Radical Scavenging and Total Phenolic and Flavonoid Contents of Garcinia lasoar PAM. Pharm. Chem. J. 2020, 53, 1151–1157. [Google Scholar] [CrossRef]
- Muzolf, M.; Szymusiak, H.; Swiglo, A.G.; Rietjens, I.M.C.M.; Tyrakowska, B. pH-dependent radical scavenging capacity of green tea catechins. J. Agric. Food Chem. 2008, 56, 816–823. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 274, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- De Mello Andrade, J.M.; Fasolo, D. Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 253–265, Chapter 20. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Korea Meteorological Administration. Available online: https://www.kma.go (accessed on 10 November 2020).
- Stracke, B.A.; Rüfer, C.E.; Weibel, F.P.; Bub, A.; Watzl, B. Three-Year Comparison of the Polyphenol Contents and Antioxidant Capacities in Organically and Conventionally Produced Apples (Malus domestica Bork. Cultivar ‘Golden Delicious’). J. Agric. Food Chem. 2009, 57, 4598–4605. [Google Scholar] [CrossRef]
- Kang, M. Genotype-Environment Interaction: Progress and Prospects. In Quantitative Genetics, Genomics and Plant Breeding; CABI: Wallingford, UK, 2002; pp. 221–243. [Google Scholar]
- Waters, A.J.; Makarevitch, I.; Noshay, J.; Burghardt, L.T.; Hirsch, C.N.; Hirsch, C.D.; Springer, N.M. Natural variation for gene expression responses to abiotic stress in maize. Plant J. 2017, 89, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.W. Eat, Drink and Be Healthy; Free Press: New York, NY, USA, 2001; p. 132. [Google Scholar]

| Genotype (G) | Year (Y) | Interaction (G × Y) | |
|---|---|---|---|
| DPPH | 11,376 *** | 185,186 *** | 6211 *** |
| ABTS | 1324.7 *** | 23,968.5 *** | 620.9 *** |
| TFC | 4253 *** | 24,469 *** | 1237 *** |
| TPC | 1725.3 *** | 49,921.6 *** | 876.4 *** |
| Year | Antioxidant Activity | Range | Mean 1 | CV (%) |
|---|---|---|---|---|
| 2018 | DPPH (mg AAE/g) | 1.1–75.2 | 30.8 ± 17.6 | 57.1 |
| ABTS (mg AAE/g) | 16.7–78.3 | 58.3 ± 15.1 | 25.9 | |
| TFC (mg QE/g) | 21.7–52.7 | 37.4 ± 6.6 | 17.6 | |
| TPC (mg GAE/g) | 48.9–154.4 | 94.1 ± 24.2 | 25.7 | |
| 2019 | DPPH (mg AAE/g) | 8.5–68.8 | 40.2 ± 13.8 | 34.3 |
| ABTS (mg AAE/g) | 36.6–79.2 | 66.4 ± 9.5 | 14.3 | |
| TFC (mg QE/g) | 22.3–54.7 | 39.8 ± 6.1 | 15.3 | |
| TPC (mg GAE/g) | 68.8–154.6 | 111.7 ± 19.4 | 17.4 |
| Year | Species | No. | DPPH (mg AAE/g) | ABTS (mg AAE/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Acc | Range | Mean ± SD | CV (%) | Range | Mean ± SD | CV (%) | ||
| 2018 | A. blitum | 3 | 23.5–43.7 | 36.3 ± 11.1 ab 1 | 30.6 | 62.7–74.2 | 69.9 ± 6.3 a | 9.0 |
| A. caudatus | 18 | 6.1–68.7 | 25.5 ± 16 ab | 63.1 | 26.9–77.8 | 51.2 ± 14.7 b | 28.7 | |
| A. crispus | 11 | 24.2–69.9 | 43.4 ± 11.9 ab | 27.4 | 56.4–74.6 | 67.7 ± 5.1 ab | 7.5 | |
| A. cruentus | 7 | 3.5–53.6 | 24.2 ± 17.8 b | 73.6 | 25.6–75.8 | 54.2 ± 18.3 ab | 33.8 | |
| A. dubius | 6 | 10.9–42.1 | 26.9 ± 15.3 ab | 56.9 | 44.2–74.8 | 59.7 ± 15.1 ab | 25.3 | |
| A. hybridus | 7 | 31.0–56.3 | 44.9 ± 10.4 a | 23.2 | 62.5–73.3 | 68.4 ± 4.5 a | 6.6 | |
| A. hypochondriacus | 31 | 6.0–65.6 | 30.1 ± 16.8 ab | 55.8 | 31.1–78.3 | 60.8 ± 13.1 ab | 21.5 | |
| A. tricolor | 30 | 1.1–75.2 | 27.6 ± 20.9 ab | 75.7 | 16.7–78.1 | 53.3 ± 17.9 ab | 33.6 | |
| A. viridis | 7 | 9.0–53.2 | 35.3 ± 15.4 ab | 43.6 | 28.7–74.0 | 60.9 ± 15.2 ab | 25.0 | |
| 2019 | A. blitum | 3 | 40.1–50.3 | 46.2 ± 5.4 b | 11.7 | 72.9–75.2 | 74.0 ± 1.15 ab | 1.6 |
| A. caudatus | 18 | 22.0–61.1 | 38.0 ± 9.5 bc | 25.1 | 49.2–78.1 | 62.8 ± 7.7 c | 12.3 | |
| A. crispus | 11 | 26.5–55.5 | 41.6 ± 8.5 b | 20.3 | 58.4–73.6 | 69.7 ± 4.5 abc | 6.5 | |
| A. cruentus | 7 | 29.9–43.9 | 36.6 ± 5.1 bc | 14.0 | 55–71.8 | 61.6 ± 6.1 c | 9.9 | |
| A. dubius | 6 | 60.1–68.8 | 64.4 ± 3.4 a | 5.3 | 77.5–79.2 | 78.4 ± 1.0 a | 0.9 | |
| A. hybridus | 7 | 33.7–66.5 | 45.5 ± 11.3 b | 24.8 | 68.9–78 | 73.5 ± 3.0 ab | 4.1 | |
| A. hypochondriacus | 31 | 19.6–67.4 | 45.5 ± 11.0 b | 24.2 | 45–77.3 | 68.1 ± 7.7 bc | 11.3 | |
| A. tricolor | 30 | 8.5–54.6 | 28.8 ± 12.8 c | 44.4 | 36.6–76.7 | 61.4 ± 11.6 c | 18.9 | |
| A. viridis | 7 | 10.3–65.6 | 44.5 ± 19.2 b | 43.1 | 43.7–77.4 | 69.1 ± 11.5 abc | 16.6 | |
| Year | Species | No. | TFC(mg QE/g) | TPC(mg GAE/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Acc | Range | Mean ± SD | CV (%) | Range | Mean ± SD | CV (%) | ||
| 2018 | A. blitum | 3 | 35.0–44.1 | 38.1 ± 5.2 ab 1 | 13.6 | 97.3–136.3 | 116.0 ± 19.5 a | 16.8 |
| A. caudatus | 18 | 21.7–46.8 | 34.9 ± 6.8 b | 19.4 | 56.2–135.2 | 84.1 ± 21.0 c | 25.0 | |
| A. crispus | 11 | 35.1–48.0 | 40.4 ± 4.2 ab | 10.3 | 96.2–137.6 | 117.0 ± 14.1 a | 12.1 | |
| A. cruentus | 7 | 27.5–47.1 | 37.1 ± 7.5 ab | 20.3 | 48.9–125.2 | 83.8 ± 28.3 c | 33.8 | |
| A. dubius | 6 | 28.7–46.1 | 36.4 ± 6.0 ab | 16.5 | 74.5–134.5 | 97.8 ± 24.6 abc | 25.2 | |
| A. hybridus | 7 | 34.4–52.7 | 42.3 ± 5.4 a | 12.8 | 103.3–126.1 | 113.0 ± 7.9 ab | 7.0 | |
| A. hypochondriacus | 31 | 23.9–51.1 | 39.3 ± 5.3 ab | 13.6 | 52.5–154.4 | 90.8 ± 25.0 bc | 27.5 | |
| A. tricolor | 30 | 24.9–49.9 | 34.7 ± 7.3 b | 21.1 | 53.5–146.4 | 89.0 ± 24.1 bc | 27.1 | |
| A. viridis | 7 | 23.6–44.1 | 37.4 ± 7.0 ab | 18.7 | 61.7–113.8 | 98.8 ± 18.1 abc | 18.3 | |
| 2019 | A. blitum | 3 | 41.7–47.6 | 44.2 ± 3.1 a | 6.9 | 128.1–138.6 | 134.0 ± 5.3 ab | 3.9 |
| A. caudatus | 18 | 25.4–44.4 | 37.8 ± 4.5 ab | 12.0 | 78.3–128.6 | 102.4 ± 12.4 de | 12.1 | |
| A. crispus | 11 | 36.1–51.4 | 42.9 ± 4.4 ab | 10.3 | 91.3–140.5 | 117.5 ± 12.8 bcde | 10.9 | |
| A. cruentus | 7 | 35.8–45.1 | 41.0 ± 3.4 ab | 8.4 | 90.4–120.8 | 100.1 ± 11.3 e | 11.3 | |
| A. dubius | 6 | 39.0–47.8 | 42.5 ± 3.1 ab | 7.4 | 132.2–152.6 | 141.9 ± 8.1 a | 5.7 | |
| A. hybridus | 7 | 34.4–50.2 | 42.2 ± 5.3 ab | 12.6 | 96.2–145.0 | 124.9 ± 17.7 abc | 14.2 | |
| A. hypochondriacus | 31 | 31.1–49.8 | 40.8 ± 4.4 ab | 10.7 | 79.7–139.8 | 111.5 ± 16.6 cde | 14.9 | |
| A. tricolor | 30 | 24.8–54.7 | 36.4 ± 7.9 b | 21.7 | 68.8–154.6 | 104.5 ± 21.5 de | 20.6 | |
| A. viridis | 7 | 22.3–49.3 | 42.6 ± 9.1 ab | 21.3 | 75.1–140.0 | 121.0 ± 21.5 bcd | 17.8 | |
| Year | Group | No. acc | DPPH (mg AAE/g) | ABTS (mg AAE/g) | TFC (mg/QE/g) | TPC (mg/GAE/g) |
|---|---|---|---|---|---|---|
| 2018 | I | 55 | 46.1 ± 12 a 1 | 70.8 ± 4.9 a | 41.7 ± 5.0 a | 115.4 ± 14.7 a |
| II | 42 | 21.1 ± 8.3 b | 53.5 ± 8.2 b | 36.5 ± 4.1 b | 80.1 ± 13.0 b | |
| III | 23 | 12.0 ± 8.4 c | 37.2 ± 12.5 c | 28.6 ± 3.4 c | 68.9 ± 12.8 c | |
| 2019 | I | 55 | 45.4 ± 11.1 a | 70.7 ± 6.3 a | 42.5 ± 4.5 a | 120.4 ± 15.5 a |
| II | 42 | 42.5 ± 11.5 a | 67.9 ± 6.2 a | 40.9 ± 4.1 a | 112.8 ± 17.3 b | |
| III | 23 | 23.7 ± 11.3 b | 53.6 ± 9.9 b | 31.5 ± 5.7 b | 88.9 ± 12.5 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, J.-H.; Lee, K.J.; Jeong, W.T.; Han, S.; Jo, I.-H.; Choi, S.H.; Cho, H.; Hyun, T.K.; Sung, J.; Lee, J.; et al. Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy 2021, 11, 1032. https://doi.org/10.3390/agronomy11061032
Bang J-H, Lee KJ, Jeong WT, Han S, Jo I-H, Choi SH, Cho H, Hyun TK, Sung J, Lee J, et al. Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy. 2021; 11(6):1032. https://doi.org/10.3390/agronomy11061032
Chicago/Turabian StyleBang, Jun-Hyoung, Kyung Jun Lee, Won Tea Jeong, Seahee Han, Ick-Hyun Jo, Seong Ho Choi, Hyunwoo Cho, Tae Kyung Hyun, Jeehye Sung, Junsoo Lee, and et al. 2021. "Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species" Agronomy 11, no. 6: 1032. https://doi.org/10.3390/agronomy11061032
APA StyleBang, J.-H., Lee, K. J., Jeong, W. T., Han, S., Jo, I.-H., Choi, S. H., Cho, H., Hyun, T. K., Sung, J., Lee, J., So, Y.-S., & Chung, J.-W. (2021). Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy, 11(6), 1032. https://doi.org/10.3390/agronomy11061032








