Comparison of the Concentration of Risk Elements in Alluvial Soils Determined by pXRF In Situ, in the Laboratory, and by ICP-OES
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experiment Description
2.3. Statistical Analysis
3. Results
3.1. Model for Arsen (As)
3.2. Model for Copper (Cu)
3.3. Model for Manganese (Mn)
3.4. Model for Nickel (Ni)
3.5. Model for Lead (Pb)
3.6. Model for Zinc (Zn)
3.7. Model Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, B.; Wu, Y.; Deng, D.; Tang, X.; Li, H. Effect of multiple factors on the adsorption of Cd in an alluvial soil from Xiba, China. J. Contam. Hydrol. 2020. [Google Scholar] [CrossRef]
- Li, C.; Sanchez, G.M.; Wu, Z.; Cheng, J.; Zhang, S.; Wang, Q.; Li, F.; Sun, G.; Meentemeyer, R.K. Spatiotemporal patterns and drivers of soil contamination with heavy metals during an intensive urbanization period (1989–2018) in southern China. Environ. Pollut. 2020, 260. [Google Scholar] [CrossRef]
- Lal, R. Soil organic matter content and crop yield. J. Soil Water Conserv. 2020, 75, 27A–32A. [Google Scholar] [CrossRef]
- Shokr, M.S.; El Baroudy, A.A.; Fullen, M.A.; El-Beshbeshy, T.R.; Ali, R.R.; Elhalim, A.; Guerra, A.J.T.; Jorge, M.C.O. Mapping of heavy metal contamination in alluvial soils of the Middle Nile Delta of Egypt. J. Environ. Eng. Landsc. Manag. 2016, 24, 218–231. [Google Scholar] [CrossRef]
- Bajčan, D.; Hegedusova, A.; Šnirc, M.; Trebichalský, P.; Jančo, I. The Impact of Former Mining Activities on the Contamination of Alluvial Soils in the Region Hont. In Proceedings of the 19th International Multidisciplinary Scientific GeoConference SGEM 2019, Albena, Bulgaria, 28 June–6 July 2019; pp. 499–506. [Google Scholar]
- Barman, M.; Datta, S.P.; Rattan, R.K.; Meena, M.C. Critical Limits of Deficiency of Nickel in Intensively Cultivated Alluvial Soils. J. Soil Sci. Plant Nutr. 2020, 20, 284–292. [Google Scholar] [CrossRef]
- Adimalla, N. Heavy metals pollution assessment and its associated human health risk evaluation of urban soils from Indian cities: A review. Environ. Geochem. Health 2020, 42, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Karande, U.B.; Kadam, A.; Umrikar, B.N.; Wagh, V.; Sankhua, R.N.; Pawar, N.J. Environmental modelling of soil quality, heavy-metal enrichment and human health risk in sub-urbanized semiarid watershed of western India. Model. Earth Syst. Environ. 2020, 6, 545–556. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, A.; Mehra, R.; Kaur, I. Quantitative assessment of exposure of heavy metals in groundwater and soil on human health in Reasi district, Jammu and Kashmir. Environ. Geochem. Health 2020, 42, 77–94. [Google Scholar] [CrossRef]
- Rostami, S.; Kamani, H.; Shahsavani, S.; Hoseini, M. Environmental monitoring and ecological risk assessment of heavy metals in farmland soils: Ecological risk assessment of heavy metals in Kamfiruz district. Hum. Ecol. Risk Assess. 2020, 1–13. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Antoniadis, V.; Kwon, E.; Song, H.; Wang, S.L.; Hseu, Z.Y.; Rinklebe, J. Soil contamination by potentially toxic elements and the associated human health risk in geo- and anthropogenic contaminated soils: A case study from the temperate region (Germany) and the arid region (Egypt). Environ. Pollut. 2020, 262, 114212. [Google Scholar] [CrossRef]
- Fazekašová, D.; Fazekaš, J. Soil Quality and Heavy Metal Pollution Assessment of Iron Ore Mines in Nizna Slana (Slovakia). Sustainability 2020, 12, 2549. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. (Eds.) Methods of Soil Analysis; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; ISBN 9780891188667. [Google Scholar]
- Bettinelli, M.; Beone, G.; Spezia, S.; Baffi, C. Determination of heavy metals in soils and sediments by microwave-assisted digestion and inductively coupled plasma optical emission spectrometry analysis. Anal. Chim. Acta 2000, 424, 289–296. [Google Scholar] [CrossRef]
- Hu, W.; Huang, B.; Weindorf, D.C.; Chen, Y. Metals analysis of agricultural soils via portable X-ray fluorescence spectrometry. Bull. Environ. Contam. Toxicol. 2014, 92, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.G.; Ferrini, M.; Manni, A. Artificial neural networks to evaluate organic and inorganic contamination in agricultural soils. Chemosphere 2017, 186, 124–131. [Google Scholar] [CrossRef]
- Al Maliki, A.; Al-lami, A.K.; Hussain, H.M.; Al-Ansari, N. Comparison between inductively coupled plasma and X-ray fluorescence performance for Pb analysis in environmental soil samples. Environ. Earth Sci. 2017, 76, 433. [Google Scholar] [CrossRef]
- Wang, B.; Yu, J.; Huang, B.; Hu, W.; Chang, Q. Fast Monitoring Soil Environmental Qualities of Heavy Metal by Portable X-ray Fluorescence Spectrometer. Spectrosc. Spectr. Anal. 2015, 35, 1735–1740. [Google Scholar] [CrossRef]
- Rouillon, M.; Taylor, M.P. Can field portable X-ray fluorescence (pXRF) produce high quality data for application in environmental contamination research? Environ. Pollut. 2016, 214, 255–264. [Google Scholar] [CrossRef]
- Konstantinova, E.; Minkina, T.; Nevidomskaya, D.; Mandzhieva, S.; Bauer, T.; Zamulina, I.; Burachevskaya, M.; Sushkova, S. Exchangeable form of potentially toxic elements in floodplain soils along the river-marine systems of Southern Russia. Eurasian J. Soil Sci. 2021, 10, 132–141. [Google Scholar] [CrossRef]
- Hu, B.; Chen, S.; Hu, J.; Xia, F.; Xu, J.; Li, Y.; Shi, Z. Application of portable XRF and VNIR sensors for rapid assessment of soil heavy metal pollution. PLoS ONE 2017, 12, e0172438. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.M.W.; Speakman, R.J. Portable XRF analysis of archaeological sediments and ceramics. J. Archaeol. Sci. 2015, 53, 626–638. [Google Scholar] [CrossRef]
- Rincheval, M.; Cohen, D.R.; Hemmings, F.A. Biogeochemical mapping of metal contamination from mine tailings using field-portable XRF. Sci. Total Environ. 2019, 662, 404–413. [Google Scholar] [CrossRef]
- McIntosh, K.G.; Guimarães, D.; Cusack, M.J.; Vershinin, A.; Chen, Z.W.; Yang, K.; Parsons, P.J. Evaluation of portable XRF instrumentation for assessing potential environmental exposure to toxic elements. Int. J. Environ. Anal. Chem. 2016, 96, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Melquiades, F.L.; Appoloni, C.R. Application of XRF and field portable XRF for environmental analysis. J. Radioanal. Nucl. Chem. 2004, 262, 533–541. [Google Scholar] [CrossRef]
- Peinado, F.M.; Ruano, S.M.; González, M.G.B.; Molina, C.E. A rapid field procedure for screening trace elements in polluted soil using portable X-ray fluorescence (PXRF). Geoderma 2010, 159, 76–82. [Google Scholar] [CrossRef]
- Kodom, K.; Preko, K.; Boamah, D. X-ray Fluorescence (XRF) Analysis of Soil Heavy Metal Pollution from an Industrial Area in Kumasi, Ghana. Soil Sediment Contam. 2012, 21, 1006–1021. [Google Scholar] [CrossRef]
- Parsons, C.; Margui Grabulosa, E.; Pili, E.; Floor, G.H.; Roman-Ross, G.; Charlet, L. Quantification of trace arsenic in soils by field-portable X-ray fluorescence spectrometry: Considerations for sample preparation and measurement conditions. J. Hazard. Mater. 2013, 262, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ. Int. 2019, 126, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Paveley, C.F.; Davies, B.E.; Jones, K. Comparison of results obtained by X-ray fluorescence of the total soil and the atomic absorption spectrometry assay of an acid digest in the routine determination of lead and zinc in soils. Commun. Soil Sci. Plant Anal. 1988, 19, 107–116. [Google Scholar] [CrossRef]
- Jenkins, R. X-ray Fluorescence Spectrometry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1999; ISBN 978-1-118-52104-5. [Google Scholar]
- Wan, M.; Hu, W.; Qu, M.; Tian, K.; Zhang, H.; Wang, Y.; Huang, B. Application of arc emission spectrometry and portable X-ray fluorescence spectrometry to rapid risk assessment of heavy metals in agricultural soils. Ecol. Indic. 2019, 101, 583–594. [Google Scholar] [CrossRef]
- Lilli, M.A.; Moraetis, D.; Nikolaidis, N.P.; Karatzas, G.P.; Kalogerakis, N. Characterization and mobility of geogenic chromium in soils and river bed sediments of Asopos basin. J. Hazard. Mater. 2015, 281, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, S.; Cai, B.; Zhang, M.; Qu, C. Application of portable X-ray fluorescence spectrometry in environmental investigation of heavy metal-contaminated sites and comparison with laboratory analysis. IOP Conf. Ser. Earth Environ. Sci. 2018, 121, 10. [Google Scholar] [CrossRef]
- Horta, A.; Malone, B.; Stockmann, U.; Minasny, B.; Bishop, T.F.A.; McBratney, A.B.; Pallasser, R.; Pozza, L. Potential of integrated field spectroscopy and spatial analysis for enhanced assessment of soil contamination: A prospective review. Geoderma 2015, 241–242, 180–209. [Google Scholar] [CrossRef]
- Sahraoui, H.; Hachicha, M. Effect of soil moisture on trace elements concentrations. J. Fundam. Appl. Sci. 2017, 9, 468–484. [Google Scholar] [CrossRef]
- Padilla, J.T.; Hormes, J.; Magdi Selim, H. Use of portable XRF: Effect of thickness and antecedent moisture of soils on measured concentration of trace elements. Geoderma 2019, 337, 143–149. [Google Scholar] [CrossRef]
- Stockmann, U.; Cattle, S.R.; Minasny, B.; McBratney, A.B. Utilizing portable X-ray fluorescence spectrometry for in-field investigation of pedogenesis. Catena 2016, 139, 220–231. [Google Scholar] [CrossRef]
- U.S. EPA. Method 6200: Field Portable X-ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Menšík, L.; Hlisnikovský, L.; Holík, L.; Nerušil, P.; Kunzová, E. Possibilities of determination of risk elements in alluvial agriculture soils in the MŽE and otava river basins by X-ray fluorescence spectrometry. Agriculture 2020, 66, 15–23. [Google Scholar] [CrossRef]
- Laiho, J.; Perämäki, P. Evaluation of portable X-ray fluorescence (PXRF) sample preparation methods. Spec. Pap. Geol. Surv. Finl. 2005, 73–82. [Google Scholar]
- Stockmann, U.; Jang, H.J.; Minasny, B.; McBratney, A.B. The Effect of Soil Moisture and Texture on Fe Concentration Using Portable X-ray Fluorescence Spectrometers. In Digital Soil Morphometrics; Springer: Cham, Switzerland, 2016; pp. 63–71. [Google Scholar] [CrossRef]
- Hangen, E.; Čermák, P.; Geuß, U.; Hlisnikovský, L. Information depth of elements affects accuracy of parallel pXRF in situ measurements of soils. Environ. Monit. Assess. 2019, 191. [Google Scholar] [CrossRef]
- Radu, T.; Diamond, D. Comparison of soil pollution concentrations determined using AAS and portable XRF techniques. J. Hazard. Mater. 2009, 171, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- McComb, J.Q.; Rogers, C.; Han, F.X.; Tchounwou, P.B. Rapid screening of heavy metals and trace elements in environmental samples using portable X-ray fluorescence spectrometer, A comparative study. Water Air Soil Pollut. 2014, 225. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, Y.; Suh, J.; Lee, S.H. Mapping copper and lead concentrations at abandoned mine areas using element analysis data from ICP-AES and portable XRF instruments: A comparative study. Int. J. Environ. Res. Public Health 2016, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.R.; Cancès, B.; Breton, C.; Ponthieu, M.; Morvan, X.; Conreux, A.; Marin, B. Comparison of field portable XRF and aqua regia/ICPAES soil analysis and evaluation of soil moisture influence on FPXRF results. J. Soils Sediments 2016, 16, 438–448. [Google Scholar] [CrossRef]
- Caporale, A.G.; Adamo, P.; Capozzi, F.; Langella, G.; Terribile, F.; Vingiani, S. Monitoring metal pollution in soils using portable-XRF and conventional laboratory-based techniques: Evaluation of the performance and limitations according to metal properties and sources. Sci. Total Environ. 2018, 643, 516–526. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO (Food and Agriculture Organization of the United Nations): Rome, Italy, 2015. [Google Scholar]
- Zbíral, J. Soil Analysis. Standard Working Procedures; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2011; ISBN 9788074010408. [Google Scholar]
- Melou, M.; Militký, J. Statistical Data Analysis, a Practical Guide with 1250 Exercises and Answer Key on CD; Woodhead Publishing: New Delhi, India, 2012; ISBN 9780857091093. [Google Scholar]
- Hess, E.; Splichal, P. Environmental Technology Verification Report: Field Portable X-ray Fluorescence Analyzer: Scitec MAP Spectrum Analyzer (EPA 600-R-97-147; NERL-LV-98-012; PB2001101792); U.S. Environmental Protection Agency: Washington, DC, USA, 1998.
- Messager, M.L.; Davies, I.P.; Levin, P.S. Development and validation of in-situ and laboratory X-ray fluorescence (XRF) spectroscopy methods for moss biomonitoring of metal pollution. MethodsX 2021, 8, 101319. [Google Scholar] [CrossRef]
- Qu, M.; Chen, J.; Li, W.; Zhang, C.; Wan, M.; Huang, B.; Zhao, Y. Correction of in-situ portable X-ray fluorescence (PXRF) data of soil heavy metal for enhancing spatial prediction. Environ. Pollut. 2019, 254, 112993. [Google Scholar] [CrossRef]
- Ran, J.; Wang, D.; Wang, C.; Zhang, G.; Yao, L. Using portable X-ray fluorescence spectrometry and GIS to assess environmental risk and identify sources of trace metals in soils of peri-urban areas in the Yangtze Delta region, China. Environ. Sci. Process. Impacts 2014, 16, 1870–1877. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, Z.; Cheng, Q.; Zhang, Z.; Yang, J. Rapid in situ determination of heavy metal concentrations in polluted water via portable XRF: Using Cu and Pb as example. Environ. Pollut. 2018, 243, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Chen, J.; Huang, B.; Zhao, Y. Resampling with in situ field portable X-ray fluorescence spectrometry (FPXRF) to reduce the uncertainty in delineating the remediation area of soil heavy metals. Environ. Pollut. 2021, 271, 116310. [Google Scholar] [CrossRef]
- Kennedy, S.A.; Kelloway, S.J. Heavy Metals in Archaeological Soils. Adv. Archaeol. Pract. 2021, 1–15. [Google Scholar] [CrossRef]
- Adler, K.; Piikki, K.; Söderström, M.; Eriksson, J.; Alshihabi, O. Predictions of Cu, Zn, and Cd Concentrations in Soil Using Portable X-ray Fluorescence Measurements. Sensors 2020, 20, 474. [Google Scholar] [CrossRef]
- Kebonye, N.M.; John, K.; Chakraborty, S.; Agyeman, P.C.; Ahado, S.K.; Eze, P.N.; Němeček, K.; Drábek, O.; Borůvka, L. Comparison of multivariate methods for arsenic estimation and mapping in floodplain soil via portable X-ray fluorescence spectroscopy. Geoderma 2021, 384, 114792. [Google Scholar] [CrossRef]
- Boruvka, L.; Vacha, R. Litavka River Alluvium as a Model Area Heavily Polluted with Potentially Risk Elements. In Phytoremediation of Metal-Contaminated Soils; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 267–298. [Google Scholar]
- Shuttleworth, E.L.; Evans, M.G.; Hutchinson, S.M.; Rothwell, J.J. Assessment of lead contamination in peatlands using field portable XRF. Water Air. Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Havukainen, J.; Hiltunen, J.; Puro, L.; Horttanainen, M. Applicability of a field portable X-ray fluorescence for analyzing elemental concentration of waste samples. Waste Manag. 2019, 83, 6–13. [Google Scholar] [CrossRef]
- Kim, S.M.; Choi, Y. Assessing statistically significant heavy-metal concentrations in abandoned mine areas via hot spot analysis of portable XRF data. Int. J. Environ. Res. Public Health 2017, 14, 16. [Google Scholar] [CrossRef]
- Nawar, S.; Delbecque, N.; Declercq, Y.; De Smedt, P.; Finke, P.; Verdoodt, A.; Van Meirvenne, M.; Mouazen, A.M. Can spectral analyses improve measurement of key soil fertility parameters with X-ray fluorescence spectrometry? Geoderma 2019, 350, 29–39. [Google Scholar] [CrossRef]
- Andrade, R.; Faria, W.M.; Silva, S.H.G.; Chakraborty, S.; Weindorf, D.C.; Mesquita, L.F.; Guilherme, L.R.G.; Curi, N. Prediction of soil fertility via portable X-ray fluorescence (pXRF) spectrometry and soil texture in the Brazilian Coastal Plains. Geoderma 2020, 357, 113960. [Google Scholar] [CrossRef]
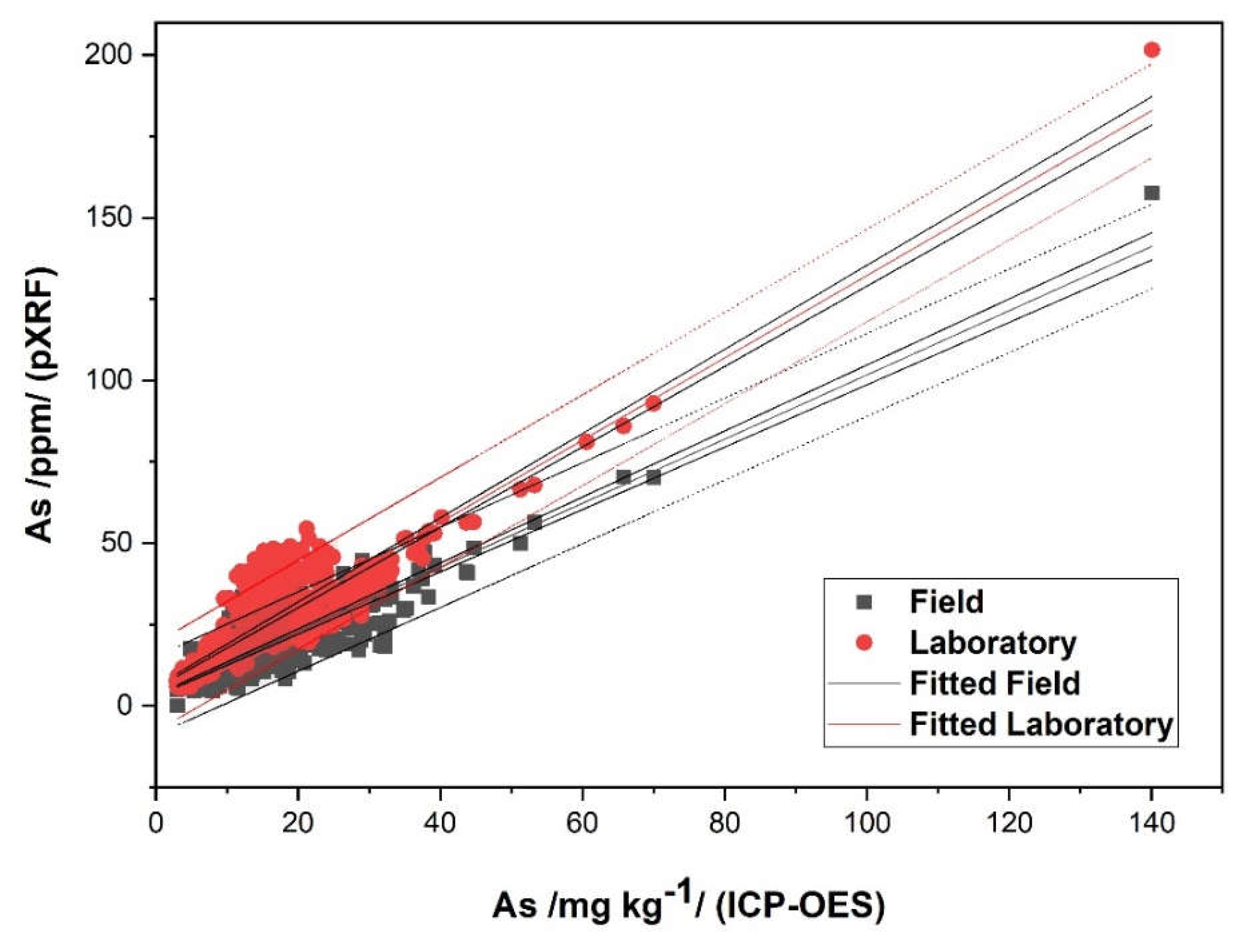



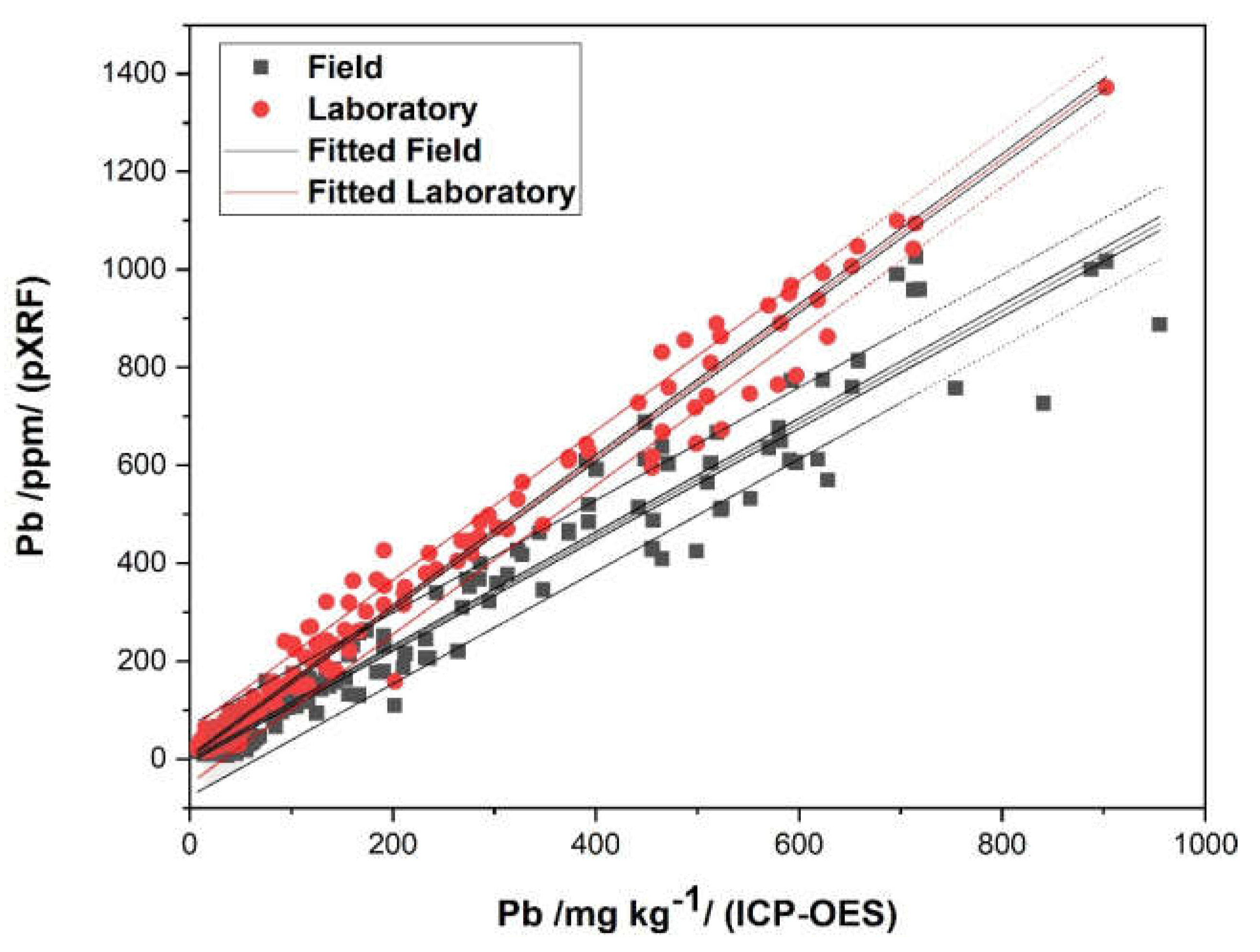
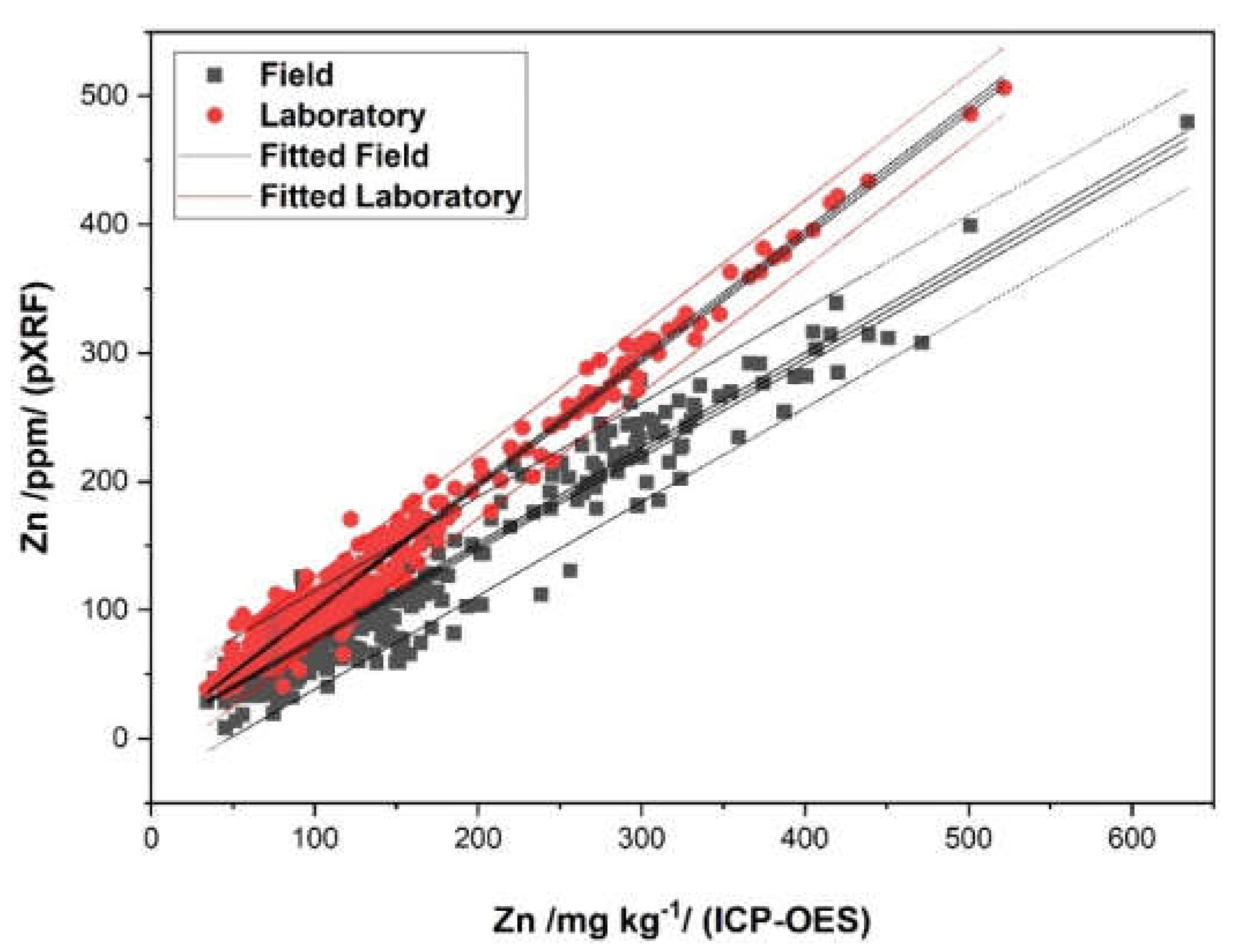
| As (pXRF) FIELD | As (pXRF) LABORATORY | Cu (pXRF) FIELD | Cu (pXRF) LABORATORY | |
| Intercept | 3.138 ± 0.584 | 5.751 ± 0.607 | 17.151 ± 0.914 | 6.970 ± 0.778 |
| Slope | 0.986 ± 0.029 | 1.265 ± 0.031 | 0.384 ± 0.035 | 1.007 ± 0.027 |
| Residual Sum of Squares | 16,463.964 | 22,110.301 | 7056.466 | 21,571.303 |
| Pearson’s r | 0.854 | 0.887 | 0.563 | 0.898 |
| R2 | 0.729 | 0.786 | 0.317 | 0.807 |
| MEP | 39.129 | 49.248 | 27.125 | 0.561 |
| AIC | 1576.698 | 1782.510 | 871.438 | 85.573 |
| Mn (pXRF) FIELD | Mn (pXRF) LABORATORY | Ni (pXRF) FIELD | Ni (pXRF) LABORATORY | |
| Intercept | 62.733 ± 15.679 | 93.056 ± 17.722 | 49.910 ± 5.468 | 22.462 ± 1.224 |
| Slope | 0.687 ± 0.024 | 1.139 ± 0.027 | −0.290 ± 0.244 | 1.713 ± 0.070 |
| Residual Sum of Squares | 5,974,539.238 | 1.09716 × 10−7 | 40,933.997 | 22,037.307 |
| Pearson’s r | 0.799 | 0.892 | −0.100 | 0.816 |
| R2 | 0.638 | 0.796 | 0.010 | 0.666 |
| MEP | 13,375.954 | 24,382.635 | - | 73.516 |
| AIC | 4276.196 | 4808.1535 | - | 1302.888 |
| Pb (pXRF) FIELD | Pb (pXRF) LABORATORY | Zn (pXRF) FIELD | Zn (pXRF) LABORATORY | |
| Intercept | −3.697 ± 1.918 | 4.431 ± 1.477 | 3.554 ± 1.567 | 2.028 ± 1.118 |
| Slope | 1.149 ± 0.010 | 1.527 ± 0.009 | 0.731 ± 0.010 | 0.977 ± 0.008 |
| Residual Sum of Squares | 660,329.315 | 382,461.348 | 180,251.141 | 80,459.575 |
| Pearson’s r | 0.981 | 0.992 | 0.960 | 0.986 |
| R2 | 0.963 | 0.984 | 0.922 | 0.973 |
| MEP | 1408.789 | 809.513 | 380.689 | 173.113 |
| AIC | 3528.776 | 3233.392 | 2839.733 | 2412.816 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menšík, L.; Hlisnikovský, L.; Nerušil, P.; Kunzová, E. Comparison of the Concentration of Risk Elements in Alluvial Soils Determined by pXRF In Situ, in the Laboratory, and by ICP-OES. Agronomy 2021, 11, 938. https://doi.org/10.3390/agronomy11050938
Menšík L, Hlisnikovský L, Nerušil P, Kunzová E. Comparison of the Concentration of Risk Elements in Alluvial Soils Determined by pXRF In Situ, in the Laboratory, and by ICP-OES. Agronomy. 2021; 11(5):938. https://doi.org/10.3390/agronomy11050938
Chicago/Turabian StyleMenšík, Ladislav, Lukáš Hlisnikovský, Pavel Nerušil, and Eva Kunzová. 2021. "Comparison of the Concentration of Risk Elements in Alluvial Soils Determined by pXRF In Situ, in the Laboratory, and by ICP-OES" Agronomy 11, no. 5: 938. https://doi.org/10.3390/agronomy11050938
APA StyleMenšík, L., Hlisnikovský, L., Nerušil, P., & Kunzová, E. (2021). Comparison of the Concentration of Risk Elements in Alluvial Soils Determined by pXRF In Situ, in the Laboratory, and by ICP-OES. Agronomy, 11(5), 938. https://doi.org/10.3390/agronomy11050938







