Diverse Physiological and Physical Responses among Wild, Landrace and Elite Barley Varieties Point to Novel Breeding Opportunities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Infra-Red Gas Exchange Measurements of Photosynthesis.
2.3. Stomatal Kinetic Responses
2.4. Stomatal Anatomical Characteristics
2.5. Growth Measurements
2.6. Statistical Analyses
3. Results
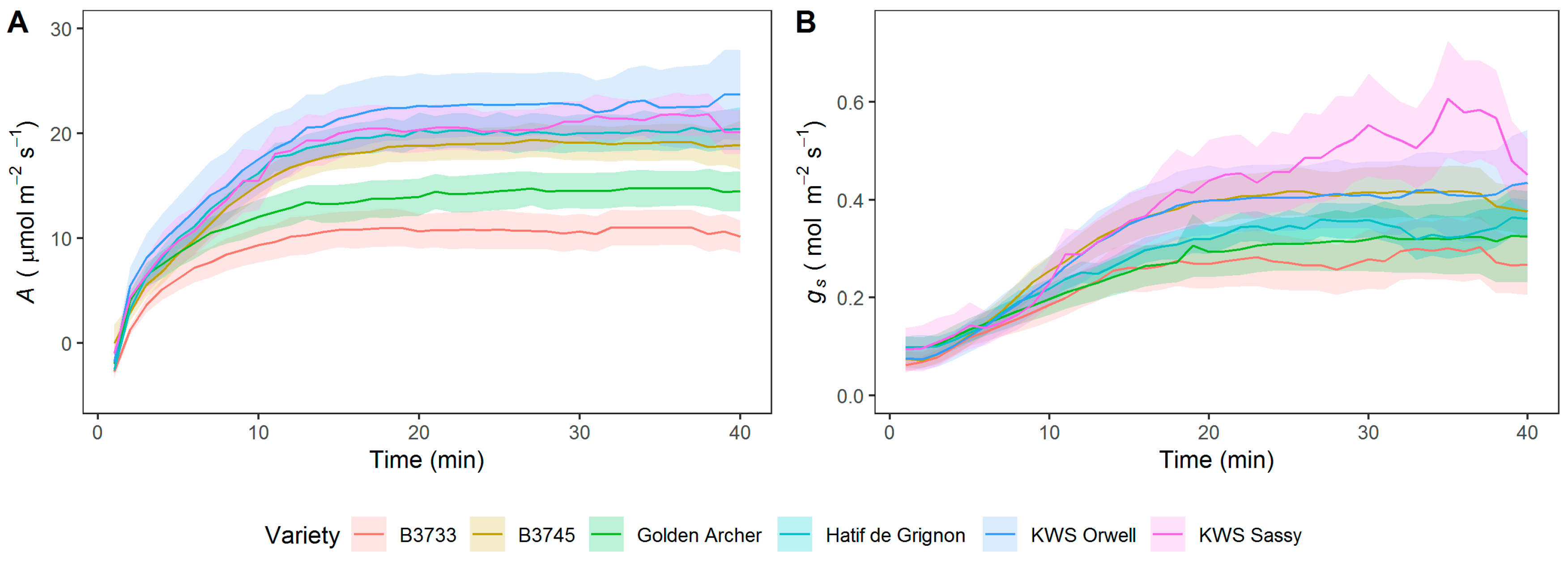
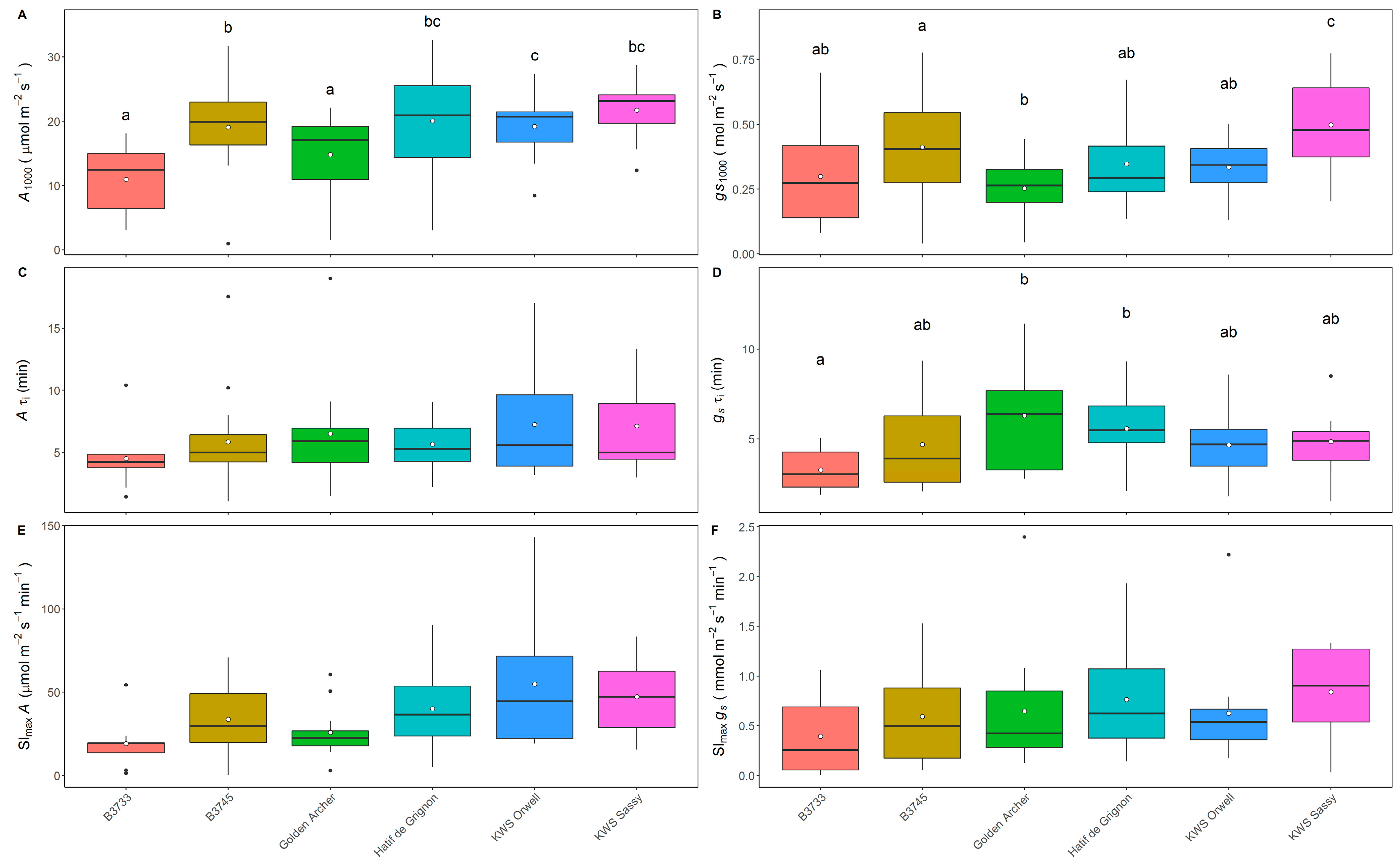
3.1. Analysis of Steady-State and Kinetics of gs and A in Field-Grown Varieties
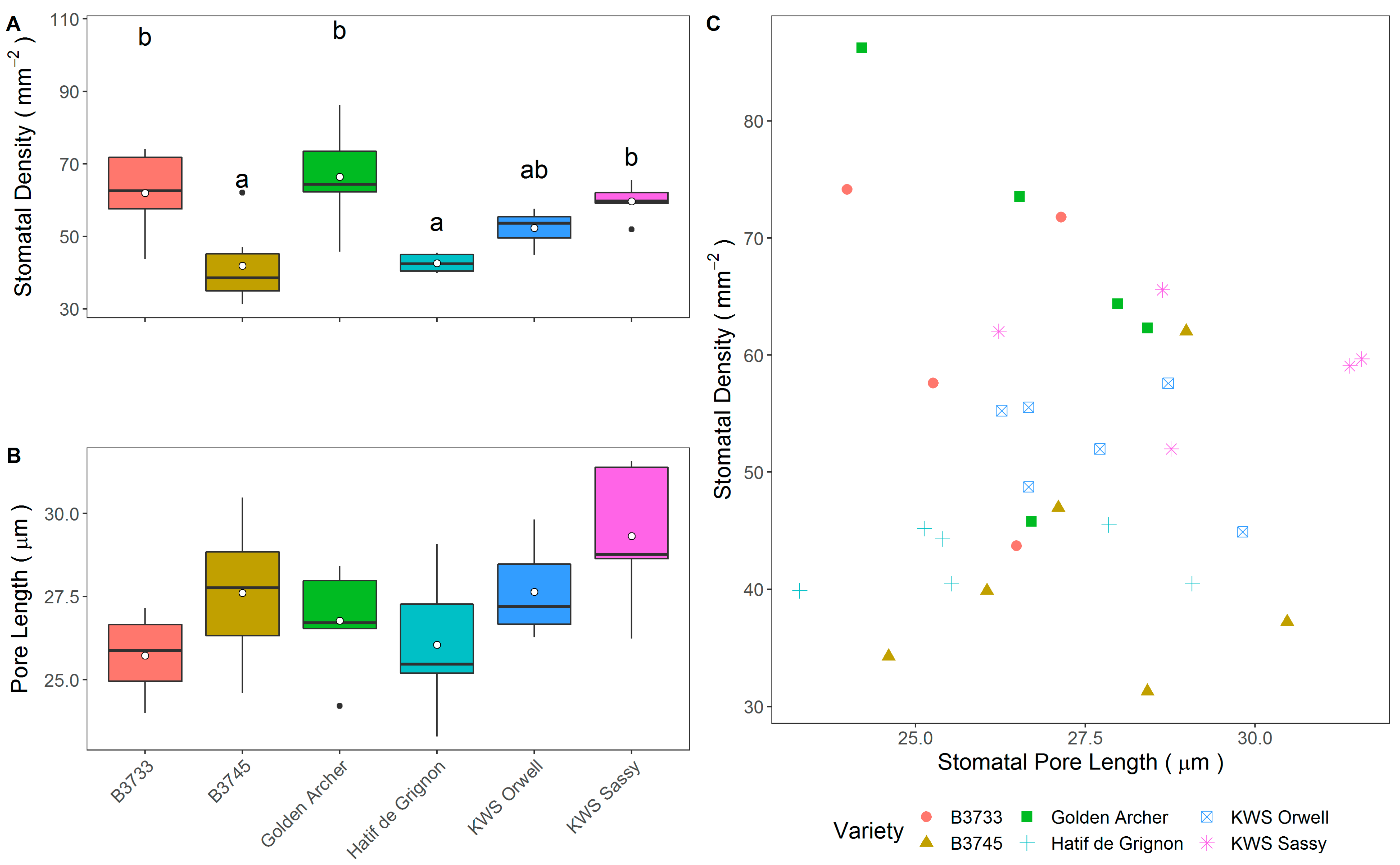
3.2. Field Trial Stomatal Anatomy
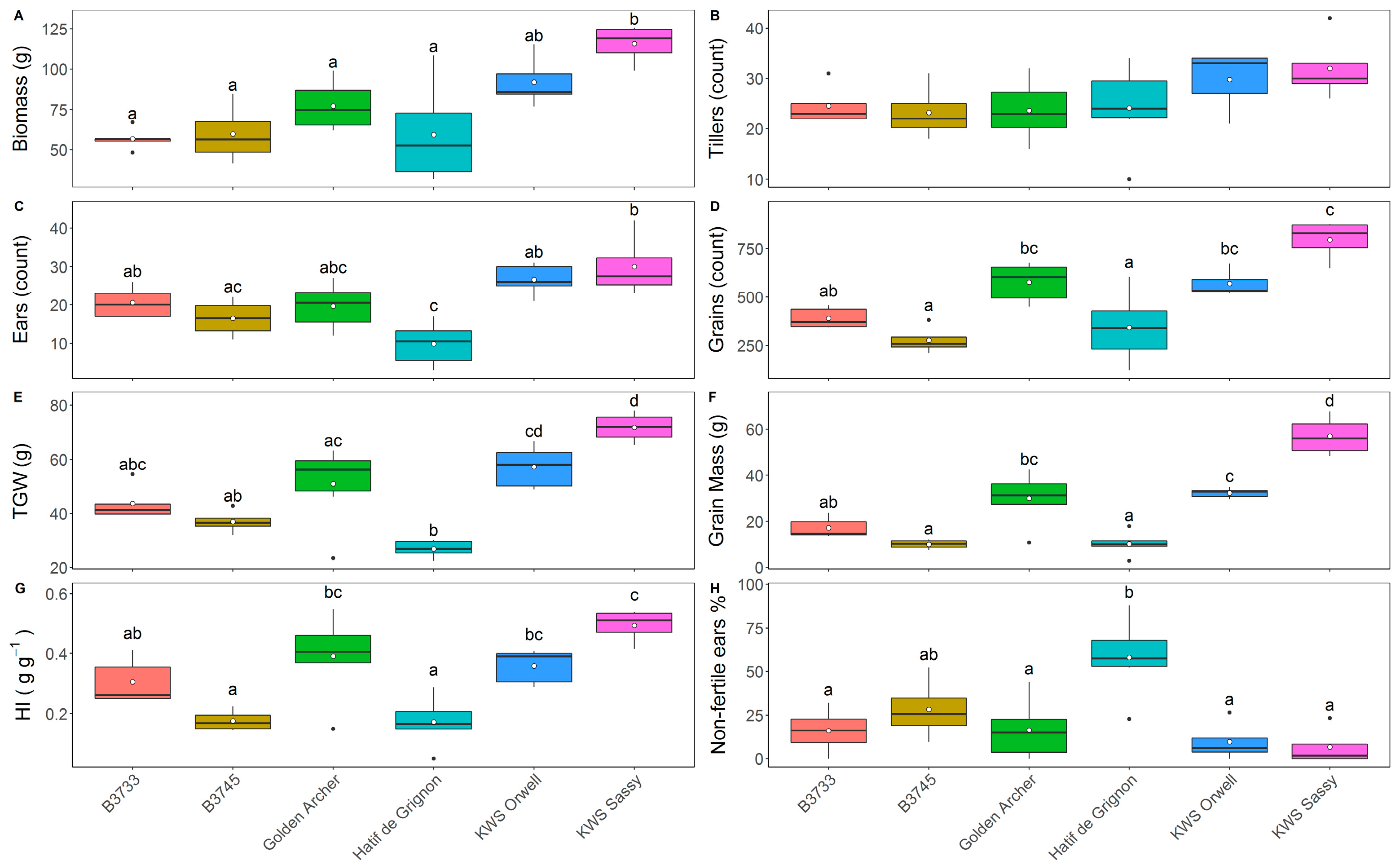
3.3. Field Trial Harvest
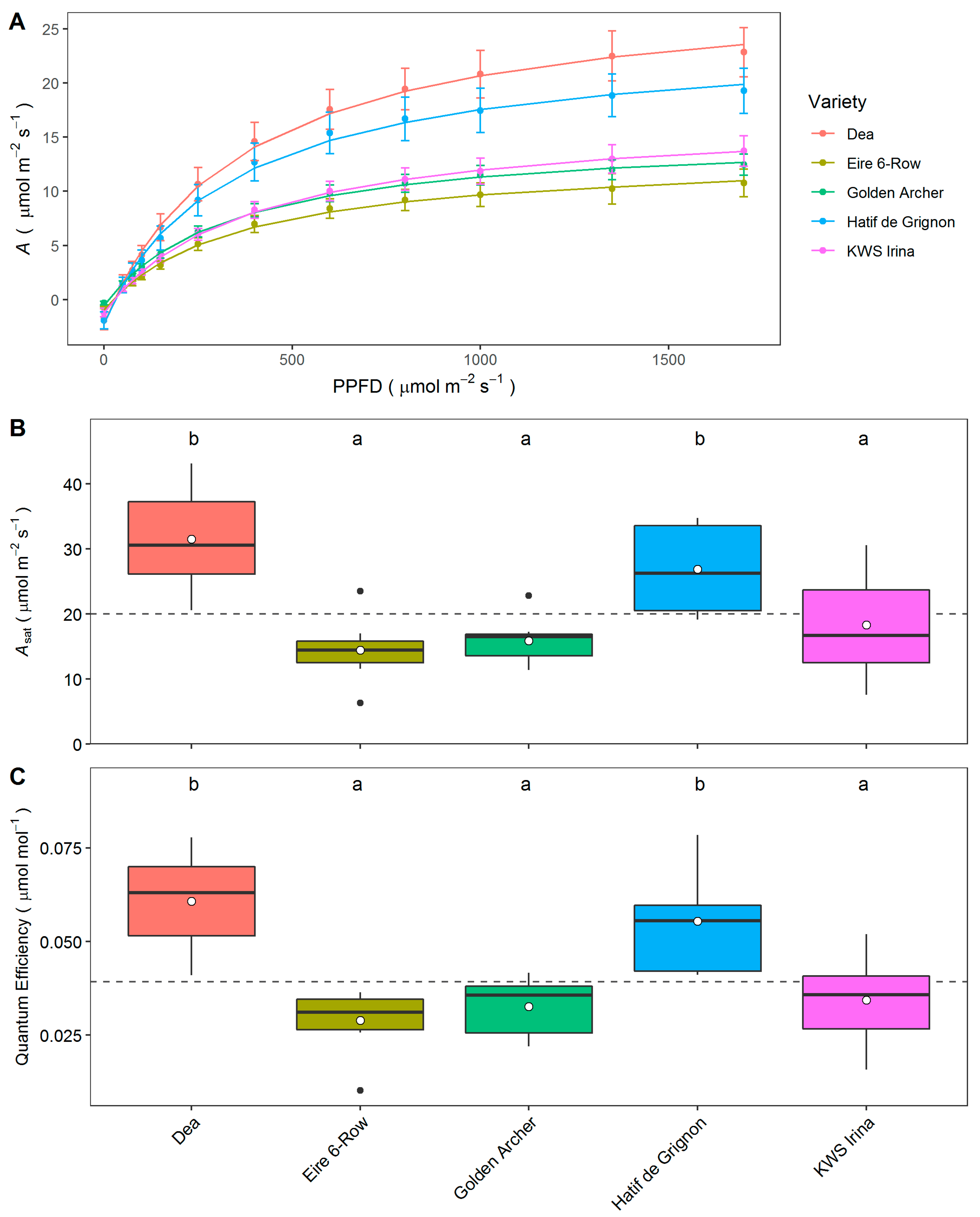
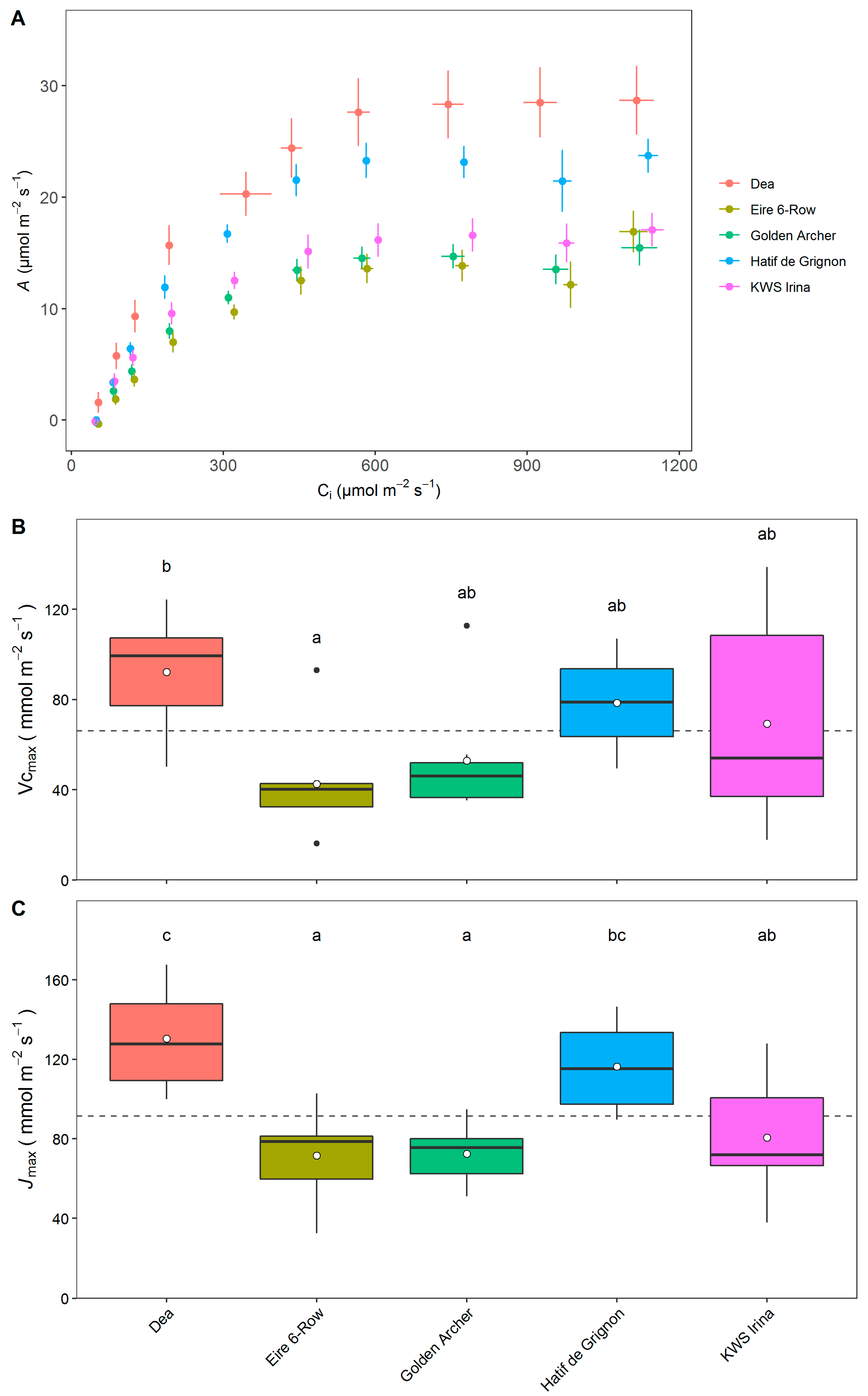
3.4. Photosynthetic Capacity of Elite and Landrace Barley Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Team, C.W., Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- UN Enviroment Programme; UNEO DTU Partnership. Emissions Gap Report; UNEP: Nairobi, Kenya, 2020; Available online: https://www.unep.org/emissions-gap-report-2020 (accessed on 21 April 2021).
- McGuire, S. WHO, World Food Programme, and International Fund for Agricultural Development. 2012. The State of Food Insecurity in the World 2012. Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. Rome, FAO. Adv. Nutr. 2013, 4, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Bongaarts, J.; McNicoll, G.; Todaro, M.P. 2006 state of the future. Popul. Dev. Rev. 2006, 32, 787. [Google Scholar]
- Jones, B.G.; Kandel, W.A. Population-growth, urbanization, and disaster risk and vulnerability in metropolitan areas—A conceptual framework. Environ. Manag. Urban Vulnerabil. 1992, 168, 51–76. [Google Scholar]
- Maclean, J.; Hardy, B.; Hettel, G. Rice Almanac: Source Book for One of the Most Important Economic Activities on Earth, 4th ed.; International Rice Research Institute (IRRI): Los Banos, Phillipines, 2013. [Google Scholar]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising CO2: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Sage, R.F.; Sharkey, T.D. The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field-grown plants. Plant Physiol. 1987, 84, 658–664. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12. [Google Scholar] [CrossRef]
- Merilo, E.; Yarmolinsky, D.; Jalakas, P.; Parik, H.; Tulva, I.; Rasulov, B.; Kilk, K.; Kollist, H. Stomatal VPD Response: There Is More to the Story Than ABA. Plant Physiol. 2018, 176, 851–864. [Google Scholar] [CrossRef]
- FAO. Towards a Water and Food Secure Future; FAO Natural Resources and Environment Department: Rome, Italy, 2015; p. 76. [Google Scholar]
- Aasamaa, K.; Sober, A.; Rahi, M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Aust. J. Plant Physiol. 2001, 28, 765–774. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The effects of climate extremes on global agricultural yields. Environ. Res. Lett. 2019, 14. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Woodward, F.I. Stomatal Numbers are sensitive to increases in CO2 from preindustrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.J.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef]
- Hubbart, S.; Smillie, I.R.A.; Heatley, M.; Swarup, R.; Foo, C.C.; Zhao, L.; Murchie, E.H. Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Calcagno, P.E.; Brown, K.L.; Simkin, A.J.; Fisk, S.J.; Vialet-Chabrand, S.; Lawson, T.; Raines, C.A. Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nat. Plants 2020, 6, 1054. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Guan, X.K.; Song, L.; Wang, T.C.; Turner, N.C.; Li, F.M. Effect of Drought on the Gas Exchange, Chlorophyll Fluorescence and Yield of Six Different-Era Spring Wheat Cultivars. J. Agron. Crop Sci. 2015, 201, 253–266. [Google Scholar] [CrossRef]
- Kromdijk, J.; Glowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Matthews, J.S.A.; Brendel, O.; Blatt, M.R.; Wang, Y.; Hills, A.; Griffiths, H.; Rogers, S.; Lawson, T. Modelling water use efficiency in a dynamic environment: An example using Arabidopsis thaliana. Plant Sci. 2016, 251, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; von Caemmerer, S.; Baroli, I. Photosynthesis and stomatal behaviour. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2010; Volume 72, pp. 265–304. [Google Scholar]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.A.P.; Jarvis, P.G. Hysteresis in the response of stomatal conductance in Pinus sylvestris L needles to light: Observations and a hypothesis. Plant Cell Environ. 1980, 3, 207–216. [Google Scholar]
- Whitehead, D.; Teskey, R.O. Dynamic response of stomata to changing irradiance in loblolly pine (Pinus taeda L.). Tree Physiol. 1995, 15, 245–251. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef]
- Graf, A.; Schlereth, A.; Stitt, M.; Smith, A.M. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc. Natl. Acad. Sci. USA 2010, 107, 9458–9463. [Google Scholar] [CrossRef]
- Haydon, M.J.; Mielczarek, O.; Robertson, F.C.; Hubbard, K.E.; Webb, A.A.R. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 2013, 502, 689. [Google Scholar] [CrossRef]
- Lehmann, P.; Or, D. Effects of stomata clustering on leaf gas exchange. New Phytol. 2015, 207, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 2019, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McAusland, L.; Vialet-Chabrand, S.; Davey, P.; Baker, N.R.; Brendel, O.; Lawson, T. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 2016, 211, 1209–1220. [Google Scholar] [CrossRef]
- Kumar, U.; Quick, W.P.; Barrios, M.; Cruz, P.C.S.; Dingkuhn, M. Atmospheric CO2 concentration effects on rice water use and biomass production. PLoS ONE 2017, 12, e0169706. [Google Scholar] [CrossRef][Green Version]
- Munns, R.; James, R.A.; Sirault, X.R.R.; Furbank, R.T.; Jones, H.G. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 2010, 61, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.N.; Hamdani, S.; Li, W.Z.; Wang, S.M.; Tang, J.Y.; Chen, Z.; Song, Q.F.; Li, M.; Zhao, H.L.; Chang, T.G.; et al. Rapid stomatal response to fluctuating light: An under-explored mechanism to improve drought tolerance in rice. Funct. Plant Biol. 2016, 43, 727–738. [Google Scholar] [CrossRef]
- Raven, J.A. Speedy small stomata? J. Exp. Bot. 2014, 65, 1415–1424. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Garmash, E. Temperature Controls a Dependence of Barley Plant Growth on Mineral Nutrition Level. Russ. J. Plant Physiol. 2005, 52, 338–344. [Google Scholar] [CrossRef]
- Michaelis, L.; Menten, M.L. Die kinetik der invertinwirkung. J. Biochem. Z 1913, 49, 352. [Google Scholar]
- Duursma, R.A. Plantecophys—An R Package for Analysing and Modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Dreyer, E.; Brendel, O. Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant Cell Environ. 2013, 36, 1529–1546. [Google Scholar] [CrossRef]
- Weyers, J.; Johansen, L. Accurate estimation of stomatal aperture from silicone rubber impressions. New Phytol. 1985, 101, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, M.; van Hintum, T.; Kik, C.; van Treuren, R.; Visser, B. Genetic diversity trends in twentieth century crop cultivars: A meta analysis. Theor. Appl. Genet. 2010, 120, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Evenson, R.E.; Gollin, D. Assessing the impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef] [PubMed]
- AHDB. Barley Growth Guide. Available online: http://cereals.ahdb.org.uk/media/186381/g67-barley-growth-guide.pdf (accessed on 24 May 2019).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 2 August 2019).
- Fischer, R.A.; Rees, D.; Sayre, K.D.; Lu, Z.M.; Condon, A.G.; Saavedra, A.L. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 1998, 38, 1467–1475. [Google Scholar] [CrossRef]
- Faralli, M.; Cockram, J.; Ober, E.; Wall, S.; Galle, A.; Van Rie, J.; Raines, C.; Lawson, T. Genotypic, Developmental and Environmental Effects on the Rapidity of g(s) in Wheat: Impacts on Carbon Gain and Water-Use Efficiency. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- McElwain, J.C. Climate-independent paleoaltimetry using stomatal density in fossil leaves as a proxy for CO2 partial pressure. Geology 2004, 32, 1017–1020. [Google Scholar] [CrossRef]
- Elliott-Kingston, C.; Haworth, M.; Yearsley, J.M.; Batke, S.P.; Lawson, T.; McElwain, J.C. Does Size Matter? Atmospheric CO2 May Be a Stronger Driver of Stomatal Closing Rate Than Stomatal Size in Taxa That Diversified under Low CO2. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kardiman, R.; Raebild, A. Relationship between stomatal density. size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Chen, G.; Dai, F.; Wang, Y.Z.; Hills, A.; Ruan, Y.L.; Zhang, G.P.; Franks, P.J.; Nevo, E.; Blatt, M.R. Molecular Evolution of Grass Stomata. Trends Plant Sci. 2017, 22, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef]
- Perera-Castro, A.V.; Nadal, M.; Flexas, J. What drives photosynthesis during desiccation? Mosses and other outliers from the photosynthesis-elasticity trade-off. J. Exp. Bot. 2020, 71, 6460–6470. [Google Scholar] [CrossRef]
- Lawson, T.; Matthews, J. Guard Cell Metabolism and Stomatal Function. Ann. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.M.; Blatt, M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef]
- Salter, W.T.; Li, S.; Dracatos, P.M.; Barbour, M.M. Identification of quantitative trait loci for dynamic and steady-state photosynthetic traits in a barley mapping population. AoB Plants 2020, 12. [Google Scholar] [CrossRef]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A.J. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef]
- McAusland, L.; Vialet-Chabrand, S.; Jauregui, I.; Burridge, A.; Hubbart-Edwards, S.; Fryer, M.J.; King, I.P.; King, J.; Pyke, K.; Edwards, K.J.; et al. Variation in key leaf photosynthetic traits across wheat wild relatives is accession dependent not species dependent. New Phytol. 2020, 228, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Kramer, D.M.; Raines, C.A. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr. Opin. Biotechnol. 2012, 23, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bezant, J.; Laurie, D.; Pratchett, N.; Chojecki, J.; Kearsey, M. Mapping QTL controlling yield and yield components in a spring barley (Hordeum vulgare L) cross using marker regression. Mol. Breed. 1997, 3, 29–38. [Google Scholar] [CrossRef]
- Bidinger, F.; Musgrave, R.B.; Fischer, R.A. Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley. Nature 1977, 270, 431–433. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.R. Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef]
- Bingham, I.J.; Karley, A.J.; White, P.J.; Thomas, W.T.B.; Russell, J.R. Analysis of improvements in nitrogen use efficiency associated with 75 years of spring barley breeding. Eur. J. Agron. 2012, 42, 49–58. [Google Scholar] [CrossRef]
- Wang, C.L.; Hu, S.L.; Gardner, C.; Lubberstedt, T. Emerging Avenues for Utilization of Exotic Germplasm. Trends Plant Sci. 2017, 22, 624–637. [Google Scholar] [CrossRef]
- Russell, J.R.; Fuller, J.D.; Macaulay, M.; Hatz, B.G.; Jahoor, A.; Powell, W.; Waugh, R. Direct comparison of levels of genetic variation among barley accessions detected by RFLPs, AFLPs, SSRs and RAPDs. Theor. Appl. Genet. 1997, 95, 714–722. [Google Scholar] [CrossRef]
- Sadras, V.O.; Slafer, G.A. Environmental modulation of yield components in cereals: Heritabilities reveal a hierarchy of phenotypic plasticities. Field Crops Res. 2012, 127, 215–224. [Google Scholar] [CrossRef]
- Chapin, F.S. The mineral nutrition of wild plants. Ann. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- GRU. John Innes Centre Germplasm Resources Unit SeedStor Database. Available online: https://www.seedstor.ac.uk/ (accessed on 28 June 2019).
- Rode, J.; Ahlemeyer, J.; Friedt, W.; Ordon, F. Identification of marker-trait associations in the German winter barley breeding gene pool (Hordeum vulgare L.). Mol. Breed. 2012, 30, 831–843. [Google Scholar] [CrossRef]
- Molina-Cano, J.; Fra-Mon, P.; Salcedo, G.; Aragoncillo, C.; De Togores, F.R.; García-Olmedo, F. Morocco as a possible domestication center for barley: Biochemical and agromorphological evidence. Theor. Appl. Genet. 1987, 73, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur.-Agric. Policy Econ. Environ. 2017, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
| Variety | Type | Rows | Habit | Use | Poly-Tunnel | Growth Chamber | Source |
|---|---|---|---|---|---|---|---|
| B3733 | Wild | 2 | Winter | - | √ | JIC GRU | |
| B3745 | Wild | 2 | Winter | - | √ | JIC GRU | |
| Dea | Landrace | 6 | Winter | Feed | √ | JIC GRU | |
| Eire-6-Row | Landrace | 6 | Spring | Malt | √ | KWS UK Ltd. | |
| Golden Archer | Landrace | 2 | Spring | Malt | √ | √ | JIC GRU |
| Hatif de Grignon | Landrace | 6 | Winter | Feed | √ | √ | JIC GRU |
| KWS Irina | Elite | 2 | Spring | Feed/Malt | √ | KWS UK Ltd. | |
| KWS Orwell | Elite | 2 | Winter | Feed | √ | KWS UK Ltd. | |
| KWS Sassy | Elite | 2 | Spring | Malt | √ | KWS UK Ltd. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, J.; Jones, M.A.; Lawson, T. Diverse Physiological and Physical Responses among Wild, Landrace and Elite Barley Varieties Point to Novel Breeding Opportunities. Agronomy 2021, 11, 921. https://doi.org/10.3390/agronomy11050921
Stevens J, Jones MA, Lawson T. Diverse Physiological and Physical Responses among Wild, Landrace and Elite Barley Varieties Point to Novel Breeding Opportunities. Agronomy. 2021; 11(5):921. https://doi.org/10.3390/agronomy11050921
Chicago/Turabian StyleStevens, Jim, Matthew Alan Jones, and Tracy Lawson. 2021. "Diverse Physiological and Physical Responses among Wild, Landrace and Elite Barley Varieties Point to Novel Breeding Opportunities" Agronomy 11, no. 5: 921. https://doi.org/10.3390/agronomy11050921
APA StyleStevens, J., Jones, M. A., & Lawson, T. (2021). Diverse Physiological and Physical Responses among Wild, Landrace and Elite Barley Varieties Point to Novel Breeding Opportunities. Agronomy, 11(5), 921. https://doi.org/10.3390/agronomy11050921







