Predicting Seedling Emergence of Three Canarygrass (Phalaris) Species under Semi-Arid Conditions Using Parametric and Non-Parametric Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Estimation of TT and HTT
2.4. Development of the Parametric Model
2.5. Development of the Non-Parametric Model
2.6. Model Accuracy
2.7. Data Validation
3. Results
3.1. Weather Conditions
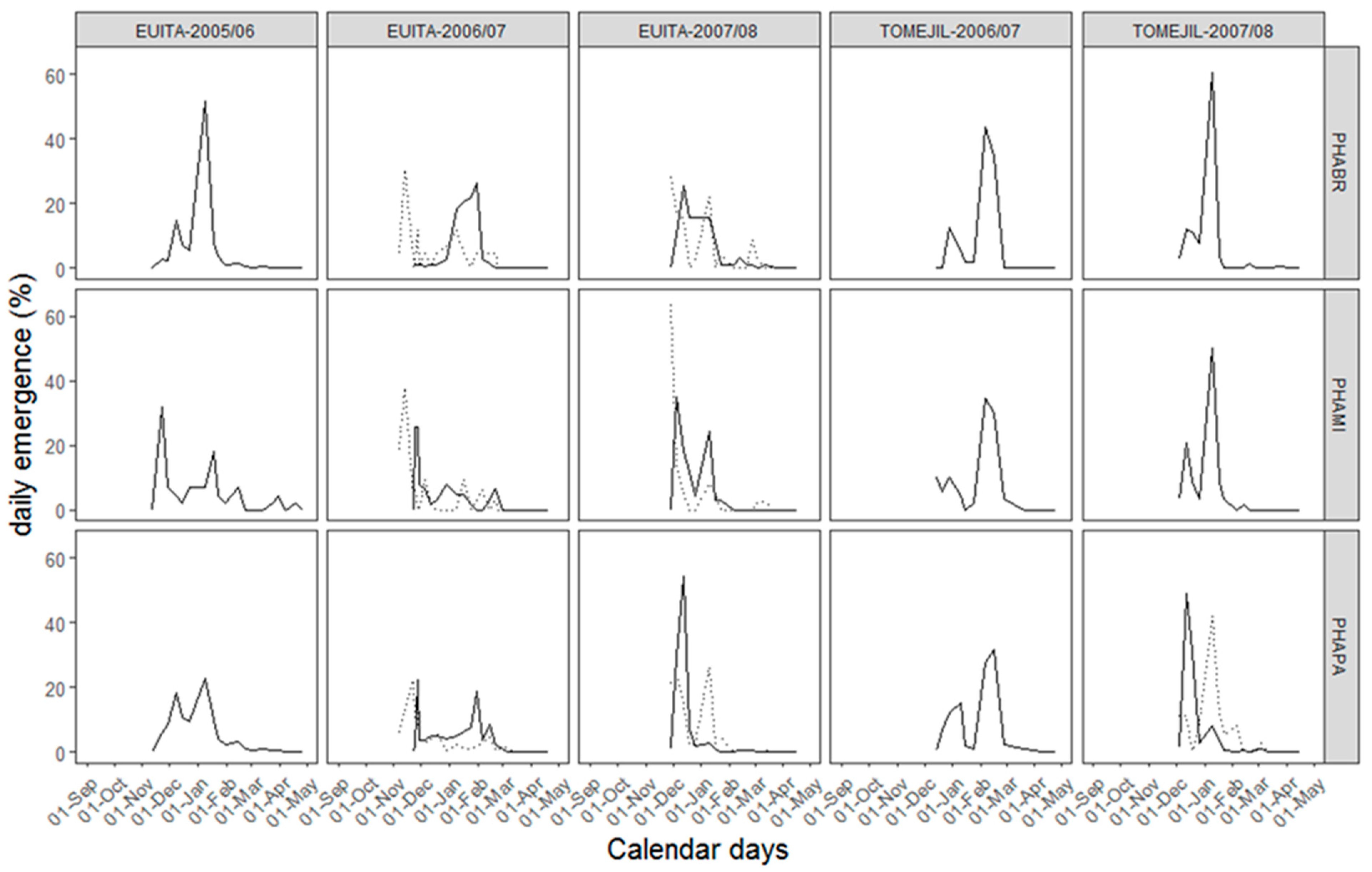
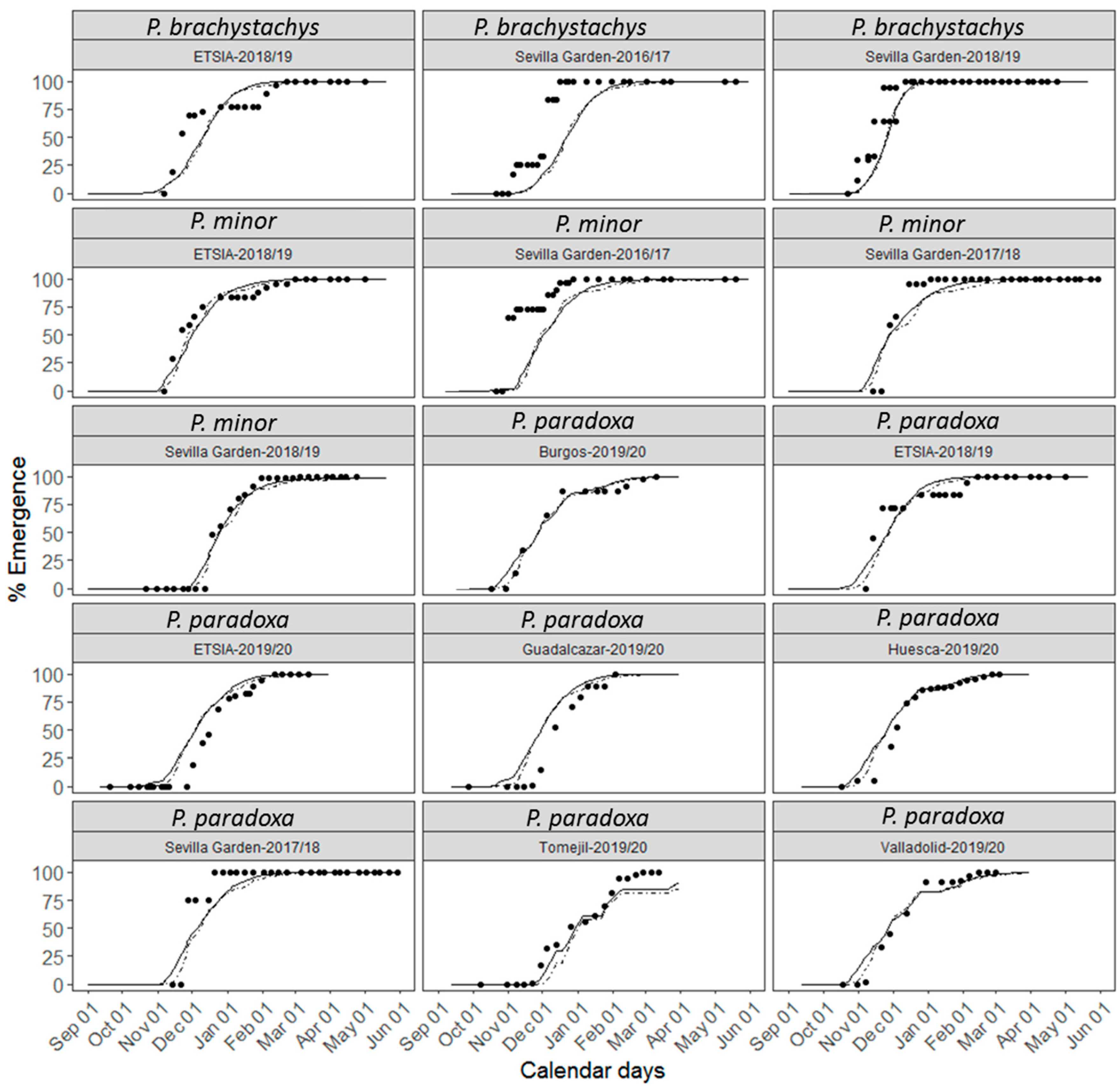
3.2. Description of the Emergence
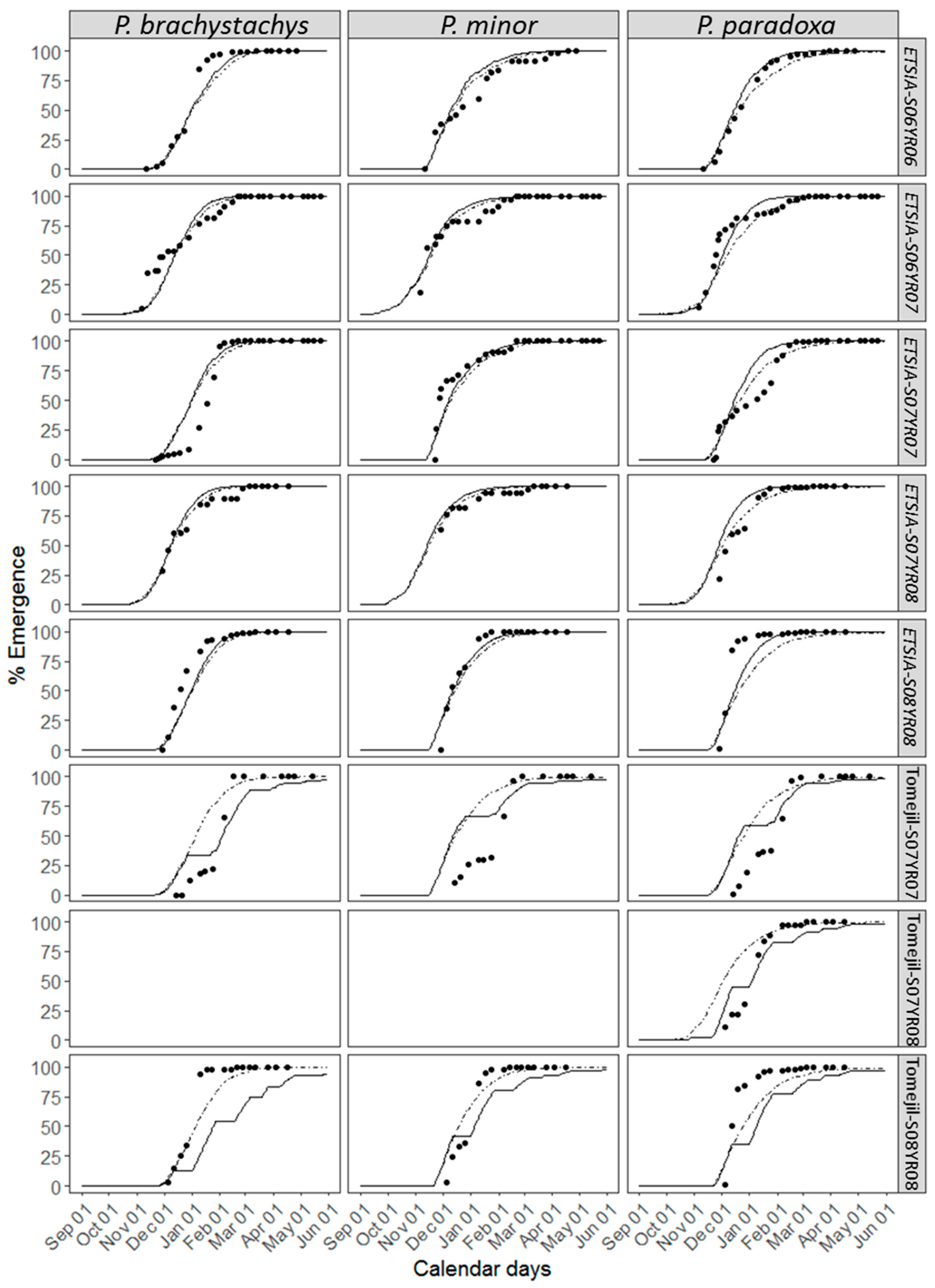
3.3. Accuracy of the Parametric Model
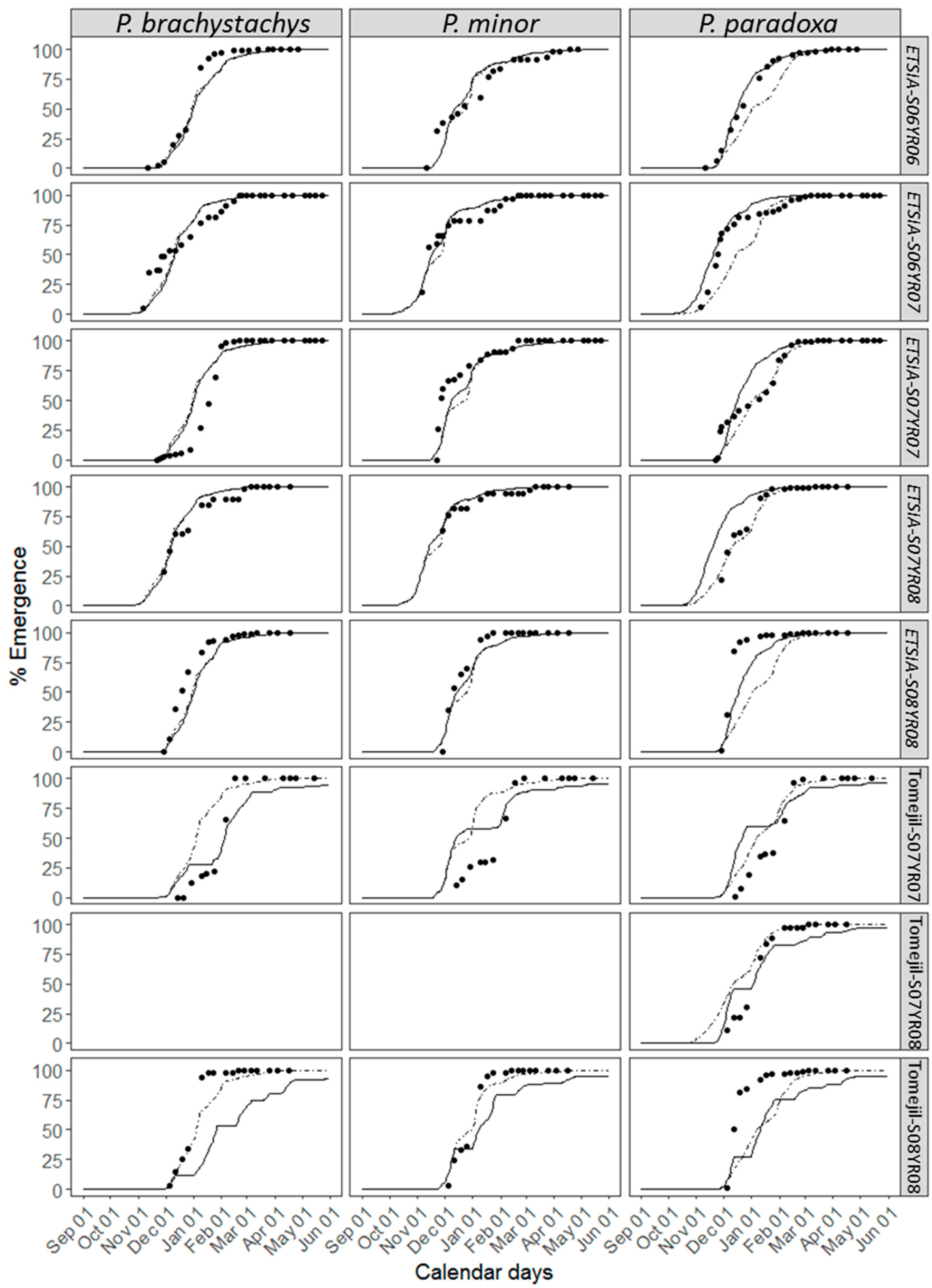
3.4. Accuracy of the Non-Parametric Model
3.5. Validation of the Models with Independent Data Sets
4. Discussion
4.1. Emergence Pattern
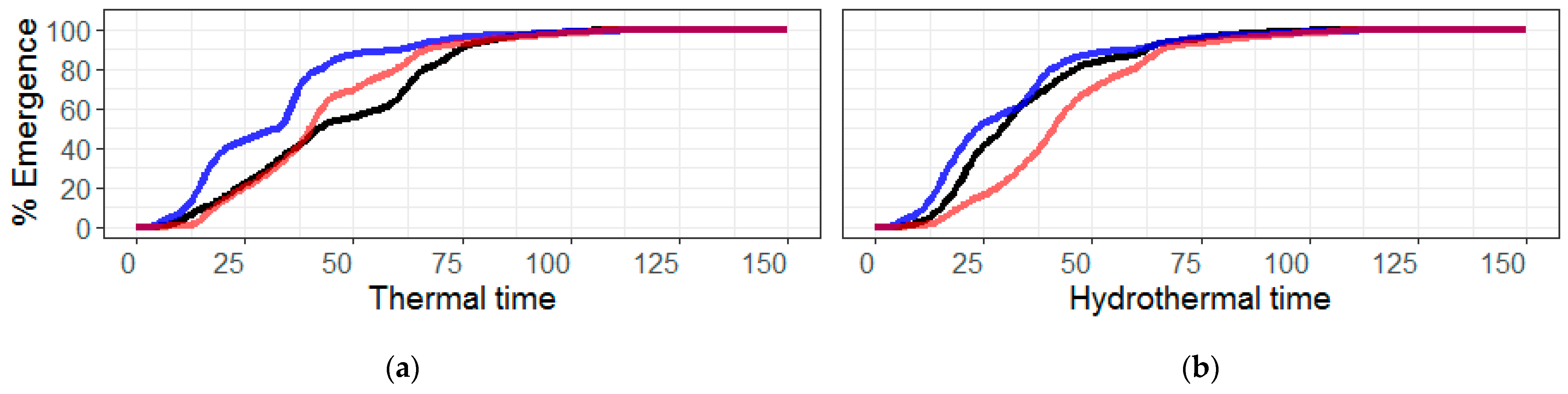
4.2. Threshold Parameters (Tb, To, Tc and Ψb)
4.3. Parametric vs. Non-Parametric Models
4.4. Applicability of the Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García Baudín, J.; Contreras de Vera, A.; Salto, T. Especies adventicias del género Phalaris, en los cereales de invierno de Andalucía Occidental. Boletín Sanid. Veg. Plagas 1982, 8, 185–191. [Google Scholar]
- Damanakis, M. Weed species in wheat fields of Greece-1982, 1983 survey. Zizaniologia 1983, 1, 85–90. [Google Scholar]
- Chhokar, R.S.; Sharma, R.K. Multiple herbicide resistance in littleseed canarygrass (Phalaris minor): A threat to wheat production in India. Weed Biol. Manag. 2008, 8, 112–123. [Google Scholar] [CrossRef]
- Bir, S.S.S.M. Observations on the weed flora of cultivable lands in Punjab- wheat fields in Patiala District. New Bot. 1979, 6, 79–89. [Google Scholar]
- Xu, G.; Shen, S.; Zhang, Y.; Clements, D.R.; Yang, S.; Li, J.; Dong, L.; Zhang, F.; Jin, G.; Gao, Y. Designing Cropping Systems to Improve the Management of the Invasive Weed Phalaris minor Retz. Agronomy 2019, 9, 809. [Google Scholar] [CrossRef]
- USDA PLANTS database. Choice Rev. Online 2006, 43. [CrossRef]
- Gonzalez-Andujar, J.L.; Jimenez-Hidalgo, M.; Garcia-Torres, L.; Saavedra, M. Demography and population dynamic of the arable weed Phalaris brachystachys L. (short-spiked canary grass) in winter wheat. Crop Prot. 2005, 24, 581–584. [Google Scholar] [CrossRef]
- Alcantara, C.; Fuentes-García, M.; Carranza, R.; Sanchez-Gamonoso, M.; Saavedra, M. Aplicaciones de Herbicidas de Postemergencia Temprana y Tardía Contra Alpisteras (Phalaris spp.) en Trigo Duro; Consejería de Agricultura, Pesca y Desarrollo Rural. Instituto de Investigación y Formación Agraria y Pesquera: Cordoba, Spain, 2017. [Google Scholar]
- Ortiz, R.; Contreras, J.M.; Ruiz, A.; Sanz, M.A.; Romero, M.; Gordillo, M.; Taberner, A.; Urbano, J.M.; Urbano Fuentes-Guerra, J.M. Malas hierbas preocupantes en españa. Actas la Soc. Española Malherbologia 2015, 1, 497–503. [Google Scholar]
- Cudney, D.W.; Hill, J.E. The response of wheat grown with three population levels of canarygrass to various herbicide treatments. Proc. West. Soc. Weed Sci. 1979, 55–56. [Google Scholar]
- Jimenez-Hidalgo, M. Incidencia y Evolución de los Alpistes (Phalaris ssp.) En el Trigo (Triticum sp.); Universidad de Córdoba: Andalusia, Spain, 1993. [Google Scholar]
- Afentouli, C.G.; Eleftherohorinos, I.G. Competition between wheat and canarygrass biotypes and their response to herbicides. Weed Sci. 1999, 47, 55–61. [Google Scholar] [CrossRef]
- González-Diaz, L.; Bastida, F.; Gonzalez-Andujar, J.L. Short comunication. Modelling of the population dynamics of Phalaris brachystachys Link under various herbicide control scenarios in a Mediterranean climate. Spanish J. Agric. Res. 2009, 7, 155–159. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org (accessed on 1 May 2020).
- Forcella, F.; Benech-Arnold, R.L.; Sánchez, R.; Ghersa, C.M. Modelling seedling emergence. Field Crops Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Torra, J.; Chantre, G.R. Weed Emergence Models. In Decision Support Systems for Weed Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 85–116. [Google Scholar]
- Bradford, K.J. Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- Grundy, A.C. Predicting weed emergence: A review of approaches and future challenges. Weed Res. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- Cao, R.; Francisco-Fernández, M.; Anand, A.; Bastida, F.; González-Andújar, J.L. Computing statistical indices for hydrothermal times using weed emergence data. J. Agric. Sci. 2011, 149, 701–712. [Google Scholar] [CrossRef]
- Gonzalez-Andujar, J.L.; Francisco-Fernandez, M.; Cao, R.; Reyes, M.; Urbano, J.M.; Forcella, F.; Bastida, F. A comparative study between nonlinear regression and nonparametric approaches for modelling Phalaris paradoxa seedling emergence. Weed Res. 2016, 56, 367–376. [Google Scholar] [CrossRef]
- Mesgaran, M.B.; Mashhadi, H.R.; Alizadeh, H.; Hunt, J.; Young, K.R.; Cousens, R.D. Importance of distribution function selection for hydrothermal time models of seed germination. Weed Res. 2013, 53, 89–101. [Google Scholar] [CrossRef]
- Derakhshan, A.; Gherekhloo, J.; Vidal, R.A.; De Prado, R. Quantitative Description of the Germination of Littleseed Canarygrass (Phalaris minor) in Response to Temperature. Weed Sci. 2014, 62, 250–257. [Google Scholar] [CrossRef]
- Singh, S.; Kirkwood, R.C.; Marshall, G. Biology and control of Phalaris minor Retz. (littleseed canarygrass) in wheat. Crop Prot. 1999, 18, 1–16. [Google Scholar] [CrossRef]
- Sousa-Ortega, C.; Chamber, E.; Urbano, J.M.; Izquierdo, J.; Loureiro, I.; Marí, A.I.; Cordero, F.; Vargas, M.; Saavedra, M.; Lezaun, J.A.; et al. Should emergence models for Lolium rigidum be changed throughout climatic conditions? The case of Spain. Crop Prot. 2020, 128, 105012. [Google Scholar] [CrossRef]
- Sousa-Ortega, C.; Royo-Esnal, A.; DiTommaso, A.; Izquierdo, J.; Loureiro, I.; Marí, A.I.; Cordero, F.; Vargas, M.; Saavedra, M.; Paramio, J.A.; et al. Modeling the Emergence of North African Knapweed (Centaurea diluta), an Increasingly Troublesome Weed in Spain. Weed Sci. 2020, 1–30. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Fuentes-Yagüe, J.; García-Legaspi, G. Técnicas de Riego. Ministerio de Agricultura, Pesca y Alimentación; Ministerio de Agricultrua, pesca y alimentación, Ed.; Mundi-Prensa México: Ciudad Cuauhtémoc, México, 1999; ISBN 968-7462-17-5. [Google Scholar]
- Duong, T. Ks: Kernel density estimation and kernel discriminant analysis for multivariate data in R. J. Stat. Softw. 2007, 21, 1–16. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Torra, J.; Conesa, J.A.; Recasens, J. Characterisation of emergence of autumn and spring cohorts of Galium spp. in winter cereals. Weed Res. 2010, 50, 572–585. [Google Scholar] [CrossRef][Green Version]
- Taylor, I.N.; Walker, S.R.; Adkins, S.W. Burial depth and cultivation influence emergence and persistence of Phalaris paradoxa seed in an Australian sub-tropical environment. Weed Res. 2005, 45, 33–40. [Google Scholar] [CrossRef]
- Sousa-Ortega, C.; Royo-Esnal, A.; Loureiro, I.; Marí, A.I.; Lezáun, J.A.; Cordero, F.; Saavedra, M.; Paramio, J.A.; Fernández, J.L.; Torra, J.; et al. Modelling Emergence of Sterile Oat (Avena sterilis spp ludoviciana) Under Semi-arid Conditions. Weed Sci. 2021, 1–29. [Google Scholar] [CrossRef]
- Mesgaran, M.B.; Onofri, A.; Mashhadi, H.R.; Cousens, R.D. Water availability shifts the optimal temperatures for seed germination: A modelling approach. Ecol. Modell. 2017, 351, 87–95. [Google Scholar] [CrossRef]
- Ahmadi, A.; Hosseini, M.; Zeidali, E. Study of ecological characteristics of canary grass (Phalaris minor). Tech. J. Eng. Appl. Sci. 2013, 3, 1835–1840. [Google Scholar]
- Alcantara, C.; Jimenez-Hidalgo, M.J.; Saavedra, M. Responses of Phalaris minor Rezt. and Phalaris brachystachys Link to different levels of soil water availability. Spanish J. Agric. Res. 2010, 8, 1074. [Google Scholar] [CrossRef]
- Taylor, I.N.; Peters, N.C.B.; Adkins, S.W.; Walker, S.R. Germination response of Phalaris paradoxa L. seed to different light quality. Weed Res. 2004, 44, 254–264. [Google Scholar] [CrossRef]
- Ohadi, S.; Rahimian Mashhadi, H.; Tavakkol-Afshari, R.; Beheshtian Mesgaran, M. Modelling the effect of light intensity and duration of exposure on seed germination of Phalaris minor and Poa annua. Weed. Res. 2010, 50, 209–217. [Google Scholar] [CrossRef]
- Izquierdo, J.; González-Andújar, J.L.; Bastida, F.; Lezaún, J.A.; del Arco, M.J.S. A Thermal Time Model to Predict Corn Poppy (Papaver rhoeas) Emergence in Cereal Fields. Weed Sci. 2009, 57, 660–664. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Variation in Seed Dormancy and Germination within and between Individuals and Populations of a Species. In Seeds (Second Edition): Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: New York, NY, USA, 2014; pp. 277–373. ISBN 9780124166776. [Google Scholar]
- García, A.L.; Recasens, J.; Forcella, F.; Torra, J.; Royo-Esnal, A. Hydrothermal Emergence Model for Ripgut Brome (Bromus diandrus). Weed Sci. 2013, 61, 146–153. [Google Scholar] [CrossRef]
- Masin, R.; Loddo, D.; Gasparini, V.; Otto, S.; Zanin, G. Evaluation of Weed Emergence Model AlertInf for Maize in Soybean. Weed Sci. 2014, 62, 360–369. [Google Scholar] [CrossRef]
- Masin, R.; Loddo, D.; Benvenuti, S.; Otto, S.; Zanin, G. Modeling Weed Emergence in Italian Maize Fields. Weed Sci. 2012, 60, 254–259. [Google Scholar] [CrossRef]

| Location | Code 1 | Sowing Date | First Relevant Rain | Total Emergence | ||
|---|---|---|---|---|---|---|
| PHABR | PHAMI | PHAPA | ||||
| ETSIA | S06YR06 | 11 November 2005 | 14 November 2005 | 1794 | 44 | 1519 |
| ETSIA | S06YR07 | 13 September 2006 | 43 | 32 | 174 | |
| ETSIA | S07YR07 | 12 November 2006 | 16 November 2006 | 1391 | 62 | 914 |
| ETSIA | S07YR08 | 21 September 2007 | 46 | 38 | 178 | |
| ETSIA | S08YR08 | 15 November 2007 | 20 November 2007 | 95 | 66 | 895 |
| Tomejil | S07YR07 | 15 November 2006 | 16 November 2006 | 55 | 90 | 473 |
| Tomejil | S07YR08 | 21 September 2007 | - | - | 36 | |
| Tomejil | S08YR08 | 20 November 2007 | 22 November 2007 | 190 | 58 | 679 |
| Location | Year | Number of Emerged Seedling | ||
|---|---|---|---|---|
| P. brachystachys | P. minor | P. paradoxa | ||
| Sevilla-Garden | 2016/17 | 12 | 29 | - |
| 2017/18 | - | 48 | 16 | |
| 2018/19 | 17 | 103 | - | |
| ETSIA | 2018/19 | 26 | 24 | 18 |
| Location | Latitude | Longitude | Sowing Date(2019) | Sand (%) | Silt (%) | Clay (%) | Emerged Seedling |
|---|---|---|---|---|---|---|---|
| Burgos | 42.4402 N | 3.7209 W | Sept 18 | 22 | 46 | 32 | 369 |
| ETSIA | 37.3524 N | 5.9392 W | Sept 20 | 38 | 30 | 32 | 242 |
| Guadalcazar | 37.7558 N | 4.9354 W | Oct 17 | 36 | 33 | 30 | 231 |
| Huesca | 42.1277 N | 0.3987 W | Oct 17 | 25 | 40 | 35 | 514 |
| Tomejil | 37.4027 N | 5.5878 W | Sept 23 | 5 | 32 | 62 | 158 |
| Valladolid | 41.7789 N | 4.8752 W | Oct 10 | 62 | 22 | 16 | 507 |
| Location | Month | Temperature (°C) | Precipitation (mm) | ||||
|---|---|---|---|---|---|---|---|
| 2005/06 | 2006/07 | 2007/08 | 2005/06 | 2006/07 | 2007/08 | ||
| ETSIA | September | 22.4 | 23.7 | 23.0 | 0.0 | 37.0 | 42.8 |
| ETSIA | October | 17.7 | 19.6 | 18.8 | 119.2 | 197.8 | 22.6 |
| ETSIA | November | 11.5 | 14.3 | 13.1 | 25.4 | 120.6 | 91.4 |
| ETSIA | December | 9.7 | 8.9 | 9.6 | 29.0 | 43.4 | 15.0 |
| ETSIA | January | 7.1 | 8.2 | 10.8 | 38.0 | 30.4 | 44.8 |
| ETSIA | February | 9.1 | 11.8 | 13.4 | 52.8 | 59.6 | 68.4 |
| ETSIA | March | 13.4 | 13.4 | 14.1 | 63.6 | 12.4 | 20.0 |
| ETSIA | April | 17.0 | 15.3 | 16.8 | 35.2 | 38.0 | 165.4 |
| Average | 13.5 | 14.4 | 14.9 | 363.2 | 539.2 | 470.4 | |
| Tomejil | September | 24.2 | 23.4 | 65.8 | 30.0 | ||
| Tomejil | October | 20.4 | 18.9 | 93.2 | 44.2 | ||
| Tomejil | November | 15.2 | 13.3 | 78.8 | 118.2 | ||
| Tomejil | December | 9.4 | 10.1 | 37.8 | 12.8 | ||
| Tomejil | January | 8.8 | 11.1 | 32.8 | 57.4 | ||
| Tomejil | February | 12.0 | 13.6 | 58.0 | 65.0 | ||
| Tomejil | March | 12.0 | 13.0 | 20.6 | 25.8 | ||
| Tomejil | April | 14.3 | 16.0 | 43.2 | 177.6 | ||
| Average | 14.5 | 14.9 | 430.2 | 531.0 | |||
| Location. | Sowing 1 | P. brachystachys | P. paradoxa | P. minor | |||
|---|---|---|---|---|---|---|---|
| 50% 2 | Emergence 3 Period | 50% 2 | Emergence 3 Period | 50% 2 | Emergence 3 Period | ||
| ETSIA | S06YR06 | Dec. 28 | 44.1 | Dec. 20 | 59.1 | Dec. 20 | 90.2 |
| ETSIA | S06YR07 | Nov. 30 | 89.9 | Nov. 23 | 86.8 | Nov. 11 | 88.8 |
| ETSIA | S07YR07 | Jan. 17 | 31.6 | Jan. 07 | 74.9 | Nov. 26 | 61.6 |
| ETSIA | S07YR08 | Dec. 07 | 88.6 | Dec. 07 | 45.7 | Mar. 18 | 46.7 |
| ETSIA | S08YR08 | Dec. 18 | 42.0 | Dec. 07 | 16.4 | Dec. 10 | 37.8 |
| Tomejil | S07YR07 | Feb. 01 | 48.0 | Jan. 29 | 54.0 | Jan. 30 | 61.8 |
| Tomejil | S07YR08 | - | - | Jan. 02 | 52.9 | - | 36.9 |
| Tomejil | S08YR08 | Dec. 30 | 30.7 | Dec. 11 | 30.4 | Dec. 30 | 36.9 |
| Model | P. brachystachys | P. paradoxa | P. minor | |||
|---|---|---|---|---|---|---|
| K | b | k | b | k | b | |
| TT | 8.264187 | 2.134985 | 5.161581 | 1.401254 | 4.589567 | 1.3303 |
| HTT | 9.231423 | 2.410334 | 6.093273 | 1.747469 | 4.711248 | 1.402724 |
| Location | Sowing | P. brachystachys | P. paradoxa | P. minor | |||
|---|---|---|---|---|---|---|---|
| TT | HTT | TT | HTT | TT | HTT | ||
| ETSIA | S06YR06 | 10.2 | 8.2 | 5.6 | 5.3 | 7.3 | 9.5 |
| ETSIA | S06YR07 | 10.0 | 11.1 | 10.7 | 8.8 | 5.4 | 6.4 |
| ETSIA | S07YR07 | 13.0 | 13.5 | 8.2 | 12.8 | 9.5 | 8.5 |
| ETSIA | S07YR08 | 5.5 | 7.0 | 7.5 | 12.3 | 3.4 | 5.3 |
| ETSIA | S08YR08 | 11.1 | 10.0 | 19.9 | 14.7 | 8.5 | 7.6 |
| Tomejil | S07YR07 | 24.7 | 15.9 | 24.4 | 22.2 | 30.4 | 26.4 |
| Tomejil | S07YR08 | - | - | 22.2 | 13.0 | - | - |
| Tomejil | S08YR08 | 13.7 | 34.8 | 17.3 | 24.5 | 11.9 | 16.5 |
| Average | 12.6 | 14.4 | 14.5 | 14.2 | 10.9 | 11.5 | |
| Location | Sowing | P. brachystachys | P. paradoxa | P. minor | |||
|---|---|---|---|---|---|---|---|
| TT | HTT | TT | HTT | TT | HTT | ||
| ETSIA | S06YR06 | 7.9 | 8.0 | 12.3 | 3.6 | 7.3 | 9.7 |
| ETSIA | S06YR07 | 9.9 | 11.1 | 16.6 | 7.1 | 5.4 | 5.5 |
| ETSIA | S07YR07 | 13.4 | 12.7 | 7.5 | 12.2 | 9.5 | 11.3 |
| ETSIA | S07YR08 | 6.5 | 6.5 | 4.3 | 12.4 | 3.4 | 3.6 |
| ETSIA | S08YR08 | 11.1 | 12.1 | 29.8 | 16.5 | 8.5 | 6.6 |
| Tomejil | S07YR07 | 26.4 | 13.9 | 13.7 | 21.0 | 30.4 | 22.3 |
| Tomejil | S07YR08 | - | - | 15.2 | 13.6 | - | - |
| Tomejil | S08YR08 | 11.4 | 36.5 | 27.1 | 28.3 | 11.9 | 17.8 |
| Average | 12.4 | 14.4 | 15.8 | 14.4 | 10.9 | 11.0 | |
| Weed | Experiment | Parametric Model | Non-Parametric Model | ||
|---|---|---|---|---|---|
| TT | HTT | TT | HTT | ||
| P. brachystachys | ETSIA-2018/19 | 15.3 | 16.3 | 15.7 | 17.1 |
| Sevilla Garden-2016/17 | 28.7 | 28.5 | 27.7 | 29.5 | |
| Sevilla Garden-2018/19 | 12.5 | 12.2 | 11.3 | 12.2 | |
| Average | 18.9 | 19.0 | 18.2 | 19.6 | |
| P. minor | ETSIA-2018/19 | 9.2 | 8.8 | 9.6 | 7.6 |
| Sevilla Garden-2016/17 | 31.9 | 30.5 | 32.7 | 31.0 | |
| Sevilla Garden-2017/18 | 12.7 | 11.2 | 14.3 | 12.3 | |
| Sevilla Garden-2018/19 | 7.5 | 5.8 | 7.6 | 5.9 | |
| Average | 15.4 | 14.1 | 16.1 | 14.2 | |
| P. paradoxa | Burgos-2019/20 | 10.6 | 6.8 | 16.9 | 4.2 |
| ETSIA-2018/19 | 12.3 | 11.6 | 19.7 | 11.2 | |
| ETSIA-2019/20 | 9.9 | 12.4 | 8.5 | 12.1 | |
| Guadalcazar-2019/20 | 14.8 | 17.7 | 11.7 | 15.8 | |
| Huesca-2019/20 | 11.1 | 9.3 | 17.0 | 8.5 | |
| Sevilla Garden-2017/18 | 18.0 | 12.9 | 25.7 | 13.6 | |
| Tomejil-2019/20 | 16.4 | 9.5 | 9.2 | 12.9 | |
| Valladolid-2019/20 | 14.6 | 8.6 | 20.2 | 6.3 | |
| Average | 13.5 | 11.1 | 16.1 | 10.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa-Ortega, C.; Royo-Esnal, A.; Urbano, J.M. Predicting Seedling Emergence of Three Canarygrass (Phalaris) Species under Semi-Arid Conditions Using Parametric and Non-Parametric Models. Agronomy 2021, 11, 893. https://doi.org/10.3390/agronomy11050893
Sousa-Ortega C, Royo-Esnal A, Urbano JM. Predicting Seedling Emergence of Three Canarygrass (Phalaris) Species under Semi-Arid Conditions Using Parametric and Non-Parametric Models. Agronomy. 2021; 11(5):893. https://doi.org/10.3390/agronomy11050893
Chicago/Turabian StyleSousa-Ortega, Carlos, Aritz Royo-Esnal, and José María Urbano. 2021. "Predicting Seedling Emergence of Three Canarygrass (Phalaris) Species under Semi-Arid Conditions Using Parametric and Non-Parametric Models" Agronomy 11, no. 5: 893. https://doi.org/10.3390/agronomy11050893
APA StyleSousa-Ortega, C., Royo-Esnal, A., & Urbano, J. M. (2021). Predicting Seedling Emergence of Three Canarygrass (Phalaris) Species under Semi-Arid Conditions Using Parametric and Non-Parametric Models. Agronomy, 11(5), 893. https://doi.org/10.3390/agronomy11050893







