Mechanism(s) of Glyphosate Resistance in a Selected Plantago lanceolata (L.) R Biotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Dose Response Trial
2.2. Shikimic Acid Analysis
Gas Chromatograph Mass Spectrometry (GC–MS)
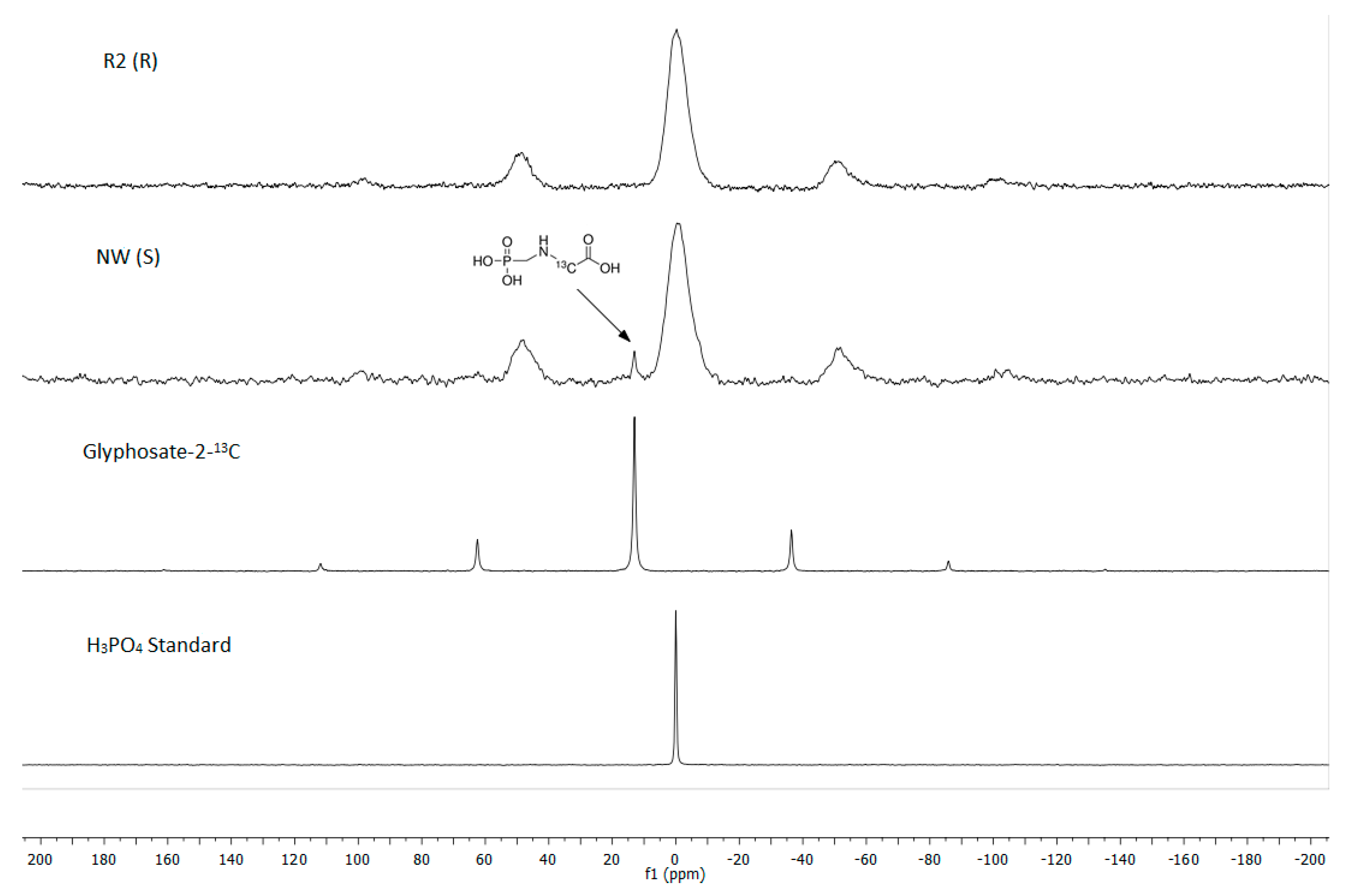
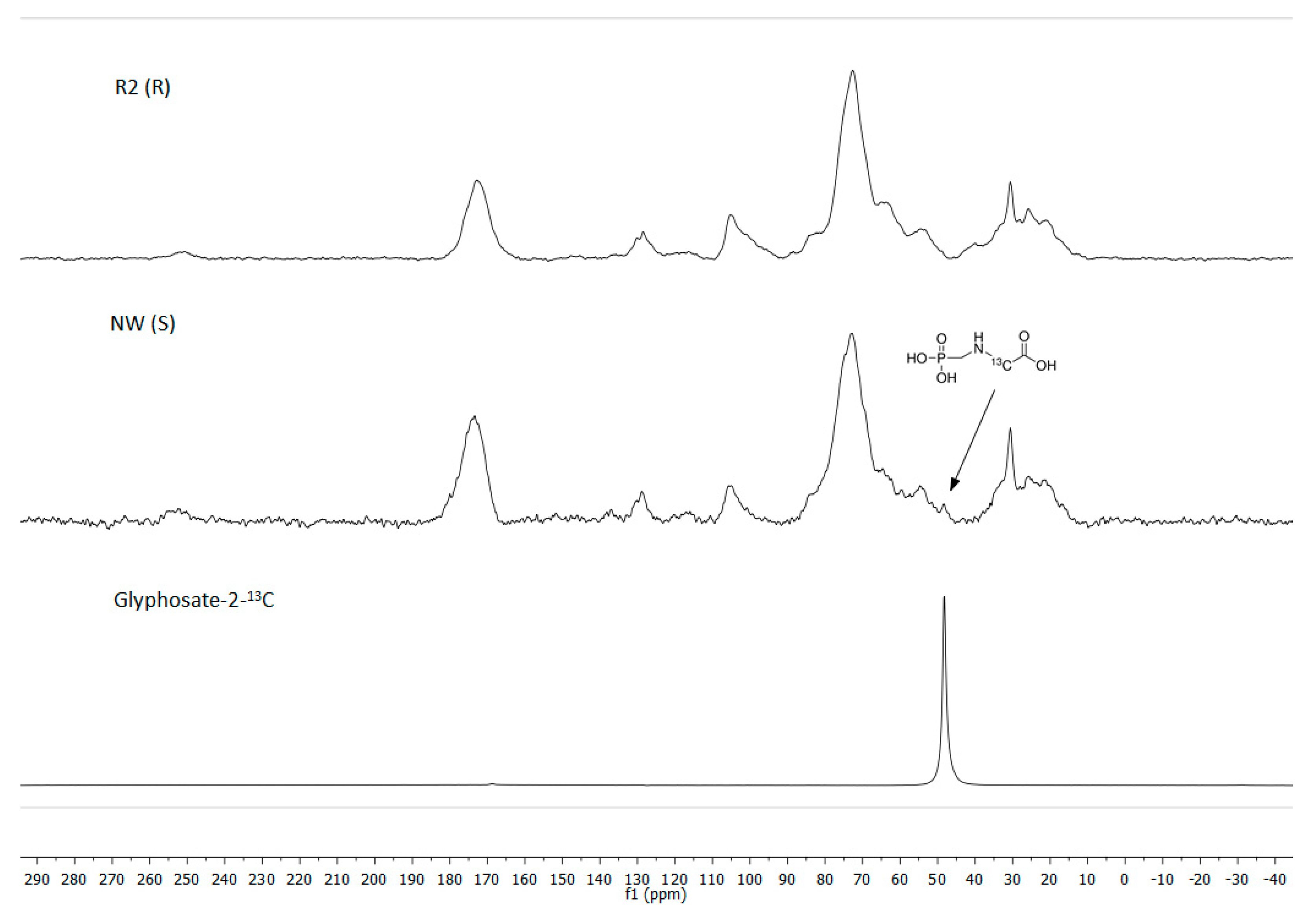
2.3. 31P and 13C NMR
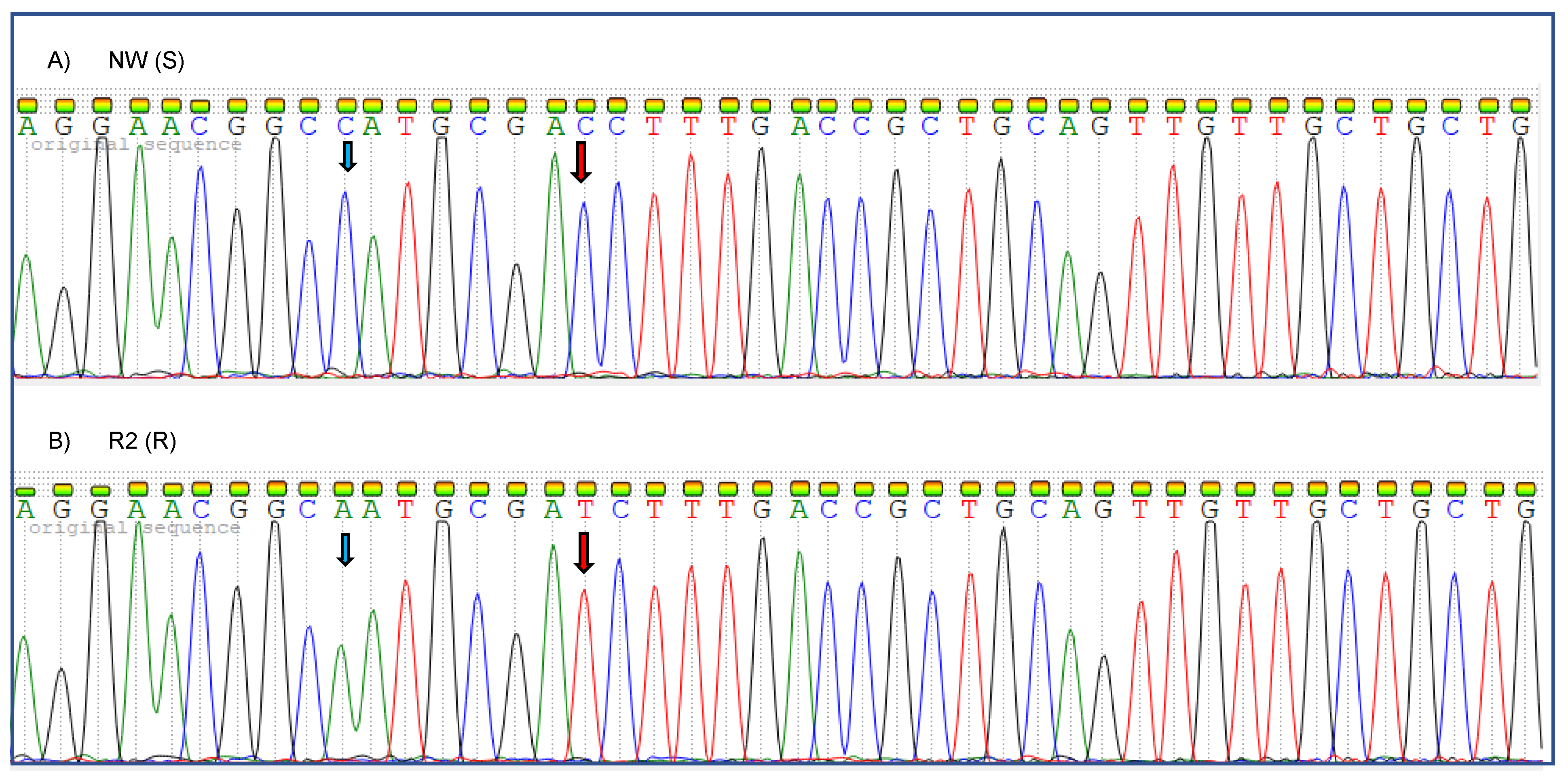
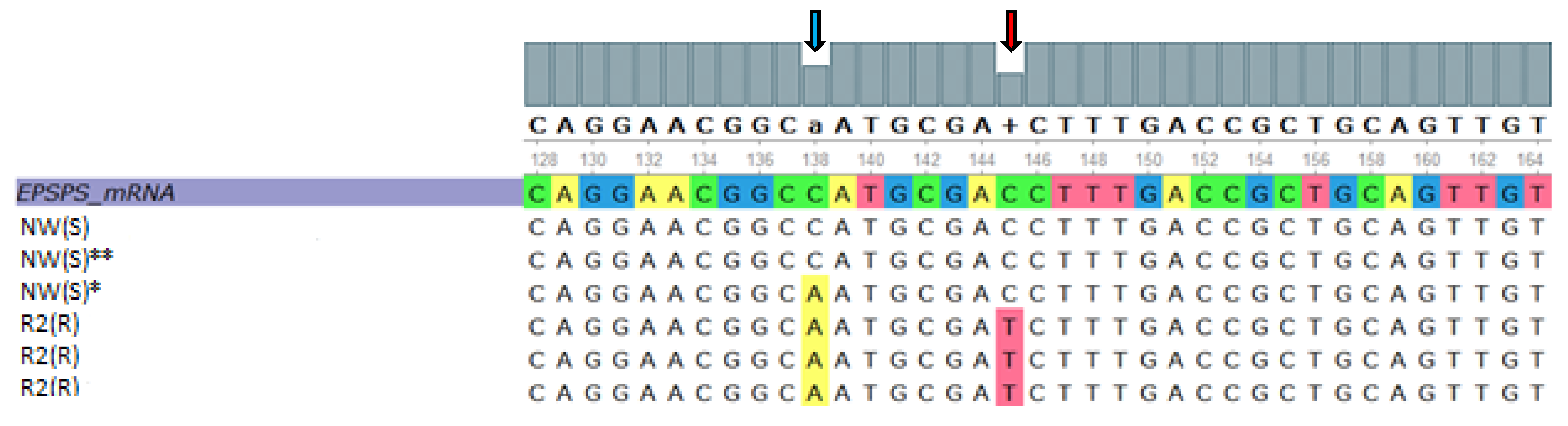
2.4. EPSPS cDNA Sequencing
2.5. Statistical Analysis
3. Results
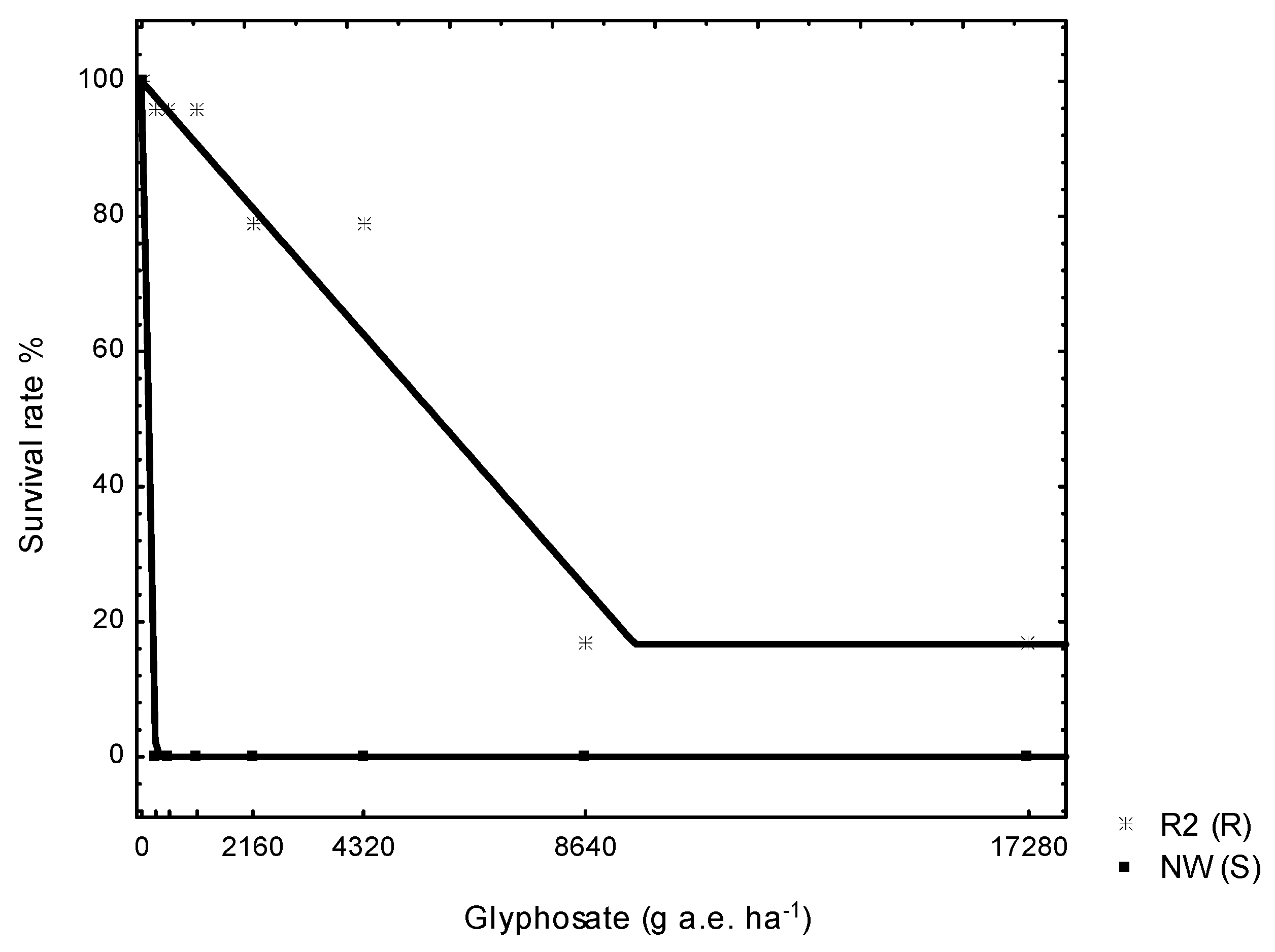
3.1. Dose Response Trial
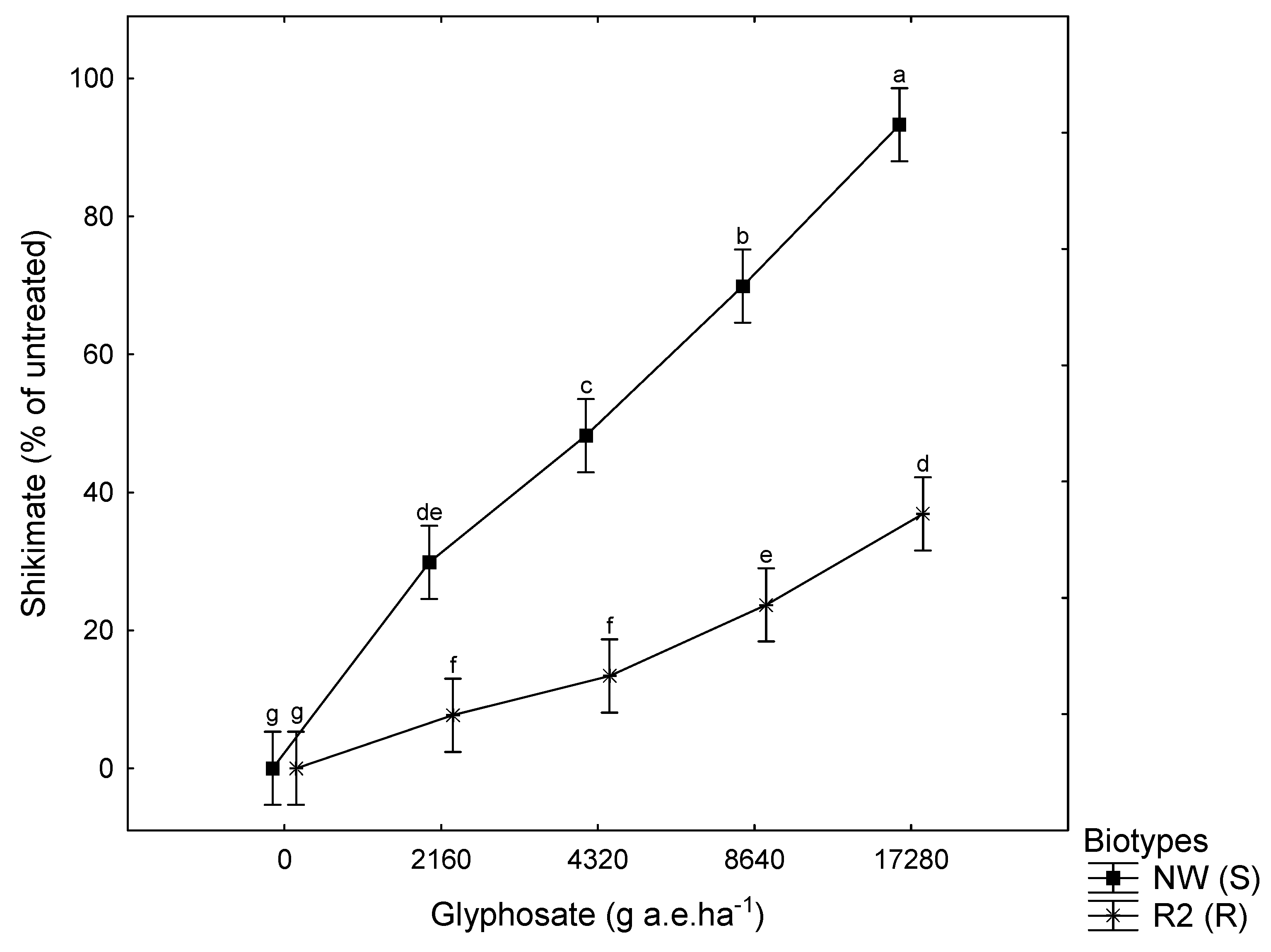
3.2. Shikimic Acid
3.3. Relationship between Shikimate, Survival Rate, and Dry Mass
3.4. Glyphosate Translocation Using 31P and 13C NMR
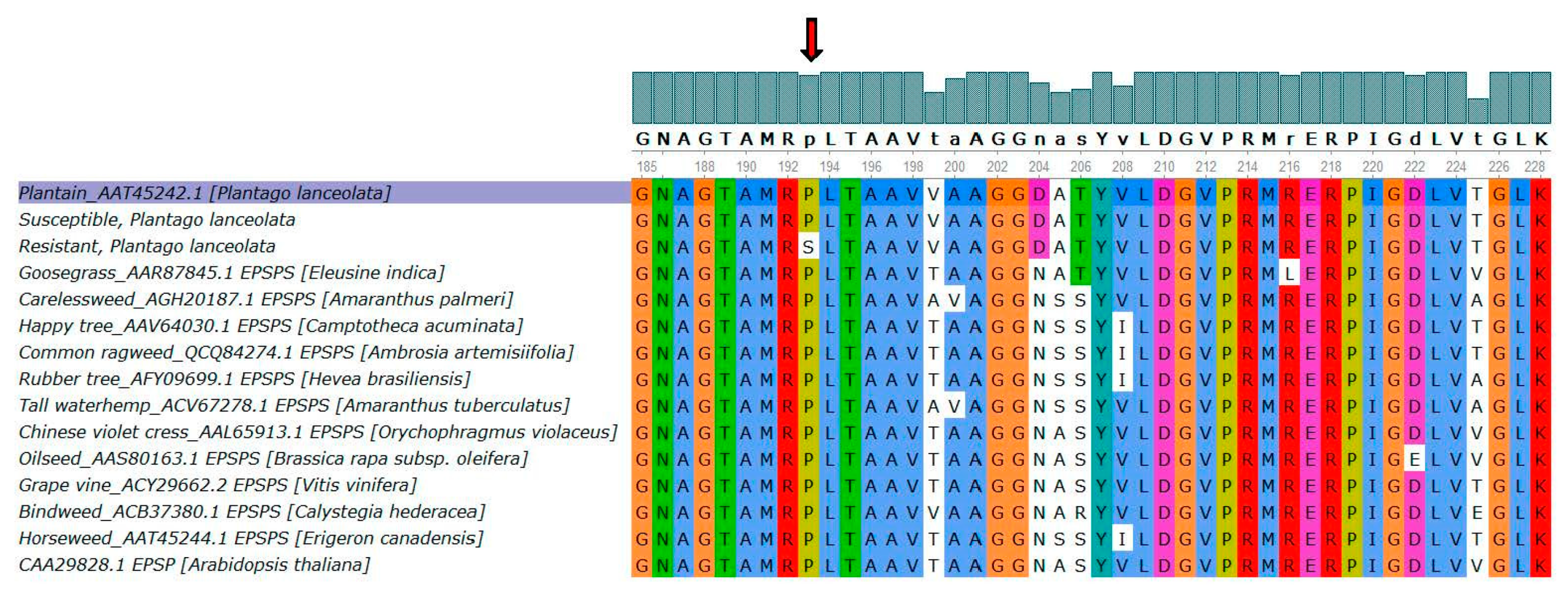
3.5. EPSPS cDNA Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duke, S.O.; Powles, S.B. Glyphosate: A once in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cairns, A.; Powles, S.B. Glyphosate, paraquat and ACCase multiple herbicide resistance evolved in a Lolium rigidum biotype. Planta 2007, 225, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Heap, I.; Duke, S.O. Overview of glyphosate–resistant weeds worldwide. Pest Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Powles, S.B. Glyphosate-resistant rigid ryegrass (Lolium rigidum) populations in the Western Australian Grain Belt. Weed Technol. 2010, 24, 44–49. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Zhang, C.; Wei, S.; Huang, Z.; Chen, J.; Wang, X. Mutations and amplification of EPSPS gene confer resistance to glyphosate in goosegrass (Eleusine indica). Planta 2015, 242, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Garcia, J.G.; Palma-Bautista, C.; Rojano-Delgado, A.M.; De Prado, R.; Menendez, J. The First Case of Glyphosate Resistance in Johnsongrass (Sorghum halepense (L.) Pers.) in Europe. Plants 2020, 9, 313. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.com (accessed on 24 March 2021).
- Ge, X.; d’Avignon, D.A.; Ackerman, J.J.; Collavo, A.; Sattin, M.; Ostrander, E.L.; Hall, E.; Sammons, R.D.; Preston, C. Vacuolar glyphosate-sequestration correlates with glyphosate resistance in ryegrass (Lolium spp.) from Australia, South America, and Europe: A 31P NMR investigation. J. Agric. Food Chem. 2012, 60, 1243–1250. [Google Scholar] [CrossRef]
- Moretti, M.L.; Hanson, B.D. Reduced translocation is involved in resistance to glyphosate and paraquat in Conyza bonariensis and Conyza canadensis from California. Weed Res. 2017, 57, 25–34. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Blabi, M.C.; Distefano, A.J.; Fernández, L.; Hopp, E.; Yu, Q.; Powles, S.B. Glyphosate resistance in perennial Sorghum halepense (Johnsongrass), endowed by reduced glyphosate translocation and leaf uptake. Pest Manag. Sci. 2012, 68, 430–436. [Google Scholar] [CrossRef]
- Nandula, V.K.; Ray, J.D.; Ribeiro, D.N.; Pan, R.Z.; Reddy, K.N. Glyphosate resistance in tall waterhemp (Amaranthus tuberculatus) from Mississippi is due to both altered target-site and nontarget-site mechanisms. Weed Sci. 2013, 61, 374–383. [Google Scholar] [CrossRef]
- Brunharo, C.; Patterson, E.L.; Carrijo, D.R.; de Melo, M.S.C.; Nicolai, M.; Gaines, T.A.; Nissen, S.J.; Christoffoleti, P.J. Confirmation and mechanism of glyphosate resistance in tall windmill grass (Chloris elata) from Brazil. Pest Manag. Sci. 2016, 72, 1758–1764. [Google Scholar] [CrossRef]
- Wakelin, A.M.; Lorraine-Colwill, D.F.; Preston, C. Glyphosate resistance in four different populations of Lolium rigidium is associated with reduced translocation of glyphosate to meristematic zones. Weed Res. 2004, 44, 453–459. [Google Scholar] [CrossRef]
- Powles, S.B.; Preston, C. Evolved glyphosate resistance in plants: Biochemical and genetic basis of resistance. Weed Technol. 2006, 20, 282–289. [Google Scholar] [CrossRef]
- Ge, X.; d’Avignon, D.A.; Ackerman, J.J.; Sammons, R.D. Rapid vacuolar sequestration: The horseweed glyphosate resistance mechanism. Pest Manag. Sci. 2010, 66, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Oromi-Farrús, M.; Minguell, J.P.; Oromi, N.; Canela-Garayoa, R. A reliable method for quantification of phosphonates and their impurities by 31P NMR. Anal. Lett. 2013, 46, 1910–1921. [Google Scholar] [CrossRef]
- Meudt, H.M. A taxonomic revision of native New Zealand Plantago (Plantaginaceae). N. Z. J. Bot. 2012, 50, 101–178. [Google Scholar] [CrossRef]
- Glen, H.F. FSA contributions 12: Plantaginaceae. Bothalia 1998, 28, 151–157. [Google Scholar] [CrossRef]
- Cranston, L.M.; Kenyon, P.R.; Morris, S.T.; Lopez-Villalobos, N.; Kemp, P.D. Morphological and physiological responses of plantain (Plantago lanceolata) and Chicory (Cichorium intybus) to water stress and defoliation frequency. J. Agron. Crop Sci. 2015, 202, 13–24. [Google Scholar] [CrossRef]
- Hammond, J. Plantago as a host of economically important viruses. Adv. Virus Res. 1982, 27, 103–138. [Google Scholar]
- Bromilow, C. Problem Plants of South Africa; Briza Publications: Pretoria, South Africa, 2001. [Google Scholar]
- Van Groenendael, J.M. Life history characteristics of two ecotypes of Plantago lanceolata L. Acta Bot. Neerl. 1986, 35, 71–86. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Becerril, J.M.; Duke, S.O.; Lydon, J. Glyphosate effects on shikimate pathway products in leaves and flowers of velvetleaf. Phytochemistry 1989, 28, 695–699. [Google Scholar] [CrossRef]
- González-Torralva, F.; Rojano-Delgado, A.M.; Luque de Castro, M.D.; Mülleder, N.; de Padro, R. Two non-target mechanisms are involved in glyphosate-resistant horseweed (Conyza canadensis L. Cronq.) biotypes. J. Plant Physiol. 2012, 169, 1673–1679. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Zhang, Y.; Zhou, Y. Analysis of (–)-Shikimic acid in chinese star anise by GC–MS with selected ion monitoring. Chromatographia 2008, 69, 339–344. [Google Scholar] [CrossRef]
- Gout, E.; Bligny, R.; Genix, P.; Tissut, M.; Douce, R. Effect of glyphosate on plant cell metabolism. 31P and 13C NMR studies. Biochemie 1992, 74, 875–882. [Google Scholar] [CrossRef]
- Christensen, A.M.; Schaefer, J. Solid-State NMR determination of intra- and intermolecular 31P-13C distances for shikimate 3-Phosphate and [l-13C] glyphosate bound to enolpyruvylshikimate-3-phosphate synthase. Biochemistry 1993, 32, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Jacob, G.S.; Schaefer, J.; Stejskal, E.O.; McKay, R.A. Solid-state NMR determination of glyphosate metabolism in a Pseudomonas sp. J. Biol. Chem. 1985, 260, 5899–5905. [Google Scholar] [CrossRef]
- Love, G.D.; Snape, C.E.; Jarvis, M.C. Comparison of leaf and stem cell-wall components in barley straw by solid-state 13C NMR. Phytochemistry 1998, 49, 1191–1194. [Google Scholar] [CrossRef]
- Gherekhloo, J.; Fernández-Moreno, P.T.; Alcántara-de la Cruz, R.; Sánchez-González, E.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; De Prado, R. Pro-106-Ser mutation and EPSPS overexpression acting together simultaneously in glyphosate-resistant goosegrass (Eleusine indica). Sci. Rep. 2017, 7, 6702. [Google Scholar] [CrossRef]
- Mucheri, T.; Pieterse, P.J.; Reinhardt, C.F.; Kleinert, A. Responses of Lolium spp. to glufosinate ammonium application at different temperatures. Weed Res. 2020, 60, 374–384. [Google Scholar] [CrossRef]
- Tehranchian, P.; Nandula, V.; Jugulam, M.; Putta, K.; Jasieniuk, M. Multiple resistance to glyphosate, paraquat and ACCase-inhibiting herbicides in italian ryegrass populations from California: Confirmation and mechanisms of resistance. Pest Manag. Sci. 2018, 74, 868–877. [Google Scholar] [CrossRef]
- Taylor, J. Investigation into the Molecular and Biochemical Mechanisms of Resistance in Two Biotypes of Glyphosate Resistant Giant Ragweed. Master’s Thesis, The University of Guelph, Guelph, ON, Canada, 2014. [Google Scholar]
- Harring, T.; Streibig, J.C.; Husted, S. Accumulation of shikimic acid: A technique for screening glyphosate efficacy. J. Agric. Food Chem. 1998, 46, 4406–4412. [Google Scholar] [CrossRef]
- Gougler, J.; Geiger, D.R. Carbon partitioning and herbicide transport in glyphosate-treated sugarbeet (Beta vulgaris). Weed Sci. 1984, 32, 546–551. [Google Scholar] [CrossRef]
- Feng, P.C.C.; Tran, M.; Chiu, T.; Sammons, R.D.; Heck, G.R.; CaJacob, C.A. Investigations into glyphosate-resistant horseweed (Conyza canadensis): Retention, uptake, translocation and metabolism. Weed Sci. 2004, 52, 498–505. [Google Scholar] [CrossRef]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.P.; Tranel, P.J. Target-site mutations conferring herbicide resistance. Plants 2019, 8, 382. [Google Scholar] [CrossRef]
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Alvarez, C.E.; Tuesca, D.; Permingeat, H.A. novel triple amino acid substitution in the EPSPS found in a high-level glyphosate-resistant Amaranthus hybridus population from Argentina. Pest Manag. Sci. 2019, 75, 1242–1251. [Google Scholar] [CrossRef]

| Locality | Coordinates | Crop |
|---|---|---|
| R2 (R) | 33°46′ S, 19°45′ E | Vineyard |
| NW (S) | 33°56′ S, 18°40′ E | Mixed vegetation |
| Glyphosate | D | b | LD50 (g a.e. ha−1) | r2 | R/S Ratio of LD/GR50 |
|---|---|---|---|---|---|
| Survival rate | |||||
| R | 100 | −0.00 | 5775 | 0.98 | 43 |
| S | 100 | −0.37 | 130 | 1.00 | |
| Dry mass | GR50 | ||||
| R | 100 | 0.00 | 784 | 0.94 | 5 |
| S | 100 | 0.10 | 134 | 1.00 |
| Shikimate | ||
|---|---|---|
| Survival Rate | Dry Mass | |
| r | −0.69 | −0.83 |
| p | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndou, V.; Pieterse, P.J.; Brand, D.J.; Vorster, A.; Louw, A.; Phiri, E. Mechanism(s) of Glyphosate Resistance in a Selected Plantago lanceolata (L.) R Biotype. Agronomy 2021, 11, 884. https://doi.org/10.3390/agronomy11050884
Ndou V, Pieterse PJ, Brand DJ, Vorster A, Louw A, Phiri E. Mechanism(s) of Glyphosate Resistance in a Selected Plantago lanceolata (L.) R Biotype. Agronomy. 2021; 11(5):884. https://doi.org/10.3390/agronomy11050884
Chicago/Turabian StyleNdou, Vhuthu, Petrus Jacobus Pieterse, Dirk Jacobus Brand, Alvera Vorster, Amandrie Louw, and Ethel Phiri. 2021. "Mechanism(s) of Glyphosate Resistance in a Selected Plantago lanceolata (L.) R Biotype" Agronomy 11, no. 5: 884. https://doi.org/10.3390/agronomy11050884
APA StyleNdou, V., Pieterse, P. J., Brand, D. J., Vorster, A., Louw, A., & Phiri, E. (2021). Mechanism(s) of Glyphosate Resistance in a Selected Plantago lanceolata (L.) R Biotype. Agronomy, 11(5), 884. https://doi.org/10.3390/agronomy11050884






