Effect of the Degree of Soil Contamination with Heavy Metals on Their Mobility in the Soil Profile in a Microplot Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Location
2.2. Study Area
2.3. Collection and Processing of Soils
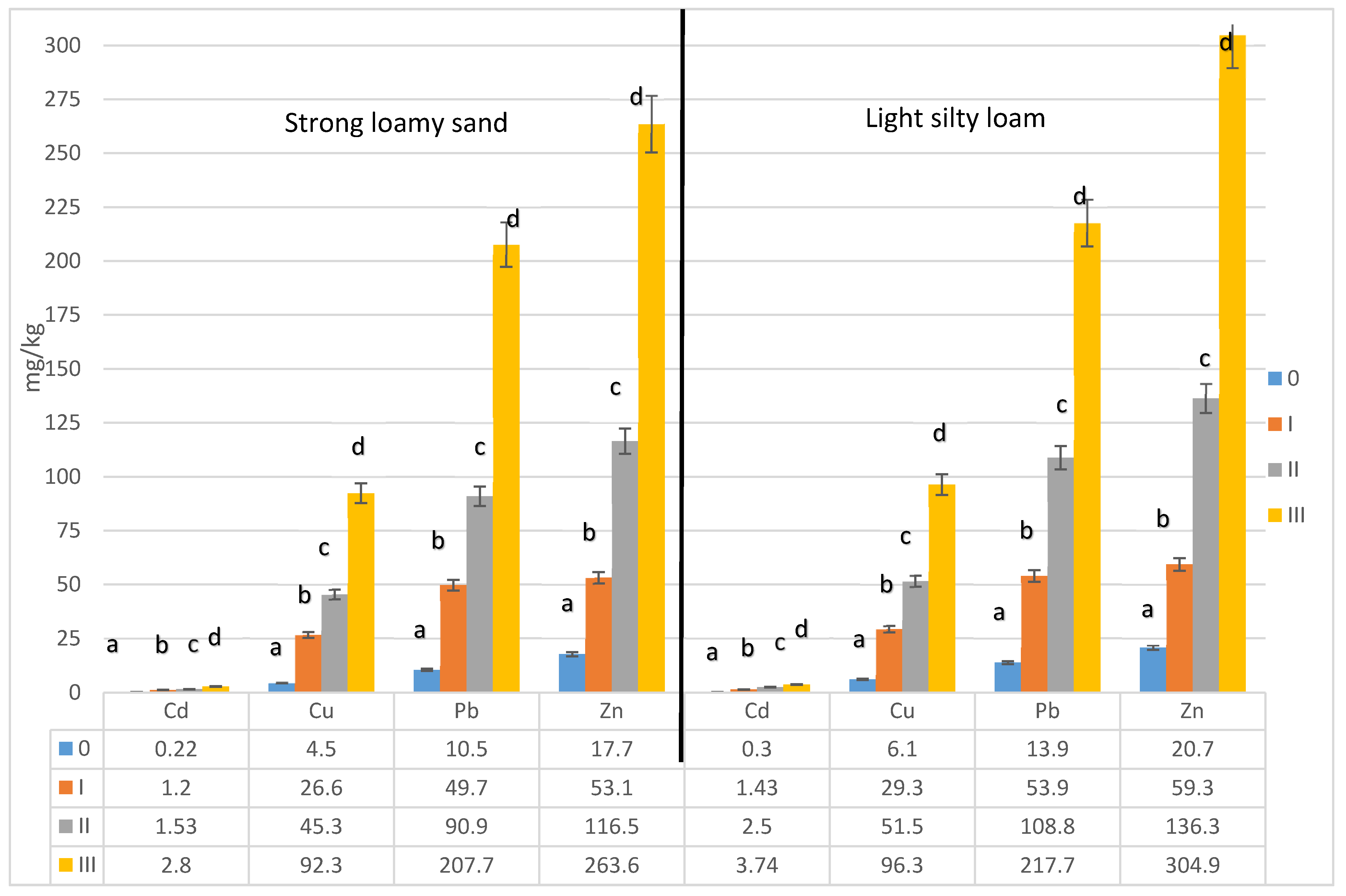
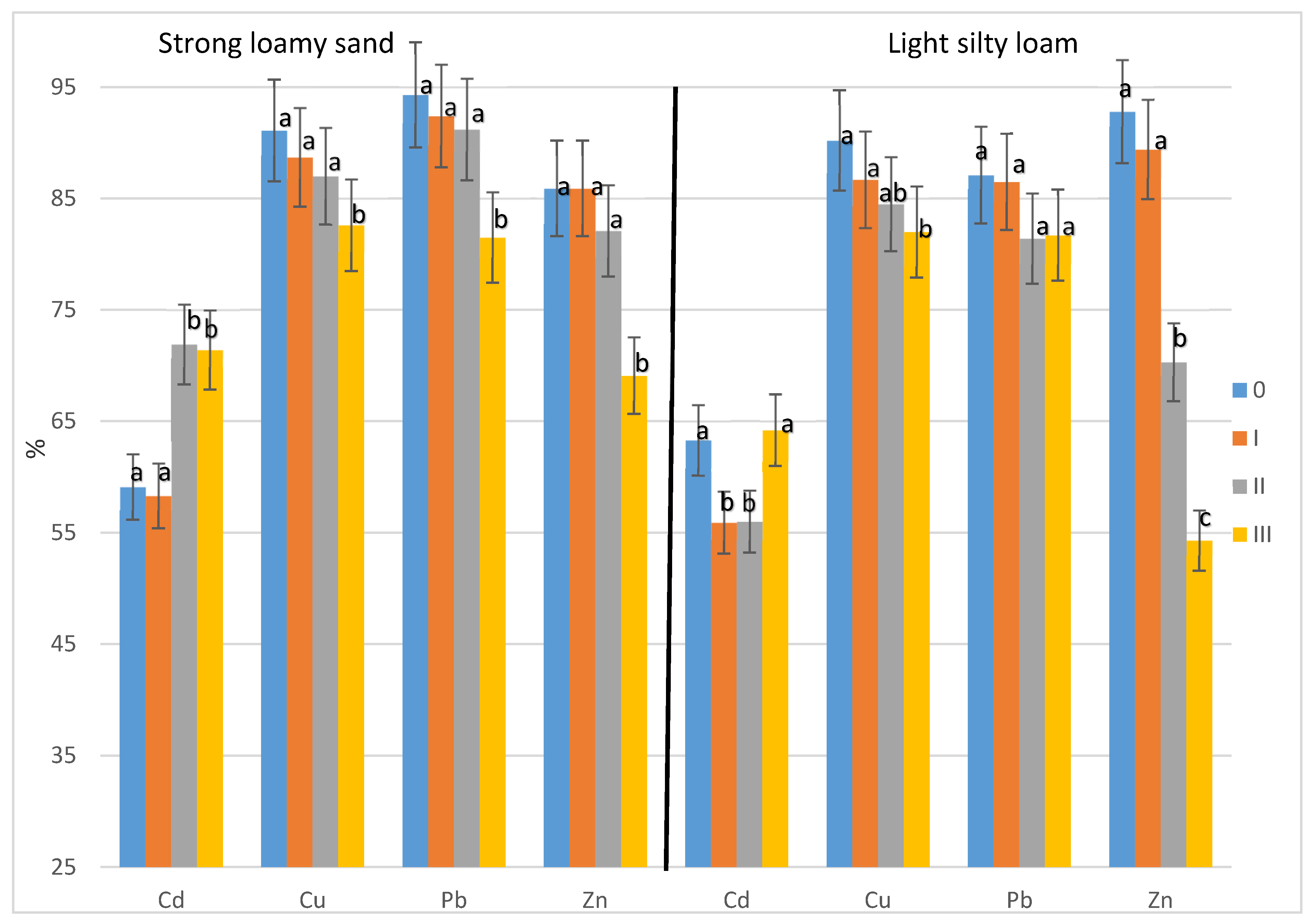
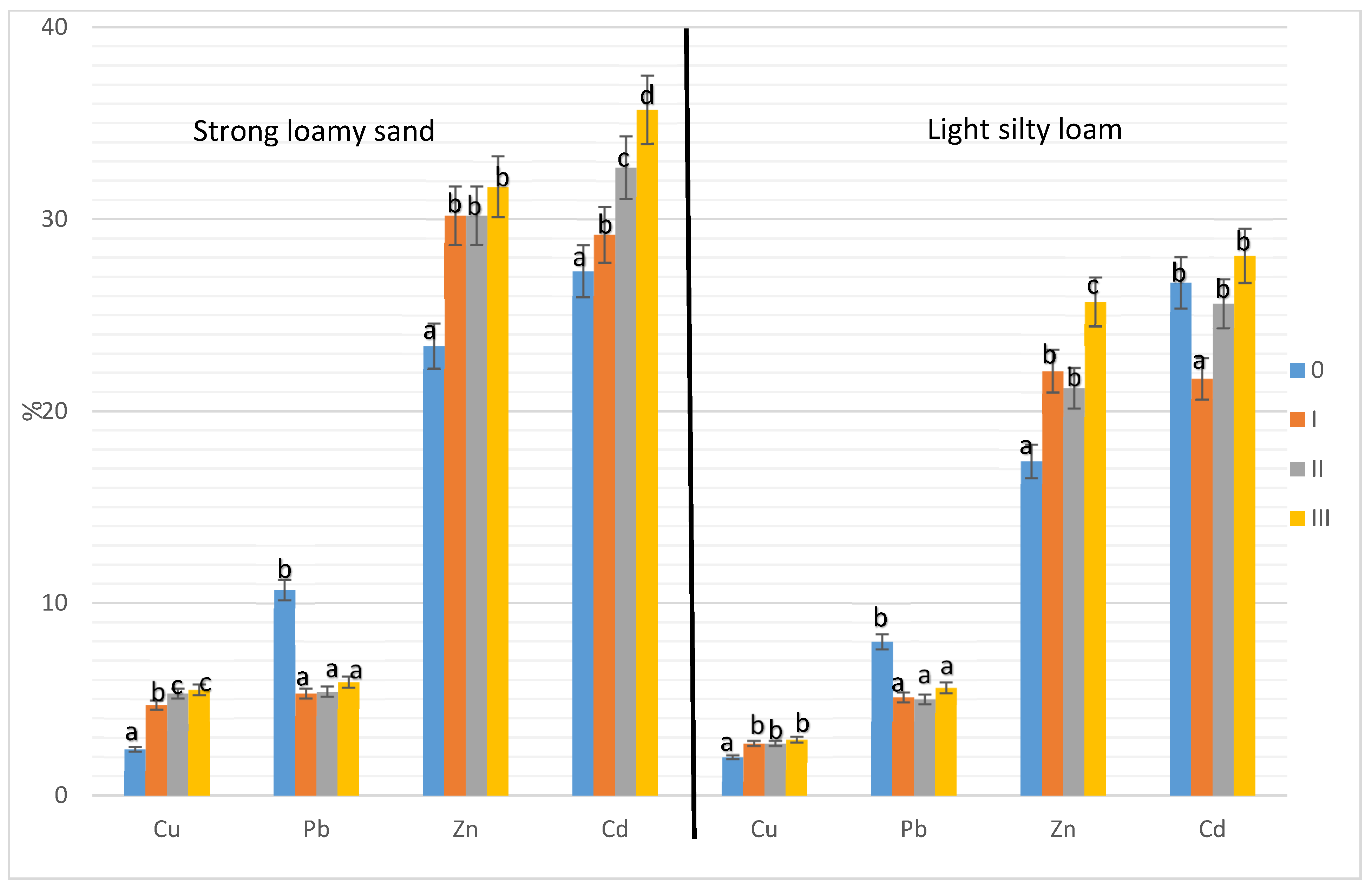
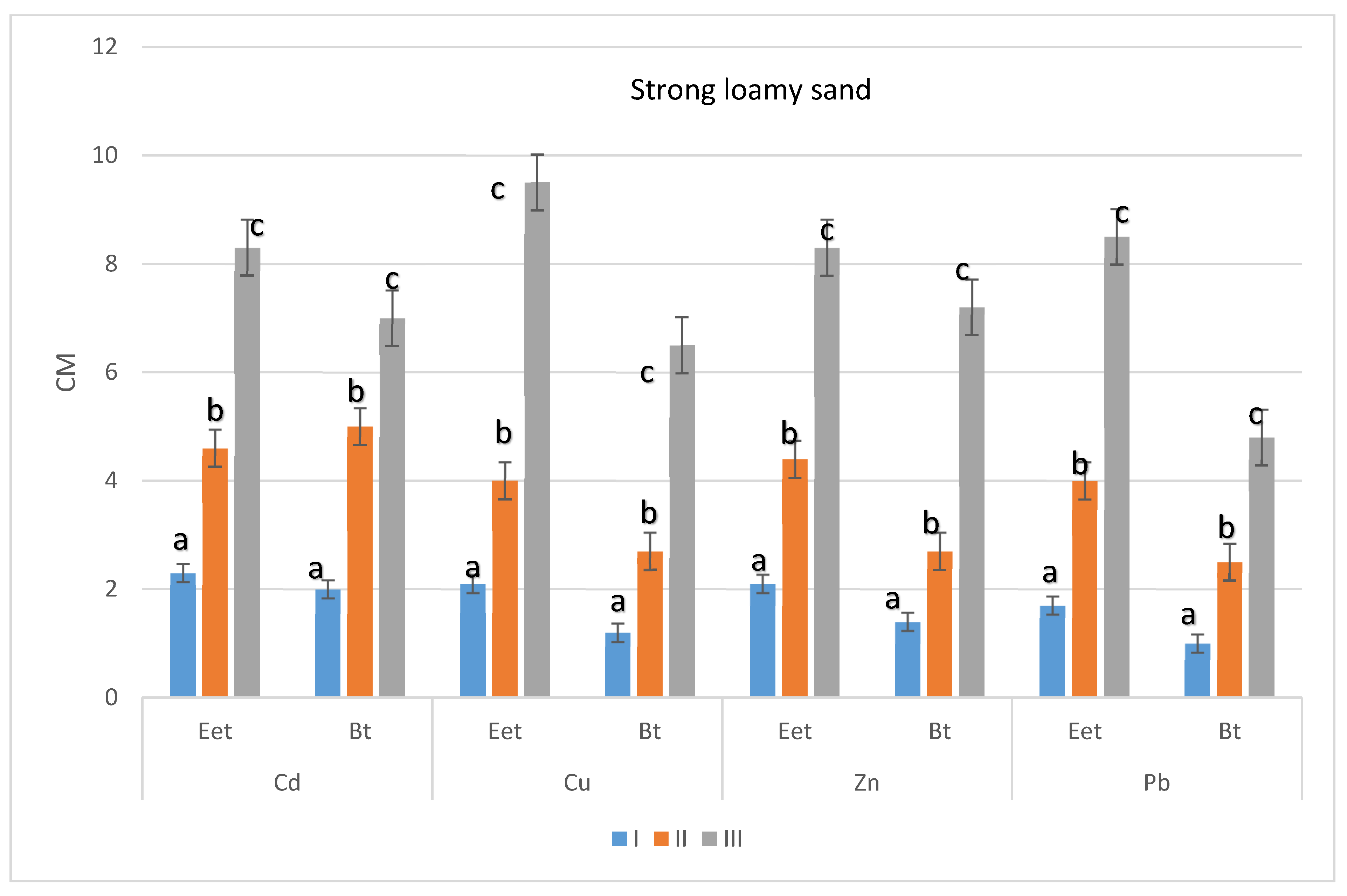
3. Results
3.1. Physico-Chemical Characteristics of Soils
3.2. Share of Soluble Forms of Heavy Metals in the Total Content of These Elements in the Soil
3.3. The Relocation of Heavy Metals in Soil Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kabata-Pendias, A. Agricultural Problems Related to Excessive Trace Metal Contents of Soils. In Heavy Metals; Förstner, U., Salomons, W., Mader, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 3–18. [Google Scholar]
- Kabata-Pendias, A. Soil-plant transfer of trace elements an environment issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Cadmium in the Human Environment: Toxicity and Carcinogenicity. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cadmium-In-The-Human-Environment-Toxicity-And-Carcinogenicity-1992 (accessed on 3 February 2020).
- Sillanpää, M. Trace elements in soil and agriculture. FAO Soils Bull. 1972, 17, 33. [Google Scholar]
- Dziadek, K.; Wacławek, W. Metals in the Environment, Vol. I Heavy Metals (Zn, Cu, Ni, Pb, Cd) in the Soils Environment; Department of Chemical Physics, University of Opole, Institute of Chemistry: Opole, Poland, 2005; Volume 10, pp. 1–2. [Google Scholar]
- Puga, S.; Sosa, M.; Lebgue, T.; Quintana, C.; Campos, A. Heavy metals pollution in soils damaged by mining industry. Ecol. Appl. 2006, 5, 149–155. [Google Scholar] [CrossRef]
- Senesil, G.S.; Baldassaree, G.; Senes, N.; Radina, B. Trace element inputs into soils by anthropogenic activities and implications for human health. Chemosphere 1999, 39, 343–377. [Google Scholar] [CrossRef]
- Koncewicz-Baran, M.; Gondek, K. Content of trace elements in agricultural soils. Infrastruct. Ecol. Rural. Areas 2010, 14, 65–74. [Google Scholar]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernandez-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Gabisu, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead and arsenic. Environ. Sci. Bio/Technol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Shiva Kumar, D.; Srikantaswamy, S. Factors Affecting on Mobility of Heavy Metals in Soil Environment. IJSRD 2014, 2, 201–203. [Google Scholar]
- Kabata-Pendias, A.; Motowicka-Terelak, T.; Piotrowska, M.; Terelak, H.; Witek, T. The assesmen of the quality and possibilities of agricultural using soils polluted with heavy metals. Assessment of the degree of polluting soils and plants with heavy metals and sulphor. In General Guidelines for the Farming; Institute of Soil Science and Plant Cultivation: Pulawy, Poland, 1993; Volume 53, p. 20. [Google Scholar]
- Gembarzewski, H.; Korzeniowska, J. The Using of 1 M HCl Solution as a Common Extractant to Assess the Abundance of Soil in Available Forms of Microelements; Works of the Scientific Committee of the Polish Soil Science Society; Scientific Committee of the Polish Soil Science Society: Warsaw, Poland, 1987; Volume 991–999. [Google Scholar]
- Fotyma, M.; Mercik, S. Agricultural Chemistry; Polish Scientific Publishing House: Warsaw, Poland, 1992; ISBN 83-01-10654-9. [Google Scholar]
- Sykut, S.; Ruszkowska, M.; Wojcieska, U.K. Collection and washing of cadmium and nickel depending on soil type and fertilization (results of lysimetric experiment). Adv. Agric. Sci. Probl. Issues 1997, 448a, 317–322. [Google Scholar]
- Gębski, M. Development of a Method of Fertilization on Light Soils Contaminated with Heavy Metals. Ph.D. Thesis, Warsaw University of Life Sciences, Warsaw, Poland, 1997; 112p. [Google Scholar]
- Mercik, S.; Stępień, W.; Gębski, M. Cadmium content in plants and soil profiles as a result of two or three applications of lime from the lead smelter. Adv. Agric. Sci. Probl. Issues 1997, 448a, 253–259. [Google Scholar]
- Borowiec, J.; Baran, S.; Turski, R. Accumulation of Pb, Zn, Cd and Cr in individual mechanical fractions of soils contaminated with dust from zinc smelters. Adv. Agricul. Sci. Probl. Issues 1997, 448a, 241–246. [Google Scholar]
- Fulekar, M.H.; Dave, J.M. Release and behaviour of Cr, Mn, Ni and Pb in a flyash/soil/water environment: Column experiment. Int. J. Environ. Stud. 1991, 38, 281–296. [Google Scholar] [CrossRef]
- Sommers, L.E.; Nelson, D.W.; Silviera, D.J. Transformation of carbon, nitrogen and metals in soils treated with waste materials. J. Environ. Qual. 1979, 8, 287–294. [Google Scholar] [CrossRef]
- Williams, D.E.; Vlamis, J.; Pukite, A.H.; Corey, J.E. Metal movement in sludge-amended soils: A nine year study. Soil Sci. 1987, 143, 124–131. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Kacprzak, M.; Grobelak, A.; Placek, A. The influence of selected soil parameters on the mobility of heavy metals in soil. Eng. Environ. Prot. 2012, 15, 81–92. [Google Scholar]
- Wessolek, G.; Fahrenhorst, C. Immobilization of heavy metals in a polluted soil of a sexage farm by application of a modified alumino-silicate: A laboratory and numerical displacement study. Soil Technol. 1994, 7, 221–232. [Google Scholar] [CrossRef]
- Bowen, H.M. Environmental Chemistry of the Elements; Academic Press: New York, NY, USA, 1979; 333p. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; Polish Scientific Publishing House: Warszawa, Poland, 1999; p. 398. [Google Scholar]
- Lipiński, W.; Bednarek, W. The presence of cadmium and nickel in soils with different granulometric compositions. Adv. Agric. Sci. Probl. Issues 1997, 448a, 231–235. [Google Scholar]
- Vaněk, A.; Borůvka, L.; Drábek, O.; Mihaljevič, M.; Komárek, M. Mobility of lead, zinc and cadmium in alluvial soils heavily polluted by smelting industry. Plant Soil Environ. 2005, 51, 316–332. [Google Scholar] [CrossRef]
- Kaniuczak, J.; Hajduk, E. Cadmium and lead in some soils of south-eastern Poland. Adv. Agric. Sci. Probl. Issuss 1995, 18, 241–246. [Google Scholar]
- Patorczyk-Pytlik, B.; Spiak, Z. Impact of Częstochowa iron-works on the soil environment and chemical composition of plants. Adv. Agric. Sci. Probl. Issues 1999, 467, 421–428. [Google Scholar]

| Soil Texture | Corg. Content in g∙kg−1 | Soil pH | S | Hh | T | V |
|---|---|---|---|---|---|---|
| mmol (+) kg−1 | % | |||||
| Strong loamy sand | 4 | 29.6 a | 43.6 c | 73.1 a | 40 | |
| 5 | 37.4 b | 36.1 b | 73.5 a | 51 | ||
| 6 | 58.1 c | 30.4 a | 88.6 b | 66 | ||
| Light silty loam | 4 | 87.8 a | 34.1 c | 121.9 a | 72 | |
| 5 | 102.4 b | 29.5 b | 131.9 b | 78 | ||
| 6 | 121.4 c | 23.5 a | 144.9 c | 84 | ||
| Strong loamy sand | 6 | 34.1 a | 30.0 a | 64.2 a | 53 | |
| 9 | 41.1 b | 35.7 b | 76.8 b | 54 | ||
| 12 | 49.8 c | 44.4 c | 94.2 c | 53 | ||
| Light silty loam | 6 | 96.1 a | 23.3 a | 119.4 a | 80 | |
| 9 | 102.7 b | 27.7 b | 130.4 b | 79 | ||
| 12 | 112.7 c | 36.1 c | 148.8 c | 76 | ||
| Soil Texture | Mean Content of Ca, Mg and K w mg∙kg −1 d.w. | ||
|---|---|---|---|
| Ca | Mg | K | |
| Light | 674.3 | 55.9 | 130.0 |
| Medium | 1440.2 | 332.9 | 159.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikuła, D.; Stępień, W. Effect of the Degree of Soil Contamination with Heavy Metals on Their Mobility in the Soil Profile in a Microplot Experiment. Agronomy 2021, 11, 878. https://doi.org/10.3390/agronomy11050878
Pikuła D, Stępień W. Effect of the Degree of Soil Contamination with Heavy Metals on Their Mobility in the Soil Profile in a Microplot Experiment. Agronomy. 2021; 11(5):878. https://doi.org/10.3390/agronomy11050878
Chicago/Turabian StylePikuła, Dorota, and Wojciech Stępień. 2021. "Effect of the Degree of Soil Contamination with Heavy Metals on Their Mobility in the Soil Profile in a Microplot Experiment" Agronomy 11, no. 5: 878. https://doi.org/10.3390/agronomy11050878
APA StylePikuła, D., & Stępień, W. (2021). Effect of the Degree of Soil Contamination with Heavy Metals on Their Mobility in the Soil Profile in a Microplot Experiment. Agronomy, 11(5), 878. https://doi.org/10.3390/agronomy11050878







