Sweet Sorghum (Sorghum bicolor) Performance in a Legume Intercropping System under Weed Interference
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Design, and Trial Management
2.2. Data, Soil Sample Collection, and Yield
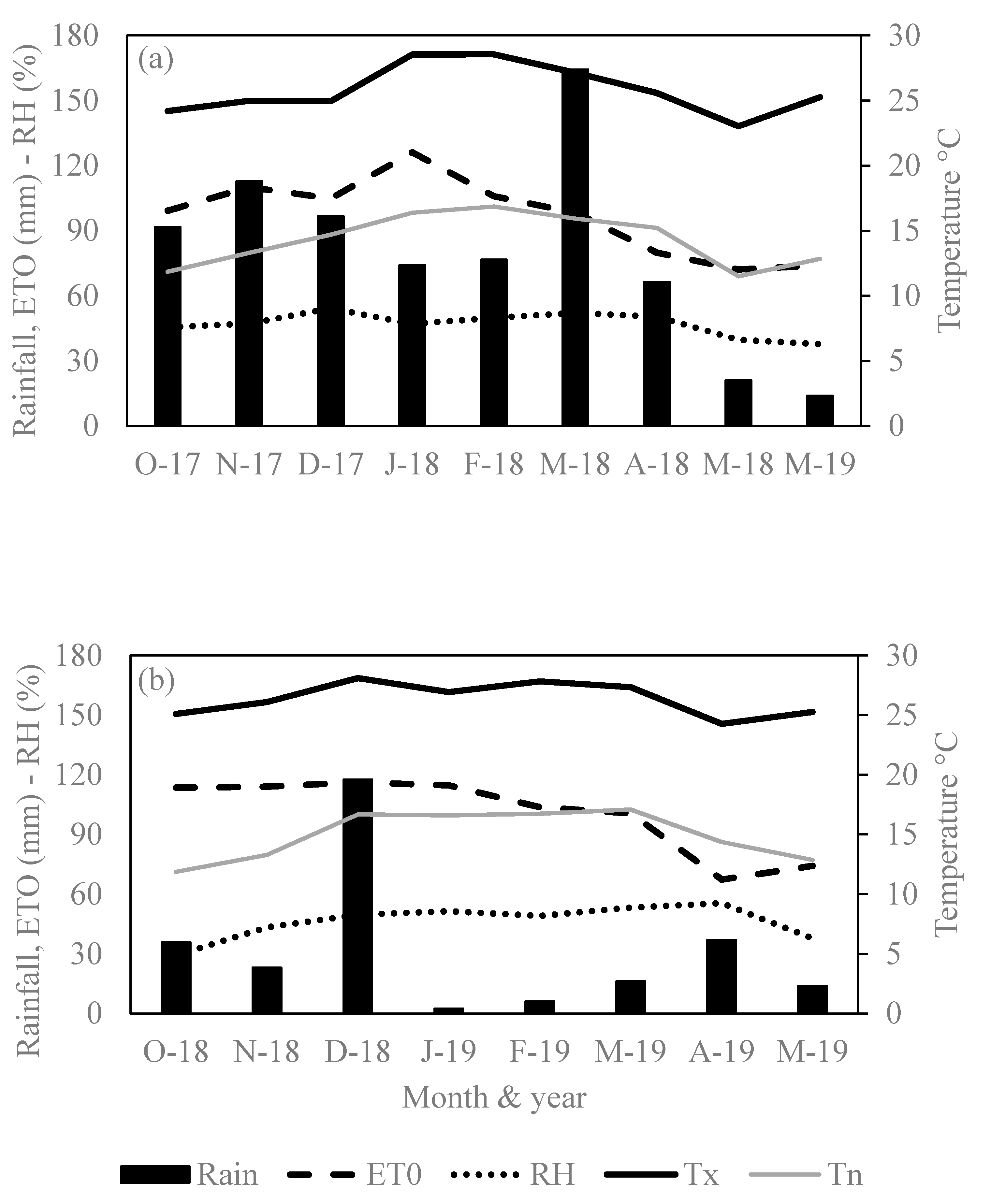
2.3. Soil Analysis and Climatic Condition
2.4. Statistical Analysis
3. Results
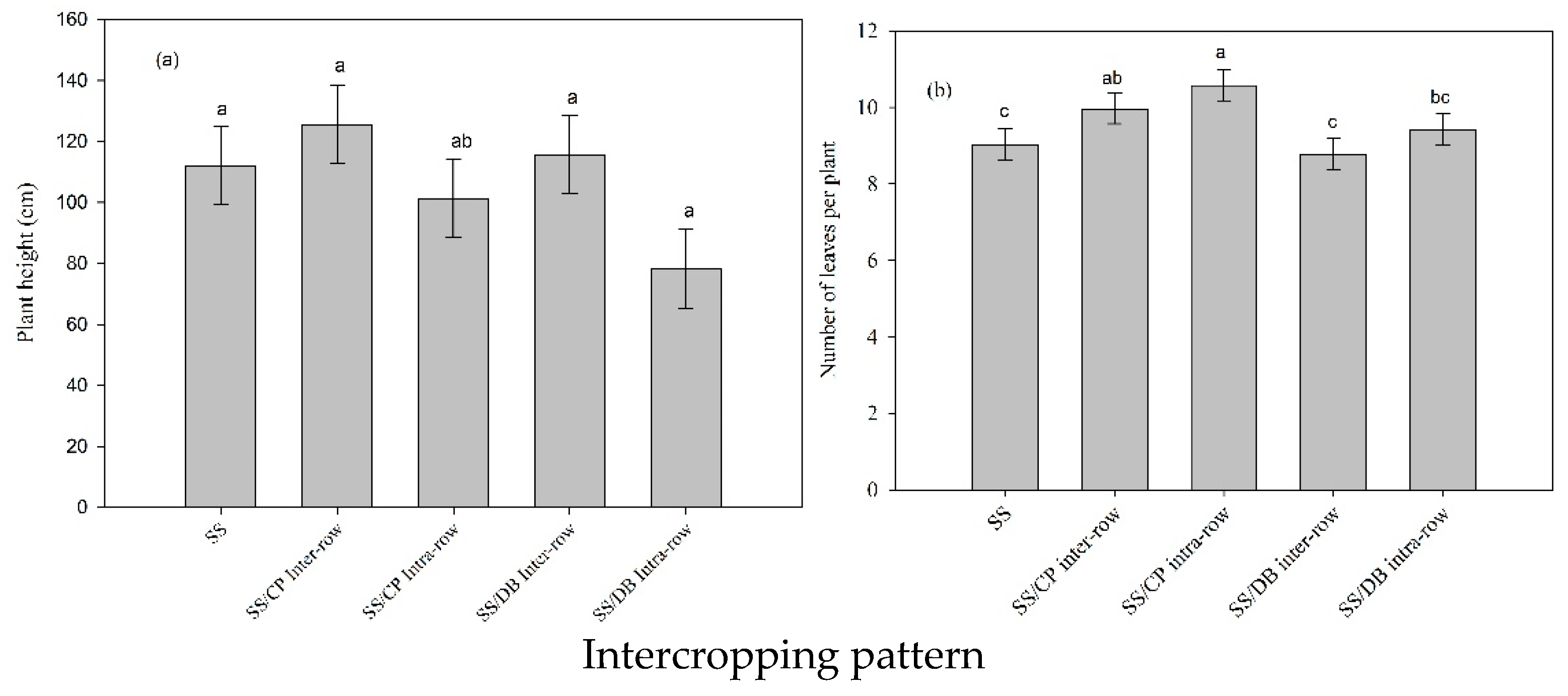
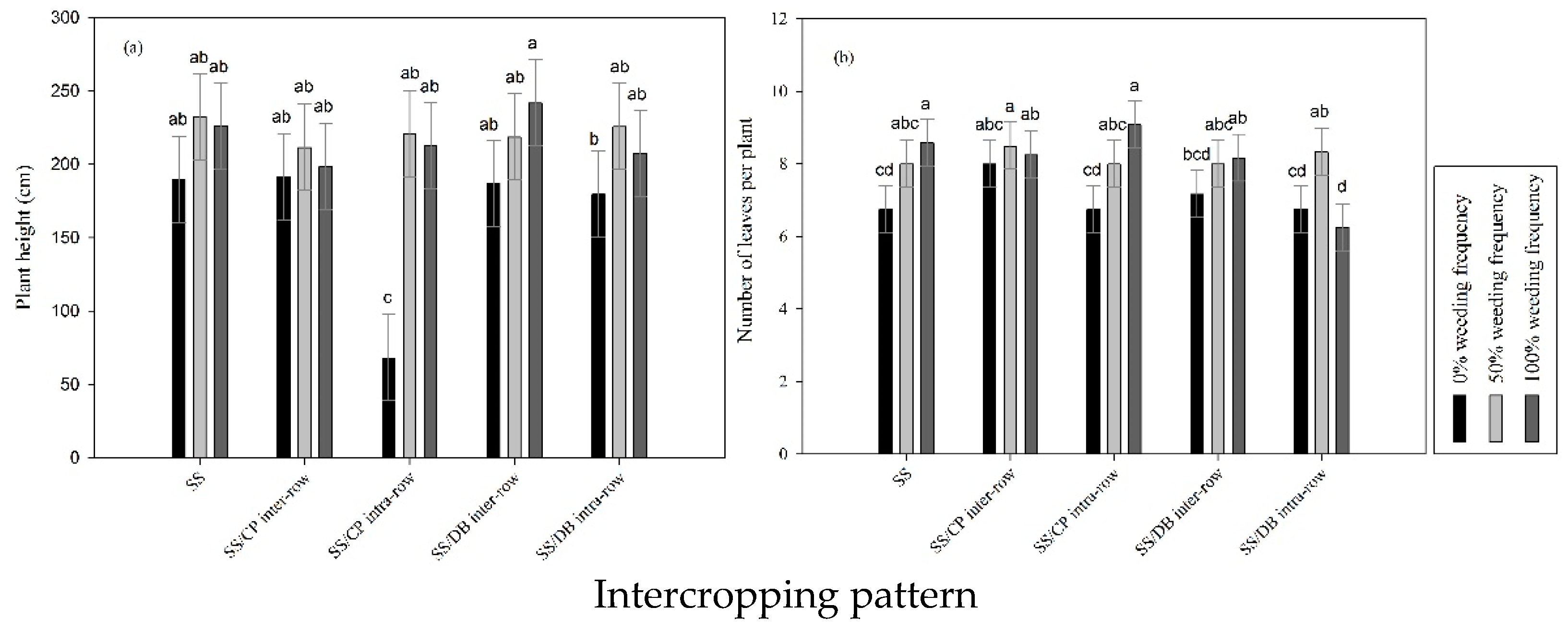
3.1. Intercropping Patterns and Weeding Frequency Effects on SS Measured Agronomic Traits
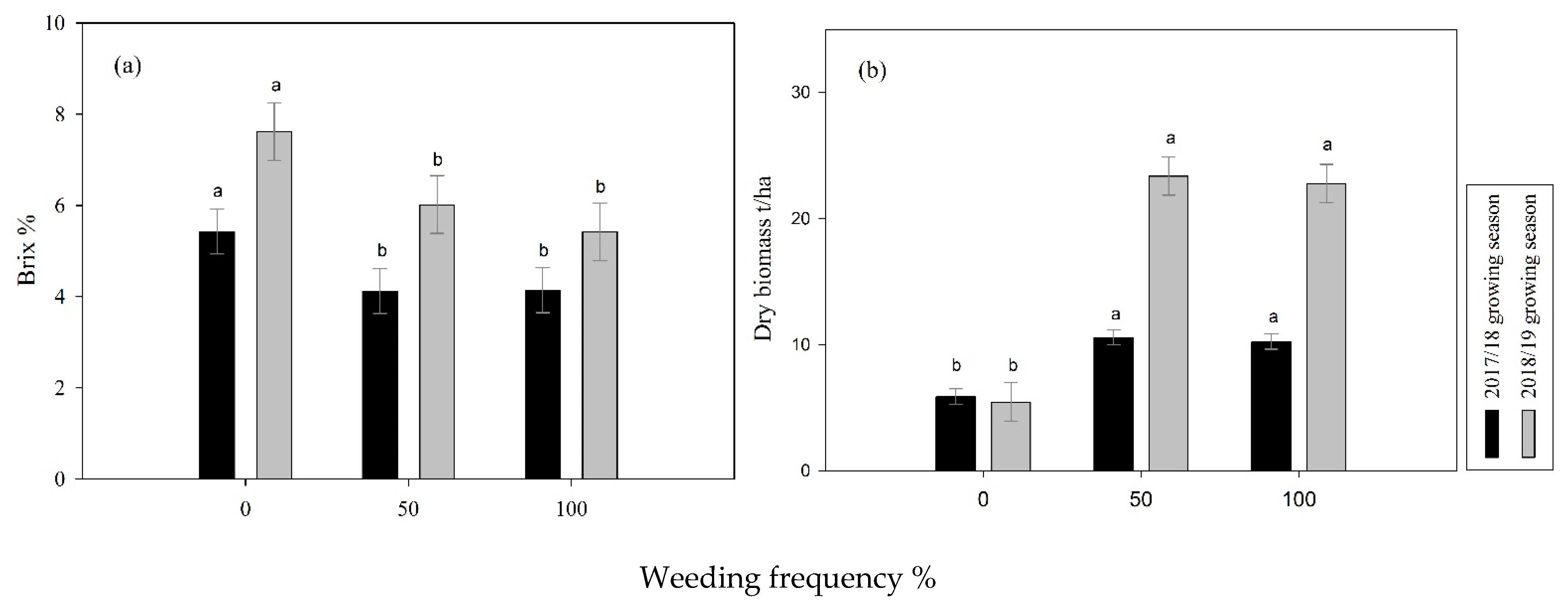
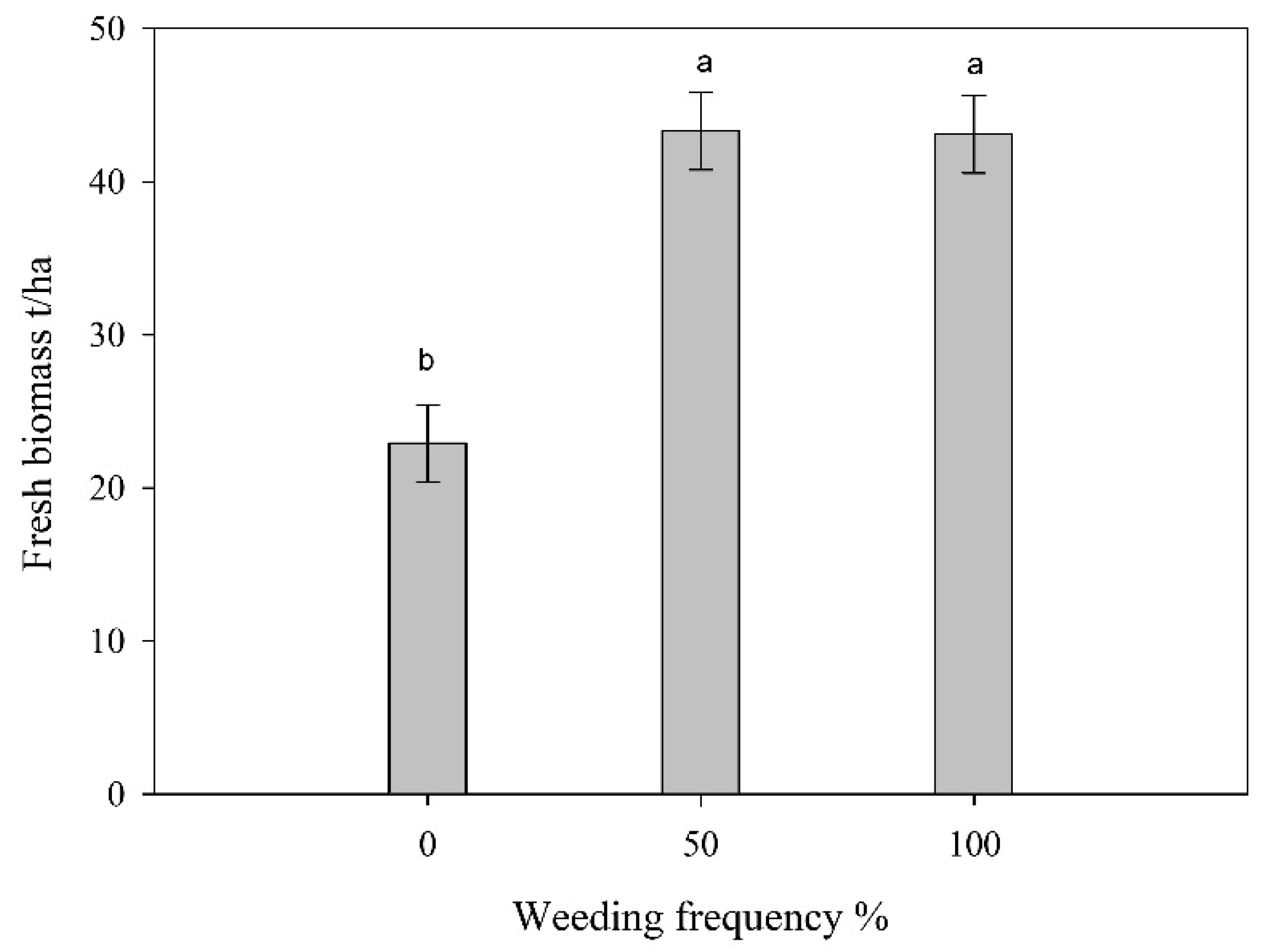
3.2. Brix, Dry and Fresh Biomass Yield Accumulation as Affected by Weeding Frequency
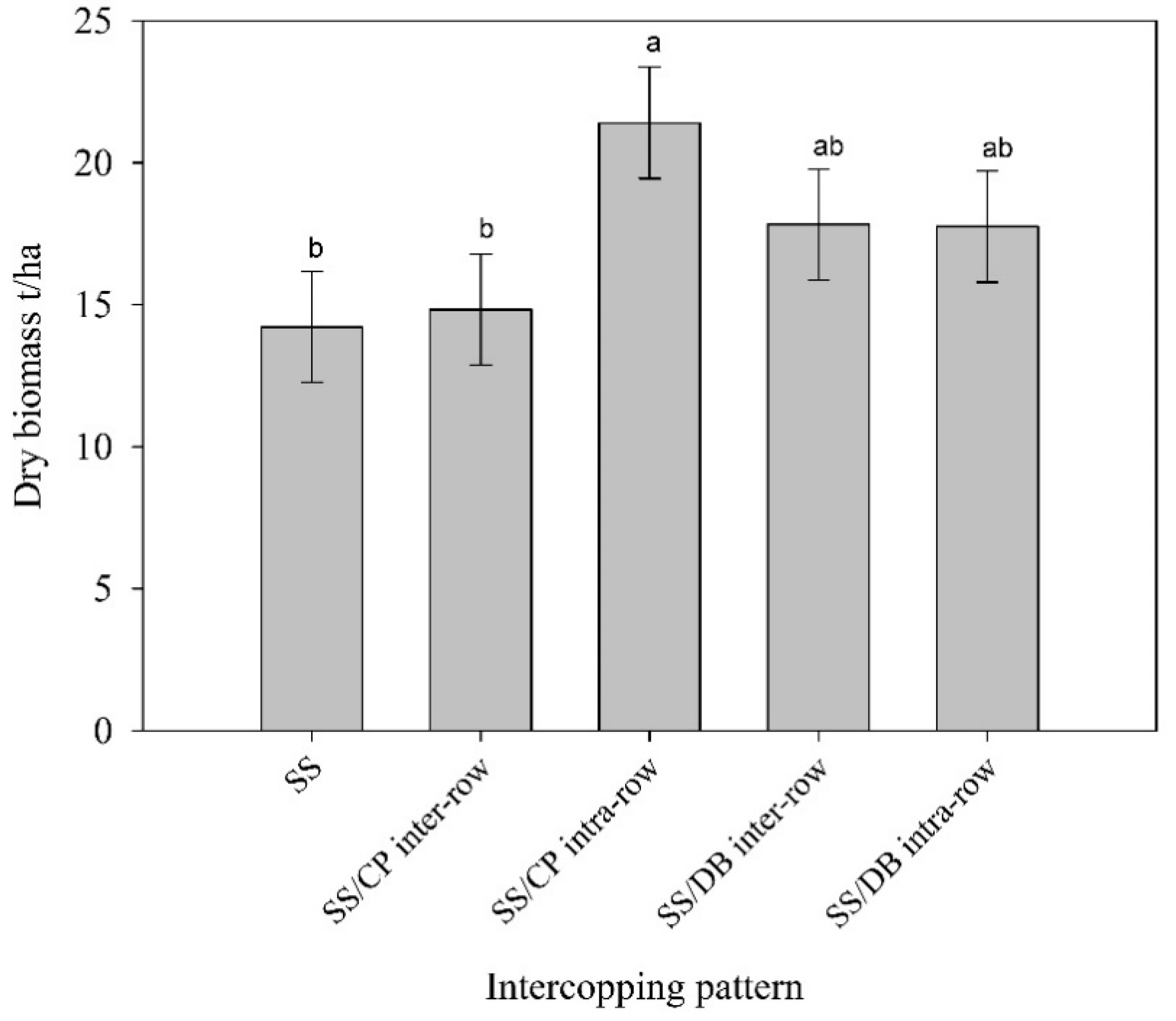
3.3. Dry and Fresh Biomass Yield Accumulation as Affected by Intercropping Pattern
3.4. Impact of Intercropping Pattern and Weeding Frequency on SS Height and Number of Leaves per Plant
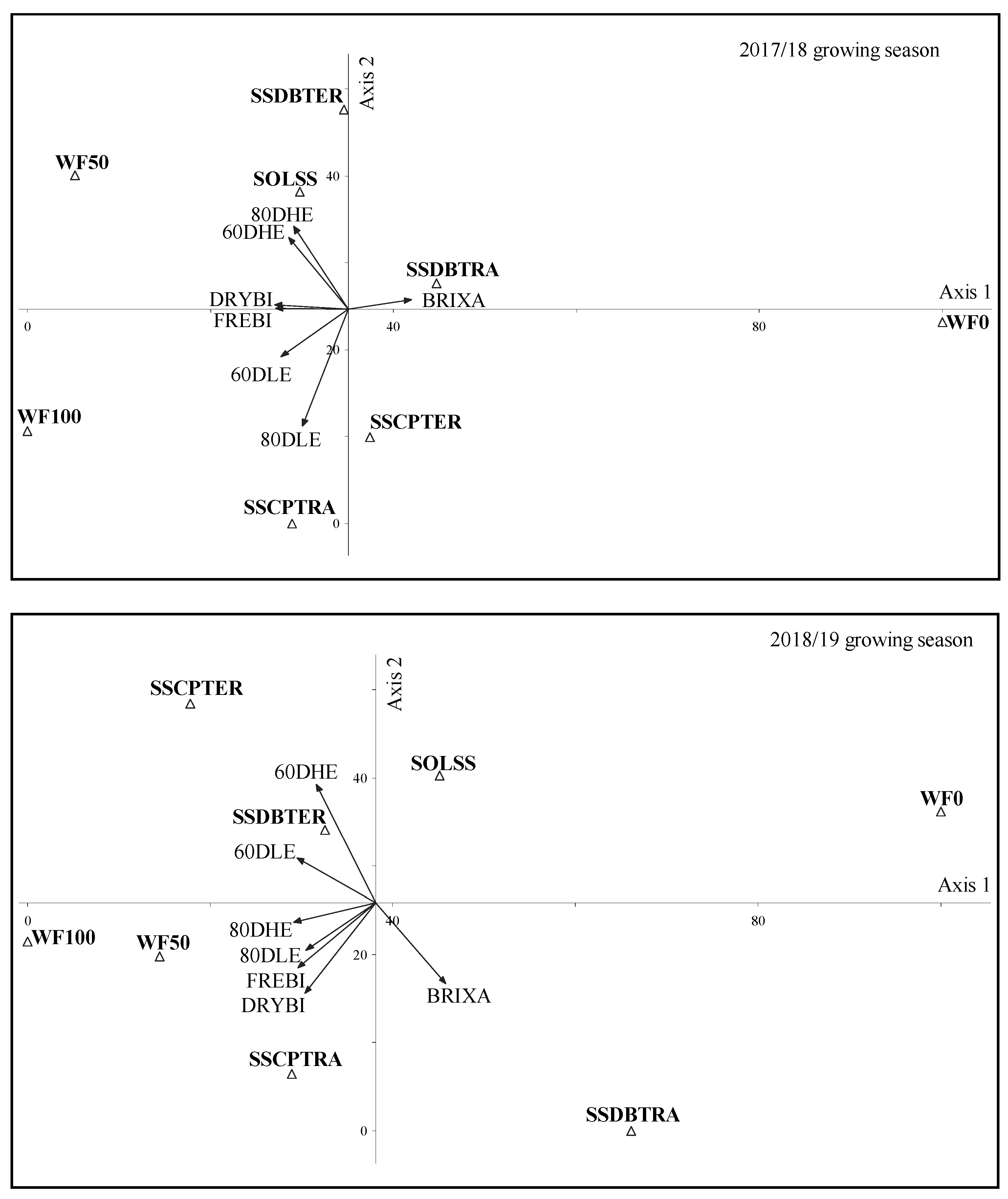
3.5. LER and PCA Analysis of SS/DB or CP Intercropping in Weed Interference
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mabhaudhi, T.; Chimonyo, V.G.P.; Chibarabada, T.P.; Modi, A.T. Developing a Roadmap for Improving Neglected and Underutilized Crops: A Case Study of South Africa. Front. Plant Sci. 2017, 8, 2143. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, Z.; Chen, C.; Xie, P.; Xie, Q. The Prospect of Sweet Sorghum as the Source for High Biomass Crop. J. Agric. Sci. Bot. 2018, 2. [Google Scholar] [CrossRef]
- Gomiero, T. Are Biofuels an Effective and Viable Energy Strategy for Industrialized Societies? A Reasoned Overview of Potentials and Limits. Sustainability 2015, 7, 8491–8521. [Google Scholar] [CrossRef]
- Hadebe, S.T.; Modi, A.T.; Mabhaudhi, T. Drought Tolerance and Water Use of Cereal Crops: A Focus on Sorghum as a Food Security Crop in Sub-Saharan Africa. J. Agron. Crop Sci. 2017, 203, 177–191. [Google Scholar] [CrossRef]
- Prasad, S.; Sheetal, K.R.; Renjith, P.S.; Kumar, A.; Kumar, S. Sweet Sorghum: An Excellent Crop for Renewable Fuels Production. In Prospects of Renewable Bioprocessing in Future Energy Systems; Rastegari, A.A., Yadav, A.N., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 291–314. [Google Scholar] [CrossRef]
- Damasceno, C.M.B.; Schaffert, R.E.; Dweikat, I. Mining Genetic Diversity of Sorghum as a Bioenergy Feedstock. In Plants and BioEnergy; McCann, M.C., Buckeridge, M.S., Carpita, N.C., Eds.; Springer New York: New York, NY, USA, 2014; pp. 81–106. [Google Scholar] [CrossRef]
- Bassam, N.E. Handbook of Bioenergy Crops: A Complete Reference to Species, Development and Applications; Routledge Studies in Bioenergy Series; Earthscan: London, UK, 2010. [Google Scholar]
- Martin-Guay, M.-O.O.; Paquette, A.; Dupras, J.; Rivest, D. The New Green Revolution: Sustainable Intensification of Agriculture by Intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of Grain Legumes and Cereals Improves the Use of Soil N Resources and Reduces the Requirement for Synthetic Fertilizer N: A Global-Scale Analysis. Agron. Sustain. Dev. 2020, 40. [Google Scholar] [CrossRef]
- Feike, T.; Doluschitz, R.; Chen, Q.; Graeff-Hönninger, S.; Claupein, W. How to Overcome the Slow Death of Intercropping in the North China Plain. Sustainability 2012, 4, 2550–2565. [Google Scholar] [CrossRef]
- Peoples, M.B.; Hauggaard-Nielsen, H.; Huguenin-Elie, O.; Jensen, E.S.; Justes, E.; Williams, M. Chapter 8—The Contributions of Legumes to Reducing the Environmental Risk of Agricultural Production; Lemaire, G., Carvalho, P.C.D.F., Kronberg, S., Recous, S.B.T.-A.D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 123–143. [Google Scholar] [CrossRef]
- Boudreau, M.A. Diseases in Intercropping Systems. Annu. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef]
- Lopes, T.; Hatt, S.; Xu, Q.; Chen, J.; Liu, Y.; Francis, F. Wheat (Triticum Aestivum L.)-Based Intercropping Systems for Biological Pest Control. Pest Manag. Sci. 2016, 72, 2193–2202. [Google Scholar] [CrossRef]
- Korres, N.E. Chapter 6—Agronomic Weed Control: A Trustworthy Approach for Sustainable Weed Management; Jabran, K., Chauhan, B.S.B.T.-N.-C.W.C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 97–114. [Google Scholar] [CrossRef]
- Burnside, O.C.; Wicks, G.A. The Effect of Weed Removal Treatments on Sorghum Growth. Weeds 1967, 15, 204–207. [Google Scholar] [CrossRef]
- Smith, B.S.; Murray, D.S.; Green, J.D.; Wanyahaya, W.M.; Weeks, D.L. Interference of Three Annual Grasses with Grain Sorghum (Sorghum bicolor). Weed Technol. 1990, 4, 245–249. [Google Scholar] [CrossRef]
- Xu, Y.; He, R.; Gao, Z.; Li, C.; Zhai, Y.; Jiao, Y. Weed Density Detection Method Based on Absolute Feature Corner Points in Field. Agronomy 2020, 10, 113. [Google Scholar] [CrossRef]
- Capinera, J.L. Relationships between Insect Pests and Weeds: An Evolutionary Perspective. Weed Sci. 2005, 53, 892–901. [Google Scholar] [CrossRef]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Yadav, P.; Priyanka, P.; Kumar, D.; Yadav, A.; Yadav, K. Bioenergy Crops: Recent Advances and Future Outlook. In Prospects of Renewable Bioprocessing in Future Energy Systems; Rastegari, A.A., Yadav, A.N., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 315–335. [Google Scholar] [CrossRef]
- Shukla, S.; Felderhoff, T.J.; Saballos, A.; Vermerris, W. The Relationship between Plant Height and Sugar Accumulation in the Stems of Sweet Sorghum (Sorghum bicolor (L.) Moench). Field Crops Res. 2017, 203, 181–191. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jensen, E.S. Facilitative Root Interactions in Intercrops. Plant Soil 2005, 274, 237–250. [Google Scholar] [CrossRef]
- Bilalis, D.; Papastylianou, P.; Konstantas, A.; Patsiali, S.; Karkanis, A.; Efthimiadou, A. Weed-Suppressive Effects of Maize–Legume Intercropping in Organic Farming. Int. J. Pest Manag. 2010, 56, 173–181. [Google Scholar] [CrossRef]
- Dayoub, E.; Naudin, C.; Piva, G.; Shirtliffe, S.J.; Fustec, J.; Corre-Hellou, G. Traits Affecting Early Season Nitrogen Uptake in Nine Legume Species. Heliyon 2017, 3, e00244. [Google Scholar] [CrossRef] [PubMed]
- Corre-Hellou, G.; Dibet, A.; Hauggaard-Nielsen, H.; Crozat, Y.; Gooding, M.; Ambus, P.; Dahlmann, C.; von Fragstein, P.; Pristeri, A.; Monti, M.; et al. The Competitive Ability of Pea–Barley Intercrops against Weeds and the Interactions with Crop Productivity and Soil N Availability. Field Crops Res. 2011, 122, 264–272. [Google Scholar] [CrossRef]
- Naudin, C.; Corre-Hellou, G.; Pineau, S.; Crozat, Y.; Jeuffroy, M.-H. The Effect of Various Dynamics of N Availability on Winter Pea–Wheat Intercrops: Crop Growth, N Partitioning and Symbiotic N2 Fixation. Field Crops Res. 2010, 119, 2–11. [Google Scholar] [CrossRef]
- Li, F.Y.; Jamieson, P.D.; Johnstone, P.R.; Pearson, A.J. Mechanisms of Nitrogen Limitation Affecting Maize Growth: A Comparison of Different Modelling Hypotheses. Crop Pasture Sci. 2009, 60, 738–752. [Google Scholar] [CrossRef]
- Fayaud, B.; Coste, F.; Corre-Hellou, G.; Gardarin, A.; Dürr, C. Modelling Early Growth under Different Sowing Conditions: A Tool to Predict Variations in Intercrop Early Stages. Eur. J. Agron. 2014, 52, 180–190. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Fundira, T.; Henley, G. Biofuels in Southern Africa: Political Economy, Trade, and Policy Environment; WIDER Working Papers; World Institute for Development Economics Research (UNU-WIDER): Helsinki, Finland; United Nations University: Tokyo, Japan, 2017. [Google Scholar] [CrossRef]
- Chimonyo, V.G.P.; Modi, A.T.; Mabhaudhi, T. Assessment of Sorghum–Cowpea Intercrop System under Water-Limited Conditions Using a Decision Support Tool. Water SA 2016, 42, 316–327. [Google Scholar] [CrossRef]
- Willey, R.W. Intercropping: Its Importance and Research Needs. Part 1, Competition and Yield Advantages; Commonwealth Agricultural Bureaux: Wallingford, Oxfordshire, UK, 1979. [Google Scholar]
- Peerzada, A.M.; Ali, H.H.; Chauhan, B.S. Weed Management in Sorghum [Sorghum Bicolor (L.) Moench] Using Crop Competition: A Review. Crop Prot. 2017, 95, 74–80. [Google Scholar] [CrossRef]
- Gholami, S.; Moeini, M.; Zand, E.; Noormohammadi, G. Non Chemical Management of Weeds Effects on Forage Sorghum Production. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 614–623. [Google Scholar]
- Xavier, K.V.; Pfeiffer, T.; Parreira, D.F.; Chopra, S.; Vaillancourt, L. Aggressiveness of Colletotrichum Sublineola Strains from Sorghum bicolor and S. halepense to Sweet Sorghum Variety Sugar Drip, and Their Impact on Yield. Plant Dis. 2017, 101, 1578–1587. [Google Scholar] [CrossRef]
- Vinutha, K.S.; Rayaprolu, L.; Yadagiri, K.; Umakanth, A.V.; Patil, J.V.; Srinivasa Rao, P. Sweet Sorghum Research and Development in India: Status and Prospects. Sugar Tech. 2014, 16, 133–143. [Google Scholar] [CrossRef]
- Mathur, S.; Umakanth, A.V.; Tonapi, V.A.; Sharma, R.; Sharma, M.K. Sweet Sorghum as Biofuel Feedstock: Recent Advances and Available Resources. Biotechnol. Biofuels 2017, 10, 146. [Google Scholar] [CrossRef]
- Silva, C.; Da Silva, A.F.; Do Vale, W.G.; Galon, L.; Petter, F.A.; May, A.; Karam, D. Weed Interference in the Sweet Sorghum Crop. Bragantia 2014, 73, 438–445. [Google Scholar] [CrossRef]
- Qazi, H.A.; Paranjpe, S.; Bhargava, S. Stem Sugar Accumulation in Sweet Sorghum—Activity and Expression of Sucrose Metabolizing Enzymes and Sucrose Transporters. J. Plant Physiol. 2012, 169, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Disasa, T.; Feyissa, T.; Admassu, B. Characterization of Ethiopian Sweet Sorghum Accessions for Brix, Morphological and Grain Yield Traits. Sugar Tech. 2017, 19, 72–82. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Yu, H.; Zhu, K.; Zhang, Z.; Zou, F.L.J. Effect of Excessive Soil Moisture Stress on Sweet Sorghum: Physiological Changes and Productivity. Pak. J. Bot. 2016, 48, 1–10. [Google Scholar]
- Cassman, K.G.; Liska, A.J. Food and Fuel for All: Realistic or Foolish? Biofuels Bioprod. Biorefin. 2007, 1, 18–23. [Google Scholar] [CrossRef]
- Bastiaans, L.; Kropff, M.J. WEEDS|Weed Competition; Elsevier: Oxford, UK, 2003; pp. 1494–1500. [Google Scholar] [CrossRef]
- Graham, P.L.; Steiner, J.L.; Wiese, A.F. Light Absorption and Competition in Mixed Sorghum-Pigweed Communities. Agron. J. 1988, 80, 415–418. [Google Scholar] [CrossRef]
- Dille, J.A.; Stahlman, P.W.; Thompson, C.R.; Bean, B.W.; Soltani, N.; Sikkema, P.H. Potential Yield Loss in Grain Sorghum (Sorghum bicolor) with Weed Interference in the United States. Weed Technol. 2020, 34, 624–629. [Google Scholar] [CrossRef]
- Ziska, L.H. Changes in Competitive Ability between a C4 Crop and a C3 Weed with Elevated Carbon Dioxide. Weed Sci. 2001, 49, 622–627. [Google Scholar] [CrossRef]
- Zhang, M.; Zeiss, M.R.; Geng, S. Agricultural Pesticide Use and Food Safety: California’s Model. J. Integr. Agric. 2015, 14, 2340–2357. [Google Scholar] [CrossRef]
- Hozayn, M.; El-Shahawy, T.A.E.G.; Sharara, F.A. Implication of Crop Row Orientation and Row Spacing for Controlling Weeds and Increasing Yield in Wheat. Aust. J. Basic Appl. Sci. 2012, 6, 422–427. [Google Scholar]
- Hussain, M.; Farooq, S.; Merfield, C.; Jabran, K. Chapter 8—Mechanical Weed Control; Jabran, K., Chauhan, B.S.B.T.-N.-C.W.C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 133–155. [Google Scholar] [CrossRef]
- Tillett, N.D.; Hague, T.; Grundy, A.C.; Dedousis, A.P. Mechanical Within-Row Weed Control for Transplanted Crops Using Computer Vision. Biosyst. Eng. 2008, 99, 171–178. [Google Scholar] [CrossRef]
- Forcella, F.; Westgate, M.E.; Warnes, D.D. Effect of Row Width on Herbicide and Cultivation Requirements in Row Crops. Am. J. Altern. Agric. 1992, 7, 161–167. [Google Scholar] [CrossRef]
- Moomaw, R.S.; Martin, A.R. Cultural Practices Affecting Season-Long Weed Control in Irrigated Corn (Zea mays). Weed Sci. 1984, 32, 460–467. [Google Scholar] [CrossRef]
- Locke, M.A.; Reddy, K.N.; Zablotowicz, R.M. Weed Management in Conservation Crop Production Systems. Weed Biol. Manag. 2002, 2, 123–132. [Google Scholar] [CrossRef]


| Growing Season | pH | P | K | Ca | Mg | Na | Org C | Total N | CEC | Bulk Density |
|---|---|---|---|---|---|---|---|---|---|---|
| (H2O) | mg/kg | % | cmol (+)/kg | (g∙cm−3) | ||||||
| 2017/18 | 6.04 | 27.65 | - | - | - | - | 1.76 | 0.17 | - | 1.10 |
| 2018/19 | 5.87 | 52.86 | 406.83 | 1801.67 | 614.83 | 51.58 | 0.1 | 21.7 | 1.12 | |
| Source of Variation | df | ◦Bx | Dry Biomass | Fresh Biomass | Height | Number of Leaves | ||
|---|---|---|---|---|---|---|---|---|
| 60 DAE | 80 DAE | 60 DAE | 80 DAE | |||||
| ms | ms | ms | ms | ms | ms | ms | ||
| Intercropping pattern | 4 | 1.88 ns | 2.39 ns | 26.56 ns | 1637 ns | 14,415 * | 0.55 ns | 4.80 ** |
| Weeding frequency | 2 | 8.39 * | 102.84 ** | 2067.7 ** | 4851.9 ** | 31,957 ** | 10.02 ** | 15.47 ** |
| Intercropping pattern × weeding frequency | 8 | 1.97 ns | 2.80 ns | 72.2 ns | 3210.4 ns | 23,802 * | 0.08 ns | 0.72 ns |
| Residual | 28 | 1.82 | 2.76 | 47.45 | 5829 | 36,199 | 0.35 | 0.73 |
| CV | 29.6 | 18.6 | 18.9 | 10.4 | 17.9 | 1.9 | 3 | |
| Source of Variation | df | ◦Bx | Dry Biomass | Fresh Biomass | Height | Number of Leaves | ||
|---|---|---|---|---|---|---|---|---|
| 60 DAE | 80 DAE | 60 DAE | 80 DAE | |||||
| ms | ms | ms | ms | ms | ms | ms | ||
| Intercropping pattern | 4 | 23.29 ns | 74.05 ** | 120.26 * | 2943.4 * | 205 ns | 1.56 ns | 1.41 ns |
| Weeding frequency | 2 | 38.8 ** | 1554.91 *** | 7168.41 *** | 1788.4 ns | 8569 * | 5.38 *** | 5.15 ** |
| Intercropping pattern × weeding frequency | 8 | 18.13 ns | 37.05 ns | 109.18 ** | 639.1 ns | 342 ns | 1.49 * | 0.63 ns |
| Residual | 28 | 84.1 | 17.26 | 30.74 | 746.3 | 1789 | 0.62 | 0.72 |
| CV | 27.3 | 24.1 | 13 | 25.6 | 22.1 | 10.2 | 8.9 | |
| Growing Season | Intercropping Pattern | Weeding Frequency % | Total Plot | ||
|---|---|---|---|---|---|
| 0 | 50 | 100 | |||
| 2017/18 | SS/CP inter-row | 0.97 | 1.91 | 1.51 | 1.61 |
| SS/CP intra-row | 1.48 | 2.69 | 2.29 | 2.26 | |
| SS/DB inter-row | 0.91 | 2.03 | 1.70 | 1.66 | |
| SS/DB intra-row | 1.12 | 2.78 | 2.1 | 2.19 | |
| 2018/19 | SS/CP inter-row | 1.16 | 1.60 | 1.28 | 1.36 |
| SS/CP intra-row | 1.65 | 2.45 | 1.85 | 2.47 | |
| SS/DB inter-row | 1.27 | 1.65 | 1.82 | 1.96 | |
| SS/DB intra-row | 2.65 | 1.84 | 1.60 | 1.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, C.; Modi, A.T.; Nciizah, A.D. Sweet Sorghum (Sorghum bicolor) Performance in a Legume Intercropping System under Weed Interference. Agronomy 2021, 11, 877. https://doi.org/10.3390/agronomy11050877
Baker C, Modi AT, Nciizah AD. Sweet Sorghum (Sorghum bicolor) Performance in a Legume Intercropping System under Weed Interference. Agronomy. 2021; 11(5):877. https://doi.org/10.3390/agronomy11050877
Chicago/Turabian StyleBaker, Conrad, Albert T. Modi, and Adornis D. Nciizah. 2021. "Sweet Sorghum (Sorghum bicolor) Performance in a Legume Intercropping System under Weed Interference" Agronomy 11, no. 5: 877. https://doi.org/10.3390/agronomy11050877
APA StyleBaker, C., Modi, A. T., & Nciizah, A. D. (2021). Sweet Sorghum (Sorghum bicolor) Performance in a Legume Intercropping System under Weed Interference. Agronomy, 11(5), 877. https://doi.org/10.3390/agronomy11050877







