Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review
Abstract
1. Introduction
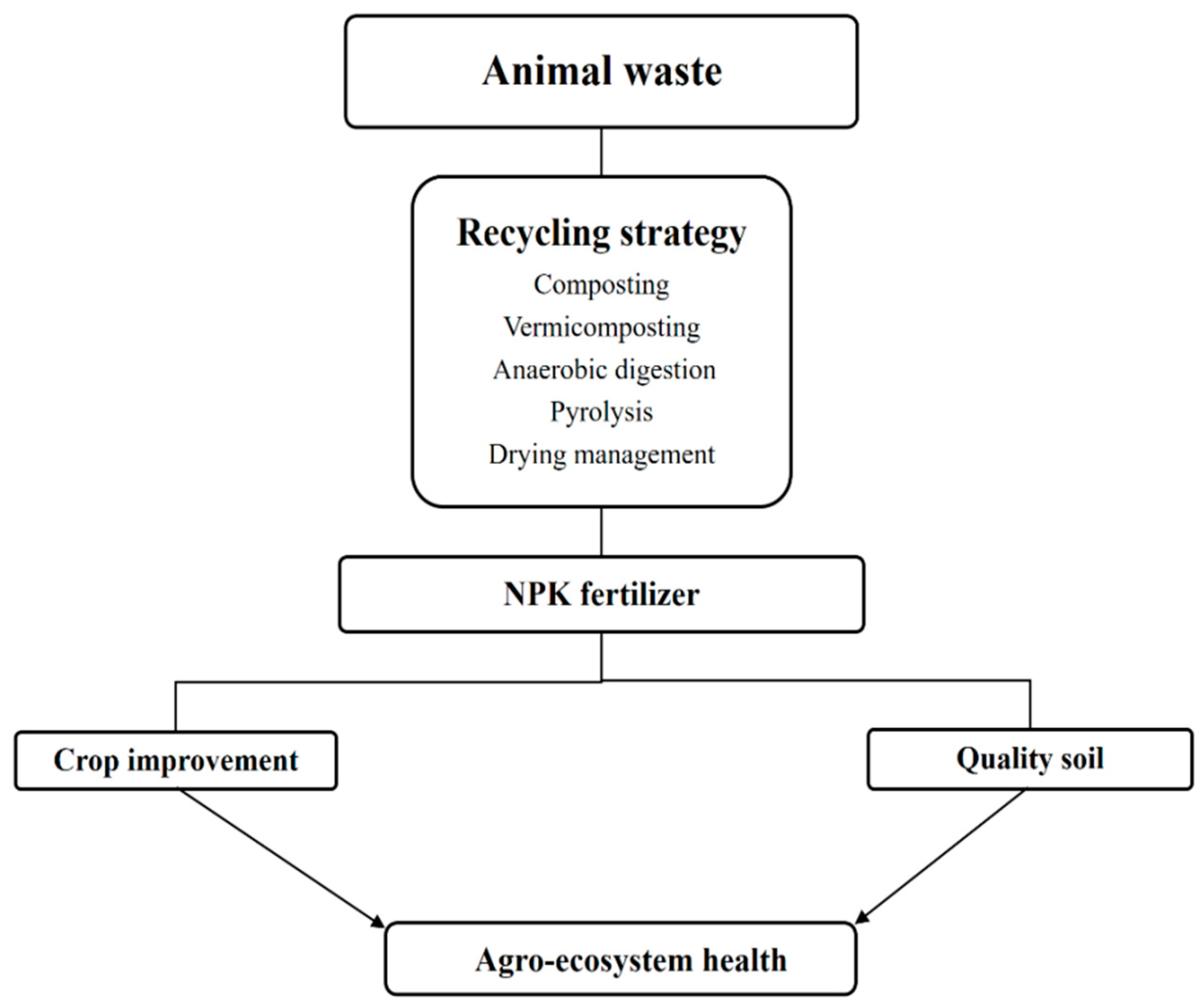
2. Recycling Animal Waste for Fertilizer Production
2.1. Composted Amendment
2.2. Vermicompost Manure
2.3. Anaerobically Produced Digestate
2.4. Pyrolysed Biochar
2.5. Dried Animal Waste
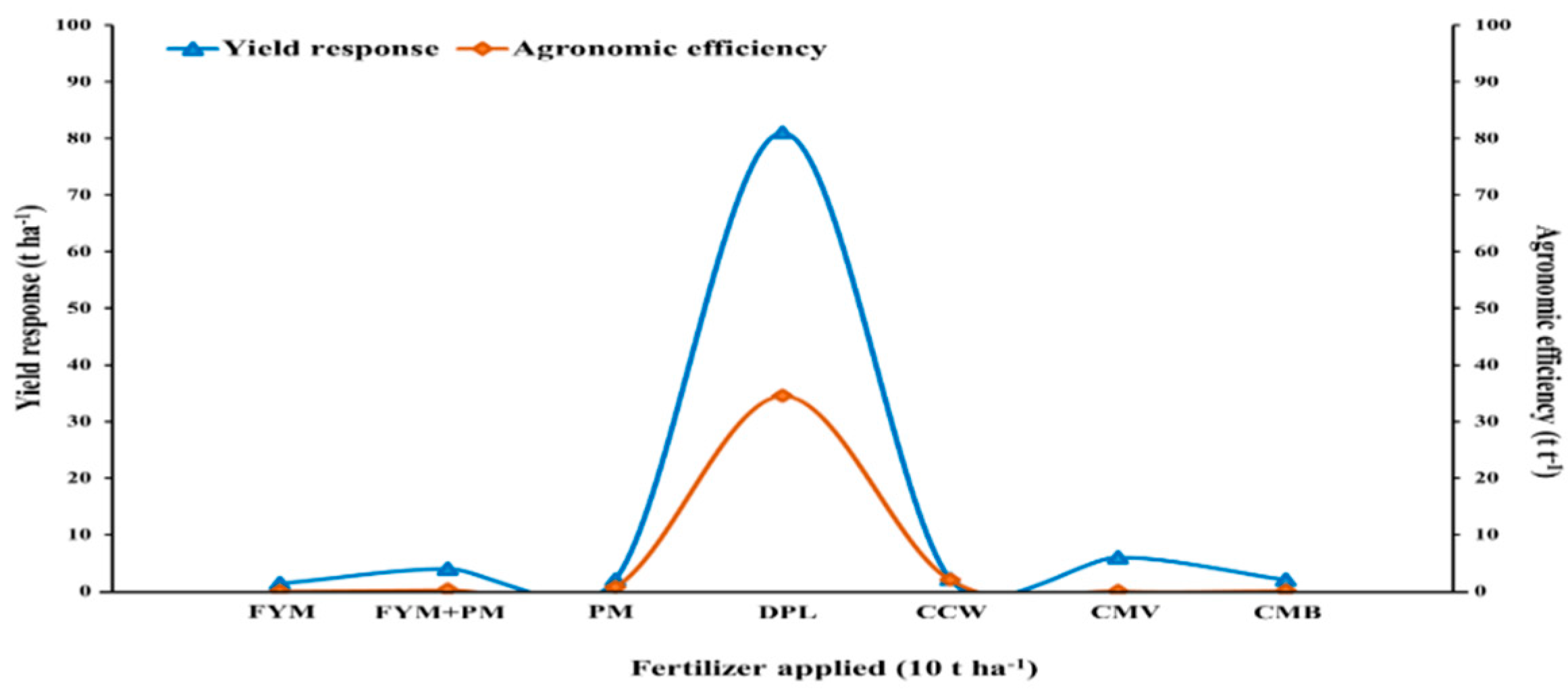
3. Dose Calculation and Yield Potential Assessment
4. Effects on Agro-Ecosystem Health
4.1. Aggregate Formation
4.2. SOM Turnover
4.3. Microbial Abundance and Community Composition
4.4. Enzymatic Activity
4.5. Disease Suppression
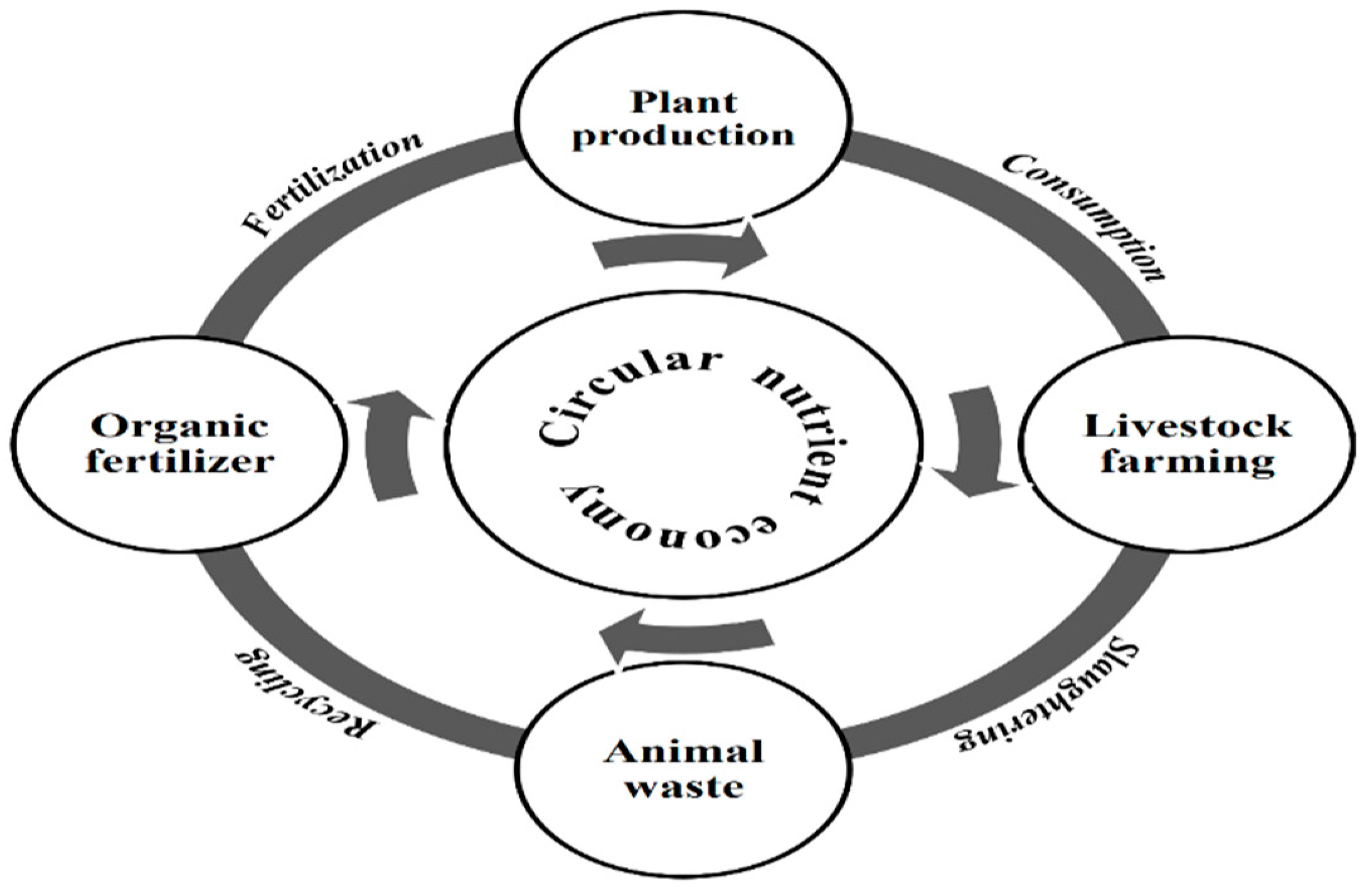
5. Circulation of Nutrients Together with Economy
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Gandhi, V.P.; Zhou, Z. Food demand and the food security challenge with rapid economic growth in the emerging economies of India and China. Food Res. Int. 2014, 63, 108–124. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. 2050: A Third More Mouths to Feed. 2009. Available online: http://www.fao.org/news/story/en/item/35571/icode/ (accessed on 25 January 2021).
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef]
- Sjauw-Koen-Fa, A. Sustainability and Security of the Global Food Supply Chain; Rabobank Group: Haarlem, The Netherlands, 2010; pp. 8–17. [Google Scholar]
- Pretty, J.; Bharucha, Z.P. Sustainable intensifcation in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Effectiveness of organic wastes as fertilizers and amendments in salt-affected soils. Agriculture 2015, 5, 221–230. [Google Scholar] [CrossRef]
- Ansari, R.A.; Mahmood, I. Optimization of organic and bio-organic fertilizers on soil properties and growth of pigeon pea. Sci. Hortic. 2017, 226, 1–9. [Google Scholar] [CrossRef]
- Pershina, E.; Valkonen, J.; Kurki, P.; Ivanova, E.; Chirak, E.; Korvigo, I.; Provorov, N.; Andronov, E. Comparative analysis of prokaryotic communities associated with organic and conventional farming systems. PLoS ONE 2015, 10, e0145072. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.L. Oligotrophs versus copiotrophs. Bioessays 2001, 23, 657–661. [Google Scholar] [CrossRef]
- Tal, A. Making conventional agriculture environmentally friendly: Moving beyond the glorification of organic agriculture and the demonization of conventional agriculture. Sustainability 2018, 10, 1078. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Kim, J.J.; John, K.M.; Hae-Kyung, M.; Jin, K.; Enkhtaivan, G.; Kim, D.H. Morphological and biochemical variation of Chinese cabbage (Brassica rapa spp. Pekinensis) cultivated using different agricultural practices. J. Food Compos. Anal. 2014, 36, 12–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Niu, S.; Kong, M.; Zhang, J.; Lu, Y.; Yao, Y. Animal wastes as fertilizers enhance growth of young walnut trees under soil drought conditions. J. Sci. Food Agric. 2020, 100, 3445–3455. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Debsarcar, A.; Mukherjee, J. Application of recycled slaughterhouse wastes as an organic fertilizer for successive cultivations of bell pepper and amaranth. Sci. Hortic. 2021, 280, 109927. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Y.; Tian, Y.; Ceng, K.; Zhao, M.; Zhao, M.; Yin, B. Increasing yield and N use efficiency with organic fertilizer in Chinese intensive rice cropping systems. Field Crops Res. 2018, 227, 102–109. [Google Scholar] [CrossRef]
- Osman, K.T. Soils: Principles, Properties and Management, 1st ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 97–110. [Google Scholar]
- Li, S.; Li, J.; Li, G.; Li, Y.; Yuan, J.; Li, D. Effect of different organic fertilizers application on soil organic matter properties. Compost Sci. Util. 2017, 25, 31–36. [Google Scholar] [CrossRef]
- Yan, D.; Wang, D.; Yang, L. Long-term effect of chemical fertilizer, straw, and manure on labile organic matter fractions in a paddy soil. Biol. Fertil. Soils 2007, 44, 93–101. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Di Silverio, N.; Carrino, L.; Cesarano, G.; Assaeed, A.M.; Abd-ElGawad, A.M. Decomposition and organic amendments chemistry explain contrasting effects on plant growth promotion and suppression of Rhizoctonia solani damping off. PLoS ONE 2020, 15, e0230925. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Gong, Y.; Yang, H.; Fan, M.; Kuzyakov, Y. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Chae, Y.; Cui, R.; Kim, S.W.; An, G.; Jeong, S.W.; An, Y.J. Exoenzyme activity in contaminated soils before and after soil washing: ß-glucosidase activity as a biological indicator of soil health. Ecotoxicol. Environ. Saf. 2017, 135, 368–374. [Google Scholar] [CrossRef]
- Bailey, K.L.; Lazarovits, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: San Diego, CA, USA, 2005; p. 803. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Boehm, M.J. Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hoitink, H.A.J.; Tuovinen, O.H. The role of microbial activity in suppression of damping-off caused by Pythium ultimum. Phytopathology 1987, 78, 314–322. [Google Scholar] [CrossRef]
- De Brito Alvarez, M.A.; Gagne, S.; Antoun, H. Effect of compost on rhizosphere microflora of the tomato and on the incidence of plant growth-promoting rhizobacteria. Appl. Environ. Microbiol. 1995, 61, 194–199. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R. Beneficial microbial allelopathies in the root zone: The management of soil quality and plant disease with rhizobacteria. Soil Tillage Res. 2003, 72, 107–123. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Insam, H. Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: A review. Crit. Rev. Microbiol. 2013, 39, 139–151. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mukherjee, J. Waste management of rural slaughterhouses in developing countries. In Advanced Organic Management: Sustainable Practices and Approaches; Hussain, C.M., Hait, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; accepted. [Google Scholar]
- Domingo, J.L.; Nadal, M. Domestic waste composting facilities: A review of human health risks. Environ. Int. 2009, 35, 382–389. [Google Scholar] [CrossRef]
- Miskiewicz, A.; Kowalczyk, P.; Oraibi, S.M.; Cybulska, K.; Misiewicz, A. Bird feathers as potential sources of pathogenic microorganisms: A new look at old diseases. Antonie Leeuwenhoek 2018, 111, 1493–1507. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Bailey, M.J. The ecology of transfer of mobile genetic elements. FEMS Microbiol. Ecol. 2002, 42, 187–197. [Google Scholar] [CrossRef]
- Bondarczuk, K.; Markowicz, A.; Piotrowska-Seget, Z. The urgent need for risk assessment on the antibiotic resistance spread via sewage sludge land application. Environ. Int. 2016, 87, 49–55. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; The Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Salminen, E.; Rintala, J. Anaerobic digestion of organic solid poultry slaughterhouse waste—A review. Bioresour. Technol. 2002, 83, 13–26. [Google Scholar] [CrossRef]
- Arbenz, M.; Gould, D.; Stopes, C. ORGANIC 3.0—The vision of the global organic movement and the need for scientific support. Org. Agric. 2017, 7, 199–207. [Google Scholar] [CrossRef]
- Rahmann, G.; Ardakani, M.R.; Bàrberi, P.; Boehm, H.; Canali, S.; Chander, M.; David, W.; Dengel, L.; Erisman, J.W.; Galvis-Martinez, A.C.; et al. Organic Agriculture 3.0 is innovation with research. Org. Agric. 2017, 7, 169–197. [Google Scholar] [CrossRef]
- Roy, M.; Karmakar, S.; Debsarcar, A.; Sen, P.K.; Mukherjee, J. Application of rural slaughterhouse waste as an organic fertilizer for pot cultivation of solanaceous vegetables in India. Int. J. Recycl. Org. Waste Agric. 2013, 2, 1–11. [Google Scholar] [CrossRef]
- Roy, M.; Das, R.; Debsarcar, A.; Sen, P.K.; Mukherjee, J. Conversion of rural abattoir wastes to an organic fertilizer and its application the field cultivation of tomato in India. Renew. Agric. Food Syst. 2016, 31, 350–360. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Solomon, D. Recycling slaughterhouse waste into fertilizer: How do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry? J. Sci. Food Agric. 2015, 95, 281–288. [Google Scholar] [CrossRef]
- Frazão, J.J.; de Melo Benites, V.; Ribeiro, J.V.S.; Pierobon, V.M.; Lavres, J. Agronomic effectiveness of a granular poultry litter-derived organomineral phosphate fertilizer in tropical soils: Soil phosphorus fractionation and plant responses. Geoderma 2019, 337, 582–593. [Google Scholar] [CrossRef]
- Nunes, W.A.G.D.A.; Menezes, J.F.S.; Benites, V.D.M.; Lima Junior, S.A.D.; Oliveira, A.D.S. Use of organic compost produced from slaughterhouse waste as fertilizer in soybean and corn crops. Sci. Agric. 2015, 72, 343–350. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Metzger, J.D.; Lucht, C. Effects of vermicomposts produced from cattle manure, food waste and paper waste on the growth and yield of peppers in the field. Pedobiologia 2005, 49, 297–306. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yen, H.W.; Nomanbhay, S.; Ho, Y.C.; Show, P.L. Transformation of biomass waste into sustainable organic fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Gentile, R.; Vanlauwe, B.; Chivenge, P.; Six, J. Trade-offs between the short-and long-term effects of residue quality on soil C and N dynamics. Plant Soil. 2011, 338, 159–169. [Google Scholar] [CrossRef]
- Anon. The Animal By-Products Regulations (EC) No. 1774/2002; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of slaughterhouse waste in value-added applications: Recent advances in the development of wood adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef]
- Akdeniz, N. A systematic review of biochar use in animal waste composting. Waste Manag. 2019, 88, 291–300. [Google Scholar] [CrossRef] [PubMed]
- National Agricultural Biosecurity Centre (NABC). Carcass Disposal: A Comprehensive Review; Report written for the USDA Animal and Plant Health Inspection Service; Kansas State University: Manhattan, KS, USA, 2004. [Google Scholar]
- Senesi, N.; Plaza, C.; Brunetti, G.; Polo, A. A comparative survey of recent results on humic-like fractions in organic amendments and effects on native soil humic substances. Soil Biol. Biochem. 2007, 39, 1244–1262. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Ragályi, P.; Kádár, I. Effect of organic fertilizers made from slaughterhouse wastes on yield of crops. Arch. Agron. Soil Sci. 2012, 58, 122–126. [Google Scholar] [CrossRef]
- Glatz, P.; Miao, Z.; Rodda, B. Handling and treatment of poultry hatchery waste: A review. Sustainability 2011, 3, 216–237. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Abbasi, S.A. Solid waste management by composting: State of the art. Crit. Rev. Environ. Sci. Technol. 2008, 38, 311–400. [Google Scholar] [CrossRef]
- Swati, A.; Hait, S. Fate and bioavailability of heavy metals during vermicomposting of various organic wastes—A review. Process Saf. Environ. Prot. 2017, 109, 30–45. [Google Scholar] [CrossRef]
- Ramnarain, Y.I.; Ansari, A.A.; Ori, L. Vermicomposting of different organic materials using the epigeic earthworm Eisenia foetida. Int. J. Recycl. Org. Waste Agric. 2019, 8, 23–36. [Google Scholar] [CrossRef]
- Tognetti, C.; Laos, F.; Mazzarino, M.J.; Hernandez, M.T. Composting vs. vermicomposting: A comparison of end product quality. Compost Sci. Util. 2005, 13, 6–13. [Google Scholar] [CrossRef]
- Maboeta, M.S.; Van Rensburg, L. Vermicomposting of industrially produced woodchips and sewage sludge utilizing Eisenia fetida. Ecotox. Environ. Saf. 2003, 56, 265–270. [Google Scholar] [CrossRef]
- Yadav, A.; Gupta, R.; Garg, V.K. Organic manure production from cow dung and biogas plant slurry by vermicomposting under field conditions. Int. J. Recycl. Org. Waste Agric. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Lores, M.; Gómez-Brandón, M.; Pérez-Díaz, D.; Domínguez, J. Using FAME profiles for the characterization of animal wastes and vermicomposts. Soil Biol. Biochem. 2006, 38, 2993–2996. [Google Scholar] [CrossRef]
- Chattopadhyay, G.N. Use of vermicomposting biotechnology for recycling organic wastes in agriculture. Int. J. Recycl. Org. Waste Agric. 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Arancon, N.Q.; Edwards, C.A.; Metzger, J.D. The influence of earthworm-processed pig manure on the growth and productivity of marigolds. Bioresour. Technol. 2002, 81, 103–108. [Google Scholar] [CrossRef]
- Garczyńska, M.; Kostecka, J.; Pączka, G.; Hajduk, E.; Mazur-Pączka, A.; Butt, K.R. Properties of vermicomposts derived from Cameroon sheep dung. Appl. Sci. 2020, 10, 5048. [Google Scholar] [CrossRef]
- Borges, Y.V.; Alves, L.; Bianchi, I.; Espíndola, J.C.; Oliveira, J.M.D., Jr.; Radetski, C.M.; Somensi, C.A. Optimization of animal manure vermicomposting based on biomass production of earthworms and higher plants. J. Environ. Sci. Health B 2017, 52, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef]
- Kumazawa, K. Beneficial Effects of Organic Matter on Rice Growth and Yield in Japan. In Organic Matter and Rice; International Rice Research Institute: Manila, Philippines, 1984; pp. 431–444. [Google Scholar]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. SpringerPlus 2012, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Ducey, T.; Ro, K.S.; Hunt, P.G. Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Gutser, R.; Ebertseder, T.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J. Plant Nutr. Soil Sci. 2005, 168, 439–446. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Li, B.; Dinkler, K.; Zhao, N.; Sobhi, M.; Merkle, W.; Liu, S.; Dong, R.; Oechsner, H.; Guo, J. Influence of anaerobic digestion on the labile phosphorus in pig, chicken, and dairy manure. Sci. Total Environ. 2020, 737, 140234. [Google Scholar] [CrossRef]
- Nkoa, R.; Coulombe, J.; Desjardins, Y.; Tremblay, N. Towards optimization of growth via nutrient supply phasing: Nitrogen supply phasing increases broccoli (Brassica oleracea var. italica) growth and yield. J. Exp. Bot. 2001, 52, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Loria, E.R.; Sawyer, J.E.; Barker, D.W.; Lundvall, J.P.; Lorimor, J.C. Use of anaerobically digested swine manure as a nitrogen source in corn production. Agron. J. 2007, 99, 1119–1129. [Google Scholar] [CrossRef]
- Collins, H.P.; Kimura, E.; Frear, C.S.; Kruger, C.E. Phosphorus uptake by potato from fertilizers recovered from anaerobic digestion. Agron. J. 2016, 108, 2036–2049. [Google Scholar] [CrossRef]
- Côté, C.; Massé, D.I.; Quessy, S. Reduction of indicator and pathogenic microorganisms by psychrophilic anaerobic digestion in swine slurries. Bioresour. Technol. 2006, 97, 686–691. [Google Scholar] [CrossRef]
- Viau, E.; Peccia, J. Survey of wastewater indicators and human pathogen genomes in biosolids produced by class A and class B stabilization treatments. Appl. Environ. Microbiol. 2009, 75, 164–174. [Google Scholar] [CrossRef]
- Massé, D.I.; Saady, N.M.C.; Gilbert, Y. Potential of biological processes to eliminate antibiotics in livestock manure: An overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef]
- Salminen, E.; Rintala, J.; Härkönen, J.; Kuitunen, M.; Högmander, H.; Oikari, A. Anaerobically digested poultry slaughterhouse wastes as fertiliser in agriculture. Bioresour. Technol. 2001, 78, 81–88. [Google Scholar] [CrossRef]
- Yadav, A.; Ansari, K.B.; Simha, P.; Gaikar, V.G.; Pandit, A.B. Vacuum pyrolysed biochar for soil amendment. Resour. Technol. 2016, 2, 177–185. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sources A 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass chars: The effects of pyrolysis conditions on their morphology, structure, chemical properties and reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, Y.; Han, L. Influence of pyrolysis temperature on chemical speciation, leaching ability, and environmental risk of heavy metals in biochar derived from cow manure. Bioresour. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Pumpanen, J. Biochar Use in Global Forests: Opportunities and Challenges. In Developments in Soil Science; Busse, M., Giardina, C.P., Morris, D.M., Page-Dumroese, D.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 427–453. [Google Scholar] [CrossRef]
- Luo, Y.; Dungait, J.A.; Zhao, X.; Brookes, P.C.; Durenkamp, M.; Li, G.; Lin, Q. Pyrolysis temperature during biochar production alters its subsequent utilization by microorganisms in an acid arable soil. Land Degrad. Dev. 2018, 29, 2183–2188. [Google Scholar] [CrossRef]
- Petruccelli, R.; Di Lonardo, S. Role of biochars in soil fertility management of fruit crops. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Cambridge, UK, 2020; pp. 431–444. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils: A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; European Commission Report No. EUR 24099 EN; European Communities: Ispara, Italy, 2010. [Google Scholar]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shinogi, Y.; Kanri, Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 2003, 90, 241–247. [Google Scholar] [CrossRef]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mukherjee, J. Use of rural slaughterhouse wastes (SHWs) as fertilizer in agriculture: A review. In Proceedings of the International Conference on Energy Management for Green Environment, Kolkata, India, 25–27 September 2019; pp. 1–6. [Google Scholar] [CrossRef]
- European Commission (EC). The Veterinary Rules for the Disposal and Processing of Animal Waste; European Commission: Brussels, Belgium, 1990.
- Kádár, I.; Hámori, V.; Morvai, B.; Petróczki, F. Soil load and pollution limit values; sewage sludge and slaughterhouse waste compost effect on sugar beet. In Cukorrépa-Termesztési/-Termeltetési Tanfolyam és Tanácskozás; Várnainé, J.A., Ed.; Cukoripari Egyesülés: Budapest, Hungary, 2002; pp. 37–40. [Google Scholar]
- Bhowmik, A.; Bhunia, S.; Mukherjee, J. An Apparatus for Recycling Slaughterhouse Waste and Method Thereof. Indian Patent 202031033116, 2020. [Google Scholar]
- Roy, M.; Das, R.; Kundu, A.; Karmakar, S.; Das, S.; Sen, P.; Debsarcar, A.; Mukherjee, J. Organic cultivation of tomato in India with recycled slaughterhouse wastes: Evaluation of fertilizer and fruit safety. Agriculture 2015, 5, 826–856. [Google Scholar] [CrossRef]
- Bonanomi, G.; Cesarano, G.; Lombardi, N.; Motti, R.; Scala, F.; Mazzoleni, S.; Incerti, G. Litter chemistry explains contrasting feeding preferences of bacteria, fungi, and higher plants. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Lazcano, C.; Arnold, J.; Zaller, J.G.; Martín, J.D.; Salgado, A.T. Compost and vermicompost as nursery pot components: Effects on tomato plant growth and morphology. Span. J. Agric. Res. 2009, 944–951. [Google Scholar] [CrossRef]
- Jackson, D.R.; Smith, K.A. Animal manure slurries as a source of nitrogen for cereals; effect of application time on efficiency. Soil Use Manag. 1997, 13, 75–81. [Google Scholar] [CrossRef]
- Sradnick, A.; Feller, C. A typological concept to predict the nitrogen release from organic fertilizers in farming systems. Agronomy 2020, 10, 1448. [Google Scholar] [CrossRef]
- Hua, W.; Luo, P.; An, N.; Cai, F.; Zhang, S.; Chen, K.; Yang, J.; Han, X. Manure application increased crop yields by promoting nitrogen use efficiency in the soils of 40-year soybean-maize rotation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Almeida, R.F.; Queiroz, I.D.S.; Mikhael, J.E.R.; Oliveira, R.C.; Borges, E.N. Enriched animal manure as a source of phosphorus in sustainable agriculture. Int. J. Recycl. Org. Waste Agric. 2019, 8, 203–210. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2018, 10, 51. [Google Scholar] [CrossRef]
- Cassman, K.G.; Gines, G.C.; Dizon, M.A.; Samson, M.I.; Alcantara, J.M. Nitrogen-use efficiency in tropical lowland rice systems: Contributions from indigenous and applied nitrogen. Field Crops Res. 1996, 47, 1–12. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Kihara, J.; Chivenge, P.; Pypers, P.; Coe, R.; Six, J. Agronomic use efficiency of N fertilizer in maize-based systems in sub-Saharan Africa within the context of integrated soil fertility management. Plant Soil. 2011, 339, 35–50. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; López-Bellido, L. Efficiency of nitrogen in wheat under Mediterranean conditions: Effect of tillage, crop rotation and N fertilization. Field Crops Res. 2001, 71, 31–46. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Antoniadis, V.; Damalas, C.A.; Fotiadis, S. Effect of organic manure on wheat grain yield, nutrient accumulation, and translocation. Agron. J. 2016, 108, 615–625. [Google Scholar] [CrossRef]
- Adediran, J.A.; Taiwo, L.B.; Akande, M.O.; Sobulo, R.A.; Idowu, O.J. Application of organic and inorganic fertilizer for sustainable maize and cowpea yields in Nigeria. J. Plant Nutr. 2005, 27, 1163–1181. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M. Effect of methods and time of poultry manure application on soil and leaf nutrient concentrations, growth and fruit yield of tomato (Lycopersicon esculentum Mill). J. Saudi Soc. Agric. Sci. 2017, 16, 383–388. [Google Scholar] [CrossRef]
- Moyin-Jesu, E.I. Use of different organic fertilizers on soil fertility improvement, growth and head yield parameters of cabbage (Brassica oleraceae L). Int. J. Recycl. Org. Waste Agric. 2015, 4, 291–298. [Google Scholar] [CrossRef]
- Evanylo, G.; Sherony, C.; Spargo, J.; Starner, D.; Brosius, M.; Haering, K. Soil and water environmental effects of fertilizer-, manure-, and compost-based fertility practices in an organic vegetable cropping system. Agric. Ecosyst. Environ. 2008, 127, 50–58. [Google Scholar] [CrossRef]
- Busari, M.A.; Salako, F.K.; Adetunji, M.T. Soil chemical properties and maize yield after application of organic and inorganic amendments to an acidic soil in Southwestern Nigeria. Span. J. Agric. Res. 2008, 6, 691–699. [Google Scholar] [CrossRef]
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy. Front. Microbiol. 2017, 8, 1702. [Google Scholar] [CrossRef] [PubMed]
- Llaven, M.A.O.; Jimenez, J.L.G.; Coro, B.I.C.; Rincon-Rosales, R.; Molina, J.M.; Dendooven, L.; Gutierrez-Miceli, F.A. Fruit characteristics of bell pepper cultivated in sheep manure vermicompost substituted soil. J. Plant Nutr. 2008, 31, 1585–1598. [Google Scholar] [CrossRef]
- Joshi, R.; Vig, A.P.; Singh, J. Vermicompost as soil supplement to enhance growth, yield and quality of Triticum aestivum L.: A field study. Int. J. Recycl. Org. Waste Agric. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock manure and the impacts on soil health: A review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; De la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Stimpfl, E.; Insam, H. Residues from renewable energy production: Their value for fertilizing pastures. Biomass Bioenergy 2012, 39, 290–295. [Google Scholar] [CrossRef]
- Karim, A.A.; Ramasamy, C. Expanding Frontiers of Agriculture: Contemporary Issues; Kalyani Publishers: Ludhiana, India, 2000. [Google Scholar]
- Chuan, L.; He, P.; Pampolino, M.F.; Johnston, A.M.; Jin, J.; Xu, X.; Zhao, S.; Qiu, S.; Zhou, W. Establishing a scientific basis for fertilizer recommendations for wheat in China: Yield response and agronomic efficiency. Field Crops Res. 2013, 140, 1–8. [Google Scholar] [CrossRef]
- Lund, Z.F.; Doss, B.D. Residual effects of dairy cattle manure on plant growth and soil properties. Agron. J. 1980, 72, 123–130. [Google Scholar] [CrossRef]
- McAndrews, G.M.; Liebman, M.; Cambardella, C.A.; Richard, T.L. Residual effects of composted and fresh solid swine (Sus scrofa L.) manure on soybean [Glycine max (L.) Merr.] growth and yield. Agron. J. 2006, 98, 873–882. [Google Scholar] [CrossRef]
- Chiti, T.; Gardin, L.; Perugini, L.; Quaratino, R.; Vaccari, F.P.; Miglietta, F.; Valentini, R. Soil organic carbon stock assessment for the different cropland land uses in Italy. Biol. Fertil. Soils 2012, 48, 9–17. [Google Scholar] [CrossRef]
- Van Bniggen, A.H.; Termorskuizen, A.J. Integrated approaches to root disease management in organic farming systems. Aust. Plant Pathol. 2003, 32, 141–156. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of organic wastes through composting: Process performance and compost application in agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Hati, K.M.; Swarup, A.; Mishra, B.; Manna, M.C.; Wanjari, R.H.; Mandal, K.G.; Misra, A.K. Impact of long-term application of fertilizer, manure and lime under intensive cropping on physical properties and organic carbon content of an Alfisol. Geoderma 2008, 148, 173–179. [Google Scholar] [CrossRef]
- Das, B.; Chakraborty, D.; Singh, V.K.; Aggarwal, P.; Singh, R.; Dwivedi, B.S.; Mishra, R.P. Effect of integrated nutrient management practice on soil aggregate properties, its stability and aggregate-associated carbon content in an intensive rice-wheat system. Soil Tillage Res. 2014, 136, 9–18. [Google Scholar] [CrossRef]
- Tripathi, R.; Nayak, A.K.; Bhattacharyya, P.; Shukla, A.K.; Shahid, M.; Raja, R.; Panda, B.B.; Mohanty, S.; Kumar, A.; Thilagam, V.K. Soil aggregation and distribution of carbon and nitrogen in different fractions after 41 years long-term fertilizer experiment in tropical rice-rice system. Geoderma 2014, 213, 280–286. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.K.; Saha, S.; Mani, P.K.; Mandal, B. Effect of organic inputs on aggregate associated organic carbon concentration under long-term rice-wheat cropping system. Geoderma 2010, 154, 379–386. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, J.; Zhu, A.; Zhang, C. Effects of long-term (23 years) mineral fertilizer and compost application on physical properties of fluvo-aquic soil in the North China Plain. Soil Tillage Res. 2016, 156, 166–172. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Hu, C.; Mooney, S.J.; Dong, W.; Peng, X. Inorganic fertilization effects on the structure of a calcareous silt loam soil. Agron. J. 2017, 109, 2871–2880. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Zhang, X.; Wei, K.; Chen, L.; Liang, W. Effects of conservation tillage on soil aggregation and aggregate binding agents in black soil of Northeast China. Soil Tillage Res. 2012, 124, 196–202. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Zhang, S.; Xing, Y.; Wang, R.; Liang, W. Organic amendment effects on aggregate-associated organic C, microbial biomass C and glomalin in agricultural soils. Catena 2014, 123, 188–194. [Google Scholar] [CrossRef]
- Davinic, M.; Fultz, L.M.; Acosta-Martinez, V.; Calderón, F.J.; Cox, S.B.; Dowd, S.E.; Allen, V.G.; Zak, J.C.; Moore-Kucera, J. Pyrosequencing and mid-infrared spectroscopy reveal distinct aggregate stratification of soil bacterial communities and organic matter composition. Soil Biol. Biochem. 2012, 46, 63–72. [Google Scholar] [CrossRef]
- Ma, B.; Lv, X.; Cai, Y.; Chang, S.X.; Dyck, M.F. Liming does not counteract the influence of long-term fertilization on soil bacterial community structure and its co-occurrence pattern. Soil Biol. Biochem. 2018, 123, 45–53. [Google Scholar] [CrossRef]
- Guo, Z.C.; Zhang, Z.B.; Zhou, H.; Rahman, M.T.; Wang, D.Z.; Guo, X.S.; Li, L.J.; Peng, X.H. Long-term animal manure application promoted biological binding agents but not soil aggregation in a Vertisol. Soil Tillage Res. 2018, 180, 232–237. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Davis, J.G.; Brummer, J.E.; Stromberger, M.E.; Mikha, M.M.; Haddix, M.L.; Booher, M.R.; Paul, E.A. Rapid changes in microbial biomass and aggregate size distribution in response to changes in organic matter management in grass pasture. Geoderma 2013, 193, 68–75. [Google Scholar] [CrossRef]
- Babalola, O.; Adesodun, J.; Olasantan, F.; Adekunle, A. Responses of some soil biological, chemical and physical properties to short-term compost amendment. Int. J. Soil Sci. 2012, 7, 28–38. [Google Scholar] [CrossRef][Green Version]
- Bertagnoli, B.G.; Oliveira, J.F.; Barbosa, G.M.; Colozzi Filho, A. Poultry litter and liquid swine slurry applications stimulate glomalin, extraradicular mycelium production, and aggregation in soils. Soil Tillage Res. 2020, 202, 104657. [Google Scholar] [CrossRef]
- Li-Xian, Y.; Guo-Liang, L.; Shi-Hua, T.; Gavin, S.; Zhao-Huan, H. Salinity of animal manure and potential risk of secondary soil salinization through successive manure application. Sci. Total Environ. 2007, 383, 106–114. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, J.A.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Smith, P.; Davies, C.A.; Ogle, S.; Zanchi, G.; Bellarby, J.; Bird, N.; Boddey, R.M.; McNamara, N.P.; Powlson, D.; Cowie, A.; et al. Towards an integrated global framework to assess the impacts of land use and management change on soil carbon: Current capability and future vision. Glob. Chang. Biol. 2012, 18, 2089–2101. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, R.; Begum, F.; Bohannan, B.J.M.; Inebert, L.; Meyer, K. Variation in soil physical, chemical and microbial parameters under different land uses in Bagrot valley, Gilgit, Pakistan. J. Chem. Soc. Pak. 2017, 39, 97–107. [Google Scholar]
- Maillard, É.; Angers, D.A. Animal manure application and soil organic carbon stocks: A meta-analysis. Glob. Chang. Biol. 2014, 20, 666–679. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, N.; Ge, T.; Kuzyakov, Y.; Wang, Z.L.; Li, Z.; Tang, Z.; Chen, Y.; Wu, C.; Lou, Y. Soil aggregation regulates distributions of carbon, microbial community and enzyme activities after 23-year manure amendment. Appl. Soil Ecol. 2017, 111, 65–72. [Google Scholar] [CrossRef]
- Balser, T.C.; Firestone, M.K. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 2005, 73, 395–415. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Tian, J.; Lou, Y.; Gao, Y.; Fang, H.; Liu, S.; Xu, M.; Blagodatskaya, E.; Kuzyakov, Y. Response of soil organic matter fractions and composition of microbial community to long-term organic and mineral fertilization. Biol. Fertil. Soils 2017, 53, 523–532. [Google Scholar] [CrossRef]
- Kong, A.Y.; Six, J.; Bryant, D.C.; Denison, R.F.; Van Kessel, C. The relationship between carbon input, aggregation, and soil organic carbon stabilization in sustainable cropping systems. Soil Sci. Soc. Am. J. 2005, 69, 1078–1085. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Liang, B.C.; Ellert, B.H.; Drury, C.F. Fertilization effects on soil organic matter turnover and corn residue C storage. Soil Sci. Soc. Am. J. 1996, 60, 472–476. [Google Scholar] [CrossRef]
- Whalen, J.K.; Benslim, H.; Jiao, Y.; Sey, B.K. Soil organic carbon and nitrogen pools as affected by compost applications to a sandy-loam soil in Québec. Can. J. Soil Sci. 2008, 88, 443–450. [Google Scholar] [CrossRef]
- Brown, S.; Cotton, M. Changes in soil properties and carbon content following compost application: Results of on-farm sampling. Compost Sci. Util. 2011, 19, 87–96. [Google Scholar] [CrossRef]
- Majumder, B.; Mandal, B.; Bandyopadhyay, P.K.; Chaudhury, J. Soil organic carbon pools and productivity relationships for a 34 year old rice-wheat-jute agroecosystem under different fertilizer treatments. Plant Soil. 2007, 297, 53–67. [Google Scholar] [CrossRef]
- Bouajila, K.; Sanaa, M. Effects of organic amendments on soil physico-chemical and biological properties. J. Mater. Environ. Sci. 2011, 2, 485–490. [Google Scholar]
- Dass, A.; Lenka, N.K.; Patnaik, U.S.; Sudhishri, S. Integrated nutrient management for production, economics, and soil improvement in winter vegetables. Int. J. Veg. Sci. 2008, 14, 104–120. [Google Scholar] [CrossRef]
- Jayakumar, M.; Sivakami, T.; Ambika, D.; Karmegam, N. Effect of turkey litter (Meleagris gallopavo L.) vermicompost on growth and yield characteristics of paddy, Oryza sativa (ADT-37). Afr. J. Biotechnol. 2011, 10, 15295–15304. [Google Scholar] [CrossRef]
- Zhao, H.T.; Li, T.P.; Zhang, Y.; Hu, J.; Bai, Y.C.; Shan, Y.H.; Ke, F. Effects of vermicompost amendment as a basal fertilizer on soil properties and cucumber yield and quality under continuous cropping conditions in a greenhouse. J. Soils Sediments 2017, 17, 2718–2730. [Google Scholar] [CrossRef]
- Compton, J.E.; Boone, R.D. Soil nitrogen transformations and the role of light fraction organic matter in forest soils. Soil Biol. Biochem. 2002, 34, 933–943. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Plaza, C.; Giannetta, B.; Fernández, J.M.; López-de-Sá, E.G.; Polo, A.; Gascó, G.; Méndez, A.; Zaccone, C. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 150–159. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, W.; Bao, Y.; Zhang, J.; Petropoulos, E.; Li, Z.; Lin, X.; Feng, Y. Fertilization shapes a well-organized community of bacterial decomposers for accelerated paddy straw degradation. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Xu, L.; Yi, M.; Yi, H.; Guo, E.; Zhang, A. Manure and mineral fertilization change enzyme activity and bacterial community in millet rhizosphere soils. World J. Microbiol. Biotechnol. 2018, 34, 1–13. [Google Scholar] [CrossRef]
- Yu, W.T.; Bi, M.L.; Xu, Y.G.; Zhou, H.; Ma, Q.; Jiang, C.M. Microbial biomass and community composition in a Luvisol soil as influenced by long-term land use and fertilization. Catena 2013, 107, 89–95. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Sunil, E.; Thomas, G.V. Amplification of plant beneficial microbial communities during conversion of coconut leaf substrate to vermicompost by Eudrilus sp. Curr. Microbiol. 2009, 59, 15–20. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Chaudhry, V.; Rehman, A.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb. Ecol. 2012, 64, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, M.; Wu, M.; Jiang, C.; Kuzyakov, Y.; Gavrichkova, O.; Feng, Y.; Dong, Y.; Li, Z. Bacterial community succession in paddy soil depending on rice fertilization. Appl. Soil Ecol. 2019, 144, 92–97. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, G.; Robinson, D.; Yang, Z.; Guo, J.; Xie, J.; Fu, S.; Zhou, L.; Yang, Y. Large amounts of easily decomposable carbon stored in subtropical forest subsoil are associated with r-strategy-dominated soil microbes. Soil Biol. Biochem. 2016, 95, 233–242. [Google Scholar] [CrossRef]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Shi, S.; Richardson, A.E.; O’Callaghan, M.; DeAngelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M. Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Pascault, N.; Ranjard, L.; Kaisermann, A.; Bachar, D.; Christen, R.; Terrat, S.; Mathieu, O.; Lévêque, J.; Mougel, C.; Henault, C.; et al. Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems 2013, 16, 810–822. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, X.; Ma, M.; Zhou, B.; Guan, D.; Zhao, B.; Zhou, J.; Cao, F.; Li, L.; Li, J. Effect of 35 years inorganic fertilizer and manure amendment on structure of bacterial and archaeal communities in black soil of northeast China. Appl. Soil Ecol. 2016, 105, 187–195. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Shanks, O.C.; Kelty, C.A.; Archibeque, S.; Jenkins, M.; Newton, R.J.; McLellan, S.L.; Huse, S.M.; Sogin, M.L. Community structures of fecal bacteria in cattle from different animal feeding operations. Appl. Environ. Microbiol. 2011, 77, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, C.; Liu, X.; Lu, Y.; Wang, Y. Saline-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Appl. Soil Ecol. 2020, 156, 103705. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Y.; Zhao, F.; He, X.; Liu, H.; Yu, K. Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Role of Enzymes in Maintaining Soil Health. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 25–42. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Shi, W. Agricultural and ecological significance of soil enzymes: Soil carbon sequestration and nutrient cycling. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 43–60. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Zhang, Y.; Hao, X.; Thomas, B.W.; Eastman, A.H.; Schwinghamer, T.D. Linking soil microbial biomass and enzyme activities to long-term manure applications and their nonlinear legacy. Pedobiologia 2019, 74, 34–42. [Google Scholar] [CrossRef]
- Effron, D.; de la Hora, A.M.; Defrieri, R.L.; Fontanive, V.; Palma, P.M. Effect of cadmium, copper, and lead on different enzyme activities in a native forest soil. Commun. Soil Sci. Plant Anal. 2004, 35, 1309–1321. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, X.; He, M.; Liu, Y.; Tian, G.; Shi, J. Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: A microcosm incubation study. Chemosphere 2016, 142, 128–135. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Dawood, M.H. Monitoring soil enzymes activity before and after animal manure application. Agriculture 2020, 10, 166. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Romeo, F.; Mallamaci, C.; Muscolo, A. Digestate application on two different soils: Agricultural benefit and risk. Waste Biomass Valor. 2021, 1–13. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Soil fertility management and insect pests: Harmonizing soil and plant health in agroecosystems. Soil Tillage Res. 2003, 72, 203–211. [Google Scholar] [CrossRef]
- Borrero, C.; Trillas, M.I.; Ordovás, J.; Tello, J.C.; Avilés, M. Predictive factors for the suppression of Fusarium wilt of tomato in plant growth media. Phytopathology 2004, 94, 1094–1101. [Google Scholar] [CrossRef]
- Yatoo, A.M.; Ali, M.N.; Baba, Z.A.; Hassan, B. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea: A review. Agron. Sustain. Dev. 2021, 41, 1–26. [Google Scholar] [CrossRef]
- Manandhar, T.; Yami, K.D. Biological control of foot rot disease of rice using fermented products of compost and vermicompost. Sci. World 2008, 6, 52–57. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Galvis, P.A.; Edwards, C.A. Suppression of insect pest populations and damage to plants by vermicomposts. Bioresour. Technol. 2005, 96, 1137–1142. [Google Scholar] [CrossRef]
- Kannangara, T.; Utkhede, R.S.; Paul, J.W.; Punja, Z.K. Effects of mesophilic and thermophilic composts on suppression of Fusarium root and stem rot of greenhouse cucumber. Can. J. Microbiol. 2000, 46, 1021–1028. [Google Scholar] [CrossRef]
- Szczech, M.; Smolińska, U. Comparison of suppressiveness of vermicomposts produced from animal manures and sewage sludge against Phytophthora nicotianae Breda de Haan var. Nicotianae. J. Phytopathol. 2001, 149, 77–82. [Google Scholar] [CrossRef]
- Pane, C.; Spaccini, R.; Piccolo, A.; Scala, F.; Bonanomi, G. Compost amendments enhance peat suppressiveness to Pythium ultimum, Rhizoctonia solani and Sclerotinia minor. Biol. Control 2011, 56, 115–124. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 1–14. [Google Scholar] [CrossRef]
- European Commission (EC). Innovating for Sustainable Growth: A Bioeconomy for Europe; European Commission: Brussels, Belgium, 2012.
- Carrez, D.; Van Leeuwen, P. Bioeconomy: Circular by Nature; The European Files. 2015. Available online: https://biconsortium.eu/sites/biconsortium.eu/files/downloads/European_Files_september2015_38.pdf (accessed on 15 March 2021).
- Sheridan, K. Making the bioeconomy circular: The biobased industries’ next goal? Ind. Biotechnol. 2016, 12, 339–340. [Google Scholar] [CrossRef]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. 2020, 6, 100029. [Google Scholar] [CrossRef]
- Valve, H.; Ekholm, P.; Luostarinen, S. The Circular Nutrient Economy: Needs and Potentials of Nutrient Recycling. In Handbook of the Circular Economy; Brandão, M., Lazarevic, D., Finnveden, G., Eds.; Edward Elgar Publishing: Cheltenham, UK, 2020; pp. 358–368. [Google Scholar] [CrossRef]
| Amendment Type | Used Feedstock | Fertilizer Value (%) | References | ||
|---|---|---|---|---|---|
| N | P | K | |||
| Composted fertilizer | Poultry hatchery waste | 1 | 2.5 | 0.2 | Glatz et al. [55] |
| Cow | 1.8 | 2 | 0.1 | Nunes et al. [44] | |
| Vermicompost manure | Cow | 2.8 | 1 | 0.9 | Yadav et al. [61] |
| Sheep | 1.7 | 1 | 1.3 | Garczyńska et al. [65] | |
| Anaerobic digestate | Poultry | 16.4 | 2.4 | 1.9 | Salminen et al. [82] |
| Pig | 2.2 | 0.4 | 0.9 | Möller and Müller [74] | |
| Pyrolysed biochar | Cow | 0.1 | 0.8 | - | Uzoma et al. [85] |
| Dried amendment | Buffalo | 4.9 | 0.6 | 0.9 | Roy et al. [40,41] |
| Study Country | Fertilizer Type | Application Rate (t ha−1) | Cultivated Crops | Yield Response (t ha−1) | References |
|---|---|---|---|---|---|
| Greece | FYM | 0 16 32 | Wheat | 3.2 3.4 4.5 | Koutroubas et al. [111] |
| Nigeria | FYM+PM (3:1) compost | 0 2.5 5 7.5 10 | Maize | 1.6 2.1 2.2 2.4 4.0 | Adediran et al. [112] |
| Nigeria | PM | 0 5 10 | Maize | 1.9 3.7 2.9 | Busari et al. [116] |
| Hungary | CCW | 0 25 50 100 200 | Triticale | 5.2 5.4 4.7 6.7 6.4 | Ragályi and Kádár [54] |
| India | CMV | 0 5 10 20 | Wheat | 2 3 3.1 3.1 | Joshi et al. [119] |
| United States | DPL | 0 2 | Maize | 2.4 16.2 | Evanylo et al. [115] |
| Japan | CMB | 0 10 15 20 | Maize | 1.2 1.3 3.1 2.4 | Uzoma et al. [85] |
| Soil Health Parameters | Type of Fertilizer Applied | References | |
|---|---|---|---|
| Chemical | Organic | ||
| Aggregate formation | Increases the proportion of micro-aggregates in soil (<250 µm) | Accumulates more SOM in macro-aggregates (>250 µm) | Lin et al. [16] |
| SOM turnover | No significant alteration in SOM turnover rate | More labile SOC pools in organically fertilized soils | Gregorich et al. [154]/Brown and Cotton [156] |
| Microbial abundance | Oligotrophic (Actinobacteria, Acidobacteria and Gemmatimonadetes) | Copiotrophic (Proteobacteria, Bacteroidetes, Firmicutes and Planctomycetes) | Bhunia et al. [15] |
| Enzymatic activity | Relatively less but greater than the control treatment | Higher | Lupwayi et al. [190] |
| Disease suppression | Poses a greater risk of pest outbreak | Protects crops from Pythium, Fusarium, Verticillium, Phytopthora and Rhizoctonia like soil-borne pathogens | Kim et al. [13]/Bailey and Lazarovits [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy 2021, 11, 823. https://doi.org/10.3390/agronomy11050823
Bhunia S, Bhowmik A, Mallick R, Mukherjee J. Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy. 2021; 11(5):823. https://doi.org/10.3390/agronomy11050823
Chicago/Turabian StyleBhunia, Shantanu, Ankita Bhowmik, Rambilash Mallick, and Joydeep Mukherjee. 2021. "Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review" Agronomy 11, no. 5: 823. https://doi.org/10.3390/agronomy11050823
APA StyleBhunia, S., Bhowmik, A., Mallick, R., & Mukherjee, J. (2021). Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy, 11(5), 823. https://doi.org/10.3390/agronomy11050823






