Abstract
Randomized complete block design was used, with three replications. Heterosis for yield and fruit quality characteristics was studied, and expressed as Relative heterosis, heterobeltiosis and Standard heterosis. It would be expected, according to the dominance model, that the heterosis recorded after crossing the recombinant lines, having only a small portion of recessive deleterious alleles, would be minimal. The results showed that the elite recombinant inbred lines became the parents of elite restructured hybrids, with increased levels of re-heterosis for all characters measured. This may prove that dominance is not the only case in explaining heterosis in tomato for yield components and fruit quality characteristics. Several recombinant lines, and most of the new reconstructed F1 hybrids, showed excellent productivity under a low input farming system. The evaluation and selection of the different types of cultivars (recombinant pure lines or reconstructed hybrids) under low input conditions could point towards the most suitable/ideal genotype for organic cultivation.
1. Introduction
1.1. Heterosis
Heterosis, or hybrid vigor, refers to the phenomenon in which the offspring of crosses between different cultivars or species show increased biomass, growth rate, and fertility compared to both parents [1]. The phenomenon of heterosis affects the F1 generation after crossing different species, cultivars, or inbred lines. Heterosis is now recognized as the main contributing factor to successful plant breeding in many crops for developing early, high-yielding, and uniform cultivars which combine several important economic characters.
Despite numerous experiments and knowledge of heterosis for more than a century, its molecular underpinnings remain a significant challenge for scientists [2,3]. Two main hypotheses have been proposed on the genetic basis of heterosis: dominance and overdominance. Tsaftaris [4] summarized these hypotheses as the following: (i) dominance, which attributes the increased vigor of heterozygosity to a complementary effect of dominant alleles from both parents, due to a correlation between recessive loci and reduced fitness; (ii) overdominance, which assumes that heterozygosity per se is essential for hybrid vigor. Overdominance is described as the combining result of high-quality alleles, presumed as advantageous to enzymatic or regulatory function, over the sum of different functions under varying conditions [5]. Jinks [6] linked epistasis to heterosis expressions, and multilocus epistatic interactions are now considered a third plausible hypothesis for heterosis [7,8].
1.2. Heterosis and Tomato Breeding
Tomato hybrids began to dominate the market in the late 1960s and early 1970s due to their considerable earliness, high-quality fruits, and pronounced adapting ability. Their use increased dramatically in the following decades. Duvick [9] reported that 100% of fresh tomatoes and 80% of industrial tomatoes were all hybrids in America. Today, in almost all countries, only hybrid seeds are used for glasshouse and early field production.
According to Powers’ [10] opinion, the number of fruits is more significant than their weight. Therefore, the heterosis effect is due to the greater number of fruits in the hybrids, not to their greater mean fruit weight. Kumar et al. [11] reported a hybrid superiority of 60%, whereas Kumar et al. [12] found 193.55% heterobeltiosis for the number of fruits per plant. Hannan et al. [13] reported heterosis of 72.9% for the number of fruits per plant, and 189% for the yield per plant. Kurian et al. [14] showed a 13.24% heterobeltiosis for yield in a tomato hybrid, and Hannan et al. [13] found that heterobeltiosis was 75.53% for the number of fruits per plant and 172% for total fruit weight per plant. Ahmad et al. [15] found heterosis up to 16.67% for fruit weight, 62.31% for total yield, and 62.31% over better parent for yield per plant in some tomato crosses. Kumari and Sharma [16] reported high heterosis for yield and fruit number. Hussien [17] reported standard heterosis and heterobeltiosis for yield per plant, up to 36.6 and 27.0%, respectively, and a maximum heterobeltiosis of 61.2% for the number of fruits per plant. Shankar et al. [18] showed a 29.22% standard heterosis for fruit weight.
The smaller number of locules per fruit indicates the fruit firmness in tomato [19]. Hence, negative standard heterosis is considered desirable. Regarding the number of locules per fruit, the estimate of heterosis varied between −61.5% and 21.4% [17]. These results are in agreement with those of Naorem et al. [20]. Shankar et al. [18] found in crosses a standard heterosis of −29.63% for the number of locules per fruit, and 11.67% for pericarp thickness. Pericarp thickness is a desirable attribute, as it imparts fruit firmness, and such fruits are suitable for long-distance transportation and canning, and are characterized by high quality [21]. Hence, positive standard heterosis was desirable. Hussien [17] reported that standard heterosis and Heterobeltiosis for pericarp thickness reached 27.5%. Heterosis for pericarp thickness was also analyzed by Patil and Patil [22], Daskaloff et al. [23], and Dod and Kale [24].
1.3. Forms of Gene Action in Tomato
Cuartero and Cubero [25] studied tomatoes’ genetic behavior and concluded that heterosis seems to be based on dominance rather than overdominance. Burdick [26] stated that dominance is the main cause of heterosis, although the possibility of dominance or epistasis could not be rejected. Studies on earliness have shown that dominance plays an essential role in this trait [27,28]. As for traits connected with productivity, additive and no additive gene action was observed. Saidi et al. [29] reported additive and non-additive effects for the characteristics of the number of fruits per plant and fruit weight, while dominance affects fruit yield. Saleem et al. [30] found that additive gene effects were responsible for fruit yield per plant, fruit weight, and number of fruits per plant, and the decision to improve those traits would be effective in early generations (F2–F3). Aisyah et al. [31] indicated that additive gene action was more significant than non-additive for a number of characteristics (yield per plant, number of fruits, individual fruit weight, number of locules, and pericarp thickness). Moreover, pericarp thickness was found to have additive and no additive gene expression [32,33].
1.4. Inbred Vigor in Tomato
In general, all individuals carry degenerative–deleterious alleles [34,35]. As most of the degenerative alleles are in a high degree recessive [36], these alleles are not expressed in organisms, unless they are in a homozygous state. Based on the genetic load of individuals and their pedigree, the offspring of closely related crosses may exhibit more degenerative alleles, resulting in a decrease in robustness and survival rate [37]. This decrease in individuals’ robustness, derived through closely related reproduction, indicates the degree of inbreeding depression [38]. So, inbreeding depression is attributable to the homozygosity of undesirable recessive deleterious genes [39].
Fasoulas [40] pointed out that the presence of degenerative recessive genes is revealed in the generation after selfing (F2). If inbreeding depression is less than 50%, selection favors an increase in the rate of self-fertilization. In comparison, when inbreeding depression is greater than 50%, selection acts in favor of cross-pollinated genotypes [41]. In the presence of deleterious genes, hybrids are superior to pure lines, while the presence of desired additive genes means the opposite. The breeder’s main tool to free a cultivar from the concentration of deleterious genes is artificial self-pollination.
Breeders have two alternatives—the extinction of deleterious genes by continuous selection, and the concealment of their negative action through heterozygosity [40]. Once deleterious genes are eliminated, pure lines become more important than hybrids. Crow [42] stated that if dominance or partial dominance are the causes of heterosis, it will be possible to produce pure lines similar to or better than hybrids. Powers [43] suggests that tomato lines equal to or superior to F1 can be created by selection. Williams [44] followed self-fertilization in two F1 commercial tomato hybrids and studied the possibility of fixing their vigor by creating inbred lines. In self-fertilized crops, such as tomato, which carry the lowest load of deleterious genes, additive genetic variation predominates, and it is always feasible to fix and transgress heterosis [26,45,46]. The predominance of inbred lines is attributed to the higher amount of gene product due to their additive homoallelic complementation, resulting in inbred vigor [47]. This becomes feasible by systematically removing deleterious genes and replacing them with favorable additive alleles [47].
Christakis and Fasoulas [48] concluded that continuous evaluation and selection could overcome hybrid vigor by exploiting additive genes. This is demonstrated by inbred lines that surpassed the performance of the original hybrids. Fixing heterosis and increasing inbred lines’ productivity has already been achieved in tomato [45,49,50,51,52].
Avdikos et al. [52], following pedigree selection and honeycomb design for the first (F2–F3) segregating generations in four commercial F1 tomato hybrids, created recombinant inbred lines with the same or better behavior as the original F1 hybrids in terms of productivity and fruit quality. This occurred by replacing the deleterious genes presented in the genome of hybrids with favorable additive genes, making inbred vigor more important than hybrid vigor.
This study attempts to address the question about whether it is possible to develop a high level of promising re–heterosis in new reconstructed hybrids, originating from crosses, among recombinant pure lines with different genetic backgrounds, when in these lines a significant load of deleterious genes has been replaced by favorable additive genes.
2. Materials and Methods
2.1. Plant Material
The commercial F1 single-cross hybrids ‘Iron’, ‘Sahara’, ‘Formula’, and ‘Elpida’ were used as starting materials. The above cultivars are considered very popular amongst growers in the Southern Mediterranean region [46]. Furthermore, cv. ‘Makedonia’, a traditional tomato cultivar which was developed at the Agricultural Research Center of Northern Greece (ARCNG) through pure line selection, was used as parent in the scheme of diallelic crossings. This tomato cultivar was widely used in traditional farming and low input cropping systems, before high-input agricultural systems prevailed.
Following a systematic breeding scheme using Mass, Pedigree and Recurrent selection [52], 11 recombinant lines, which were produced under an organic farming system—4 from ‘Formula’, 5 from ‘Elpida’, 1 from ‘Iron’, and 1 from ‘Sahara’—were selected for having all the desirable characteristics of earliness, productivity, and fruit quality.
Between recombinant lines and ‘Makedonia’ cultivar, diallelic crosses were made to produce second cycle new reconstructed hybrids (Figure 1).
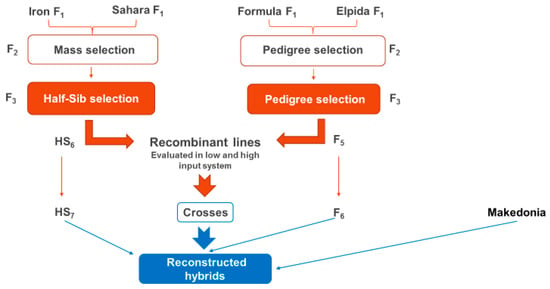
Figure 1.
The breeding process that was applied for the production of new recombinant lines and reconstructed hybrids.
2.2. Selection and Assessment Procedure
The selected recombinant lines and reconstructed hybrids produced as described previously were evaluated under organic (low input) conditions in a non-heated greenhouse in Thermi-Thessaloniki, at the Agricultural Research Center of Northern Greece (ARCNG). Uniform seedlings were hand transplanted on 15 April 2016, at an intra-row distance of 0.5 m and an inter-row distance of 1 m (2 plants/m2). The greenhouse was shaded in the period from June to August. An RCB design was used, with 3 replications, each consisting of 15 plants. Organic cropping practices were followed for fertilizer, irrigation, and pesticide application. The cropping practices applied were identical to organic farming principles (field rotation with legumes, manure, soil mulching using a biodegradable film, and no chemical or agrochemical applications). Composted poultry manure was applied at 3 t ha−1 (dry weight). All the observations and measurements were obtained on an individual plant basis. For each entry, earliness, yield components, and fruit quality characteristics of table-ripe fruit were evaluated.
2.3. Traits Evaluated
Table-ripe fruit yield was recorded on a per plant basis over 6 harvest dates. Earliness and total yield were estimated based on production (g), based on harvests until 75 and up to 105 days after transplanting, respectively. All table-ripe fruits harvested were transferred to the laboratory of ARCNG, where they were counted and weighed. Fruit pericarp thickness (mm) and the number of locules per fruit were also measured.
2.4. Statistical Analyses
Data were subjected to an analysis of variance procedures (ANOVA) and a Scott–Knott cluster test at 5% probability and significance level [53]. The behavior of a hybrid compared to its parents can be attributed in two ways: (i) heterosis concerning the median value, i.e., the behavior of the hybrid in relation to the average behavior of both parents (Relative heterosis); and (ii) heterosis concerning the best parent, i.e., the behavior of the hybrid in relation to the behavior of the best parent (heterobeltiosis). Another approach with practical value in the improvement of self-pollinating species, when used hybrids, is the comparison of their response to the best commercial variety that adapts well to a given cultivated region. This expression of heterosis in relation to the best local/commercial variety is called Standard heterosis [54], and it presents significant economic value. In this study, heterosis for the characteristics studied was estimated for 21 reconstructed hybrids, and was expressed as an increase or decrease over mid parental value (Relative heterosis), over better parent value (heterobeltiosis), and over ‘Makedonia’ and mid value of ‘Formula’ and ‘Elpida’ commercial hybrids (Standard heterosis).
A total of 19 agro-morphological characteristics were recorded, which included the following: (i) 12 ordinal and (ii) 7 continuous measurements. A nonlinear (categorical) principal component analysis (PCA) with optimal scaling was applied. This approach results in reducing observed variables to a smaller number of new, uncorrelated synthetic variables (6) which describe most of the original variability [55]. A Hierarchical cluster analysis (HCA) was carried out based on those new synthetic variables. A dendrogram of HCA was constructed using the unweighted pair group method, with arithmetic mean analysis and the square Euclidean distance as the genetic distance measure [56,57].
The characteristics used to make the dendrogram were as follows: (1) inflorescence type: 1. mainly uniparous, 2. equally uniparous and multiparous, 3. mainly multiparous; (2) inflorescence arrangement: 1. sparse, 2. dense; (3) number of inflorescences in a specific date; (4) plant height (cm); (5) height of 9th inflorescence (cm); (6) plant attitude: 1. leaves do not cover the main stem, 2. medium situation, 3. leaves cover the main stem; (7) leaf attitude: 1. erect, 3. horizontal, 5. drooping; (8) leaf angle: 1. small, 2. medium, 3. big, 4. very big; (9) leaf length (cm); (10) leaf width (cm); (11) size of leaflets: 1. small, 3. medium, 5. large; (12) intensity of leaf’s green color: 3. light, 5: medium, 7: large; (13) attitude of petiole of leaflet in relation to main axis: 3. semi-erect, 5. horizontal, 7. semi-drooping; (14) leaf blistering: 3. weak, 5. medium, 7. strong; (15) pubescence of flower’s style: 1. absent, 9. present; (16) extent of fruit’s green shoulder: 1. very small, 3. small, 5. medium, 7. large; (17) fruit blotching: 1. absent, 2. small, 3. large; (18) plant height/inflorescences; (19) stem length between 1st and 4th inflorescences.
3. Results
3.1. Comparisons between Genetic Materials
Regarding early yield, three out of four recombinant lines originating from ‘Formula’ F1 reached this hybrid’s levels and, also, all recombinant lines derived from ‘Elpida’ did not differ significantly from the original hybrid, possessing a depression from 19% to 39% (Table 1). However, recombinant line derived from ‘Iron’ hybrid and traditional cultivar ‘Makedonia’ lagged significantly behind ‘Formula’ and ‘Elpida’ (Table 1). In contrast, recombinant line obtained from the ‘Sahara’ hybrid did not differ significantly from the hybrids mentioned above. In total, 19 out of 21 reconstructed hybrids reached the ‘Formula’ and ‘Elpida’ F1 hybrids in early yield performance (Table 1). Regarding F2 generation, both ‘Formula’ and ‘Elpida’ exhibited inbreeding depression of 27% and 41%, respectively, in relation to the hybrids.

Table 1.
Early production for yield per plant (g), fruit number per plant, and fruit weight (g) of the original F1 commercial hybrids, new recombinant inbred lines, and the new reconstructed hybrids.
In the fruit number per plant characteristic, all recombinant lines originated from ‘Formula’ had the same performance (Table 1). Only two out of five recombinant lines which originated from ‘Elpida’ F1 reached the hybrid’s early yield fruit number per plant (Table 1). However, ‘Iron-1′, ‘Sah-1′ and ‘Makedonia’ had a smaller fruit number than the ‘Formula’ and ‘Elpida’ F1 hybrids. In all, 18 out of 21 reconstructed hybrids were characterized by a high number of fruits per plant, whereas all genotypes had similar behavior in early fruit weight, varying from 76.3 to 172.3 g (Table 1).
However, ‘Iron-1, ‘Sah-1′ and ‘Makedonia’ had a smaller fruit number than the ‘Formula’ and ‘Elpida’ F1 hybrids. A total of 18 out of 21 reconstructed hybrids were characterized by a high number of fruits per plant (Table 1).
All genetic material displayed similar behavior in earliness for fruit weight, with values from 76.3 to 172.3 g/fruit (Table 1).
In total yield, recombinant lines derived from ‘Formula’ were less productive, by 22% to 43%, than the original F1 hybrid, while only one of the recombinant lines originating from the ‘Elpida’ hybrid (Elp-4) reached the yield of its original hybrid (Figure 2). Recombinant lines ‘Iron’-1, ‘Sah’-1 and the cultivar ‘Makedonia’ yielded behind the ‘Formula’ and ‘Elpida’ hybrids (Table 2). Twelve reconstructed second cycle hybrids performed better than or equal to the ‘Formula’ and ‘Elpida’ F1 hybrids in terms of total yield (Table 2). Likewise, the reconstructed hybrid ‘For-1 × Elp-1′ yielded 28% more than the most productive commercial hybrid (‘Elpida’ F1) (Table 2). Regarding inbreeding depression in the F2 generation, in the case of ‘Formula’, it reached only 16%, while ‘Elpida’ achieved slightly higher with 28%.
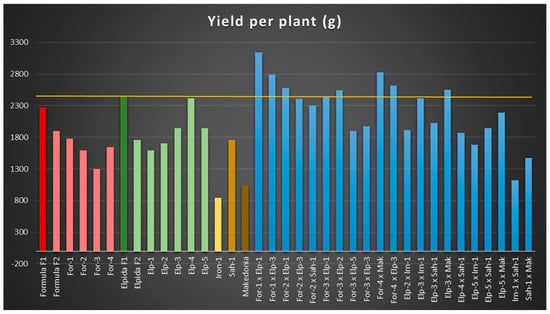
Figure 2.
Total production for yield per plant (g), of the original F1 commercial hybrids, new recombinant inbred lines, and the new reconstructed hybrids.

Table 2.
Total production for yield per plant (g), fruit number per plant, and fruit weight (g) of the original F1 commercial hybrids, new recombinant inbred lines, and the new reconstructed hybrids.
For fruit number per plant, both ‘Formula’ F2 and the recombinant lines yielded less than the original hybrid, as happened in total yield (Table 2). Similarly, the recombinant line ‘Elp-4′ performed the same as ‘Elpida’ F1, while the other recombinant lines showed a significant inbreeding depression. (Table 2). Recombinant lines ‘Iron-1′, Sah-1′ and the traditional cultivar ‘Makedonia’ produced fewer fruits per plant than the commercial hybrids and many other entries, whereas eight reconstructed hybrids showed significantly better behavior than the commercial hybrids of ‘Formula’ and ‘Elpida’.
In the second yield component, i.e., fruit weight, all genetic material showed similar behavior without having significant differences. It was observed that the average fruit weight had small values, which were obviously affected by the low input farming system.
Regarding pericarp thickness, the commercial hybrids ‘Formula’ and ‘Elpida’, their F2 generations, most of their recombinant lines, ‘Iron-1′, and five reconstructed hybrids showed the highest values (Table 3). In the characteristic of locule number, the highest values had the recombinant line ‘Sah-1′ (8.9 locules) and the traditional cultivar ‘Makedonia’ (7.5 locules).

Table 3.
Pericarp thickness (mm) and locule number of the original F1 commercial hybrids, new recombinant inbred lines, and the new reconstructed hybrids.
3.2. Heterosis
Heterosis is expressed as the superiority of F1 hybrid over parents. In case that this superiority is expressed over the mid-parent value, it is called Relative heterosis. Usually, in plant breeding programs, Relative heterosis is not as significant as the other terms of heterosis, since it does not offer the hybrid any advantage over the better parent. Therefore, heterosis is estimated over the better parent, which is often called heterobeltiosis. It is also desirable to estimate heterosis in relation to a popular commercial variety of the crop (‘Makedonia’). Such an estimate is known as Standard, Useful, or Economic heterosis. Estimates of Relative, heterobeltiosis, and Standard heterosis for early and total yield (g/plant) are presented in Table 4 and Table 5.

Table 4.
Relative heterosis, heterobeltiosis, and standard heterosis of the reconstructed new hybrids for early yield (g/plant).

Table 5.
Relative heterosis, heterobeltiosis, and standard heterosis of the reconstructed new hybrids for total yield (g/plant).
Regarding early yield, crosses with high Relative heterosis were ‘For-4 × Mak’ (140%) and ‘For-1 × Elp-3′ (69%) (Table 4). Estimates of heterobeltiosis for this trait ranged from −47% to 120% (Table 4). The average Standard heterosis over ‘Makedonia’ (M) was 146%. Reconstructed hybrids ‘For-1 × Elp-3′ and ‘For-2 × Elp-3′ showed the highest heterosis over ‘Makedonia’, at 267% and 218%, respectively. In Standard heterosis over the average value of the two commercial hybrids ‘Formula’ and ‘Elpida’ (FE), 6 out of the 21 reconstructive hybrids showed heterosis from 7% to 36% (Table 4).
The new reconstructed hybrids ‘For-4 × Mak’, ‘For-1 × Elp-1′, ‘Elp-3 × Mak’, ‘For-3 × Elp-1′, and ‘For-3 × Elp-2′ showed the highest Relative heterosis for total yield (Table 5). Among all crosses, only two did not present Relative heterosis (Table 4). The average heterosis over best parent was 21%. The top three best crosses based on heterobeltiosis were ‘For-1 × Elp-1′ (77%), ‘For-4 × Mak’ (72%) and ‘For-2 × Elp-1′ (62%) (Table 4). The average Standard heterosis over ‘Makedonia’ was 112%. The reconstructed hybrids ‘For-1 × Elp-1′ and ‘For-4 × Mak’ recorded the highest Standard heterosis (M) estimates (200% and 170%, respectively) (Table 5). The Standard heterosis (FE) estimates varied from −53% to 33% (Table 5). The top two best reconstructed hybrids based on Standard heterosis (FE) were ‘For-1 × Elp-1′ and ‘For-1 × Elp-3′ (33% and 18%, respectively) (Table 5).
The fruit number per plant in earliness was maximum for the reconstructed hybrids ‘For-4 × Mak’ and ‘For-1 × Elp-3′ over the average value of both parents and the best parent (Table 6). The level of heterobeltiosis ranged from a decrease of up to −31% (‘Irn-1 × Sah-1′) to an increase of up to 80% (‘For-4 × Mak’) (Table 6). The best reconstructed hybrids over ‘Makedonia’ and commercial F1 hybrids were ‘For-1 × Elp-3′ (198% and 48%), ‘Elp-3 × Sah-1′ (183% and 40%) and ‘For-2 × Elp-3′ (170% and 34%). The average Standard heterosis ‘M’ and ‘FE’ for fruit number per plant in earliness, were 125% and 12%, respectively (Table 6).

Table 6.
Relative heterosis, heterobeltiosis, and standard heterosis of the reconstructed new hybrids for early yield (fruit number/plant).
The average Relative heterosis and Heterobeltiosis for fruit number per plant in total yield was 38% and 17%. The best reconstructed hybrids for both type of heterosis were ‘For-1 × Elp-1′ and ‘For-4 × Mak’ (Table 7). Among reconstructed hybrids, the best for Standard heterosis over both ‘Makedonia’ and commercial hybrids were ‘For-4 × Mak’, ‘For-1 × Elp-1′, ‘Elp-3 × Mak’ and ‘For-1 × Elp-3′. ‘For-1 × Elp-1′ hybrid reached 31% Standard heterosis (FE) levels (Table 7).

Table 7.
Relative heterosis, heterobeltiosis, and standard heterosis of the reconstructed new hybrids for total yield (fruit number/plant).
Overall, 6 out of the 21 reconstructed hybrids possessed Relative heterosis for the fruit weight characteristic in early production (Table 8). Hybrids ‘For-4 × Mak’ and ‘Sah-1 × Mak’ were the only ones which scored positive values in heterobeltiosis. The average values for Standard heterosis over ‘Makedonia’ and commercial hybrids were 1% and −19%, respectively (Table 8). The crosses ‘For-2 × Sah-1′ and ‘For-3 × Elp-2′ showed the highest heterosis over ‘Makedonia’ for fruit weight in early production (23%). All reconstructed hybrids showed negative values in Standard heterosis (FE), which reached up to −42% (Table 8).

Table 8.
Relative heterosis, heterobeltiosis, and standard heterosis of the reconstructed new hybrids for early yield (g/fruit).
In term of fruit weight, reconstructed hybrids ‘Elp-3 × Irn-1′ and ‘Sah-1 × Mak’ expressed highest Relative heterosis (13%) and heterobeltiosis (10% and 8%, respectively) (Table 8). The best heterotic combinations, for Standard heterosis (M and FE) were the new reconstructed hybrids ‘For-1 × Elp-1′ ‘For-2 × Elp-1′ and ‘Sah-1 × Mak’ (Table 9). The average Standard heterosis (FE) for this trait was −10%. The high levels of Standard heterosis (FE) in total yield are related to an analogous standard heterosis (FE) in fruit number. Fruit weight did not contribute to this improvement.

Table 9.
Relative heterosis, heterobeltiosis, and standard heterosis of the original commercial hybrids, the new recombinant inbred lines, and the reconstructed new hybrids, as for total yield (g/fruit).
Estimates of Relative heterosis and heterobeltiosis for pericarp thickness ranged from −31% to 11% (Table 10). A total 3 out of 21 reconstructed hybrids showed heterobeltiosis from 3% to 10%. The top two best reconstructed hybrids based on Standard heterosis (M) were ‘Elp-2 × Irn-1′ and ‘Elp-3 × Irn-1′, with 31% and 27%, respectively). Standard heterosis (FE) for pericarp thickness had negative values (−33% up to −3%).

Table 10.
Relative heterosis, heterobeltiosis, and standard heterosis of the original commercial hybrids, the new recombinant inbred lines, and the reconstructed new hybrids, as for pericarp thickness (mm).
The best heterotic combination for locule number was ‘For-3 × Elp-5′, with 45% and 33% for Relative heterosis and heterobeltiosis, respectively. The average values for Standard heterosis (M) and (FE) were −37% and 1%, respectively. Regarding Standard heterosis (FE), 9 out of 21 reconstructed hybrids showed positive values ranging from −25% to 40% (Table 11).

Table 11.
Relative heterosis, heterobeltiosis, and standard heterosis of the original commercial hybrids, the new recombinant inbred lines, and the reconstructed new hybrids, as for locule number.
3.3. Dendrogram of Genetic Distances for Morphological Characteristics
In this dendrogram, accessions were classified into two groups: A and B. Regarding group (A), there are two subgroups (A1) and (A2). Group (A1) consists of twelve reconstructed hybrids and three recombinant lines classified into two subgroups—(A1-1) and (A1-2). Subgroup (A1-1) includes ten reconstructed hybrids, nine of which include a parent of the ‘Elpida’ family, with ‘Elp-3′ being in five of them. In the same group, there is also the domestic cultivar ‘Makedonia’ and the recombinant line ‘Elp-4′. Most of the restructured hybrids, in which the series Makedonia participates as a parent, are in the same group (A1-1). Regarding the group (A1-2), two recombinant lines (‘Irn-1′ and ‘Sah-1′) and two reconstructed hybrids, which were produced from the above inbred lines, were classified.
Group (A2) consists of three recombinant lines and nine reconstructed hybrids, classified into two groups—(A2-1) and (A2-2). Subgroup (A2-1) consists of the three recombinant lines of the ‘Elpida’ F1 family, (‘Elp-2′, ‘Elp-3′, ‘Elp-5′), ‘Elpida’ F2, as well as the original ‘Elpida’ F1 hybrid, indicating that the phenotypes of the above materials are similar in terms of the specific characteristics studied. In the eight reconstructed hybrids classified in the group (A2-1), the above inbred lines from the ‘Elpida’ family participated as parents. The group (A2-2) consists of only one genotype—the reconstructed hybrid (‘For-4 × Mak’)—which seems to differ in morphological characteristics from the other genetic materials.
Group B includes ‘Formula F1′ hybrid, ‘Formula F2′, all recombinant inbred lines originating from ‘Formula’, and recombinant line ‘Elp-1′ (Figure 3). This clustering of the second group (B) indicates that the ‘Formula’ F1 family’s genetic materials have little variability between them (fixed materials).
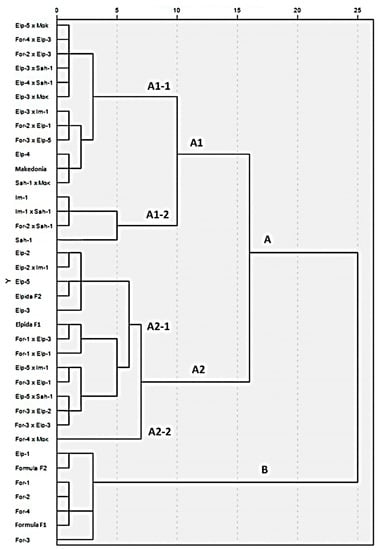
Figure 3.
Dendrogram of hierarchical clustering of the original commercial hybrids, the recombinant inbred lines, and the reconstructed new hybrids according to Ward linkage and squared Euclidean distance method, based on 19 morphological characteristics.
4. Discussion
Since 1970, significant efforts have been made to explore agricultural practices for reducing aggravating plant protection substances, both for consumers and for the environment, without reducing the quantity and quality of the end product. The need to reduce external inputs in agricultural systems is a challenge for breeders and growers due to reduced production costs [58]. Thus, researchers’ interest turned to alternative production systems and methods, such as organic farming and low input agriculture, which were considered more sustainable than conventional farming [59]. However, few studies have been published on production’s economic viability in low input systems [60]. This information is critical for those wishing to adopt this system, especially in crops that require high inputs, such as tomato cultivation [61]. The use of appropriate cultivars to meet specific cultivation systems is essential, considering that many cultivars which developed under high input conditions have failed to meet producers’ requirements in low input systems [62]. This fact necessitates the creation of suitable cultivars as, according to Lammerts van Bueren, etc. [63], more than 95% of organic farming is based on high-input cultivars. For this reason, the creation of cultivars intended for cultivation in conditions of lower inputs requires their breeding process to take place in similar conditions [64,65,66]. Our study focused on the production of tomato recombinant pure lines under low input farming conditions, targeting these kind farming systems (low inputs and organic cultivation). Tomato commercial hybrids Formula F1 and Elpida F1, the recombinant lines derived from them and from the hybrids ‘Iron’ and ‘Sahara’, the cultivar ‘Makedonia’, and the second cycle reconstructed hybrids, produced after crossing these inbred lines, were evaluated under low input conditions.
Nowadays, there is a demand for the development of high-yielding tomato inbred cultivars or, in some cases, hybrids for increased yield, even in the least productive areas [67]. Over the last few years, inbred lines’ replacement by hybrids has remarkably increased yield [68,69]. In general, hybrids are preferred over pure line varieties in tomato because of their superiority in marketable fruit yield, component traits, and fruit quality [70]. The exploitation of heterosis proves an efficient approach for the improvement of tomato, with the tomato’s hybrid varieties showing high yield potential and gaining popularity among growers [71]. In many countries, including Greece, the hybrid is the predominant type of commercial cultivar. Tomato hybrids dominate in high-input agricultural systems (greenhouse cultivation/hydroponics) and may be extended to the lower-input systems, where economic benefit can be demonstrated [72]. In our study, we have proven that hybrids which are structured from recombinant lines and developed under low input conditions are suitable for such cultivation conditions.
Earliness is one of the primary criteria in every breeding program [73]. This study’s genetic materials did not differ significantly regarding fruit weight, as it was shown for the characteristic of fruit number per plant. As such, the latter seems to be the reason why, in total yield, there were statistically significant differences. The lines having high number of fruits per plant are characterized by high yielding potential. Recombinant inbred lines ‘For-2′ and ‘Elp-3′ are promising genetic materials for earliness. Furthermore, four reconstructed hybrids exceeded both original hybrids, ‘Formula’ and ‘Elpida’, in absolute values
The ‘Elpida’ F1 hybrid possessed a higher level of inbreeding depression (28%) than the ‘Formula’ F1 hybrid (16%), both of which presented tolerance to inbreeding and were not prohibitive in designing breeding programs for the production of inbred lines.
Patel et al. [71] stated that the crosses exhibiting high heterobeltiosis with low inbreeding depression were characterized by the accumulation of favorable additive genes for the traits evaluated. The inbred line ‘Elp-4′ reached the productivity in total yield of the original hybrid ‘Elpida’ F1, and surpassed the ‘Formula’ F1 hybrid by 6%. This reinforces the view of it being possible to isolate inbred lines superior to hybrids [52,74]. Although tomato hybrids are the dominant type of variety, studies conducted by many researchers confirm the ’recovery’ of inbred lines through selection by hybrids, thus supporting inbred vigor. Experimental studies on the ‘isolation’ of inbred lines by breeding and selecting tomato hybrids reinforce the above position [45,49,52,75]. At the same time, it has been found that, through selection methodologies, the frequency of alleles with additive action increases, resulting in inbred lines that could yield, theoretically, as much as hybrids [76]. According to Syukur et al. [77], the characters which are controlled by additive genes will be easier to select, mainly for improving inbred line varieties. Sharma et al. [78], Kumaravel et al. [79], and Hosamani [80] found that additive variance was consistently greater than non-additive variation, indicating that the major portion of the genetic variation for the characters number of fruits per plant and total fruits yield per plant was controlled by genes with additive effects [81]. Analogous results are shown in the present study. New breeding objectives should consider the concept of agricultural sustainability by maintaining high yield and fruit quality under low input farming practices, especially under adverse climate conditions [82]. Each of the recombinant inbred lines developed under low input farming systems in this study are characterized by earliness, high yield, and desirable fruit quality traits, which could be cultivars targeted to low input farming systems.
In the case of the isolation of recombinant inbred lines with a yielding potential equivalent to hybrids, a question arises about the behavior of hybrids resulting from these superior inbred lines. According to Guan Yi-Xin, et al. [83], homozygous parental lines produce restructured F1 hybrids with a high degree of heterosis in many characteristics. This was the primary task of this study. The recombinant lines produced with base material dynamic commercial hybrids under honeycomb pedigree methodology were proven to be equal to, or even better than, the original hybrids. This means that additive genes replaced deleterious recessives, and inbred vigor surpassed hybrid vigor. In such a situation, it is necessary to check whether the specific genetic materials can, by crossing them, create additional heterosis; therefore, if there is still a sufficiently high genetic load of recessive deleterious genes, they, in the state of heterozygosity, will cover their action behind the action of dominant genes.
The study of the total yield of genetic materials revealed no significant superiority of seven reconstructed hybrids from the original F1 hybrids ‘Formula’ and ‘Elpida’, with ‘For1 × Elp1′ reaching 3140.9 g per plant. These reconstructed hybrids produced under low input farming systems could be an alternative type of cultivar suitable for such systems.
An increase in production is associated with an increase in the number of fruits per plant or/and in fruit weight [75]. Avdikos et al. [52] reported a high positive correlation between yield per plant and number of fruits per plant (r = 0.97), while a low correlation was observed between yield per plant and fruit weight per plant (r = 0.27). The results indicated the importance of the characteristic of the number per plant in a breeding program. Solieman et al. [84] reported a positive correlation between the total yield and the number of fruits per plant, while the correlation was negative between the number of fruits per plant and fruit weight. This is confirmed by our results, as the reconstructed hybrids, (‘For-1 × Elp-1′) and (‘For-4 × Mak’), scored the highest yield, with the highest number of fruits per plant. Similarly, the recombinant ‘Elp-4′ line, provided a high yield and a high number of fruits per plant, whereas the ‘Irn-1′ recombinant line showed the lowest yield and the lowest number of fruits per plant. It is noteworthy that the inbreeding depression for fruit number per plant in F2 generation of ‘Formula’ and ‘Elpida’ hybrids is similar to that recorded for total yield (‘Formula’ F2: 16% total yield—19% fruit number, ‘Elpida’ F2: 28% total yield—27% fruit number).
Almost all reconstructed hybrids possessed positive Relative heterosis regarding early yield, reaching 140% (‘For 4 × Mak’). A total of 13 out of 21 reconstructed hybrids showed positive heterobeltiosis, with ‘For 4 × Mak’ reaching 120%. All reconstructed hybrids presented high Standard Heterosis over ‘Makedonia’ in earliness. Over the mid-value of ‘Formula’ and ‘Elpida’ hybrids, we produced six reconstructed hybrids that had positive values reaching 36%. Thus, it was shown that, in second cycle there were new reconstructed hybrids produced which provided higher yields in earliness than the commercial hybrids.
Total fruit yield per plant is one of the most important traits, and it deserves the highest attention in any breeding program. Almost all hybrids showed positive Relative heterosis in total yield, reaching 86% (‘For1 × Elp1′). In heterobeltiosis, 15 reconstructed hybrids showed positive heterosis, from 1% to 77%. All second cycle reconstructed hybrids exhibited standard heterosis over domestic cultivar ‘Makedonia’, which varied from 7% to 200%. When compared with commercial hybrids, the reconstructed hybrids possessed Standard heterosis from 2% to 33%. The reconstructed hybrids ‘For1 × Elp1′, ‘For4 × Mak’ and ‘For1 × Elp3′ recorded the highest values in Standard heterosis, and appear as promising material for cultivation, at least in low input farming systems. Rehana et al. [73] found six crosses exhibiting the highest positive heterosis for yield per plant, having values ranging from 26.46% to 88.46% in heterobeltiosis and from 16.09% to 48.44% in Relative heterosis. Rasheed et al. [85] reported that the maximum better parent heterosis was 120.6%. Khan and Jindal [67], with relevance to total yield, found that the crosses revealed significant and positive heterosis over better parent, ranging from 19.6% to 103.1%, and heterosis over standard check, ranging from 26.4% to 130.7%. Solieman et al. [84] reported heterosis ranging from 22.29% to 64.33% for total fruit yield per plant. Heterosis for fruit yield was also reported by Thakur et al. [86], Premalakshme et al. [87], Harer et al. [88], and Sharma and Thakur [89].
The number of fruits per plant is a major yield contributing character [16]. Fageria et al. [90], Kurian et al. [14], and Singh et al. [91] observed a significant positive heterosis for a higher number of fruits per plant in tomato, suggesting good scope for yield improvement through its components. Regarding fruit number per plant, both in earliness and total production, almost all genetic materials showed positive Relative heterosis (reaching 101%). A similar situation was noticed for heterobeltiosis, with the best hybrid in earliness, ‘For 4 × Mak’, possessing 80%, and the best hybrid in total production, ‘For1 × Elp1′, possessing 74%. The Standard heterosis over ‘Makedonia’ was extremely high in both earliness and total production (reaching 198%). Eighteen and fourteen reconstructed hybrids out of twenty-one showed positive Standard heterosis over commercial hybrids in early and total production, respectively. Ahmad et al. [15] found for the trait of fruit number that the range of positive heterosis was 3.8% to 83.9% over the better parent. Rehana et al. [73] recorded the trait of fruit number per plant, heterobeltiosis, and Relative heterosis from 17.26% to 49.25%, respectively. Kumari and Sharma [16] found 66.08% maximum heterosis over the better parent. Similar findings were also reported by Legon et al. [92], Jamwal et al. [93], Mirshamssi et al. [94], Rani et al. [95], and Ahmad et al. [15], for higher fruit number per plant.
In terms of fruit weight, the results were not the same as in yield and number of fruits per plant. Most reconstructed hybrids showed negative values in Relative heterosis and heterobeltiosis, revealing that the higher yields shown for yield are only due to the characteristic number of fruits per plant. Some hybrids showed negative values for Standard heterosis over ‘Makedonia’ and all commercial hybrids. Heterotic analysis by Rasheed et al. [85] depicted positive and significant heterosis, as well as heterobeltiosis for individual fruit weight in cross combinations. Maximum heterosis and heterobeltiosis were 88.5% and 54.3%, respectively. Ghadage et al. [96] found that heterosis over better parent for average fruit weight ranged from −46.1% to 34.7%, whereas heterosis over standard check ranged from −35.4% to 32.9%. Rehana et al. [73] recorded fruit weight maximum Relative heterosis and heterobeltiosis at 63.5% and 48.3%, respectively. Significant positive heterosis for this character has been reported by Mondal et al. [97], Kumari and Sharma [16], Savale et al. [98], Tamata and Singh [99], Gautam et al. [100], Kattegoudar et al. [101], and Mohammad l. Al-Daej, [102].
A morphological feature of the tomato fruit is pericarp thickness [103]. Large pericarp thickness expresses a fruit’s firmness and is considered a desirable characteristic for those fruits which are intended to be transported, as the duration of post-harvest life is extended. However, consumers favor fruits with a small pericarp thickness. The largest pericarp thickness was found in the original ‘Elpida’ F1 hybrid, followed by the reconstructed hybrid (‘Elp-2 × Irn-1′). Most recombinant lines, from both the Formula and the ‘Elpida’ family, produced fruits with a large pericarp size, with no statistically significant differences from the original Elpida F1 hybrid. It is worth noting that the reconstructed hybrid (‘For-2 × Elp-1′), recorded the lowest pericarp thickness (4.7 mm), although the parental lines For-2 and Elp-1 exhibited, as individuals, a large pericarp thickness (6 and 6.8 mm, respectively). Almost all reconstructed hybrids recorded negative values for Relative heterosis, heterobeltiosis, and Standard heterosis over commercial hybrids regarding pericarp thickness. Ghadage et al. [96] found a range of heterosis over better parent for pericarp, from −22.9% to 14.7%, whereas heterosis over standard check ranged from −10.9% to 25.3%. Kumari and Sharma [16] reported a maximum heterotic effect of pericarp thickness over better parent by 19.37%. Significant positive heterosis for this character has been reported by Joshi et al. [104], Rattan [105], Garg et al. [106], and Sureshkumara et al. [107].
From a quality point of view, reducing locule number is desirable and a negative estimate of heterosis is valuable [108]. Thus, to reduce the number of locules per fruit, heterosis breeding can be exploited efficiently. The locule number of the fruit is an indicator of fruit firmness. A smaller number of locules per fruit increases fruit firmness and vice versa. Wild tomato species produce fruit with 2 to 4 locules, while cultivated varieties can develop more than 15 locules. This affects fruit shape dramatically and leads to a 50% increase in fruit size [109]. Consumers usually prefer fruits with a small number of locules. Among the genetic materials studied, the largest locule number was noted by the recombinant line ‘Sah-1′, followed by the domestic cultivar ‘Makedonia’, with 8.9 and 7.5 locules, respectively. Primarily, statistically significant differences were observed in both recombinant lines and reconstructed hybrids for the fruit’s locule number, not consistent with the materials’ fruit weight, where no statistically significant differences were observed. As for this trait, the majority of hybrids had negative estimates for all types of heterosis. Ahmad et al. [15] found that about 50% of combinations showed positive heterosis over the better parent, ranging from 5.4% to 27.5%. Rasheed et al. [85] recorded that nine hybrids exhibited positive heterosis, while six hybrids revealed negative heterosis regarding the number of locules per fruit, with a maximum heterosis of 32.4% and a heterobeltiosis of 22.7%. Maximum negative heterosis and heterobeltiosis were −39.39% and −46.42%, respectively.
The dominance model (masking of deleterious recessive alleles) presents heterosis as a simple reversal of inbreeding depression (unmasking of deleterious recessive alleles). The dominance model suffers from certain limitations, which suggest that it is only a partial explanation for the phenomenon of heterosis. An essential criticism of this model is that, if complementation of deleterious alleles is causal for heterosis, then the potential to generate heterosis by crossing commercially available inbred lines should decrease over time [110]. Elite germplasm has been exploited in breeding programs for many years and, during this period, the majority of slightly deleterious alleles would have been expected to have been purged [111]. Models of heterosis relying entirely on the concept of dominance would predict that the potential for heterosis should also have decreased over the same period [112]. Tomato is an autogamous species which has a small load of deleterious recessive genes. Besides, the continuous selfing and selection of the desired genotypes in the breeding process resulted in inbred recombinant lines, with inbred vigor equal to or even higher than hybrid vigor [52]. This means that a large portion of the small pool of recessive deleterious alleles has been replaced by a desirable additive. It would be expected that, according to the dominance model, the heterosis recorded after crossing the recombinant lines would be minimal. However, as the results of this study showed, the elite genotypes of recombinant inbred lines became the parents of elite restructured hybrids, with increased levels of re-heterosis for all characters measured. This may prove that dominance is not the only case in explaining heterosis in tomato for yield components and fruit quality characteristics.
5. Conclusions
Breeding under low input conditions could lead to tomato cultivars suitable for organic and low input agriculture. In our study, several recombinant lines, and most of the new reconstructed F1 hybrids, showed excellent productivity under a low input farming system.
In terms of heterosis, reconstructed hybrids displayed high rates, indicating the possibility of creating elite F1 hybrids using improved recombinant lines. Entries originating from hybrids, which indicated low inbreeding depression at the early stages of selection, produced superior hybrids with greater heterosis (re-heterosis), possessing a high combining ability.
The ability to produce inbred recombinant lines with similar performance to the original hybrids indicates the possible removal of deleterious genes and their replacement with favorable additive alleles. However, the extent of re-heterosis generated in this breeding program when elite recombinant lines were used as parents was not reduced, suggesting that heterosis is more than a simple complementation of deleterious recessive alleles by dominant ones.
In general, through the overall breeding effort, it was possible to create new recombinant lines from F1 hybrids with low inbreeding depression at the early stages of selection, and, afterwards, to produce new reconstructed F1 hybrids using the best recombinant line combinations as parents. The evaluation and selection of the different types of cultivars (recombinant pure lines or reconstructed hybrids) under low input conditions could point towards the most suitable/ideal genotype for organic cultivation.
Author Contributions
Conceptualization, I.D.A. and A.G.M.; data curation, I.D.A., G.-M.N., A.A., R.T., I.M., I.N.X., F.P., P.K. and A.G.M.; formal analysis, I.D.A., A.A., R.T., I.M. and I.N.X.; investigation, I.D.A., G.-M.N., A.A., R.T. and A.G.M.; methodology, I.D.A. and A.G.M.; project administration, I.D.A. and A.G.M.; software, I.D.A. and I.M.; supervision, I.D.A. and A.G.M.; validation, I.D.A. and G.-M.N.; visualization, I.D.A.; writing—original draft, I.D.A., R.T., I.M., I.N.X., F.P., P.K. and A.G.M.; writing—review and editing, I.D.A., I.M., I.N.X., F.P., P.K. and A.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Birchler, J.A.; Yao, H.; Chudalayandi, S.; Vaiman, D.; Veitia, R.A. Heterosis. Plant Cell. 2010, 22, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, B.; Georgiev, H. Expression of Heterosis by Hybridization. In Genetic Improvement of Solanaceous, 1st ed.; Razdan, M.K., Mattoo, A.K., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Volume 2, pp. 113–151. [Google Scholar] [CrossRef]
- Timberlake, W.E. Heterosis. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 3, ISBN 9780080961569. [Google Scholar]
- Tsaftaris, A.S. Molecular aspects of heterosis in plants. Physiol. Plant 1995, 94, 362–370. [Google Scholar] [CrossRef]
- Goff, S.A. A unifying theory for general multigenic heterosis: Energy efficiency, protein metabolism, and implications for molecular breeding. N. Phytol. 2011, 189, 923–937. [Google Scholar] [CrossRef]
- Jinks, J.L. The analysis of continuous variation in a diallel cross of Nicotiana rustica varieties. Genetics 1954, 39, 767–788. [Google Scholar] [CrossRef]
- Stuber, C.W. Biochemistry, molecular biology and physiology of heterosis. In Genetics and Exploitation of Heterosis in Crops, 1st ed.; Coors, J.G., Pandey, S., Eds.; American Society of Agronomy Inc.: Madison, WI, USA, 1999; pp. 173–183. [Google Scholar] [CrossRef]
- Monforte, A.J.; Tanksley, S.D. Fine mapping of a quantitative trait loci (QTL) from Lycopersicon hirsutum chromosome 1 affecting fruit characteristics and agronomic traits: Breaking linkage among QTLs affecting different traits and dissection of heterosis for yield. Theor. Appl. Genet. 2000, 100, 471–479. [Google Scholar] [CrossRef]
- Duvick, D.N. Heterosis: Feeding people and protecting natural resources. In Book of Abstracts of the International Symposium, Proceedings of the Genetics and Exploitation of Heterosis in Crop, Mexico City, Mexico, 17–22 August 1997; International Maize and Wheat Improvement Center: El Batán, Mexic, 1997; ISBN 968-6923-90-X. [Google Scholar]
- Powers, L. Relative yields of inbred lines and F1 hybrids of tomato. Bot. Gaz. 1945, 106, 247–268. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Kumar, S.; Singh, M.; Banerjee, M.K.; Rai, M. Hybrid Seed Production of Solanaceous Vegetables. In A Practical Manual; Central Board of Secondary Education: Delhi, India, 2003; Volume 9, pp. 1–34. [Google Scholar]
- Kumar, S.; Banerjee, M.K.; Partap, P.S. Studies on heterosis for various characters in tomato. Haryana J. Hort. Sci. 1995, 24, 54–60. [Google Scholar]
- Hannan, M.M.; Ahmed, M.B.; Razvy, M.A.; Karim, R.; Khatun, M.; Haydar, A.; Hossain, M.; Roy, U.K. Heterosis and correlation of yield and yield components in Tomato (Lycopersicon esulentum Mill.). Am. Eurasian J. Sci. Res. 2007, 2, 146–150. [Google Scholar]
- Kurian, A.; Peter, K.V.; Rajan, S. Heterosis for yield components and fruit characters in tomato. J. Trop. Agric. 2001, 39, 5–8. [Google Scholar]
- Ahmad, S.; Quamruzzaman, A.K.M.; Islam, M.R. Estimation of heterosis in tomato (Solanum lycopersicum L.). Bangladesh J. Agril. Res. 2011, 36, 521–527. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, M.K. Exploitation of heterosis for yield and its contributing traits in tomato, Solanum lycopersicum L. Int. J. Farm Sci. 2011, 1, 45–55. [Google Scholar]
- Hussien, A.H. Combining ability, heterosis and path coefficient analyses for yield and its components in tomato. Egypt. J. Plant Breed. 2014, 18, 737–753. [Google Scholar] [CrossRef]
- Shankar, A.; Reddy, R.V.S.K.; Sujatha, M.; Pratap, M. Development of superior F1 hybrids for com-mercial exploitation in tomato (Solanum lycopersicum L.). Int. J. Farm Sci. 2014, 4, 58–69. [Google Scholar]
- Dundi, K.B.; Madalageri, B.B. Heterosis for shelf life and its components in tomato (Lycoperscion esculentum Mill). South Indian Hortic. 1991, 39, 353–355. [Google Scholar]
- Naorem, B.S.; Shabir, H.W.; Haribhushan, A.; Rita, N. Heterosis studies for yield and its components in tomato (Solanum lycopersicum L.) under valley conditions of Manipur. LS Int. J. Life Sci. 2012, 25, 257–265. [Google Scholar] [CrossRef]
- Kalloo, G. Vegetable Breeding; CRC Press: Boca Raton, FL, USA, 1988; Volume 1, p. 239. [Google Scholar]
- Patil, A.A.; Patil, S.S. Heterosis for some quality attributes in tomato. J. Maharashtra Agric. Univ. 1988, 13, 241. [Google Scholar]
- Daskaloff, C.; Konstantinova, M.; Molle, E.; Baralieva, D. Genetic Studies on Tomato Quality, 1st ed.; Bulg. Academy of Sciences Press: Sofia, Bulgaria, 1990. [Google Scholar]
- Dod, V.N.; Kale, P.B. Heterosis for certain quality traits in tomato Lycopersicon esculentum Mill. Crop Res. 1992, 5, 302–308. [Google Scholar]
- Cuartero, J.; Cubero, J.I. Genetics of four economically important characters in tomato (Lycopersicon esculentum, Mill.). Z. Pflanzenziicht. 1981, 87, 330–338. [Google Scholar]
- Burdick, A. Genetics of heterosis for earliness in the tomato. Genetics 1954, 39, 488–505. [Google Scholar] [CrossRef]
- Banerjee, M.K.; Kalloo, G. The inheritance of earliness and fruit weight in crosses between cultivated tomatoes and two wild species of Lycopersicon. Plant Breed. 1989, 102, 140–152. [Google Scholar] [CrossRef]
- Kemble, J.M.; Gardner, R.G. Inheritance of shortened fruit maturation in the cherry tomato Cornell 871213-1 and its relation to fruit size and other components of earliness. J. Am. Soc. Hort. Sci. 1992, 117, 646–650. [Google Scholar] [CrossRef]
- Saidi, M.; Warade, S.D.; Prabu, T. Combining ability estimates for yield and its contributing traits in tomato (Lycopersicon esculentum). Int. J. Agri. Biol. 2008, 10, 238–240, Record Number: 20083140765. [Google Scholar]
- Saleem, M.Y.; Asghar, M.; Iqbal, Q.; Rahman, A.; Akram, M. Diallel analysis of yield and some yield components in tomato (Solanum lycopersicum L.). Pak. J. Bot. 2013, 45, 1247–1250, Record Number: 20173252960. [Google Scholar]
- Aisyah, S.I.; Wahyuni, S.; Syukur, M.; Witono, J.R. The estimation of combining ability and heterosis effect for yield and yield components in tomato (Solanum lycopersicum Mill.) at Lowland. Ekin J. Crop Breed. Genet. 2016, 2, 23–29, Record Number: 20173252960. [Google Scholar]
- Dobhal, V.K.; Kohli, U.K.; Mehta, D. Genetic analysis of fruit firmness and related traits in tomato. J. Hill Res. 1999, 12, 31–33. [Google Scholar]
- Singh, S.; Dhaliwal, M.S.; Cheema, D.S.; Brar, G.S. Diallel analysis of some processing attributes in tomato. J. Genet. Breed. 1998, 52, 265–269. [Google Scholar]
- Kärkkäinen, K.; Kuittinen, H.; Van Treuren, R.; Vogl, C.; Oikarinen, S.; Savolainen, O. Genetic basis of inbreeding depression in Arabis petrea. Evolution 1999, 53, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.H. Inbreeding load, average dominance and the mutation rate for mildly deleterious alleles in Mimulus guttatus. Genetics 1999, 153, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Schemske, D.W.; Schultz, S.T. High inbreeding depression, selective interference among loci, and the threshold selfing rate for purging recessive lethal mutations. Evolution 1994, 48, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.F.; Kimura, M. The Theory of Genetic Loads. In Proceedings of the XI International Congress on Genetics, Hague, The Netherlands, September 1963; Volume 3, pp. 495–506. [Google Scholar]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman Group Ltd.: Harlow, UK, 1996. [Google Scholar]
- Charlesworth, B.; Charlesworth, D. The genetic basis of inbreeding depression. Genet. Res. 1999, 74, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Fasoulas, A.C. The Honeycomb Methodology of Plant Breeding; Deptartment of Genetics and Plant Breeding, Aristotelian University of Thessaloniki: Thessaloniki, Greece, 1988; pp. 42–49. [Google Scholar]
- Schemske, D.W.; Lande, R. The evolution of self- fertilization and inbreeding depression in plants. II. Empirical observations. Evolution 1985, 39, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.F. The rise and fall of overdominance. Plant Breed. Rev. 2000, 17, 225–257, Record Number: 20001616424. [Google Scholar]
- Powers, L. Gene recombination and heterosis. In Heterosis; Gowen, J.M., Ed.; Iowa State College Press: Ames, IA, USA, 1952; pp. 298–316. [Google Scholar]
- Williams, W. Isolation of “pure lines” from F1 Hybrids of tomato and the problem of heterosis in breeding crop species. J. Agric. Sci. 1959, 53, 347–353. [Google Scholar] [CrossRef]
- Christakis, P.A.; Fasoulas, A.C. The recovery of recombinant inbreds outyielding the hybrid in tomato. J. Agric. Sci. 2001, 137, 179–183. [Google Scholar] [CrossRef]
- Koutsika-Sotiriou, M.S.; Traka-Mavrona, E.A.; Evgenidis, G.L. Assessment of tomato source breeding material through mating designs. J. Agric. Sci. 2008, 146, 301–310. [Google Scholar] [CrossRef]
- Fasoula, D.A.; Fasoula, V.A. Bridging the productivity gap between maize inbreds and hybrids by replacing gene and genome dichotomization with gene and genome integration. Maydica 2005, 50, 49–61. [Google Scholar]
- Christakis, P.A.; Fasoulas, A.C. The effects of the genotype by environmental interaction on the fixation of heterosis in tomato. J. Agric. Sci. 2002, 139, 55–60. [Google Scholar] [CrossRef]
- Cuartero, J.; Baguena, M.; Costa, J.; Diezm, J.; Nuez, F. The use of tomato hybrids or varieties. Acta Hort. 1986, 191, 347–354. [Google Scholar] [CrossRef]
- Bakalis, N. The Possibility to Fix Heterosis for Yield and Earliness in the Tomato Hybrid GC-204 (Lycopersicon esculentum Miller). Ph.D. Thesis, Department of Genetics and Plant Breeding, Aristotelian University of Thessaloniki, Thessaloniki, Greece, 1987; p. 23. [Google Scholar]
- Traka-Mavrona, E.; Fasoulas, A. Fixing hybrid superiority for yield and other trails in tomato. Agric Res. Cent. North. Greece Sci. Bull. 1987, 3, 9–22. [Google Scholar]
- Avdikos, I.D.; Tagiakas, R.; Mylonas, I.; Xynias, I.N.; Mavromatis, A.G. Assessment of Tomato Recombinant Lines in Conventional and Organic Farming Systems for Productivity and Fruit Quality Traits. Agronomy 2021, 11, 129. [Google Scholar] [CrossRef]
- Scott, A.J.; Knott, M. A cluster analysis method for grouping means in the analysis of variance. Biometrics 1974, 30, 507–512. [Google Scholar] [CrossRef]
- Meredith, W.R.; Bridge, R.R. Heterosis and gene action in cotton. Gossypium Hirsute Crop Sci. 1972, 12, 304–310. [Google Scholar] [CrossRef]
- Gifi, A. Non-Linear Multivariate Analysis; John Willey & Sons Ltd.: Chichester, UK, 1990. [Google Scholar]
- Hair, J.; Black, W.; Babin, B.; Anderson, R. Multivariate Data Analysis: A Global Perspective, 7th ed.; Pearson Education Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Menexes, G.; Angelopoulos, S. Proposals for the financing and development of Greek farms based on a clustering method for categorical data. EuroMed J. Bus. 2008, 3, 263–285. [Google Scholar] [CrossRef]
- Sajid, A.; Riaz, K.A.; Mairaj, G.; Arif, M.; Fida, M.; Bibi, S. Assessment of different crop nutrient management practices for yield improvement. J. Crop Sci. 2008, 2, 150–157. [Google Scholar]
- Seufert, V.; Ramankutty, N.; Foley, J. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Ponti, T.; Rijk, B.; Van Ittersum, K. The crop yield gap between organic an conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Neto, J.; Regina, K.; Schwan-Estrada, F.; Ozinaldo, J.; de Sena, A.; Telles, T. Economic Viability of Tomato Cultivation in Organic Farming System. Braz. Arch. Biol. Technol. 2017, 60. [Google Scholar] [CrossRef]
- Vlachostergios, D.; Lithourgidis, A.; Korkovelos, A.; Baxevanos, D.; Lazaridou, T.; Khah, A.; Mavromatis, A. Mixing ability of conventionally bred common vetch (Vicia sativa L.) cultivars for grain yield under low-input cultivation. AJCS 2011, 5, 1588–1594. [Google Scholar]
- Van Bueren, E.T.L.; Jones, S.; Tamm, L.; Murphy, K.; Myers, J.; Leifert, C.; Messmer, M. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS Wagening. J. Life Sci. 2011, 58, 193–205. [Google Scholar] [CrossRef]
- Ceccarelli, S. Yield potential and drought tolerance of segregating populations of barley in contrasting environments. Euphytica 1987, 36, 265–273. [Google Scholar] [CrossRef]
- Lammerts van Bueren, E.T. Organic Plant Breeding and Propagation: Concepts and Strategies. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2002. [Google Scholar]
- Murphy, K.; Campbell, K.; Lyon, S.; Jones, S. Evidence of varietal adaptation to organic culture systems. Field Crop. Res. 2007, 102, 172–177. [Google Scholar] [CrossRef]
- Khan, A.; Jindal, S.K. Exploiting yield potential in tomato (Solanum lycopersicum L.) through heterosis breeding. Plant Gene Trait. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Grandillo, S.; Ku, H.M.; Tanksley, S.D. Identifying the loci responsible for natural variation in fruit size and shape in tomato. Theor. Appl. Genet. 1999, 99, 978–987. [Google Scholar] [CrossRef]
- Dagade, S.B.; Barad, A.V.; Dhaduk, L.K. Studies on Hybrid Vigour in F1 and Its Retention in F2 for Fruit Firmness and Related Traits in Tomato. Int. J. Appl. Biol. Pharm. 2015, 6, 193–198. [Google Scholar]
- Kumar, P.; Bora, L.; Kumar, S.M. Heterotic studies for yield and quality traits in tomato (Lycopersicon esculentum Mill.). J. Pharm. Phytochem. 2019, 8, 1370–1375. [Google Scholar]
- Patel, U.J.; Kathiria, K.B.; Patel, J.S.; Saiyad, I.M. Heterobeltiosis and inbreeding depression in tomato (Lycopersicon esculentum Mill.). Int. J. Plant Sci. 2010, 5, 636–638. [Google Scholar]
- Cheema, D.S.; Dhaliwal, M.S. Hybrid Tomato Breeding. J. N. Seeds 2008, 6, 1–14. [Google Scholar] [CrossRef]
- Rehana, S.; Ullah, M.Z.; Zeba, N.; Narzis, N.; Husna, A.; Siddique, A.B. Estimation of heterosis for yield and yield attributing traits in tomato crossed with line and tester method. Prog. Agric. 2019, 30, 179–185. [Google Scholar] [CrossRef]
- Avdikos, I.D.; Tsivelika, N.; Gallidou, A.; Koutsika-Sotiriou, M.; Traka-Mavrona, E. Exploitation of heterosis through recurrent selection scheme applied in segregating generations of a tomato breeding program. Sci. Hortic. 2011, 130, 701–707. [Google Scholar] [CrossRef]
- Avdikos, E. Selection Criteria for Tomato Hybrids as Source of Recombinant Lines. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2013. [Google Scholar]
- Fasoula, V.A.; Fasoula, D.A. Principles underlying genetic improvement for high and stable crop yield potential. Field Crop. Res. 2002, 75, 191–209. [Google Scholar] [CrossRef]
- Syukur, M.; Sujiprihati, S.; dan Yunianti, R. Teknik Pemuliaan Tanaman, 1st ed.; Penebar Swadaya: Jakarta, Indonesia, 2012. [Google Scholar]
- Sharma, K.C.; Verma, S.; Pathak, S. Combining ability effects and components of genetic variation in tomato (Lycopersicon esculentum Mill). Indian J. Agric. Sci. 2002, 72, 496–497. [Google Scholar]
- Kumaravel, S.; Parimala, K.; Sekar, K. Combining ability studies in tomato (Lycopersicon esculentum Mill.). Int. J. Trop. Agric. 2003, 21, 21–27. [Google Scholar]
- Hosamani, R.M. Biometrical and Transformation Studies in Tomato (Solanum lycopersicum L.). Ph.D. Thesis, University of Agricultural Sciences, Dharwad, Karnataka, India, 2010. [Google Scholar]
- El-Gabry, M.A.H.; Solieman, T.I.H.; Abido, A.I.A. Combining ability and heritability of some tomato (Solanum lycopersicum L) cultivars. Sci. Hort. 2014, 164, 153–157. [Google Scholar] [CrossRef]
- Rongaa, D.; Franciaa, E.; Rizzab, F.; Badeckb, F.W.; Montevecchia, F.C.G.; Pecchionica, N. Changes in yield components, morphological, physiological and fruit quality traits in processing tomato cultivated in Italy since the 1930′s. Sci. Hortic. 2019, 257, 108726. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, B.; Feng, Y.; Li, P. Development and application of marker-assisted reverse breeding using hybrid maize germplasm. J. Integr. Agric. 2015, 14, 2538–2546. [Google Scholar] [CrossRef][Green Version]
- Solieman, T.; El-Gabry, M.; Abido, A.I. Heterosis, potence ratio and correlation of some important characters in Tomato (Solanum lycopersicum L.). Sci. Hort. 2013, 150, 25–30. [Google Scholar] [CrossRef]
- Rasheed, A.; Shahoor, A.; Ghulam, M.W.; Abdul, M.S.; Muhammad, A.; Hira, K.; Ayaz, A.K.; Abdul, Q.; Israr, A. Estimation of hybrid vigor for yield and yield related traits in tomato (Solanum lycopersicon Mill). Int. J. Biosci. 2018, 12, 160–167. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kholi, U.K.; Joshi, A. Evaluation of diallel progeny and heterosis for yield and yield components in tomato (Lycopersicon esculentum Mill). Haryana J. Hort. Sci. 2004, 33, 106–108. [Google Scholar]
- Premalakshme, V.; Thangaraj, T.; Veeraragavathatham, D.; Arumugam, T. Heterosis and combining ability in tomato. Veg. Sci. 2005, 32, 47–50. [Google Scholar]
- Harer, P.N.; Kulkarni, R.V.; Deeptashri, B. Heterosis for yield components, TSS and ascorbic acid contents in tomato (Lycopersicon esculentum Mill.). Res. Crop. 2006, 7, 270–274. [Google Scholar]
- Sharma, D.; Thakur, M.C. Evaluation of diallel progenies for yield and its contributing traits in tomato under mid hill conditions. Indian J. Hortic. 2008, 65, 297–301. [Google Scholar]
- Fageria, M.S.; Kohli, U.K.; Dhaka, R.S. Studies on heterobeltiosis for fruit yield and yield attributing traits in tomato (Lycopersicon esculentum Mill.). Haryana J. Hort. Sci. 2001, 30, 131–133. [Google Scholar]
- Singh, A.K.; Pandey, R.S.; Rai, M. Heterosis for fruit yield and its components in tomato (Solanum lycopersicon Mill.). Veg. Sci. 2007, 34, 108–111. [Google Scholar]
- Legon, M.C.; Diaz, N.; Pereg, G.G. Performance of tomato hybrids and their parents in summer. Cent. Agric. 1984, 11, 35–44. [Google Scholar]
- Jamwal, R.S.; Pattan, R.S.; Saini, S.S. Hybrid vigour and combining ability in tomato. South Indian Hort. 1984, 32, 69–74. [Google Scholar]
- Mirshamssi, A.; Farsi, M.; Shahriari, F.; Nemati, H. Estimation of heterosis and combining ability for yield components and crossing method. Agric. Sci. Technol. 2006, 20, 3–12. [Google Scholar]
- Rani, I.; Veeraragavathatham, D.; Sanjutha, D. Studies on correlation and path coefficient analysis on yield attributes in root knot nematodes resistant F1 hybrids of tomato. J. Appl. Sci. Res. 2008, 4, 287–295. [Google Scholar]
- Ghadage, N.C.; Kulkarni, G.U.; Meta, H.R.; Raju, S. Heterosis Studies for Fruit Yield and its Components in Tomato (Solanum lycopersicum L.). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1011–1020. [Google Scholar] [CrossRef]
- Mondal, C.; Sarkar, S.; Hazra, P. Line × Tester analysis of combining ability in tomato (Lycopersicon esculentum Mill.). J. Crop. Weed 2009, 5, 53–57, Record Number: 20093248215. [Google Scholar]
- Savale, S.V.; Patel, A.I.; Sante, P.R. Study of heterosis over environments in tomato (Solanum lycopersicum L.). Int. J. Chem. Stud. 2017, 5, 284–289. [Google Scholar]
- Tamata, S.; Singh, J.P. Heterosis in Tomato for Growth and Yield Traits. Int. J. Veg. Sci. 2017, 24, 169–179. [Google Scholar] [CrossRef]
- Gautam, N.; Kumar, M.; Sandeep, K.; Sharma, S. Heterosis studies for yield and its components in tomato (Solanum lycopersicum L.) under North Western Himalayan Region. India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1949–1957. [Google Scholar] [CrossRef]
- Kattegoudar, J.; Lingaiah, H.B.; Mamtha, N.C.; Arunkumar, B. Heterosis studies for yield and yield attributing characters of tomato (Solanum lycopersicum L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1073–1080. [Google Scholar] [CrossRef]
- Mohammad, I.A. Line×Tester Analysis of Heterosis and Combining Ability in Tomato (Lycopersicon esculentum Mill.) Fruit Quality Traits. Pak. J. Biol. Sci. 2018, 21, 224–231. [Google Scholar] [CrossRef]
- Cheniclet, C.; Rong, W.Y.; Causse, M.; Frangne, N.; Bolling, L.; Carde, J.P.; Renaudin, J.P. Cell Expansion and Endoreduplication Show a Large Genetic Variability in Pericarp and Contribute Strongly to Tomato Fruit Growth. Plant. Physiol. 2005, 139, 1984–1994. [Google Scholar] [CrossRef]
- Joshi, A.; Thakur, M.C.; Kohli, U.K. Heterosis and combining ability for shelf life, whole fruit firmness and related traits in tomato. Indian J. Hort. 2005, 62, 33–36. [Google Scholar]
- Rattan, P. Line × Tester Analysis Involving Bacterial Wilt Resistant Genotypes Across Environments in Tomato (Lycopersicon esculentum Mill.). Ph.D. Thesis, Department of Vegetable Science and Floriculture, Csk Himachal Pradesh Agriculture University Palampur, Pradesh, India, 2007; p. 248. [Google Scholar]
- Garg, N.; Cheema, D.S.; Chawla, N. Manifestation of heterosis for fruit yield, alleles in main- and late-seasons of north Indian plains quality and shelf-life in Tomato (Solanum lycopersicum L.) hybrids incorporating rin, nor or alc. Veg. Sci. 2013, 40, 28–33. [Google Scholar]
- Sureshkumara, B.; Lingaiah, H.B.; Shivapriya, M.; Pavithra, H.B. Evaluation of tomato genotypes for growth, yield and quality attributes under eastern dry zone of Karnataka, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1922–1930. [Google Scholar] [CrossRef]
- Reddy, G.; Eswara, R.; Nandan, A.; Vaishampayan, A.; Srivastava, K. Heterosis and genetic analysis for fruit quality traits in tomato. Progr. Res. Int. J. 2016, 11, 1210–1213. [Google Scholar]
- Tanksley, S.D. The genetic, developmental and molecular bases of fruit size and shape variation in tomato. Plant Cell. 2004, 16, S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Springer, N.M.; Stupar, R.M. Allelic variation and heterosis in maize: How do two halves make more than a whole? Genome Res. 2007, 17, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Duvick, D.N. Biotechnology in the 1930s: The development of hybrid maize. Nat. Rev. Genet. 2001, 2, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Birchler, J.A.; Auger, D.L.; Riddle, N.C. In search of the molecular basis of heterosis. Plant Cell. 2003, 15, 2236–2239. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).